SUMMARY
We report the implantation of patient-derived midbrain dopaminergic progenitor cells, differentiated in vitro from autologous induced pluripotent stem cells (iPSCs), in a patient with idiopathic Parkinson’s disease. The patient-specific progenitor cells were produced under Good Manufacturing Practice conditions and characterized as having the phenotypic properties of substantia nigra pars compacta neurons; testing in a humanized mouse model (involving peripheral-blood mononuclear cells) indicated an absence of immunogenicity to these cells. The cells were implanted into the putamen (left hemisphere followed by right hemisphere, 6 months apart) of a patient with Parkinson’s disease, without the need for immunosuppression. Positron-emission tomography with the use of fluorine-18-L-dihydroxyphenylalanine suggested graft survival. Clinical measures of symptoms of Parkinson’s disease after surgery stabilized or improved at 18 to 24 months after implantation. (Funded by the National Institutes of Health and others.)
TISSUE TRANSPLANTATION IN PATIENTS WITH PARKINSON’S DISEASE TO replace the lost dopaminergic neurons of the substantia nigra pars compacta has been studied since the 1980s. Sources of these cells have included adrenal medulla1,2 and fetal midbrain tissues from allogeneic or xenogeneic sources.3–7 Inconsistent symptomatic benefit of various durations has been reported with these approaches.8–11 Fetal cell-based therapies have been considered impractical because of ethical constraints, difficulty in producing a defined cell product, and graft-induced dyskinesias, which may be caused by contaminating serotonergic neurons, excess dopamine production, or both.8,12 Pluripotent stem cell-derived midbrain dopaminergic progenitor cells (mDAPs) represent a possible cell source that can be produced with greater consistency.13,14 Advances in the technology for creating induced pluripotent stem cells (iPSCs) and for guiding their in vitro differentiation toward a midbrain dopaminergic fate offer the hope of achieving the benefits of fetal-tissue transplantation without its inherent practical and ethical concerns.15–17 An autologous cell source may additionally obviate the need for immunosuppression. We report data from a patient treated by our group with this method; no other patients were similarly treated.
METHODS
OVERSIGHT
Informed consent included a discussion of the risks associated with first-in-human use of this method in Parkinson’s disease, with a review of currently available medical and surgical therapeutic options, including deep-brain stimulation. The study was conducted under regulatory guidance from the Food and Drug Administration (FDA) under Individual Patient Expanded Access–Investigational New Drug application 17145. Approval was obtained from institutional review boards at Weill Cornell Medical Center and at Massachusetts General Hospital. All procedures in animals were conducted with approval from the McLean Hospital Animal Care and Use Committee. All the authors vouch for the completeness and accuracy of the data reported, including adverse events, and for the adherence of the study to the protocol, available with the full text of this article at NEJM.org. There was no industry involvement in the study.
CREATION OF AUTOLOGOUS IPSCS
Fibroblasts that were harvested from a skin biopsy were used to generate multiple iPSC lines that were tested for pluripotent differentiation potential in vitro and in vivo and screened by whole-exome sequencing for the presence of protein-coding mutations.18 A single iPSC clone (designated C4) was selected for further characterization and for production of mDAPs under Good Manufacturing Practice conditions.
CHARACTERIZATION OF IN VITRO DIFFERENTIATED MDAPS
The C4 iPSC-derived cells showed normal karyotype and were characterized as mDAPs with dopamine neuron–specific and other neural markers, in two validation experiments.18 Whole-genome sequencing of both the C4 iPSCs and C4-derived progenitors was performed, and the progenitors were compared with the original source fibroblasts; the results confirmed the absence in the progenitors of known cancer-associated and neurodegeneration-associated mutations (Fig. S1 and Tables S1 and S2 in the Supplementary Appendix, available at NEJM.org). Before clinical use, neurons that were derived from these progenitor cells showed dopamine secretion and electrophysiological properties in vitro characteristic of substantia nigra pars compacta dopaminergic neurons, showed functional efficacy in animal models similar to that of fetal midbrain-derived tissue,18 and passed FDA-mandated release criteria (Figs. S2 through S4 and Tables S4 through S9). After treatment with quercetin, the final cell products (at day 28) had no detectable remaining undifferentiated iPSCs (with an upper boundary of the 95% confidence interval of ≤1 undifferentiated cell per 1 billion day-28 differentiated cells),18 on the basis of immunostaining and real-time polymerase chain reaction-based assays. Serotonergic neurons, a potential cause of graft-induced dyskinesia,12 were not detected in the final product (Figs. S3 and S4).
GRAFT SURVIVAL UNDER AUTOLOGOUS OR ALLOGENEIC CONDITIONS IN HUMANIZED MICE
Patient-derived iPSCs (C4) and allogeneic human embryonic stem cells (H9) were differentiated to day-28 mDAPs (C4-mDAPs and H9-mDAPs), and 1×105 cells of each line were grafted into the striatum of nonobese diabetic mice with severe combined immunodeficiency (NOD SCID) and depletion of the interleukin-2 receptor γ (NOD SCID gamma mice), patient-humanized NOD SCID gamma mice (C4-hu; using patient’s peripheral-blood mononuclear cells obtained 24 months [left hemisphere] and 18 months [right hemisphere] after surgery), and allogeneic humanized mice (K1-hu). Animals were killed at 2 weeks and examined histologically for graft survival by labeling for human neural-cell adhesion molecule (hNCAM+) cells, for the presence within the graft of neurons expressing markers for dopaminergic neurons (tyrosine hydroxylase [TH+] neurons), and for cellular immune response (CD4+ cells).
CLINICAL MEASURES
Neurologic examinations were performed and Parkinson’s disease-specific measures assessed at baseline and at 1, 3, 6, 9, and 12 months after each implantation and at 6-month intervals thereafter. At each examination, the neurologist recorded the “off” time, when medication did not adequately control motor symptoms, as reported by the patient. Prespecified assessments included the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), part III (scores range from 0 to 132, with higher scores indicating worse parkinsonian motor signs),19 and the 39-item Parkinson’s Disease Questionnaire (PDQ-39; scores range from 0 to 156, with higher scores indicating worse quality of life).20 (A complete list of clinical assessments is provided in Table S3.)
BRAIN IMAGING
Computed tomographic (CT) scans were performed intraoperatively to confirm accurate placement of the cell injection in the putamen and immediately postoperatively for hemorrhage screening at or near the site of implantation. Serial magnetic resonance imaging (MRI) scans and magnetic resonance spectroscopic findings were reviewed for any evidence of tumor, stroke, or hemorrhage. Serial fluorine-18-L-dihydroxyphenylalanine (18F-DOPA) positron-emission tomography (PET)-CT was performed to assess for the presence of presynaptic dopamine terminal activity in the engrafted putaminal regions. Changes in radioisotope uptake were judged semiquantitatively by 18F-DOPA standardized uptake value ratios.
SAFETY MONITORING
Serial clinical neurologic examination to detect adverse neurologic events, along with imaging reviews, was performed by two study neurologists and a study radiologist (all of whom are authors). The patient continued to be treated independently by an author who is his community neurologist.
CASE REPORT
The patient was a 69-year-old right-handed man with a 10-year history of progressive idiopathic Parkinson’s disease. He was receiving extended-release carbidopa-levodopa (in capsules containing 23.75 mg and 95 mg, respectively, at a dose of three capsules four times daily), rotigotine (4 mg daily), and rasagiline (1 mg daily) (total daily dose, 904 mg of levodopa equivalents). He reported poor control of his symptoms, with 3 hours of “off” time per day, characterized by worsening tremor, posture, and fine motor control; increases in the levodopa dose beyond these doses caused orthostatic hypotension. The patient had not had dyskinesias.
The patient underwent two MRI-guided stereotactic surgical procedures for implantation in the putamen, left hemisphere followed by right hemisphere, separated by 6 months, conforming to regulatory guidance from the FDA. At each operation, three trajectories were made in the putamen posterior to the level of the anterior commissure, each spanning the superior–inferior extent of the nucleus.21 A total of 4 million cells were delivered at each operative procedure, divided equally among the three tracks. Intravenous cefazolin was administered perioperatively. No immunosuppressants, glucocorticoids, or anticonvulsants were used at any time. After each surgery, the patient was monitored overnight and discharged the following day.
RESULTS
IMMUNOGENICITY OF GRAFTS AFTER IMPLANTATION IN HUMANIZED MICE
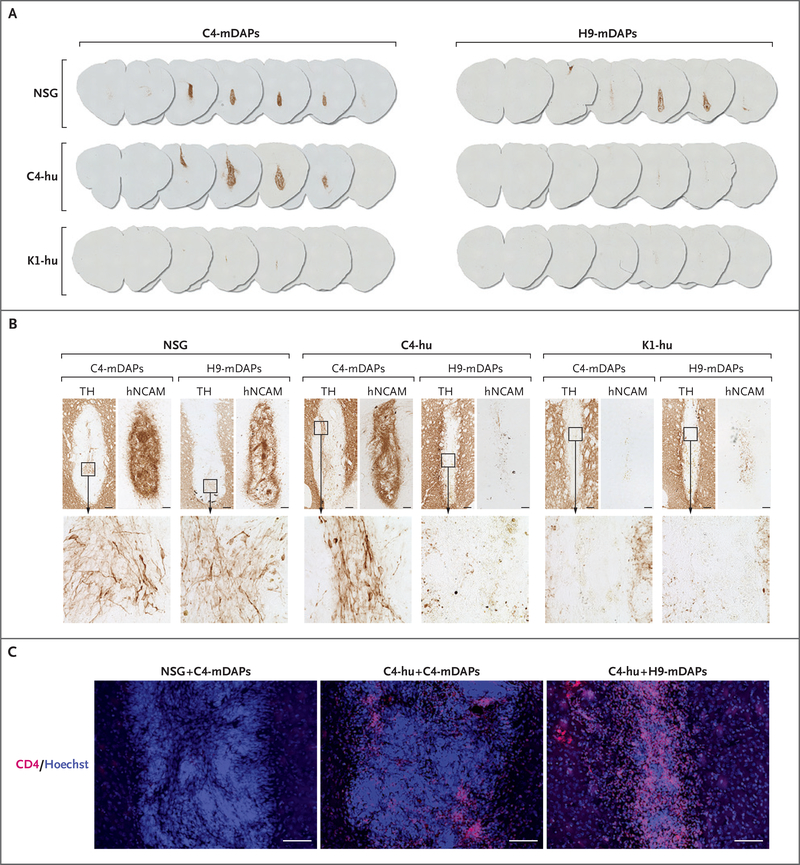
As shown in Figure 1A and Figure S5, both patient-derived mDAPs (C4-mDAPs) and allogeneic mDAPs (H9-mDAPs) survived in NOD SCID gamma mice, and both graft types were rejected when transplanted to allogeneic humanized mice (K1-hu). Patient-humanized mice (C4-hu) permitted the survival of autologous C4-mDAPs, with grafts staining positively for hNCAM+ cells and containing TH+ neurons at 2 weeks after implantation, whereas C4-hu mice rejected allogeneic H9-mDAPs, with prominent CD4+ lymphocytic infiltrates (Fig. 1B and 1C).
Figure 1. Immunogenicity of Midbrain Dopaminergic Progenitor Cells (mDAPs) in Humanized Mice.
Panels A and B show mouse brain sections stained with antibodies against human neural-cell adhesion molecule (hNCAM) (Panel A) and tyrosine hydroxylase (TH) with hNCAM (Panel B) at 2 weeks after transplantation of autologous and allogenic mDAPs, to detect the presence and dopaminergic differentiation of surviving grafts. In Panel B, the bottom row of images depicts magnified areas of the TH images. Panel C shows staining with anti-CD4 antibodies, with major cell loss and T-cell infiltration only in the allogeneic grafts placed in the patient-humanized animals. Cells were counterstained with Hoechst 33342. All scale bars indicate 100 μm. C4-hu denotes an NSG mouse (nonobese diabetic mouse with severe combined immunodeficiency and depletion of the interleukin-2 receptor γ) humanized with patient-derived peripheral-blood mononuclear cells, C4-mDAPs patient-derived mDAPs, H9-mDAPs human embryonic cell line–derived mDAPs, and K1-hu an NSG mouse humanized with volunteer-derived peripheral-blood mononuclear cells.
IMAGING AT 0 TO 24 MONTHS AFTER IMPLANTATION IN THE PATIENT
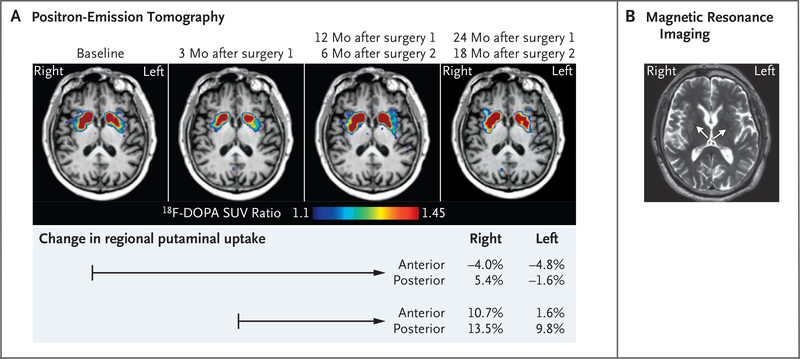
At 3 months after the first implantation, 18F-DOPA PET-CT imaging showed an initial decline in 18F-DOPA uptake from baseline in the putamina, followed by small increases in 18F-DOPA uptake at subsequent times up to 18 months and 24 months after implantations on the right side and left side, respectively. Increased activity was greater on the right (second implant) than on the left and was most prominent in the posterior putamen near the graft sites, as seen on the color intensity scale and on quantitative comparisons of selected subregions (Fig. 2A and Fig. S6). Semiquantitative changes from baseline in uptake of the radioisotope are shown in Figure 2 and varied from −4.0% to 13.5% on the right and from −4.8% to 9.8% on the left.
Figure 2. Imaging.
Panel A shows axial fluorine-18-L-dihydroxyphenylalanine (18F-DOPA) positron-emission tomographic images at the level of the basal ganglia at the time points indicated: baseline (4 months before first surgery), 3 months after the left implantation, 12 months after the left implantation and 6 months after the right implantation, and 24 months after the left implantation and 18 months after the right implantation. A decrease in 18F-DOPA uptake 3 months after the initial left implantation was followed by a modest increase in dopamine uptake on both sides (right greater than left), mainly in the posterior putamen near the graft sites. The table below the images shows the percentage change in 18F-DOPA uptake (by standardized uptake value [SUV] ratio) with respect to baseline before surgery or to the lowest uptake measured 3 months after the first (left side) implantation, as indicated by the timeline arrows. Anterior and posterior denote the position of the measured region of interest within the putamen. Panel B is a T2-weighted BLADE magnetic resonance image at 18 months after the left implantation and 12 months after the right implantation. Arrows indicate the location of the implants. Hyperintensity on T2-weighted imaging was associated with the graft sites, more prominently on the right.
MRI at 6 months after the first implantation and subsequent time points showed areas of increased T2-weighted signal intensity approximating the locations of the graft sites in the putamina, as well as along parts of the surgical tracts within the white matter, more pronounced on the right (Fig. 2B and Fig. S7). No contrast enhancement was seen at the six putaminal implantation sites. At 6 months after the second surgery, a 4-mm region of enhancement was observed 3 cm above the target in one tract; CT and MRI including arterial spin labeling magnetic resonance perfusion imaging and magnetic resonance spectroscopy showed changes consistent with postsurgical gliosis (Fig. S7).
CLINICAL ASSESSMENTS
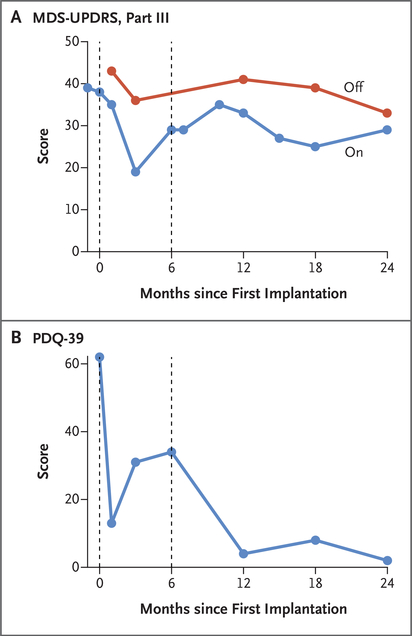
At 24 months after the first (left) implantation and 18 months after the second (right) implantation, the patient reported no adverse events or decline in function. Scores on the MDS-UPDRS, part III (assessing parkinsonian motor signs), after overnight withdrawal of dopamine replacement therapy (“off”) were not measured before the first implantation because the patient declined to cease medications owing to worsened symptoms. Scores in the off period were 43 at 4 weeks after the first implantation, 33 to 41 at subsequent follow-up times, and 33 at 24 months. Scores on the MDS-UPDRS, part III, at the peak dose of dopamine replacement therapy (“on”) were 38 at the time of implantation, 19 to 35 during follow-up, and 29 at 24 months. PDQ-39 scores (assessing Parkinson’s disease–related quality of life, with lower scores indicating better quality) were 62 at the time of implantation, 2 to 34 during follow-up, and 2 at 24 months (Fig. 3 and Table S3).
Figure 3. Longitudinal Clinical Assessments of Parkinson’s Disease–Related Motor Function and Quality of Life.
Panel A shows scores on the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), part III. Scores (range, 0 to 132, with higher scores indicating worse parkinsonian motor signs) are shown both after overnight withdrawal of levodopa (“off”) and at the peak dose of levodopa (“on”). Panel B shows scores on the 39-item Parkinson’s Disease Questionnaire (PDQ-39) assessing quality of life. Scores range from 0 to 156, with higher scores indicating worse quality of life. In both panels, the dashed line at 6 months indicates the time of the second implantation.
At 24 months, the patient’s Parkinson’s disease medications were extended-release carbidopa-levodopa (in capsules containing 23.75 mg and 95 mg, respectively, at a dose of three, three, two, and three capsules four times daily), rotigotine (4 mg daily), rasagiline (1 mg daily), and droxidopa (100 mg daily) (total daily dose, 847 mg of levodopa equivalents); this represented a 6% decrease in levodopa equivalents as compared with before the implantations. He reported less than 1 hour of “off” time per day. Dyskinesias were not reported by the patient or observed during clinical examination — similar to their absence preoperatively.
DISCUSSION
We describe the application of a personalized cell-therapy strategy using autologous, iPSC-derived dopaminergic progenitor cells in a patient with Parkinson’s disease. Imaging suggested that the two iPSC-derived grafts were delivered to the target putaminal sites and survived for 24 months (left side) and 18 months (right side). Improvements in the 18F-DOPA PET signal were modest but were most prominent near the graft sites in the posterior putamen, the subregion in which decreased uptake is typical in Parkinson’s disease.22,23
The improvements in motor assessments and patient-rated symptom scales in our patient should be interpreted with caution, because both he and the raters were aware of the intervention and there were no control comparisons. Clinical changes appeared gradually during the 18 to 24 months after implantation, a time frame consistent with gradual reinnervation of the putamen by projections from dopaminergic neurons. During this time, the levodopa equivalent daily dose decreased by 6%, a reduction of uncertain clinical importance. Studies of fetal-tissue transplantation have suggested that conclusions about long-term graft survival and clinical effects may require longer follow-up than we report.10,11 At the dose of cells implanted, after 18 to 24 months of follow-up, we did not observe dyskinesias or other adverse neurologic effects. MRI, PET, and clinical improvements were all more prominently associated with the right (second) procedure than the left, possibly related to improved procedural efficiency with the second implantation, including a shorter time from cell harvesting to implantation (Fig. S8).
It is uncertain whether the immune system in the central nervous system would react differently to iPSC-derived autologous as compared with allogeneic mDAPs.24 One goal of autologous-cell therapy is to avoid the requirement for an indeterminate period of immunosuppression inherent in the use of nonautologous tissue. No immunosuppression was used in the course of this patient’s treatment, given the hypothesis that the autologous source of the original fibroblasts would yield an implantable cell product recognized as self. The graft-survival experiments in autologous as compared with allogeneic humanized mice reported here support this hypothesis. An autologous-cell approach is costlier and more labor intensive than the use of generally available, pre-characterized allogeneic cell lines, requiring production and safety testing for each use.
We report the production and implantation of iPSC-derived autologous dopaminergic progenitor cells in a patient with Parkinson’s disease, with clinical and imaging results suggesting possible benefit over a period 24 months. Further studies are warranted to address how this approach will perform in a variety of patients with diverse genetic backgrounds and disease phenotypes over a period longer than 24 months.
Supplementary Material
Acknowledgments
Supported by grants (K23NS099380, NS070577, NS084869, and OD024622) from the National Institutes of Health and by the philanthropic support of the Parkinson’s Cell Therapy Research Fund at McLean Hospital and Massachusetts General Hospital and the William and Elizabeth Sweet Endowed Professorship in Neuroscience at Harvard Medical School.
We thank present and past members of the molecular neurobiology laboratory (including Melissa Feitosa, Dabin Hwang, Yeahan Kim, Shibo Cao, Jacob W. Feldmann, María José Luna, and Jin Hyuk Jung) for their technical assistance; Philip E. Stieg of the Weill Cornell Brain and Spine Center at the New York-Presbyterian/Weill Cornell Medical Center and staff (Alyson Hignight, Mary O’Hehir, and Kristin Strybing); Blagovest Nikolov for his help in filing the application to the Cornell institutional review board; Stephen M. Kaminsky and Hyunmi Lee, directors of the Belfer Gene Therapy Core Facility; and the personnel of the Dana-Farber/Harvard Cancer Center Cell Manipulation Core Facility, especially Sarah Nikiforow, Hélène Nègre, and Laëtitia Pinte, for their technical and administrative help in setting up the cell production in accordance with Good Manufacturing Practice conditions.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Jeffrey S. Schweitzer, Department of Neurosurgery, Massachusetts General Hospital
Bin Song, Molecular Neurobiology Laboratory, McLean Hospital, Belmont Massachusetts
Todd M. Herrington, Department of Neurology, Massachusetts General Hospital
Tae-Yoon Park, Molecular Neurobiology Laboratory, McLean Hospital, Belmont Massachusetts
Nayeon Lee, Molecular Neurobiology Laboratory, McLean Hospital, Belmont Massachusetts
Sanghyeok Ko, Molecular Neurobiology Laboratory, McLean Hospital, Belmont Massachusetts
Jeha Jeon, Molecular Neurobiology Laboratory, McLean Hospital, Belmont Massachusetts
Young Cha, Molecular Neurobiology Laboratory, McLean Hospital, Belmont Massachusetts
Kyungsang Kim, Department of Radiology, Massachusetts General Hospital Gordon Center for Medical Imaging, Massachusetts General Hospital.
Quanzheng Li, Department of Radiology, Massachusetts General Hospital Gordon Center for Medical Imaging, Massachusetts General Hospital.
Claire Henchcliffe, Department of Neurology, Weill Cornell Medical College, New York
Michael Kaplitt, Department of Neurosurgery, Weill Cornell Medical College, New York
Carolyn Neff, Department of Neurology, Kaiser Permanente, Irvine, CA
Otto Rapalino, Division of Neuroradiology, Massachusetts General Hospital
Hyemyung Seo, Molecular Neurobiology Laboratory, McLean Hospital, Belmont Massachusetts Department of Molecular and Life Sciences, Hanyang University, Seoul, South Korea.
In-Hee Lee, Department of Pediatrics, Computational Health Informatics Program, Boston Children’s Hospital
Jisun Kim, Molecular Neurobiology Laboratory, McLean Hospital, Belmont Massachusetts
Taewoo Kim, Molecular Neurobiology Laboratory, McLean Hospital, Belmont Massachusetts
Gregory A. Petsko, Brain and Mind Research Institute, Weill Cornell Medical College, New York
Jerome Ritz, Connell and O’Reilly Families Cell Manipulation Core Facility, Dana-Farber/Harvard Cancer Center, Boston
Bruce M. Cohen, Department of Psychiatry, McLean Hospital, Belmont, Massachusetts
Sek-Won Kong, Department of Pediatrics, Computational Health Informatics Program, Boston Children’s Hospital
Pierre Leblanc, Molecular Neurobiology Laboratory, McLean Hospital, Belmont Massachusetts
Bob S. Carter, Department of Neurosurgery, Massachusetts General Hospital
Kwang-Soo Kim, Molecular Neurobiology Laboratory, McLean Hospital, Belmont Massachusetts
REFERENCES
- 1.Lindvall O, Backlund EO, Farde L, et al. Transplantation in Parkinson’s disease: two cases of adrenal medullary grafts to the putamen. Ann Neurol 1987;22:457–68. [DOI] [PubMed] [Google Scholar]
- 2.Goetz CG, Olanow CW, Koller WC, et al. Multicenter study of autologous adrenal medullary transplantation to the corpus striatum in patients with advanced Parkinson’s disease. N Engl J Med 1989;320:337–41. [DOI] [PubMed] [Google Scholar]
- 3.Lindvall O, Brundin P, Widner H, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science 1990;247:574–7. [DOI] [PubMed] [Google Scholar]
- 4.Freed CR, Breeze RE, Rosenberg NL, et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson’s disease. N Engl J Med 1992;327:1549–55. [DOI] [PubMed] [Google Scholar]
- 5.Spencer DD, Robbins RJ, Naftolin F, et al. Unilateral transplantation of human fetal mesencephalic tissue into the caudate nucleus of patients with Parkinson’s disease. N Engl J Med 1992;327:1541–8. [DOI] [PubMed] [Google Scholar]
- 6.Widner H, Tetrud J, Rehncrona S, et al. Bilateral fetal mesencephalic grafting in two patients with parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). N Engl J Med 1992;327:1556–63. [DOI] [PubMed] [Google Scholar]
- 7.Deacon T, Schumacher J, Dinsmore J, et al. Histological evidence of fetal pig neural cell survival after transplantation into a patient with Parkinson’s disease. Nat Med 1997;3:350–3. [DOI] [PubMed] [Google Scholar]
- 8.Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med 2001;344:710–9. [DOI] [PubMed] [Google Scholar]
- 9.Olanow CW, Goetz CG, Kordower JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol 2003;54:403–14. [DOI] [PubMed] [Google Scholar]
- 10.Kefalopoulou Z, Politis M, Piccini P, et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol 2014;71:83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Englund E, Widner H, et al. Extensive graft-derived dopaminergic innervation is maintained 24 years after transplantation in the degenerating parkinsonian brain. Proc Natl Acad Sci U S A 2016;113:6544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Politis M, Wu K, Loane C, et al. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci Transl Med 2010;2:38ra46. [DOI] [PubMed] [Google Scholar]
- 13.Lindvall O Clinical translation of stem cell transplantation in Parkinson’s disease. J Intern Med 2016;279:30–40. [DOI] [PubMed] [Google Scholar]
- 14.Barker RA, Drouin-Ouellet J, Parmar M. Cell-based therapies for Parkinson disease — past insights and future potential. Nat Rev Neurol 2015;11:492–503. [DOI] [PubMed] [Google Scholar]
- 15.Sonntag KC, Song B, Lee N, et al. Pluripotent stem cell-based therapy for Parkinson’s disease: current status and future prospects. Prog Neurobiol 2018;168:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kikuchi T, Morizane A, Doi D, et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 2017;548:592–6. [DOI] [PubMed] [Google Scholar]
- 17.Kriks S, Shim JW, Piao J, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011;480:547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song B, Cha Y, Ko S, et al. Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson’s disease models. J Clin Invest 2020;130:904–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–70. [DOI] [PubMed] [Google Scholar]
- 20.Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res 1995;4:241–8. [DOI] [PubMed] [Google Scholar]
- 21.Schweitzer JS, Song B, Leblanc PR, Feitosa M, Carter BS, Kim KS. Columnar injection for intracerebral cell therapy. Oper Neurosurg (Hagerstown) 2019;18:321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks DJ. Neuroimaging in Parkinson’s disease. NeuroRx 2004;1:243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y, Tang C, Chaly T, et al. Dopamine cell implantation in Parkinson’s disease: long-term clinical and (18)F-FDOPA PET outcomes. J Nucl Med 2010;51:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature 2011;474:212–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.