Abstract
Malaria vaccine approaches can be divided into ‘subunit’ and ‘whole parasite’, and these can be directed at the sporozoite, liver stage, asexual or sexual stages. All combinations of approach and stage are under development with the exception of a whole parasite sexual stage (gametocyte) vaccine. A gametocyte vaccine would aim primarily to block transmission of malaria from the human host to the mosquito vector and as such is referred to as a ‘transmission-blocking vaccine’. An immunological feature of whole parasite vaccines for the sporozoite/liver stage and for the asexual blood stage is the reliance on cellular immunity involving T-cells to control parasite growth. T-cells can also respond vigorously to gametocytes and kill them in the vertebrate host and/or arrest their development. To date, cellular immunity has not been exploited in transmission-blocking vaccine development. Here, the data supporting a gametocyte whole parasite vaccine are reviewed and a strategy for vaccine development and testing is outlined.
Keywords: cellular immunity, malaria, transmission-blocking vaccine, whole parasite vaccine
Malaria continues to exact a huge global toll on human life with up to 300 million new cases and over 400 000 deaths each year [1]. While the number of deaths has reduced significantly over the last decade, further reductions will depend on the availability of effective drugs to combat emerging drug resistance and the development of an effective vaccine.
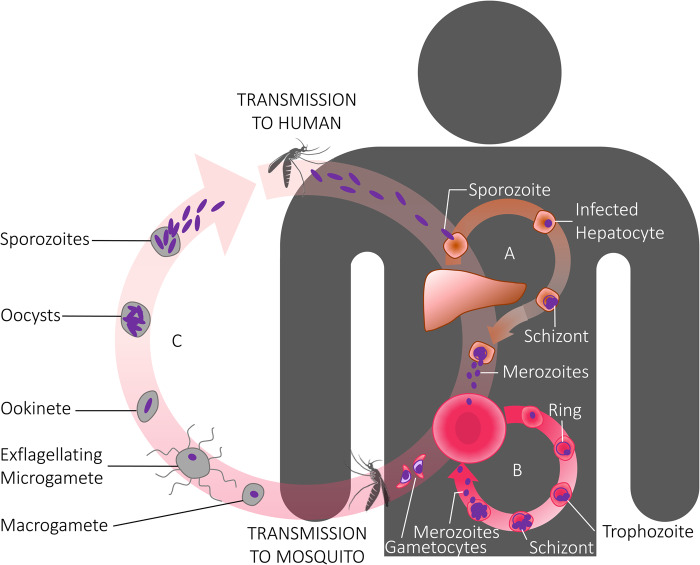
A malaria vaccine aims to block parasite growth and development at one or more stages in the life cycle. The life cycle in the vertebrate host commences with the bite of an infected Anopheles mosquito which injects tens to hundreds of sporozoites (Figure 1). Those reaching the liver within ∼30 min develop into exoerythrocytic schizonts within hepatocytes. After a further 8 days of growth, 30 000–40 000 merozoites emerge from each infected liver cell, with each one capable of entering a new red blood cell to continue the life cycle in the blood. For the dominant species of human malaria parasites (Plasmodium falciparum and P. vivax), there is a 48 h life cycle in the red cell with the asexual forms progressing from ‘rings’ to trophozoites and then segmented schizonts which burst to liberate new merozoites that invade fresh red cells. For P. falciparum, there is an approximate 10-fold increase in parasite numbers over the 48 h period. The exponential growth in parasite biomass leads directly and indirectly to the ensuing symptoms and pathology of malaria.
Figure 1. Stylized cartoon of the life cycle of the malaria parasite.
The life cycle commences in the vertebrate host with the bite of an infectious mosquito injecting sporozoite stages, and ends in the vertebrate host with the mosquito taking up sexual stages (gametocytes) to continue the life cycle in the definitive host — a female Anopheline mosquito. Gametocytes, within red blood cells, exist in the vertebrate host, whereas in the mosquito they emerge from the red cells to form female and male gametes, with the latter exflagellating to form microgametes which fertilize the female macrogametes.
The sexual parasite forms in the vertebrate host are the gametocytes. These are believed to develop continuously during the asexual life cycle with ∼10% of merozoites committed to sexual stage development during each round of asexual stage growth [2]. All merozoites from a particular schizont are committed to gametocytogenesis with all those from a given schizont becoming either male or female gametocytes [3]. The immature gametocytes, which remain within red cells, protect themselves from the innate immune defences of the host by sequestering in bone marrow in the extravascular spaces, only to reappear in the circulation after ∼8–12 days as mature gametocytes [4]. They are then taken up by mosquitoes to continue the life cycle in the primary host and emerge from within the red cell as male and female gametes which fertilize, giving rise to zygotes, then ookinetes, oocysts and eventually sporozoites which migrate to the salivary glands for final maturation.
Each stage of the life cycle is potentially vulnerable to vaccine-induced killing. However, mechanisms of immunity differ between each stage, and thus, malaria vaccine research is siloed and focused on different approaches for each stage. One vaccine (RTS,S — an anti-sporozoite recombinant subunit vaccine) has been licensed, but the efficacy window is only of several months' duration [5,6]. Antibodies need to be at very high titres at the time of sporozoite inoculation by mosquitoes, but the high levels that are present immediately after vaccination wane quickly due to the inability of ongoing malaria exposure to boost or maintain high titres [7,8]. Vaccines to the other parasite stages have not progressed beyond Phase II trials. Many of these candidate vaccines are also subunit vaccines that similarly rely on a very high antibody titre for efficacy. These vaccines aim to inhibit merozoite invasion of red cells (asexual stage vaccines) or block gamete fertilization/zygote development in the midgut of the mosquito following ingestion of the antibody during the blood meal, thus stopping the life cycle in the mosquito (transmission-blocking vaccines, TBVs).
The development of subunit TBVs stems from studies by Carter et al. [9] who demonstrated that chickens vaccinated with gametocytes from an avian malaria species developed antibodies that blocked transmission of malaria to other birds. Major immunodominant target antigens of transmission-blocking antibodies were subsequently defined for P. falciparum and P. vivax and are currently (or have been) in clinical trials as vaccine candidates [10]. The most advanced TBV candidate is Pfs25, a protein that is only expressed from the time of gamete emergence in the mosquito. Monoclonal antibodies to Pfs25 or antibodies produced in the human host following vaccination and taken up by the mosquito target zygotes and ookinetes in the mosquito, blocking parasite development. Vaccines based on recombinant Pfs25 and Pvs25 have progressed to Phase I trials [11,12]. However, the titres required for blocking transmission may not be attainable using current adjuvants. Cheru et al. [13] examined affinity-purified anti-Pfs25 antibodies from human vaccinees (vaccinated with Pfs25 and montanide ISA51) and found that a concentration of antibody greater than 80 μg/ml would only reduce the number of oocysts by 50% in a standard membrane-feeding assay. Conjugation of Pfs25 to EPA with Alhydrogel improved the immunogenicity of the vaccine, and a median concentration of 88 μg/ml was observed in volunteers in a Phase I trial [12]. Antibody levels correlated with a reduction in the number of oocysts (based on the membrane-feeding assay), but they declined to baseline within 1 year of vaccination. This is likely attributed to a lack of boosting since the protein is translationally repressed in female gametocytes and therefore not expressed in the human host. As such, natural infection of humans is not able to boost anti-Pfs25 antibody levels following vaccination. Titres will, thus, fall gradually post-vaccination. Other pre-fertilization and post-fertilization target antigens are under development as vaccine candidates. Pfs48/45 protein, another leading vaccine candidate, is expressed in the human host [14]. However, a stumbling block with this protein, which is slowing clinical development, is the difficulty in correctly folding the protein due to the large number of cysteine residues that it possesses. This protein is also under immune pressure due to its exposure to the human immune system, and the effect of known polymorphisms on vaccine efficacy against a heterogeneous population of parasites is unknown. These difficulties presented by subunits are not unique to sexual stage vaccines. To date, both asexual and transmission-blocking subunit vaccines have shown limited efficacy [10,15], with ongoing research aimed largely at identifying ways to improve immunogenicity and identify new antigen targets.
The limited success of subunit vaccines is the major driving force behind the development of ‘whole parasite’ approaches to the sporozoite and asexual blood stages. These vaccines contain all the proteins of the parasite, thereby lessening problems of antigenic polymorphism, and exploit fundamentally different types of immune responses. Current candidate whole parasite vaccines are obtained by dissecting sporozoites from mosquitoes or harvesting asexual blood stages from culture. The most advanced approach is the irradiated sporozoite vaccine (PfSPZ) which has been administered to over 800 volunteers [16]. This vaccine primarily induces a CD8+ T-cell response which targets infected liver cells. While not yet fully optimized, it has shown very encouraging success against challenge with homologous and heterologous parasites [17]. Whole parasite asexual stage vaccines [18] are only now entering clinical trials. Whereas CD8+ T-cells are the major effector cells with a sporozoite vaccine, CD4+ T-cells are primarily responsible for immunity induced by whole blood stage vaccines. CD4+ T-cells cells are also known to play a significant role in mediating anti-gametocyte immunity. However, the opportunities to exploit this class of cell for TBV development using whole gametocyte vaccines have been ignored. The remainder of the present study will discuss the evidence supporting the concept of a whole parasite TBV and explain why this approach might have the added advantage of also controlling the growth of asexual stage parasites.
In 1985, Harte et al. [19] demonstrated that T-cells from mice immunized intravenously with gametes could reduce transmission of malaria by over 95% and that this immunity was long-lasting (at least 6 months). By performing adoptive transfer experiments using spleen cells from immunized mice, they showed that the splenic CD4+ T-cell population was solely responsible for this transmission-blocking activity. A major function of the T-cells was to reduce the number of gametocytes following infection. A further additive function was to significantly reduce the ability of male gametocytes to exflagellate into microgametes.
It is not known whether human T-cells would have similar effects on human malaria parasites. However, T-cells from malaria-exposed individuals respond vigorously to gametocytes and to a major gametocyte antigen, Pfs 48/45 [20]. Furthermore, CD4+ T-cells from non-exposed donors can also respond to gametocytes [21,22], presumably as a result of immunological cross-reactivity between parasite antigens and common environmental antigens [23,24]. The majority of T-cell clones that respond to gametocytes also respond to asexual trophozoites [21,24], demonstrating that the antigens are not stage-specific, with many likely to be conserved ‘housekeeping’ antigens such as metabolic enzymes [25]. The effect that these cells have on gametocyte carriage is unknown; however, it should be noted that the carriage rates differ significantly between individuals in the same population and between different populations [26,27], which could be due to the differences in T-cell responses between individuals [21,24].
How might T-cells kill intra-erythrocytic gametocytes? Red cells do not express MHC molecules and thus cannot be directly targeted by T-cells. However, supernatants from parasite-stimulated peripheral blood mononuclear cells (PBMCs) and paroxysmal serum from P. vivax-infected individuals, in the presence of white blood cells, can quickly render gametocytes non-infective to mosquitoes and this effect is mediated, in large part, by nitric oxide liberated from the white blood cells [28,29]. TNF was a critical component in the supernatant used to stimulate the white blood cells. Furthermore, the killing was not species-specific in that supernatants from P. vivax-stimulated PBMCs were as effective at killing P. falciparum gametocytes in the presence of white blood cells as they were at killing P. vivax gametocytes. This mechanism of action and lack of specificity at the gametocyte-killing stage are identical with the reported mechanism and nature of killing of asexual stage parasites by cellular immunity following vaccination with whole parasite asexual stage vaccines [30–32].
The obvious similarities in immune mechanisms between killing gametocytes and asexual stage parasites suggest that whole gametocyte vaccines mimicking the design of whole asexual stage vaccines might be successful. Furthermore, it is possible that existing whole parasite asexual stage vaccines now entering clinical trials will have dual efficacy and induce gametocytocidal responses, given the extensive cross-reactivity of T-cells between asexual stages and gametocytes [21,22] and the fact that PBMCs stimulated by a crude asexual stage parasite extract can release cytokines capable of killing gametocytes [33]. If not, an attenuated whole gametocyte vaccine (or a whole gamete vaccine) or a killed/adjuvanted vaccine could be tested against both the asexual and sexual stages. Gametocytes used in a vaccine could be inactivated [31] or killed and presented in liposomes or another suitable formulation [34]. While gametocyte culture is tedious, it is possible to produce populations of pure gametocytes by culture and enrichment [35] which could form the basis for a novel vaccine.
Such vaccines would be tested for safety in Phase I trials and could then be tested for efficacy in early stage Phase Ib/II trials using controlled human malaria infections [36] where a reduction in peripheral gametocytaemia and a reduction in the ability of male gametocytes to exflagellate in the presence of T-cells might be expected. These are both mechanisms for which T-cells are thought to play critical roles [19,28,33]. These assays could complement standard membrane-feeding assays.
What level of gametocyte killing would be required for a vaccine to be effective? This is likely to depend on host and mosquito factors as well as the level of malaria endemicity. There is a non-linear relationship between gametocytaemia and the percentage of mosquitoes that are infected [37], suggesting that the infectiousness of gametocytes is an important determinant of transmission potential. While there are many biological and environmental factors that contribute to infectiousness, mathematical modelling predicts a role for inflammatory cytokines and the level of gametocytaemia [38]. However, there are also significant regional differences in transmissibility. Lin et al. [39] showed that in Cambodia, transmission rarely occurs when gametocytes are not detectable microscopically, whereas in areas with higher endemicity (e.g. Burkina Faso) it is estimated that almost one-third of all transmission occurs from people with sub-microscopic levels of gametocytaemia [40]. Recent data demonstrating that asexual parasites can inhibit the infectiousness of gametocytes (possibly through the activation of the mosquito immune system by hemozoin) [41] may, in part, explain these observations. In a low transmission setting, Churcher et al. [37] argue that if an intervention can keep gametocyte levels below 200 per μl, then this may be sufficient to eliminate disease. This should be a minimum goal of any vaccine. It is encouraging then that in the rodent study of cellular immunity [19], immune mice were able to reduce gametocytaemia from 0.01 to 0.001% (equivalent to ∼50 gametocytes per μl of blood).
A gametocyte vaccine designed to stimulate cellular immunity could be combined with a subunit candidate to induce a synergistic level of protection reliant on both cellular immunity and antibodies, with the potential for cross-killing between strains and even species [33,42]. Such a vaccine may well be capable of significantly reducing gametocytaemia and play an important role in malaria elimination. A successful transmission-blocking vaccine would ideally be combined with a vaccine to block sporozoite/liver stage development and/or asexual blood stage development.
Acknowledgements
We thank Hannah Brooks [43] for kindly providing Figure 1 and for reviewing the paper. M.F.G. acknowledges the National Health and Medical Research Council (Australia) for a Fellowship grant.
Abbreviations
- PBMCs
parasite-stimulated peripheral blood mononuclear cells
- TBVs
transmission-blocking vaccines
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.WHO (2016) cartographer World Malaria Report , WHO, Geneva [Google Scholar]
- 2.Eksi S., Morahan B.J., Haile Y., Furuya T., Jiang H. and Ali O. et al. (2012) Plasmodium falciparum gametocyte development 1 (Pfgdv1) and gametocytogenesis early gene identification and commitment to sexual development. PLoS Pathog. 8, e1002964 10.1371/journal.ppat.1002964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinden R. E. (2009) Malaria, sexual development and transmission: retrospect and prospect. Parasitology 136, 1427–1434 10.1017/S0031182009990667 [DOI] [PubMed] [Google Scholar]
- 4.Gardiner D.L. and Trenholme K.R. (2015) Plasmodium falciparum gametocytes: playing hide and seek. Ann. Transl. Med. 3, 45 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olotu A., Fegan G., Wambua J., Nyangweso G., Leach A., Lievens M. et al. (2016) Seven-year efficacy of RTS,S/AS01 malaria vaccine among young African children. N. Engl. J. Med. 374, 2519–2529 10.1056/NEJMoa1515257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rts SCTP (2015) Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386, 31–45 10.1016/S0140-6736(15)60721-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bojang K.A., Milligan P.J.M., Pinder M., Vigneron L., Alloueche A., Kester K.E. et al. (2001) Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 358, 1927–1934 10.1016/S0140-6736(01)06957-4 [DOI] [PubMed] [Google Scholar]
- 8.Kester K.E., Cummings J.F., Ofori-Anyinam O., Ockenhouse C.F., Krzych U., Moris P. et al. (2009) Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J. Infect. Dis. 200, 337–346 10.1086/600120 [DOI] [PubMed] [Google Scholar]
- 9.Carter R., Gwadz R.W. and McAuliffe F.M. (1979) Plasmodium gallinaceum: transmission-blocking immunity in chickens. I. Comparative immunogenicity of gametocyte- and gamete-containing preparations. Exp. Parasitol. 47, 185–193 PMID: [DOI] [PubMed] [Google Scholar]
- 10.Nikolaeva D., Draper S.J. and Biswas S. (2015) Toward the development of effective transmission-blocking vaccines for malaria. Expert. Rev. Vaccines 14, 653–680 10.1586/14760584.2015.993383 [DOI] [PubMed] [Google Scholar]
- 11.Wu Y., Ellis R.D., Shaffer D., Fontes E., Malkin E.M., Mahanty S. et al. (2008) Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS ONE 3, e2636 10.1371/journal.pone.0002636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talaat K.R., Ellis R.D., Hurd J., Hentrich A., Gabriel E., Hynes N.A. et al. (2016) Safety and immunogenicity of Pfs25-EPA/Alhydrogel®, a transmission blocking vaccine against Plasmodium falciparum: an open label study in malaria Naïve adults. PLoS ONE 11, e0163144 10.1371/journal.pone.0163144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheru L., Wu Y., Diouf A., Moretz S.E., Muratova O.V., Song G. et al. (2010) The IC50 of anti-Pfs25 antibody in membrane-feeding assay varies among species. Vaccine 28, 4423–4429 10.1016/j.vaccine.2010.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theisen M., Jore M.M. and Sauerwein R. (2017) Towards clinical development of a Pfs48/45-based transmission blocking malaria vaccine. Expert. Rev. Vaccines 16, 329–336 10.1080/14760584.2017.1276833 [DOI] [PubMed] [Google Scholar]
- 15.Tuju J., Kamuyu G., Murungi L.M. and Osier F.H.A. (2017) Vaccine candidate discovery for the next generation of malaria vaccines. Immunology 152, 195–206 10.1111/imm.12780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richie T.L., Billingsley P.F., Sim B.K.L., James E.R., Chakravarty S., Epstein J.E. et al. (2015) Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine 33, 7452–7461 10.1016/j.vaccine.2015.09.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyke K.E., Ishizuka A.S., Berry A.A., Chakravarty S., DeZure A., Enama M.E. et al. (2017) Attenuated PfSPZ vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc. Natl Acad. Sci. U.S.A. 114, 2711–2716 10.1073/pnas.1615324114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanisic D.I. and Good M.F. (2015) Whole organism blood stage vaccines against malaria. Vaccine 33, 7469–7475 10.1016/j.vaccine.2015.09.057 [DOI] [PubMed] [Google Scholar]
- 19.Harte P.G., Rogers N.C. and Targett G.A. (1985) Role of T cells in preventing transmission of rodent malaria. Immunology 56, 1–7 PMID: [PMC free article] [PubMed] [Google Scholar]
- 20.Riley E.M., Ong C.S., Olerup O., Eida S., Allen S.J., Bennett S. et al. (1990) Cellular and humoral immune responses to Plasmodium falciparum gametocyte antigens in malaria-immune individuals. Limited response to the 48/45-kilodalton surface antigen does not appear to be due to MHC restriction. J. Immunol. 144, 4810–4816 PMID: [PubMed] [Google Scholar]
- 21.Good M.F., Quakyi I.A., Saul A., Berzofsky J.A., Carter R. and Miller L.H. (1987) Human T clones reactive to the sexual stages of Plasmodium falciparum malaria. High frequency of gamete-reactive T cells in peripheral blood from nonexposed donors. J. Immunol. 138, 306–311 PMID: [PubMed] [Google Scholar]
- 22.Goodier M.R. and Targett G.A. (1997) Evidence for CD4+ T cell responses common to Plasmodium falciparum and recall antigens. Int. Immunol. 9, 1857–1865 10.1093/intimm/9.12.1857 [DOI] [PubMed] [Google Scholar]
- 23.Currier J., Sattabongkot J. and Good M.F. (1992) 'Natural' T cells responsive to malaria: evidence implicating immunological cross-reactivity in the maintenance of TCRαβ+ malaria-specific responses from non-exposed donors. Int. Immunol. 4, 985–994 10.1093/intimm/4.9.985 [DOI] [PubMed] [Google Scholar]
- 24.Goodier M.R. and Targett G.A.T. (1997) Polyclonal T-cell responses to Plasmodium falciparum gametocytes in malaria nonexposed donors. Parasite Immunol. 19, 419–425 10.1046/j.1365-3024.1997.d01-238.x [DOI] [PubMed] [Google Scholar]
- 25.Makobongo M.O., Riding G., Xu H., Hirunpetcharat C., Keough D., de Jersey J. et al. (2003) The purine salvage enzyme hypoxanthine guanine xanthine phosphoribosyl transferase is a major target antigen for cell-mediated immunity to malaria. Proc. Natl Acad. Sci. U.S.A. 100, 2628–2633 10.1073/pnas.0337629100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stepniewska K., Price R.N., Sutherland C.J., Drakeley C.J., von Seidlein L., Nosten F. et al. (2008) Plasmodium falciparum gametocyte dynamics in areas of different malaria endemicity. Malar. J. 7, 249 10.1186/1475-2875-7-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Degefa T.L., Zeynudin A., Zemene E. and Yewhalaw D. (2016) High prevalence of gametocyte carriage among individuals with asymptomatic malaria: implications for sustaining malaria control and elimination efforts in Ethiopia. Hum. Parasit. Dis. 8, 17–25 [Google Scholar]
- 28.Naotunne T.S., Karunaweera N.D., Del Giudice G., Kularatne M.U., Grau G.E., Carter R. et al. (1991) Cytokines kill malaria parasites during infection crisis: extracellular complementary factors are essential. J. Exp. Med. 173, 523–529 10.1084/jem.173.3.523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karunaweera N.D., Carter R., Grau G.E., Kwiatkowski D., Del Giudice G. and Mendis K.N. (1992) Tumour necrosis factor-dependent parasite-killing effects during paroxysms in non-immune Plasmodium vivax malaria patients. Clin. Exp. Immunol. 88, 499–505 10.1111/j.1365-2249.1992.tb06478.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinzon-Charry A., McPhun V., Kienzle V., Hirunpetcharat C., Engwerda C., McCarthy J. et al. (2010) Low doses of killed parasite in CpG elicit vigorous CD4+ T cell responses against blood-stage malaria in mice. J. Clin. Invest. 120, 2967–2978 10.1172/JCI39222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Good M.F., Reiman J.M., Rodriguez I.B., Ito K., Yanow S.K., El-Deeb I.M. et al. (2013) Cross-species malaria immunity induced by chemically attenuated parasites. J. Clin. Invest. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raja A.I., Stanisic D.I. and Good M.F. (2017) Chemical attenuation in the development of a whole-organism malaria vaccine. Infect. Immun. 85 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naotunne T.S., Karunaweera N.D., Mendis K.N. and Carter R. (1993) Cytokine-mediated inactivation of malarial gametocytes is dependent on the presence of white blood cells and involves reactive nitrogen intermediates. Immunology 78, 555–562 PMID: [PMC free article] [PubMed] [Google Scholar]
- 34.Giddam A.K., Reiman J.M., Zaman M., Skwarczynski M., Toth I. and Good M.F. (2016) A semi-synthetic whole parasite vaccine designed to protect against blood stage malaria. Acta Biomater. 44, 295–303 10.1016/j.actbio.2016.08.020 [DOI] [PubMed] [Google Scholar]
- 35.Delves M.J., Straschil U., Ruecker A., Miguel-Blanco C., Marques S., Dufour A.C. et al. (2016) Routine in vitro culture of P. falciparum gametocytes to evaluate novel transmission-blocking interventions. Nat. Protoc. 11, 1668–1680 10.1038/nprot.2016.096 [DOI] [PubMed] [Google Scholar]
- 36.Stanisic D.I., McCarthy J.S. and Good M.F. (2017) Controlled human malaria infection: applications, advances and challenges. Infect. Immun. epub ahead of print PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Churcher T.S., Bousema T., Walker M., Drakeley C., Schneider P., Ouédraogo A.L. et al. (2013) Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. eLife 2, e00626 10.7554/eLife.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckhoff, P. (2012) P. falciparum infection durations and infectiousness are shaped by antigenic variation and innate and adaptive host immunity in a mathematical model. PLoS ONE 7, e44950 10.1371/journal.pone.0044950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin J.T., Ubalee R., Lon C., Balasubramanian S., Kuntawunginn W., Rahman R. et al. (2016) Microscopic Plasmodium falciparum gametocytemia and infectivity to mosquitoes in Cambodia. J. Infect. Dis. 213, 1491–1494 10.1093/infdis/jiv599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouédraogo A.L., Gonçalves B.P., Gnémé A., Wenger E.A., Guelbeogo M.W., Ouédraogo A. et al. (2016) Dynamics of the human infectious reservoir for malaria determined by mosquito feeding assays and ultrasensitive malaria diagnosis in Burkina Faso. J. Infect. Dis. 213, 90–99 10.1093/infdis/jiv370 [DOI] [PubMed] [Google Scholar]
- 41.Kumar N. (2017) Modulation of transmission success of Plasmodium falciparum gametocytes (sexual stages) in various species of anopheles by erythrocytic asexual stage parasites. Acta Trop. 176, 263–269 10.1016/j.actatropica.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao Y., Bansal G.P., Merino K. and Kumar N. (2016) Immunological cross-reactivity between malaria vaccine target antigen P48/45 in Plasmodium vivax and P. falciparum and cross-boosting of immune responses. PLoS ONE 11, e0158212 10.1371/journal.pone.0158212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brooks H.M. (2017) Anti-malarial strategies among vulnerable populations: exploring bed net underutilization among internally displaced persons and novel adjunctive therapies for cerebral malaria. MSc Thesis, University of Alberta [Google Scholar]