Abstract
Methanogens are anaerobic archaea that grow by producing methane gas. These microbes and their exotic metabolism have inspired decades of microbial physiology research that continues to push the boundary of what we know about how microbes conserve energy to grow. The study of methanogens has helped to elucidate the thermodynamic and bioenergetics basis of life, contributed our understanding of evolution and biodiversity, and has garnered an appreciation for the societal utility of studying trophic interactions between environmental microbes, as methanogens are important in microbial conversion of biogenic carbon into methane, a high-energy fuel. This review discusses the theoretical basis for energy conservation by methanogens and identifies gaps in methanogen biology that may be filled by undiscovered or yet-to-be engineered organisms.
Keywords: archaea, bioenergetics, metabolism, methane, methanogenesis
Methanogens from the beginning
What is a methanogen?
Methane-producing archaea, or methanogens, are characterized by their ability to conserve energy for ATP (adenosine triphosphate) synthesis by producing methane gas. The first indication that methane gas could be biologically produced is credited to Alesandro Volta in 1776, who discovered flammable freshwater swamp gas and hypothesized it was derived from decaying organic matter [1]. It was not until 1933, however, that methanogens were first cultured [2]. Methanogens can be found in anaerobic habitats, and especially in low-sulfate environments such as in freshwater pond sediment. Other examples of environments that can harbor methanogens are digestive tracts of animals (for example, ruminants and humans), insects, marine sediment, and terrestrial subsurface environments. Methanogens can be isolated from across a wide range of thermochemical gradients, from acidophilic to alkaliphilic (pH 3.0–10.2), from (hyper)thermophilic to psychrophilic temperature (from −2°C to 122°C), and from freshwater estuarine to halophilic environments. While methanogens have been associated with polymicrobial diseases, such as intestinal dysbiosis and periodontal inflammation, there are no reports of methanogens being directly involved in pathogenesis using virulence factors or toxins [3].
To date, methanogens are strict anaerobic archaea and are obligate methane-producers. Methanogens can grow by reducing one-carbon (C1) compounds [CO2 (carbon dioxide), CO (carbon monoxide), methanol, methylamines, and methyl sulfides], acetate, or coal to methane gas through one of several methanogenesis pathways (Figure 1) [4–6]. Regardless of the substrate used, a methyl-coenzyme M molecule is ultimately reduced to methane by the methyl-coenzyme M reductase enzyme, Mcr [7]. Methanogens are distinguished from bacteria and archaea that may produce methane as a byproduct of metabolism by their obligate need to synthesize methane to conserve energy using the Wolfe Cycle [8]. By this distinction, all known methanogens, to date, belong to the euryarchaeal domain.
Figure 1. The Wolfe Cycle.
Arrows represent direction of biochemical reactions. Black, reaction steps and directions common to all five methanogenesis pathways from C1 compounds or acetate. (a) Hydrogenotrophic (red) and carboxydotrophic (blue) methanogenesis pathways. Formic acid and primary or secondary alcohols are oxidized to CO2 and hence methanogens that grow on these substrates use the hydrogenotrophic pathway. (b) Methyl respiration pathway (orange) and methylotrophic pathway (green). (c) Acetoclastic pathway (fuchsia). Purple, reactions are found only in Methanosarcina species; gray, proposed reactions. Shaded, electron bifurcation/confurcation reaction steps; CoB-SH, coenzyme B thiol; CoM-SH, coenzyme M thiol; CoM-S-S-CoB, coenzyme M-coenzyme B heterodisulfide; Fd, ferredoxin; Fdred, reduced ferredoxin; H4MPT, tetrahydromethanopterin; MFR, methanofuran; MPh, methanophenazine; MPhH2, reduced methanophenazine. See Table 2 for reactions and enzyme names.
Other organisms have been shown to produce small amounts of methane. Some species of pelagic bacteria release methane by using C–P lyases to liberate P from algal lipids which can comprise methylphosphonate head groups [9,10]. Reduction in the C–P methylphosphonate bond releases methane, but this process is used to obtain P, and does not directly contribute to energy conservation. The P is hypothesized to be used for biomass synthesis, but the metabolic fluxes are not high enough to support energy conservation.
Methanogenic archaea release large quantities of methane to the atmosphere and play a significant role in controlling global climate. Methane, whether produced abiotically, anthropogenically (through mining or agriculture), or biologically, has a lifetime greenhouse gas impact 28 times higher than CO2 [11]. Methane produced by methanogens is primarily oxidized to CO2 by anaerobic methanotrophic archaea or bacteria, or by aerobic methanotrophic bacteria. It is estimated that <1% of biological methane from the subsurface is released to the atmosphere [12]. Overall, it is estimated that up to 2% of carbon in the global carbon cycle (or 450 Tg annually) is mineralized by methanogens per year [13]. It has been proposed that increased global temperatures may result in increased subsurface methanogenic activity in melting subarctic tundra and wetlands, thus accelerating atmospheric methane emissions which could further increase climate warming [14]. The effect of increased methanogenesis on global climate may have been foreshadowed by the theory that methanogens may have contributed to the largest extinction in Earth's history [15]. According to molecular phylogeny, nickel deposition, C isotope ratios, and the fossil record, it has been proposed that methanogens may have acquired the ability to use acetate as a substrate from anaerobic Clostridia [16,17]. The subsequent expansion into a new ecological niche and the sharp rise in greenhouse methane seem to have caused global warming and climate changes that drove 99% of all vertebrate life on Earth to extinction, ultimately selecting for radiation of mammals. Thus, there is compelling evidence that unchecked methanogenesis can alter global climate. Fortunately, since the Permian extinction, microbes have evolved to mitigate most of the climate effects of methanogen metabolism by developing efficient means to rapidly convert methane to the less harmful CO2. Today, it is thought that aerobic and anaerobic methane oxidation by subsurface microbes reduces natural atmospheric methane emissions [12,13].
While atmospheric methane emissions may be undesirable, harnessing methanogens is useful to produce methane from renewable carbon feedstock. Anaerobic digestion of waste to produce biogas is a highly efficient process. Biogas comprises 30–90% methane and can be combusted to generate electricity or refined and compressed to power transportation [18,19]. Methanogens have been used for decades to reduce the biological oxygen demand of wastewater (agricultural or municipal) while simultaneously producing renewable methane (as biogas) that can be captured from anaerobic digesters and used as fuel. Implementation of anaerobic digestion and biogas recovery technologies has the potential to produce 7.9 million metric tons of methane or 4940 million kWh annually in the U.S.A. though less than 10% of US wastewater treatment facilities have implemented their use [20–22]. In Europe, which has a goal of producing 20% energy through renewable sources by 2020, renewable energy infrastructure is more advanced, and the European Union is on track to obtain >25% energy in the form of biogas from anaerobic digestion [23].
Despite the recognition that methane, a valuable fuel, can be produced by biological organisms, culturing and characterization of methanogens has proceeded at a slow but steady pace. The pace of discovery can be understood in the context of several technological and historical hurdles that, when overcome, have produced significant advances in Biology. First, methanogens are strict anaerobes that require not only removal of oxygen from the culture medium but also a reducing environment with a redox potential below 50 mV [24]. Thus, reliable isolation and characterization of methanogens required significant advances in microbial culturing including anaerobic liquid handling (Hungate) techniques and specialized glassware and culturing equipment (Hungate and Balch tubes) [25,26]. These anaerobic culture techniques have contributed to the study of many diverse microbes that could otherwise not be cultured. Second, before it was understood that many methanogens are lithoautotrophs that use C1 compounds or acetate to grow, addition of heterotrophic carbon sources enriched syntrophic bacterial/methanogen communities that were impossible to culture separately [27]. Early methanogenic enrichments were considered ‘contaminated’ and lost or discarded before there was an appreciation for coupled metabolism or an understanding of syntrophy. Syntrophy is a special case of mutualistic metabolism between two (or more) organisms [28]. In the strictest sense, a syntroph is an organism that cannot conserve energy on its own, but requires a second organism such as a methanogen to maintain the concentrations of substrate environment such that the thermodynamic equilibrium allows enough energy to be conserved to produce a transmembrane ion gradient for ATP synthesis. Methanogens are thought to improve the metabolism of fermenting bacteria or syntrophic partners by consuming hydrogen or acetate fermentation byproducts that, if concentrations are too high, eventually inhibit growth. To facilitate coupled metabolism, methanogens and bacteria have been observed to form layered granule structures and mats formed from interconnected pili [29,30]. Studying methanogens and the metabolic relationships they form with other microbes has resulted in significant breakthroughs in understanding cellular bioenergetics and metabolism, such as the discovery of syntrophy, and has the potential to lead to future insights about bacterial/archaeal metabolic relationships and interspecies communication.
‘Alien’ biochemistry and the tree of life
Methanogens exhibit an unusual metabolism that continues to challenge what we know about genes, biological information, and the chemistry that cells can harness to conserve energy. The unique biochemistry of methanogens provided critical supporting evidence that ribosomal gene homology can be used as a molecular clock to understand the evolution and heredity of living organisms. To test his theory that 16S ribosomal rRNA sequences could be used to build a tree of life [31], Carl Woese needed outlier microbes that had very different 16S rRNA sequences that were correspondingly biochemically different from other known microbes. Ralph Wolfe, a colleague at the University of Illinois Urbana-Champaign, volunteered his methanogen cultures to test this hypothesis [32]. Not only were methanogens found to have very different 16S rRNA sequences, supporting Woese's idea [33], but methanogens (at the time named archaebacteria or methanobacteria) were found to possess a stunning array of new biological features including isoprenoid membrane lipids [34–37], S-layer protein cell walls, or pseudomurein instead of peptidoglycan [38–40], a large proportion of genes that had little to no homology to other bacterial gene sequences, distinct viruses [41–43], a new genetically encoded amino acid pyrrolysine [44–47], and several unique biochemical cofactors [48].
Methanogens were instrumental in supporting Carl Woese's theory that living organisms can be classified into three domains in the Tree of Life: the Bacteria, Archaea, and Eukarya. Current hypotheses based on metagenomics of uncultured genomes assembled from marine subsurface environments have 16S rRNA and other eukaryotic gene signatures (such as actin, Ras GTPases, and ESCRT vesicular trafficking) that suggest candidatus Lokiarchaeon appears to be a eukaryal hydrogenotrophic methanogen [49]. While evidence of a methyl-S-CoM reductase mcr gene was not detected in the incomplete metagenome, genes for other methanogenesis enzymes, H4MPT-dependent carbon fixation, and tricarboxylic acid cycle (TCA) enzymes were most closely related to methanogen genes (cdhβδε, mtxC corrinoid methyltransferase, fwdA, and fmdBCDEF formyl-methanofuran dehydrogenases, ftr, mch, mtd, mtrH, mvhADG, hdrABCD, frhB, hyp hydrogenase maturation enzymes, ATP synthase, fructose-bisphosphate aldolase, phosphoglycerate kinase, hydroxypyruvate reductase, sucC ATP-citrate synthase α, acnA aconitate hydratase, acnA/leuB 3-isopropylmalate dehydratase, and sdhAB fumarate reductase), or are closely related to euryarchaeal genes (mer) [49]. This discovery suggests the Last Eukaryotic Common Ancestor, LECA, could have evolved from an organism that had a very methanogen-like metabolism in many respects but which had lost the ability to synthesize methane. In a slowly unfolding story, uncultured organisms belonging to the Asgard superphylum may be instrumental in unifying the archaeal and eukaryal domains [50,51].
Currently, there are seven orders of methanogens recognized on the basis of mcrA and ribosomal gene phylogeny (Table 1) [52,53]. Five of the methanogen guilds (Methanopyrales, Methanococcales, Methanobacteriales, Methanomicrobiales, and Methanocellales) so far only contain hydrogenotrophic methanogens, while the Methanomasiliiicoccales guild is so far defined as having obligate methyl-respiring methylotrophic methanogens. The Methanosarcinales have the most diverse membership, with many members capable of more than one methanogenesis pathway, as well as obligate hydrogenotrophic or obligate acetoclastic members. Three orders of closely related Euryarchea (Halobacteriales, Archaeoglobales, and Thermoplasmatales) are considered to have evolved from methanogen ancestors, but lost the ability to grow by methanogenesis. The halophilic archaea are aerobic heterotrophs that evolved from methanogens but learned how to use oxygen as a terminal electron acceptor. Hydrogenotrophic methanogens are likely overrepresented in culture collections because anaerobic heterotrophs tend to grow faster and can out-compete methanogens. This is because culture media that contain complex heterotrophic carbon substrates run the risk of enriching heterotrophs at the expense of methanogens. This reinforces the perception that methanogens grow at such a slow rate that they are impractical to experiment with. However, there are several methanogens that are known to grow rapidly, with generation times on the order of minutes (Methanocaldococcus jannaschii, 25 min) to a few hours (Methanosarcina barkeri, 6 h), which is not prohibitively long for laboratory study [12,54]. Some methanogens have also been isolated that exhibit nutritional requirements, such as increased Ni2+ (for synthesis of Ni tetrapyrrole F430 cofactor), or addition of coenzyme M or rumen fluid [55–58]. Therefore, while enriching new methanogens can require long careful effort, once they are isolated in axenic cultures, the growth rates can often be accelerated by optimizing culture medium and growth conditions. Exploration of different culture media and cultivation conditions may yet yield new methanogen guilds as well as expand the metabolic capabilities of existing guilds.
Table 1. Methanogen orders and methanogenesis pathways.
| Order | Representative organism | Methanogenesis pathways | Comments |
|---|---|---|---|
| Methanopyrales | Methanopyrus kandleri | Hydrogenotrophic | Deepest branching |
| Methanococcales | Methanococcus maripaludis | Hydrogenotrophic | Genetic systems |
| Methanobacteriales | Methanobacterium thermoautotrophicum | Hydrogenotrophic | |
| Methanosarcinales | Methanosarcina mazei | Hydrogenotrophic, methylotrophic, carboxydotrophic, acetoclastic | Largest archaeal genome, genetic systems, closely related to ANME-2 methanotrophs |
| Methanomicrobiales | Methanospirillum hungatei | Hydrogenotrophic | |
| Methanocellales | Methanocella paludicola | Hydrogenotrophic | |
| Methanomassiliicoccales | Methanomassiliicoccus luminyensis | Methylotrophic | Was ANME-1 methanotrophs |
| Halobacteriales | Halobacterium salinarum | None, aerobic halophilic heterotrophs | Genetic systems |
| Thermoplasmatales | Thermoplasma volcanium | None, thermophilic heterotrophs | |
| Archaeoglobales | Archaeoglobus fulgidus | None, anaerobic sulfate-reducers |
How do methanogens grow?
Methanogens are said to exist near the ‘thermodynamic edge of life’ [12,59]. What this means is that methanogens are among the special few organisms known that can grow autotrophically in sealed glass vessels on inorganic substrates in the absence of light. To do so, they have evolved to efficiently convert chemical energy from substrate into biomass. It is widely thought that the Gibbs' free energy equation (eqn 1) can be used to predict whether enough chemical energy is available in a metabolic reaction to allow ATP synthesis to occur should it perfectly biochemically coupled to an energy-conserving process (Figure 2) [60].
| 1 |
Figure 2. Factors that limit methanogen growth.
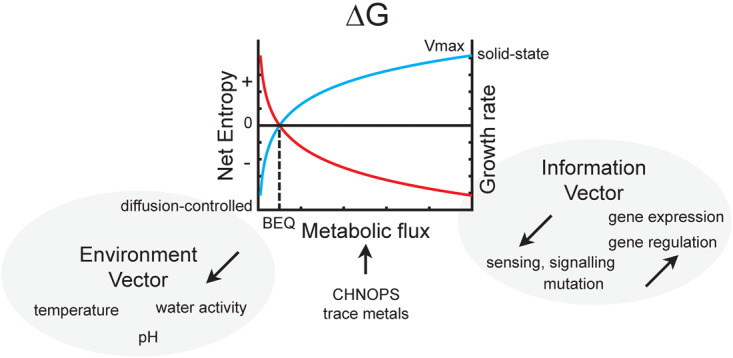
In a closed system such as in sealed anaerobic glass culture tubes, the metabolic productivity of any organism can be estimated by the Gibbs' free energy (ΔG°′) of the rate-limiting biochemical transformations occurring. For most methanogens, this is C and/or H2 metabolism. Other factors, such as physical stress (pH, temperature, and water activity) and net metabolite fluxes, also affect population growth by increasing entropy of the cell systems, thus exerting a negative vector on ΔG°′ and resulting in increased BEQ. Finally, informational entropy in the form of spatial organization, gene content, and gene regulation also affects whether cells optimally convert chemical energy into biomass. At the extremum are non-growing diffusion-controlled cell systems and at the other are compact solid-state cells in which metabolism is flux-controlled. Red, net entropy (chemical, informational); blue, specific growth rate.
The Gibbs' free energy equation (eqn 1) is applicable to any chemical equation and can be used to derive eqn 2 to describe the favorability of oxidation/reduction reactions (eqn 2).
| 2 |
Metabolism can be conceptualized as the flow of electrons from electron donor to acceptor. Whether an organism or system of organisms can conserve energy for growth can be estimated by determining whether the Gibbs' free energy of the system is overall negative and favorable (−ΔG°), and the magnitude of −ΔG° can be used to estimate the number of moles of ATP that can be synthesized per mole substrate consumed. Methanogens grow by the general equation (eqn 3), which, depending on the chemical bond energies, numbers of electrons, and the redox potential difference of the electron donor and electron acceptors, can be favorable or unfavorable (eqn 2).
| 3 |
If the Gibbs' free energy is negative and the cell has a biochemical mechanism to convert that energy into the ability to generate ATP, then we can predict that a cell with the ability to consume the substrate and produce methane can grow [61,62]. The magnitude of the Gibbs' free energy yield can indicate how much substrate must be consumed to produce a molecule of ATP, the biological energy quantum (BEQ) [63]. For instance, based on the studies of chloroplast ATP synthase, an average of 4.0 ± 0.3 protons are required to convert ADP + Pi to one molecule of ATP [64]. Transit of four protons through a membrane is equivalent to ΔG°′ (change in Gibbs’ free energy) of 31.8 kJ mol−1 under physiological conditions, with a range of −25–45 kJ mol−1 (e.g. chloroplast ATP synthase exhibits a ΔG′p of the reaction equal to 36 ± 3 kJ mol−1 [60,64,65]). Hence, a Gibbs' free energy value of an average −36 kJ mol−1 for a reaction can be hypothesized to yield one mole ATP per mole substrate oxidized.
Depending on the substrates, methanogens can obtain from 0.5 to 2 moles ATP per mole substrate, which is the lowest theoretical yield except for acetogens that grow by producing acetate from H2 + CO2 (0.25 moles ATP), and syntrophs which must have a syntrophic partner to couple metabolism such that the overall Gibbs' free energy yield for both organisms is favorable. There are several key considerations that should be taken into account when using Gibbs' free energy to predict methanogen metabolism. Chief among these considerations are that the predicted energy yields suppose methanogens use a chemiosmotic ATP synthase. To date, all methanogens use a transmembrane ion gradient to generate ATP via ATP synthase. Organisms such as fermentative bacteria can use substrate-level phosphorylation to produce ATP, such as in the synthesis of phosphoenolpyruvate in glycolysis that can be directly used to phosphorylate ADP to ATP, releasing pyruvate as a byproduct. To date, no such examples of substrate-level ATP synthesis have been demonstrated by a methanogen. Several mechanisms for coupling substrate catabolism to generating a chemiosmotic gradient exist in methanogens, such as coupled transport, scalar translocation, and active pumping by membrane-bound oxidoreductase enzymes. Several of these strategies are used by methanogens to conserve energy including proton and/or sodium-pumping enzyme complexes (Mtr, Rnf) coupled to H+/Na+ antiporter (Mrp), and scalar proton translocation. Scalar proton translocation is accomplished via the membrane electron carrier methanophenazine (MPh), which uses a quinone loop-type mechanism and hydrogenases to produce a ‘hydrogen cycle’ (Table 2) [68–71]. Using Gibbs' free energy to estimate methanogen metabolism also assumes a 100% ion pumping efficiency for generating the chemiosmotic gradient. A metabolic reaction may have enough energy to pump four protons out of the cell, but the chemical bond energy of the substrate may not be perfectly coupled to ATP synthesis. The theory also assumes negligible metabolic entropy at the optimal growth temperature. For instance, a cell may perfectly couple the substrate energy into ATP synthesis, but if cells have a high rate of ATP consumption for non-growth-associated maintenance, the ATP could be consumed rapidly without appreciable growth. The maintenance energy is defined as the flux of energy from substrate catabolism needed to maintain a unit of biomass [72]. Finally, the Gibbs' free energy gives a measure of spontaneity/favorability, but in itself cannot be used to predict the rate of substrate consumption or of cellular growth.
Table 2. Methanogenesis pathway enzymes and energy conservation1.
| Wolfe Cycle step2 | Reaction2 | ΔG°′ (kJ mol−1)2 | Enzyme | Energy-converting?3 |
|---|---|---|---|---|
| a | CO2 + MFR + 2H+ → Formyl-MFR | 16 | Formyl-methanofuran dehydrogenase, Fmd | No, but physically associated with electron-bifurcating Mvh and Hdr in Methanococcus |
| b | Formyl-MFR + H4MPT → Formyl-H4MPT + MFR | −4.4 | Formyl-methanofuran:H4MPT formyl transferase, Ftr | No |
| c | Formyl-H4MPT + H+ → Methenyl-H4MPT+ + H2O | −4.6 | Methenyl-H4MPT cyclohydrolase, Mch | No |
| d | Methenyl-H4MPT + F420H2 → Methylene-H4MPT + F420 + H+ | 5.5 | F420-dependent Methylene-H4MPT dehydrogenase, Mtd | No |
| d | Methenyl-H4MPT + H2 → Methylene-H4MPT + H+ | −5.5 | H2-forming methylene-H4MPT dehydrogenase, Hmd | No |
| e | Methylene-H4MPT + F420H2 → CH3-H4MPT + F420 | −6.2 | F420-dependent Methylene-H4MPT reductase, Mer | No, but physically associated with Hdr and Acs/Cdh in Methanosarcina |
| f | CH3-H4MPT + CoM-SH → CH3-S-CoM + H4MPT | −30 | Methyl-H4MPT:coenzyme M methyltransferase, Mtr | Yes, Na+ pumping |
| g | CH3-S-CoM + CoB-SH → CoM-S-S-CoB + CH4 | −45 | Methyl-coenzyme M reductase, Mcr | No |
| h | CoM-S-S-CoB + H2 → CoM-SH + CoB-SH | −40 | Electron-bifurcating hydrogenase:heterodisulfide reductase complex, Mvh:HdrABC | Yes |
| h | CoM-S-S-CoB + 2H+ → CoM-SH + CoB-SH | −40 | (Electron-bifurcating) ferredoxin:F420:heterodisulfide reductase, HdrABC | Yes/No in M. acetivorans |
| i | H2 + F420 → F420H2 + H+ | −11 | F420-reducing hydrogenase, Frh | Yes, hydrogen cycle in Methanosarcina |
| j | H2 + Fdox → H+ + Fdred | 22.4 (−2.7)4 | Energy-converting sodium pumping ferredoxin hydrogenase | Yes |
| k | H2 + Fdox → H+ + Fdred | 22.4 (−2.7)4 | Ferredoxin reducing hydrogenase, Eha/Ech | Yes |
| l | CoM-S-S-CoB + MPhH2 → CoM-SH + CoB-SH + MPh | −4.2 | Proton-translocating methanophenazine:heterodisulfide reductase, HdrED | Yes |
| m | Na+(in) + H+(out) → Na+(out) + H+(in) | 0 | Sodium–proton antiporter, MrpA | No |
| n | F420H2 + MPh → F420 + MPhH2 | −37.6 | F420 proton-pumping methanophenazine reductase, Fpo | Yes |
| ADP + Pi → ATP + H2O | 30.3 | ATP synthase | No |
Adapted from refs [8,66,67]. Note that the Wolfe Cycle is the product of studies of the biochemistry and genetics of Methanobacterium, Methanococcus, and Methanosarcina organisms in pure cultures. Some reactions (d, h, k) are to be catalyzed by different enzymes in different methanogen lineages. Some reactions are catalyzed by enzymes encoded by multiple gene copies on the chromosome (i.e. hydrogenases and methyltransferases), while other enzymes are typically encoded by a single gene copy (i.e. mcr, hdrED). As the diversity of methanogen isolates increases, we can expect the Wolfe Cycle to expand to reflect broader metabolic versatility of methanogens.
Reactions named in the hydrogenotrophic methanogenesis convention.
See Figure 2 to determine whether the reaction is energy-conserving or energy-consuming.
Assuming the same polyferredoxin as in step a with a redox potential of −530 mV. Values in parentheses show the ΔG° if the polyferredoxin has a more typical redox potential of −400 mV.
Ultimately, whether the chemical energy of the substrates can be harnessed to conserve energy for the cell requires that electrons from the substrate flow from a higher energy reduced state to a lower energy oxidized state while producing ATP (Table 3). For methanogens grown in axenic culture, the electron donor is either hydrogen (for hydrogenotrophic or methyl respiration pathways) or the carbon source itself (for methylotrophic, carboxydotrophic, or acetoclastic fermentation or respiration pathways). In each of these pathways, the energy state of the electrons from the electron donor is higher than that of the electrons donated to the electron acceptor. In all methanogens, a substrate is converted to methyl-Coenzyme M, which reacts with a Ni(I)F430 cofactor to produce a methyl radical and CoM-S-Ni(II)F430 in the active site of methyl-coenzyme M reductase (Figure 1) [7,75]. The nickel-tetrapyrrole coenzyme F430 is unique to methanogens and evolved to form a strong reducing agent necessary to activate carbon to accept electrons to form methane [76,77]. In the last step of methanogenesis, a coenzyme B thiol (the terminal electron donor) enters the Mcr active site, donates an electron to the methyl radical to produce methane, forms a coenzyme M-coenzyme B heterodisulfide to regenerate the Ni(I)F430 cofactor to release methane, and forms a coenzyme M-coenzyme B heterodisulfide. The CoM-S-S-CoB serves as the terminal electron acceptor for electrons flowing down the electron transport chain, which are funneled through the CoM-S-S-CoB heterodisulfide reductase enzyme, Hdr [78–82]. Thus, methanogens have evolved to be so efficient as to synthesize their own terminal electron donor and terminal electron acceptor that must be recycled to the free CoM-SH and CoB-SH thiols for subsequent rounds of methanogenesis.
Table 3. C1 and alkane methanogenesis reactions.
| Substrate | Reaction | ΔG°′ (kJ mol−1) | Favorable for methanogenesis? | Observed metabolism? |
|---|---|---|---|---|
| Carbon dioxide (bicarbonate) | 4H2 + HCO3− + H+ → CH4 + 3H2O | −135.6 | Yes | Yes |
| Formate | 4HCOO− + H2O + H+ → CH4 + 3HCO3− | −32.5 | Yes | Yes |
| Methanol + hydrogen | CH3OH + H2 → CH4 + H2O | −112.5 | Yes | Yes |
| Methanol | 4CH3OH → 3CH4 + HCO3− + H2O + H+ | −78.6 | Yes | Yes |
| Acetate | CH3COO− + H+ → CO2 + CH4 | −36 | Yes | Yes |
| Acetic acid (<pH 4) | CH3COOH → CH4 + CO2 | −31 | Yes | No |
| Carbon monoxide | CO + 3H2 → CH4 + H2O | −142 | Yes | Yes? |
| Carbon monoxide | 2CO + 2H2 → CH4 + CO2 | −171 | Yes | Yes? |
| Carbon/graphite | C + 2H2 → CH4 | −50.7 | Yes | No |
| Ethane | C2H6 + H2 → 2CH4 | −68.6 | Yes | No |
| Benzene | C6H6 + 9H2 → 6CH4 | −434 | Yes | No |
| Acetone | CH3COCH3 + H2O → 2CH4 + CO2 | −115 | Yes | No |
| Amino acids | H2NCH2COOH + 5H2 → 2CH4 + NH3 + 2H2O | −204 | Yes | No |
| Glucose | C6H12O6 → 3CO2 + 3CH4 | −418.1 | Yes | Yes, syntrophic multi-step |
Hydrogenotrophic methanogens have evolved to grow by apparently ‘cheating’ thermodynamics, in a sense. In the first step of the hydrogenotrophic pathway, CO2 must be reduced by a low-potential Fe/S ferredoxin (−530 mV) to produce formyl-methanofuran in an ‘uphill’ unfavorable reaction (Figure 1). To get over this significant energy barrier, the cells have coupled unfavorable ferredoxin reduction to favorable reduction in CoM-S-S-CoB (+140 mV) by using flavin-based electron bifurcation (FBEB) [12,83,84]. FBEB is distinguished from quinone-based electron bifurcation that plays an essential role in mitochondrial respiration and photosynthesis [85,86]. FBEB is catalyzed by multi-subunit enzyme complexes, one of which contains a flavin cofactor, and is increasingly recognized as essential for coupling redox reactions to energy conservation mechanisms in diverse bacteria and archaea [87,88]. In methanogens, FBEB is achieved by a multienzyme complex comprising formyl-methanofuran dehydrogenase (Fmd), a hydrogenase (Mvh or Vht) that donates the electrons to a flavin in a three subunit heterodisulfide reductase enzyme, HdrABC [89,90]. The HdrA polypeptide with bound flavin cofactor accepts both electrons from the hydrogenase. The high-spin electron (−250 mV) is used to reduce the CoM-S-S-CoB heterodisulfide in the HdrC active site in two one-electron reductions. The remaining low-spin electron (−450 mV) is used to reduce a low-potential 2Fe/2S cluster ferredoxin center (HdrB? A separate ferredoxin?) in 2 one-electron reductions. The low-spin electrons are donated to Fmd that reduces CO2 to formyl-methanofuran. Because the redox reactions are physically linked by one enzyme complex that couples the reactions, the redox potentials of the partial reactions can be summed. The net reaction is overall negative (−120 mV) and the Gibbs' free energy is favorable by −23.16 kJ mol−1. By linking the first and last steps of hydrogenotrophic methanogenesis to regenerate the terminal electron donors CoM-SH and CoB-SH, the pathway is cyclic and has been named the ‘Wolfe Cycle’ after Rouvière and Wolfe who first proposed the pathway [8,91].
Methylotrophic, carboxydotrophic, and acetoclastic methanogens all possess Fmd and HdrABC, but not all methanogens express functional hydrogenases and depending on the substrate and ‘wiring’ of central metabolism, electron bifurcation may not be necessary for all methanogens [92,93]. For instance, during methylotrophic methanogenesis for Methanosarcina acetivorans, which does not express functional hydrogenase enzymes, methanol is used as the electron donor and the electron acceptor in a branched anaerobic respiration pathway. In this example, methanol is oxidized to CO2, and electrons are abstracted from formyl-methanofuran in the opposite direction; hence, reduction in CoM-S-S-CoB, flavins, and any other electron acceptor is in the downhill favorable direction. Likewise, during acetoclastic methanogenesis, acetate is cleaved into a corrinoid-bound CO that is oxidized to CO2 with the production of two low-potential electrons and the methyl group is donated to methyl-tetrahydromethanopterin by the acetyl-CoA synthase/carbon monoxide dehydrogenase (Acs/Cdh, ACDS) complex. In the example of acetoclastic methanogenesis, reduction in intracellular electron carriers is also favorable and should not require FBEB. The same can be said for carboxydotrophic methanogenesis, though experiments with M. acetivorans suggest that carboxydotrophic growth is not balanced [6,94,95]. Examples of how redox balance could be maintained include releasing a product (formate/formaldehyde, acetate, or other molecules), donating electrons to a partner organism, producing a synthetically designed bioproduct, or perhaps an alternative electron acceptor such as N2, minerals, or through electrosynthesis.
New frontiers in methanogenesis
Reconsidering Gibbs' free energy
Gibbs' free energy is a useful guide in predicting whether or not an organism could exist given the overall metabolic fermentation or respiration reaction they use to generate a chemiosmotic gradient [59] (Figure 2). There are a few caveats to consider. First, the Gibb's free energy ΔG° is typically calculated from formation energies ΔfG° under standard conditions (1 bar, 1 M, 25°C) at constant temperature and pressure; conditions that may have nothing to do with what cells will experience in the environment or the intracellular conditions where enzymes do work. Unless care is taken to account for BEQ, growth temperature, concentrations, molecular phase changes, and considering biochemical fluxes (maintenance energy) it can be tricky to use the Gibb's equation to attempt to predict microbial physiology. Gibbs' free energy is most useful for determining whether one or more organisms may grow using an overall chemical reaction in a closed system (as in a sealed glass culture tube) without making assumptions about the rates or biochemical mechanisms that may be used to grow. Second, Gibbs' free energy is easier to apply to transmembrane ion pumping during respiration or fermentation reactions than to substrate-level phosphorylation or certain versions of scalar ion translocation modes of energy conservation. Substrate-level phosphorylation to generate ATP is not easily described by Gibb's free energy because metalloids can hybridize molecular orbitals depending on the bond interactions in the molecule. Unless the possible oxidation states of the carbon, phosphorus, and sulfur atoms are accounted for in the Gibb's free energy calculation, it can be prohibitively complicated to predict whether enough energy is available to synthesize ATP. Therefore, it is formally possible that organisms could exist that grow by producing methane gas by coupling it to substrate-level phosphorylation through phosphoenolpyruvate, 1,3-bisphosphoglycerate, acetyl-phosphate, or other activated phosphotransfer substrate. As long as the phosphotransfer molecule can then react with ADP to produce ATP, a cell could also dispense with ATP synthase and membrane electron carriers. Such a methanogenic organism could appear to be an uncultured archaeon with genes similar to a bacterial heterotrophic fermenter. Admittedly, it is difficult to imagine such a metabolism for a single organism, as the substrate and product fluxes would have to be quite high. However, invoking syntrophic partnerships that could balance substrate and product fluxes opens completely new horizons for untapped biochemical diversity.
Metabolism of higher-carbon compounds, such as carbohydrates, glucose, fatty acids, or alcohols to CO2 using Wolfe Cycle enzymes (with or without methane production), has been observed, albeit requiring a syntrophic association between two or more organisms [96]. This is because activation of the carbon substrate to an alkyl-thiol and subsequent C–C bond breakage is thermodynamically unfavorable under standard conditions unless metabolic fluxes are coupled to favorable reduction in the terminal electron acceptor such as sulfate. While syntrophic partnerships are typically described between two or more separate organisms organized in granules or mats, it is possible that syntrophs or other organisms and methanogens could permanently combine in a single host–symbiont system [97]. Such a symbiotic system may be impossible to identify using current shotgun metagenomic approaches or could appear as a contaminated genome assembly from single-cell genomic experiments.
It is also formally possible that a fermentation co-product could be coupled to methane synthesis, similar to bacterial mixed fermentation where multiple products are secreted. According to the Gibb's free energy prediction, as long as the half-reactions sum such that the overall reaction is favorable, an organism may have the possibility to grow. Methanogens have used this trick, via FBEB, extensively [98]. As long as the total reaction is energetically favorable, a seemingly ‘unfavorable’ reduction reaction can be coupled to a highly favorable reduction reaction. By this principle, methanogens could be theoretically coaxed to produce higher-chain alkanes or other incompletely oxidized carbon molecules in addition to methane so long as the net chemical reaction is exergonic.
Some methanogens use hydrogen as a means to generate a transmembrane ion gradient using a ‘hydrogen cycle’ [68,69,99,100]. In these methanogenic pathways, electrons derived from oxidized substrate are donated to protons, producing intracellular hydrogen gas that crosses the membrane and as reoxidized on the outer surface of the cell membrane by external-facing hydrogenases, thus resulting in scalar proton translocation that can be used to drive ATP synthesis. Methylotrophic methanogens such as Methanosarcina acetivorans that can grow without an additional electron donor (distinguished from methanogens like Methanomasiliiicoccales that grow by reducing MeOH with H2) have evolved to route electrons through the cell via an unknown network of flavins and Fe/S clusters or other organometallic cofactors [101–104]. These protein : protein interactions are essential for conserving energy, increasing the rate of metabolism, and would be critical to identify and engineer in order to manipulate or overcome substrate channeling to achieve high-yield bioproduct synthesis.
Prediction of microbial metabolism from Gibbs' free energy says nothing of how energy is conserved, only that enough energy may be available. When considering the overall metabolic reaction, as long as the rate of biomass synthesis and respiration/fermentation is net favorable and greater than the reverse degradation/lysis rate, then there will be overall net positive growth. However, the generation time of the cell is not specified (minutes, hours, weeks, or years!). Ultimately, the rate of growth is determined by the sum of the substrate uptake and secretion fluxes less the maintenance energy, which is a lower-bound estimation if the ATP used to maintain cell integrity. The tightness of coupling to pumping oxidoreductases by protein : protein interactions, the intrinsic thermodynamic stability of intracellular metabolites (aldehydes and amines), and the ‘leakiness’ of membrane transporters all contribute to the net metabolic flux of the cell. Methanogens could yet be discovered that have any combination of high/low BEQ and high/low maintenance energy requirements.
Methanogens have also been shown to grow by electrosynthesis [105–109]. In electrosynthetic respiration, the Gibbs' reaction may be unfavorable unless electrons are balanced, such as by coupling to consumption or generation of an electrical current. Through an unknown mechanism that could include diffusion of extracellular electron carriers, electrically conductive nanowires or pili, or direct membrane/mineral or electrode contact with anodes or cathodes, methanogens could possibly grow using chemistry that would be unfavorable by the Gibbs' free energy criterion in an enclosed culture tube so long as the current generated at the electrode satisfies the electron input, in so-called unbalanced fermentation [110]. Finally, it is formally possible that an organism could grow methano-photosynthetically by using photons to conserve energy or to photocatalyze metabolic reactions. Methanogens synthesize photoactive cofactors, such as cytochromes, corrinoids, hemes, F430 tetrapyrrole, methanophenazine, and deazaflavin F420, and have predicted carotenoid biosynthetic genes, all with the potential to act as chromophores that could be coupled to transmembrane ion pumping or photocatalytic redox reactions.
Reversibility
Unlike most organisms, methanogens have a ‘reversible’ metabolism. The reversibility of methanogenesis is due to the dependence on hydrogenases and thiol cofactors CoM-SH and CoB-SH that are used extensively in methanogenesis pathways [111]. Hydrogenases use Fe/Fe or Ni/Fe clusters that have a low energetic barrier in either the forward or reverse directions. The thermodynamics of H2 is also highly concentration dependent, which means that under high H2 concentrations, a hydrogenase will function in the forward direction, and under low H2 partial pressures, the same enzyme can catalyze the reverse biochemical reaction. Sulfur is a reactive metalloid element that can form atomic orbital hybridization states that facilitate rapid π → π* electronic transitions. Thus, the energetic barrier for interconverting S-C, S-S, S-H, S-Ni, S-Fe, and S-Co bonds is low and easily reversible. Reversibility could also be facilitated by close proximity of electron donors and acceptors in large oxidoreductase complexes [89,90,104]. By forming complexes, substrates could efficiently channel from one end of a pathway to the other with minimal entropic decay at intermediate reaction steps. Methanogens are not studied enough to know the extent of feedback inhibition in regulating metabolism, though genomic evidence (relative dearth of phosphotransfer-mediated cell signaling molecules) suggests that metabolism may be primarily controlled by the rates of input and output fluxes, and is minimally constrained by diffusion-mediated signal transduction or feedback inhibition.
Reverse of methanogenesis, methane oxidation to CO2, has been demonstrated by several groups [112–114]. In these cases, it is unresolved if growth occurred in the reverse direction. However, electron bifurcation suggests that while confurcation may be entropically constrained, thus forming a high temporal/spatial barrier, it is not impossible if substrate channeling and electron bifurcation by Hdr are invoked [92,115]. Phylogenomic evidence suggests anaerobic methanotrophic archaea 2a (ANME-2a), organisms that grow by oxidizing methane, solved this problem and were able to successfully evolve from Methanosarcina methanogens [116]. To do so, it is thought they have reversed the direction of Mcr, Hdr, and coupled methane oxidation to reduction of sulfur [115,117]. Theoretically, it is also possible that Fe or other heavy metals could be used as an electron sink. It is also possible that anaerobic methanotrophs could produce intermediate-oxidation state fermentation products such as organic acids, so long as there is a biochemical mechanism to generate a transmembrane ion gradient for ATP synthesis. Subsurface metagenomic data suggest an ever-widening methanogen branch of the tree of life as more mcr homologs are discovered, but the reversibility of methanogenesis enzymes leaves open the question of which of those organisms are operating in the methanogenic or methanotrophic direction. The number of possible electron sinks in the methanotrophic direction is greater than in the methanogenic direction, suggesting that a greater biodiversity is possible for methanotrophs than methanogens. However, methanogens are known to use linear, branched, or circular fermentative or respiratory metabolisms, which allow considerable metabolic diversity and complexity than what one might otherwise consider possible. To make matters more complicated, it is also formally possible that electron bifurcation or the reverse confurcation could allow organisms to grow either way depending on the relative concentrations of methanogenic or methanotrophic substrates in the environment [115].
Methanogens past or yet to be…
Improvements in DNA sequencing technology are allowing researchers to uncover a widening methanogen branch of the tree of life, but there is also an increasing appreciation that we may know very little of the ecology or biochemistry of these ‘new’ methanogens. Beyond requirements for certain metals or lithotrophic substrates, other C sources should also be considered. Recent developments on this front are encouraging with heterotrophic and coal-degrading methanogens being described [4]. Methanogens may also grow using multiple trophic modes, as other organisms can. Many methanogen isolates, to date, are restricted to using one or a few substrates to grow, but Methanosarcina species are capable of using several substrates using different methanogenesis pathways. New methanogens may yet be discovered from other clades with similar substrate flexibility.
Within the past few years, methanogenesis genes have been detected in uncultured archaea outside the Euryarchaea. Based on metagenomic reconstruction of environmental samples, it has been proposed that organisms in the Bathyarchaeota and Verstraetearchaeota phyla are capable of methanogenesis [118,119]. If true, this would support the idea that methanogenesis may have evolved independently more than once in Earth's history [120].
The most complete Bathyarchaeal genomes, to date, BA1 (∼92% complete) and BA2 (∼94% complete), indicate that both organisms contain homologs of methanogenesis genes [118]. BA1 and BA2 genomes were compiled from metagenomic assemblies of DNA isolated from subsurface waters in the Surat Basin. While BA1 appears to definitively contain methyl-S-coenzyme M reductase mcrABG genes, only a putative assignment can be made for mcrCD genes. Similar to Lokiarchea, BA1 has mtrH, but is missing the mtrABCDEFG subunits that couple methyltransferase activity to generation/consumption of a transmembrane sodium gradient. Bathyarchaea contain mvh:hdrABC, hdrD, but lack hdrE as well as the genes for CoM or CoB biosynthesis, fpo, frh, or rnf. While energy-converting hydrogenase ech is present, no other mechanisms for energy conservation or the genes for V/A-type ATP synthase are apparent. The BA2 genome has a complete mcrABGCD operon, but the Wood–Ljungdahl pathway genes are absent or are more closely related to bacterial genes. From these characteristics, it is difficult to assign Bathyarchaea as methanogens. Based on the metagenome sequences and what we know of energy conservation in known methanogens, Bathyarchaea would somehow need to couple methanogenesis to very high substrate fluxes and substrate-level phosphorylation in order to grow. Without isolates or a low-complexity enrichment, we cannot yet definitively confirm that BA1 or BA2 can grow by methanogenesis, and we cannot exclude the possibility that BA1 and BA2 are (methane-oxidizing) acetogens.
Verstraetearchaeota genomes V1, V2, V3, V4, and V5 have been assembled from metagenome datasets obtained from diverse habitats including cellulose-fed or palm oil-fed anaerobic digesters, iso-alkane-degrading methanogenic enrichments from tailing ponds, as well as from the same coal bed waters in the Surat Basin that produced the Bathyarchaea BA1 and BA2 metagenomes [119]. V1, V2, V3, and V4 genomes are 90.3–99.1% complete, while V5 is 60.2% complete. Like Bathyarchaea, Verstraetearchea have a mtrH only, methyltransferase genes, mvh, hdrABC, and an ehb energy-converting hydrogenase. However, Verstraetearchea have a complete set of mcrABGCD genes, ATP synthase, and fpo, but lack cdhabcde, acs, and all of the other genes required for hydrogenotrophic or acetoclastic methanogenesis. Instead, it seems that Verstraetearchea have flexible metabolic capabilities including for fermentation (Embden–Meyerhof–Parnas, EMP), pyruvate:ferredoxin oxidoreductase, an intermediate type II/III ribulose 1,5-biphosphate carboxylase/oxygenase (RuBisCO) for carbon fixation, peptide transporters, and keto acid fermentation genes, as well as archaeal-type adenosine diphosphate (ADP)-forming acetate synthetase (Acd). The metagenome data alone suggest that Verstraetearchea could be methyl-respiring methanogens that generate ATP via chemiosmotic coupling to a transmembrane proton gradient in addition to using substrate-level phosphorylation by sugar or peptide fermentation. However, key details, such as the identity of a membrane electron carrier substrate for fpo, or whether methanogenesis or anaerobic fermentation genes are coexpressed, remain to be resolved.
Predicting methanogen metabolism from mined metagenome sequences tests the limit of what can be predicted by sequence homology, which is especially difficult with respect to hydrogenases, electron transfer proteins, and multienzyme complexes comprising multiple subunits that may only form transient protein:protein interactions or have a level of substrate non-specificity built into optimal function of the cell system. Without isolates or enrichments of newly discovered methanogens, we cannot yet tell if methanogenesis genes are being lost or acquired, when they are expressed, or whether they encode functional proteins. While the biochemistry of methanogenesis in new organisms is fascinating and challenging to decipher, there is a broader world of methanogen capabilities and phenotypes that are yet to be described. Methanogens are known to produce archaella (functionally similar to bacterial flagella yet akin to the bacterial Type IV pilus in structure), and are known to have viruses [43,121]. Other areas of methanogen biology to be explored include finding spore-formers, identifying and characterizing other extracellular appendages, capsule and cell wall synthesis, defining mechanisms for conjugation and genetic recombination, or finding methanogen predators or methanogen pathogens. These areas are wide open for discovering new biological diversity [43,121].
When is an organism no longer a methanogen?
A methanogen is defined by the ability to conserve energy by producing methane gas. To date, all known methanogens are archaea based on 16S rRNA phylogeny, but being an archaeon is not a formal requirement for being a methanogen. Phylogenomic data comparing information processing (16S rRNA gene) and metabolism suggest that methanogens have an ancient metabolism that traces back to the Last Universal Common Ancestor, LUCA [122]. Phylogenetic evidence also suggests that ancient methanogens diversified, and several non-methanogen lineages are thought to have evolved from ancestral methanogens. Archaeoglobales are heterotrophic sulfate-reducing archaea that appear to have lost the ability to grow by methanogenesis because they no longer have the mcr gene, but they retain the rest of the core methanogenesis pathway genes. Halophilic archaea such as Halobacterium that use bacteriorhodopsin and light to conserve energy are also closely related to methanogens by 16S rRNA phylogeny [123]. ANME are also closely related to methanogens. Metagenomic data suggest that ANME methanotrophs use ‘reverse methanogenesis’, using all of the same core methanogenesis enzymes but in the opposite direction, to donate electrons to nitrate, sulfate, or metal oxides through anaerobic respiration [117]. Bathyarchaea and Verstraetearchea, while still uncultured, have several methanogenesis genes that suggest they may be capable of either methylotrophic methanogenesis or methane oxidation [118–120]. Finally, partial genomes of newly discovered Lokiarchaea in the Asgard phylum suggest an ancient link between methanogens and Eukarya, teasing the possibility that the first eukaryote may have been a hydrogenotrophic archeaon with methanogen-like metabolic capabilities that engulfed an Alphaproteobacterium that would become mitochondria [124,125].
Studying methanogen evolution in the past allows us to understand how life has evolved and continues to evolve into the future. However, the span of time that our universe has existed is not so great as to sample all the molecular and biochemical diversity that is chemically feasible. New methanogens, given the right encouragement, are yet to evolve. Metabolic engineering and synthetic biology tools are becoming increasingly sophisticated in methanogens, and because they are astoundingly prolific at producing methane and capable of growing on abundant and inexpensive non-food and waste carbon sources, methanogens hold great promise as a biotechnology platform [126]. Well-developed genetic methods are available in Methanosarcina and Methanococcus species, with numerous shuttle vectors, reporter genes, and antibiotic selection markers available [127,128]. Methanosarcinales have comparatively diverse metabolic modes with the ability to grow using several methanogenesis pathways and lithoautotrophic or heterotrophic substrates, while Methanococcus species have short generation times and are highly efficient at hydrogenotrophic methanogenesis. Methanogens can grow by respiration or fermentation in which a metabolite derived from a substrate is used as the terminal electron acceptor, albeit unlike in many bacterial fermenters, all methanogens, to date, couple fermentation to generation of a chemiosmotic membrane potential for ATP synthesis [129]. Methanogens harness very little substrate for growth, but convert 60–99% of substrate into methane that can be used to provide electricity and transportation fuel. Therefore, as a biochemical platform, very little substrate is wasted as unwanted host biomass, but a maximum amount of substrate and energy is diverted to respiration or fermentation products [126]. Methanogens can already grow by producing or using methane by taking advantage of the low energy barrier of C–S bond formation and coupling that energy to synthesis of central metabolites and biomass. By creatively applying the knowledge of redox biochemistry, microbial physiological diversity, and bioenergetics, methanogens have the potential to be coaxed into producing industrially important chemicals from non-food substrates using non-potable water with the specificity that can only be achieved by biological catalysts [126,130–132]. The discoveries of ‘reverse methanogen’ methanotrophs [117], ex-methanogen sulfate-reducing archaea, and heterotrophic aerobic archaea show us that Nature has already laid the blueprint for effective strategies to expand the metabolic diversity of methanogens.
Summary
Methanogens are defined as any organism that conserves energy by producing methane gas. To date, all methanogens are archaea.
The Wolfe Cycle is used by methanogenic archaea to grow from one-carbon substrates or acetate.
Thermodynamics can be used to explain methanogen metabolism and also to predict or design new methanogenic pathways.
Studying methanogen metabolism and biochemistry has produced groundbreaking discoveries that continue to push the boundaries of Biology today.
New methanogens are waiting to be cultured or created using synthetic biology approaches.
Abbreviations
- ANME
anaerobic methanotrophic archaea
- ATP
adenosine triphosphate
- CO2
carbon dioxide
- CO
carbon monoxide
- CH4
methane
- kJ
kilojoule
- mV
millivolts
- ΔG°′
change in Gibbs' free energy
- ΔH
change in enthalpy
- T
temperature
- ΔS
change in entropy
- e−
electron
- n
number of electrons
- F
Faraday constant
- E°′
redox potential
- Fd
ferredoxin
- Fdox
oxidized ferredoxin
- Fdred
reduced ferredoxin
- F420
deazaflavin coenzyme F420
- F420H2
reduced coenzyme F420
- F430
nickel-tetrapyrrole coenzyme F430
- H4MPT
tetrahydromethanopterin
- Mtr
methylene-H4MPT:CoM methyl transferase
- Rnf
ferredoxin:methanophenazine oxidoreductase
- Mrp
sodium/proton antiporter
- CoM-SH
coenzyme M thiol
- CoB-SH
coenzyme B thiol
- CoM-S-S-CoB
coenzyme M-coenzyme B heterodisulfide
- Hdr
CoM-S-S-CoB heterodisulfide reductase
- MPh
methanophenazine
- MPhH2
reduced methanophenazine
- ME
maintenance energy
- BEQ
biological energy quotient
- Tg
teragrams
Funding
N.R.B. has disclosed a significant financial interest in RollingCircle Biotech, LLC and Molecular Trait Evolution, LLC. This work was supported by a Cycle 12 award from the Nebraska Center for Energy Sciences. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the funding agency.
Competing Interests
The Author declares that there are no competing interests associated with this manuscript.
References
- 1.Wolfe R.S. (ed) (1993) An Historical Overview of Methanogenesis, Chapman & Hall Microbiology Series, Boston, MA [Google Scholar]
- 2.Stephenson M. and Stickland L.C.H. (1933) Hydrogenase: the bacterial formation of methane by the reduction of one-carbon compounds by molecular hydrogen. Biochem. J. 27, 1517–1527 10.1042/bj0271517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Macario E.C. and Macario A.J.L. (2009) Methanogenic archaea in health and disease: a novel paradigm of microbial pathogenesis. Int. J. Med. Microbiol. 299, 99–108 10.1016/j.ijmm.2008.06.011 [DOI] [PubMed] [Google Scholar]
- 4.Mayumi D., Mochimaru H., Tamaki H., Yamamoto K., Yoshioka H., Suzuki Y. et al. (2016) Methane production from coal by a single methanogen. Science 354, 222–225 10.1126/science.aaf8821 [DOI] [PubMed] [Google Scholar]
- 5.Daniels L., Fuchs G., Thauer R.K. and Zeikus J.G. (1977) Carbon-monoxide oxidation by methanogenic bacteria. J. Bacteriol. 132, 118–126 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rother M. and Metcalf W.W. (2004) Anaerobic growth of Methanosarcina acetivorans C2A on carbon monoxide: an unusual way of life for a methanogenic archaeon. Proc. Natl Acad. Sci. U.S.A. 101, 16929–16934 10.1073/pnas.0407486101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ermler U., Grabarse W., Shima S., Goubeaud M. and Thauer R.K. (1997) Crystal structure of methyl coenzyme M reductase: the key enzyme of biological methane formation. Science 278, 1457–1462 10.1126/science.278.5342.1457 [DOI] [PubMed] [Google Scholar]
- 8.Thauer R.K. (2012) The Wolfe cycle comes full circle. Proc. Natl Acad. Sci. U.S.A. 109, 15084–15085 10.1073/pnas.1213193109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metcalf W.W., Griffin B.M., Cicchillo R.M., Gao J.T., Janga S.C., Cooke H.A. et al. (2012) Synthesis of methylphosphonic acid by marine microbes: a source for methane in the aerobic ocean. Science 337, 1104–1107 10.1126/science.1219875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karl D.M., Beversdorf L., Bjorkman K.M., Church M.J., Martinez A. and DeLong E.F. (2008) Aerobic production of methane in the sea. Nat. Geosci. 1, 473–478 10.1038/ngeo234 [DOI] [Google Scholar]
- 11.Ramanathan V., Cicerone R.J., Singh H.B. and Kiehl J.T. (1985) Trace gas trends and their potential role in climate change. J. Geophys. Res.-Atmos. 90, 5547–5566 10.1029/JD090iD03p05547 [DOI] [Google Scholar]
- 12.Thauer R.K., Kaster A.K., Seedorf H., Buckel W. and Hedderich R. (2008) Methanogenic archaea: ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 6, 579–591 10.1038/nrmicro1931 [DOI] [PubMed] [Google Scholar]
- 13.Dlugokencky E.J., Nisbet E.G. Fisher R. and Lowry D. (2011) Global atmospheric methane: budget, changes and dangers. Philos. Trans. A Math. Phys. Eng. Sci. 369, 2058–2072 10.1098/rsta.2010.0341 [DOI] [PubMed] [Google Scholar]
- 14.Xue K., Yuan M.M., Shi Z.J., Qin Y.J., Deng Y., Cheng L. et al. (2016) Tundra soil carbon is vulnerable to rapid microbial decomposition under climate warming. Nat. Clim. Change 6, 595–600 10.1038/nclimate2940 [DOI] [Google Scholar]
- 15.Rothman D.H., Fournier G.P., French K.L., Alm E.J., Boyle E.A., Cao C.Q. et al. (2014) Methanogenic burst in the end-Permian carbon cycle. Proc. Natl Acad. Sci. U.S.A. 111, 5462–5467 10.1073/pnas.1318106111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rampino M.R., Rodriguez S., Baransky E. and Cai Y. 2017. Global nickel anomaly links Siberian Traps eruptions and the latest Permian mass extinction. Sci. Rep. 7, 12416 10.1038/S41598-017-12759-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnhart E., McClure M., Johnson K., Cleveland S., Hunt K. and Fields M. 2015. Potential role of acetyl-coa synthetase (ACS) and malate dehydrogenase (MAE) in the evolution of the acetate switch in bacteria and archaea. Sci. Rep. 5, 12498 10.1038/srep12498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahring B.K. (1995) Methanogenesis in thermophilic biogas reactors. Antonie Van Leeuwenhoek 67, 91–102 10.1007/BF00872197 [DOI] [PubMed] [Google Scholar]
- 19.Weiland P. (2010) Biogas production: current state and perspectives. Appl. Microbiol. Biotechnol. 85, 849–860 10.1007/s00253-009-2246-7 [DOI] [PubMed] [Google Scholar]
- 20.Stillwell A., Hoppock D. and Webber M. (2010) Energy recovery from wastewater treatment plants in the United States: a case study of the energy-water nexus. Sustainability 2, 945–962 10.3390/su2040945 [DOI] [Google Scholar]
- 21.Shen Y.W., Linville J.L., Urgun-Demirtas M., Mintz M.M. and Snyder S.W. (2015) An overview of biogas production and utilization at full-scale wastewater treatment plants (WWTPs) in the United States: challenges and opportunities towards energy-neutral WWTPs. Renew. Sust. Energy Rev. 50, 346–362 10.1016/j.rser.2015.04.129 [DOI] [Google Scholar]
- 22.NREL (2013) Energy analysis: biogas potential in the United States. NREL, United States Department of Energy [Google Scholar]
- 23.Holm-Nielsen J.B., Al Seadi T. and Oleskowicz-Popiel P. (2009) The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 100, 5478–5484 10.1016/j.biortech.2008.12.046 [DOI] [PubMed] [Google Scholar]
- 24.Fetzer S. and Conrad R. (1993) Effect of redox potential on methanogenesis by Methanosarcina barkeri. Arch. Microbiol. 160, 108–113 10.1007/BF00288711 [DOI] [Google Scholar]
- 25.Hungate R.E. (1950) The anaerobic mesophilic cellulolytic bacteria. Bacteriol. Rev. 14, 1–49 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balch W.E. and Wolfe R.S. (1976) New approach to cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl. Environ. Microbiol. 32, 781–791 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryant M.P., Wolin E.A., Wolin M.J. and Wolfe R.S. (1967) Methanobacillus omelianskii a symbiotic association of 2 species of bacteria. Arch. Mikrobiol. 59, 20–31 10.1007/BF00406313 [DOI] [PubMed] [Google Scholar]
- 28.Sieber J.R., McInerney M.J. and Gunsalus R.P. (2012) Genomic insights into syntrophy: the paradigm for anaerobic metabolic cooperation. Ann. Rev. Microbiol. 66, 429–452 10.1146/annurev-micro-090110-102844 [DOI] [PubMed] [Google Scholar]
- 29.Kato S., Hashimoto K. and Watanabe K. (2012) Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environ. Microbiol. 14, 1646–1654 10.1111/j.1462-2920.2011.02611.x [DOI] [PubMed] [Google Scholar]
- 30.Ishii S., Kosaka T., Hori K., Hotta Y. and Watanabe K. (2005) Coaggregation facilitates interspecies hydrogen transfer between Pelotomaculum thermopropionicum and Methanothermobacter thermautotrophicus. Appl. Environ. Microbiol. 71, 7838–7845 10.1128/AEM.71.12.7838-7845.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woese C.R. and Fox G.E. (1977) Phylogenetic structure of prokaryotic domain – primary kingdoms. Proc. Natl Acad. Sci. U.S.A. 74, 5088–5090 10.1073/pnas.74.11.5088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balch W.E., Fox G.E., Magrum L.J., Woese C.R. and Wolfe R.S. (1979) Methanogens: re-evaluation of a unique biological group. Microbiol. Rev. 43, 260–296 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox G.E., Magrum L.J., Balch W.E., Wolfe R.S. and Woese C.R. (1977) Classification of methanogenic bacteria by 16S ribosomal RNA characterization. Proc. Natl Acad. Sci. U.S.A. 74, 4537–4541 10.1073/pnas.74.10.4537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koga Y., Nishihara M., Morii H. and Akagawa-Matsushita M. (1993) Ether polar lipids of methanogenic bacteria: structures, comparative aspects, and biosyntheses. Microbiol. Rev. 57, 164–182 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comita P.B. and Gagosian R.B. (1983) Membrane lipid from deep-sea hydrothermal vent methanogen: a new macrocyclic glycerol diether. Science 222, 1329–1331 10.1126/science.222.4630.1329 [DOI] [PubMed] [Google Scholar]
- 36.Tornabene T.G. and Langworthy T.A. (1979) Diphytanyl and dibiphytanyl glycerol ether lipids of methanogenic archaebacteria. Science 203, 51–53 10.1126/science.758677 [DOI] [PubMed] [Google Scholar]
- 37.Tornabene T.G., Wolfe R.S., Balch W.E., Holzer G., Fox G.E. and Oro J. (1978) Phytanyl-glycerol ethers and squalenes in the archaebacterium Methanobacterium thermoautotrophicum. J. Mol. Evol. 11, 259–266 10.1007/BF01734487 [DOI] [PubMed] [Google Scholar]
- 38.König H. and Kandler O. (1979) The amino acid sequence of the peptide moiety of the pseudomurein from Methanobacterium thermoautotrophicum. Arch. Microbiol. 121, 271–275 10.1007/BF00425067 [DOI] [PubMed] [Google Scholar]
- 39.König H. and Kandler O. (1980) 2-amino-2-deoxytaluronic acid and 2-amino-2-deoxyglucose from the pseudomurein of Methanobacterium termoautotrophicum possess the l- and d-configurations, respectively. Hoppe Seylers Z. Physiol. Chem. 361, 981–983 PMID: [PubMed] [Google Scholar]
- 40.König H., Kandler O., Jensen M. and Rietschel E.T. (1983) The primary structure of the glycan moiety of pseudomurein from Methanobacterium thermoautotrophicum. Hoppe Seylers Z. Physiol. Chem. 364, 627–636 10.1515/bchm2.1983.364.1.627 [DOI] [PubMed] [Google Scholar]
- 41.Eiserling F., Pushkin A., Gingery M. and Bertani G. (1999) Bacteriophage-like particles associated with the gene transfer agent of Methanococcus voltae PS. J. Gen. Virol. 80, 3305–3308 10.1099/0022-1317-80-12-3305 [DOI] [PubMed] [Google Scholar]
- 42.Nolling J., Groffen A. and Devos W.M.. 1993. Phi-F1 and Phi-F3, 2 novel virulent, archaeal phages infecting different thermophilic strains of the genus Methanobacterium. J. Gen. Microbiol. 139, 2511–2516. 10.1099/00221287-139-10-2511 [DOI] [Google Scholar]
- 43.Meile L., Jenal U., Studer D., Jordan M. and Leisinger T. (1989) Characterization of Psi-M1, a virulent phage of Methanobacterium thermoautotrophicum Marburg. Arch. Microbiol. 152, 105–110 10.1007/BF00456085 [DOI] [Google Scholar]
- 44.Gaston M.A., Jiang R. and Krzycki J.A. (2011) Functional context, biosynthesis, and genetic encoding of pyrrolysine. Curr. Opin. Microbiol. 14, 342–349 10.1016/j.mib.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaston M.A., Zhang L., Green-Church K.B. and Krzycki J.A. (2011) The complete biosynthesis of the genetically encoded amino acid pyrrolysine from lysine. Nature 471, 647–650 10.1038/nature09918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rother M. and Krzycki J.A. (2010) Selenocysteine, pyrrolysine, and the unique energy metabolism of methanogenic archaea. Archaea 2010, 1 10.1155/2010/453642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srinivasan G., James C.M. and Krzycki J.A. (2002) Pyrrolysine encoded by UAG in archaea: charging of a UAG-decoding specialized tRNA. Science 296, 1459–1462 10.1126/science.1069588 [DOI] [PubMed] [Google Scholar]
- 48.DiMarco A.A., Bobik T.A. and Wolfe R.S. (1990) Unusual coenzymes of methanogenesis. Annu. Rev. Biochem. 59, 355–394 10.1146/annurev.bi.59.070190.002035 [DOI] [PubMed] [Google Scholar]
- 49.Sousa F.L., Neukirchen S., Allen J.F., Lane N. and Martin W.F. (2016) Lokiarchaeon is hydrogen dependent. Nat. Microbiol. 1, 16034 10.1038/nmicrobiol.2016.34 [DOI] [PubMed] [Google Scholar]
- 50.Spang A., Eme L., Saw J.H., Caceres E.F., Zaremba-Niedzwiedzka K., Lombard J. et al. (2018) Asgard archaea are the closest prokaryotic relatives of eukaryotes. PLoS Genet. 14, e1007080 10.1371/journal.pgen.1007080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaremba-Niedzwiedzka K., Caceres E.F., Saw J.H., Bäckström D., Juzokaite L., Vancaester E. et al. (2017) Asgard archaea illuminate the origin of eukaryotic cellular complexity. Nature 541, 353–358 10.1038/nature21031 [DOI] [PubMed] [Google Scholar]
- 52.Hug L.A., Baker B.J., Anantharaman K., Brown C.T., Probst A.J., Castelle C.J. et al. 2016. A new view of the tree of life. Nat. Microbiol. 1, 16048 10.1038/nmicrobiol.2016.48 [DOI] [PubMed] [Google Scholar]
- 53.Adam P.S., Borrel G., Brochier-Armanet C. and Gribaldo S. (2017) The growing tree of archaea: new perspectives on their diversity, evolution and ecology. ISME J. 11, 2407–2425 10.1038/ismej.2017.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller J.F., Shah N.N., Nelson C.M., Ludlow J.M. and Clark D.S. (1988) Pressure and temperature effects on growth and methane production of the extreme thermophile Methanococcus jannaschii. Appl. Environ. Microbiol. 54, 3039–3042 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor C.D., Mcbride B.C., Wolfe R.S. and Bryant M.P. (1974) Coenzyme-M, essential for growth of a rumen strain of Methanobacterium ruminantium. J. Bacteriol. 120, 974–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bryant M.P., Tzeng S.F., Robinson I.M. and Joyner A.E. (1971) Nutrient requirements of methanogenic bacteria. In Anaerobic Biological Treatment Processes Advances in Chemistry Series 105 (Pohland F.G., ed.), pp. 23–40, American Chemistry Society, Washington, DC: 10.1021/ba-1971-0105.ch003 [DOI] [Google Scholar]
- 57.Schönheit P., Moll J. and Thauer R.K. (1979) Nickel, cobalt, and molybdenum requirement for growth of Methanobacterium thermoautotrophicum. Arch. Microbiol. 123, 105–107 10.1007/BF00403508 [DOI] [PubMed] [Google Scholar]
- 58.Winter J., Lerp C., Zabel H.P., Wildenauer F.X., König H. and Schindler F. (1984) Methanobacterium wolfei, sp. nov., a new tungsten-requiring, thermophilic, autotrophic methanogen. Syst. Appl. Microbiol. 5, 457–466 10.1016/S0723-2020(84)80003-X [DOI] [Google Scholar]
- 59.Tijhuis L., Vanloosdrecht M.C.M. and Heijnen J.J. (1993) A thermodynamically based correlation for maintenance Gibbs energy requirements in aerobic and anaerobic chemotrophic growth. Biotechnol. Bioeng. 42, 509–519 10.1002/bit.260420415 [DOI] [PubMed] [Google Scholar]
- 60.Thauer R.K., Jungermann K. and Decker K. (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 41, 100–180 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoehler T.M. (2007) An energy balance concept for habitability. Astrobiology 7, 824–838 10.1089/ast.2006.0095 [DOI] [PubMed] [Google Scholar]
- 62.Hoehler T.M. (2004) Biological energy requirements as quantitative boundary conditions for life in the subsurface. Geobiology 2, 205–215 10.1111/j.1472-4677.2004.00033.x [DOI] [Google Scholar]
- 63.Schink B. (1997) Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 61, 262–280 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turina P., Samoray D. and Gräber P. (2003) H+/ATP ratio of proton transport-coupled ATP synthesis and hydrolysis catalysed by CF0F1-liposomes. EMBO J. 22, 418–426 10.1093/emboj/cdg073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Müller V. and Hess V. (2017) The minimum biological energy quantum. Front. Microbiol. 8, 2019 10.3389/fmicb.2017.02019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thauer R.K. (1998) Biochemistry of methanogenesis: a tribute to Marjory Stephenson. 1998 Marjory Stephenson Prize Lecture. Microbiology 144, 2377–2406 10.1099/00221287-144-9-2377 [DOI] [PubMed] [Google Scholar]
- 67.Tietze M., Beuchle A., Lamla I., Orth N., Dehler M., Greiner G. et al. (2003) Redox potentials of methanophenazine and CoB-S-S-CoM, factors involved in electron transport in methanogenic archaea. ChemBioChem 4, 333–335 10.1002/cbic.200390053 [DOI] [PubMed] [Google Scholar]
- 68.Odom J.M. and Peck H.D. (1981) Hydrogen cycling as a general mechanism for energy coupling in the sulfate-reducing bacteria, Desulfovibrio sp. FEMS Microbiol. Lett. 12, 47–50 10.1111/j.1574-6968.1981.tb07609.x [DOI] [Google Scholar]
- 69.Kulkarni G., Kridelbaugh D.M., Guss A.M. and Metcalf W.W. (2009) Hydrogen is a preferred intermediate in the energy-conserving electron transport chain of Methanosarcina barkeri. Proc. Natl Acad. Sci. U.S.A. 106, 15915–15920 10.1073/pnas.0905914106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gottschalk G. and Thauer R.K. (2001) The Na+-translocating methyltransferase complex from methanogenic archaea. Biochim. Biophys. Acta 1505, 28–36 PMID: [DOI] [PubMed] [Google Scholar]
- 71.Schlegel K., Welte C., Deppenmeier U. and Müller V. (2012) Electron transport during aceticlastic methanogenesis by Methanosarcina acetivorans involves a sodium-translocating RNF complex. FEBS J. 279, 4444–4452 10.1111/febs.12031 [DOI] [PubMed] [Google Scholar]
- 72.Harder J. (1997) Species-independent maintenance energy and natural population sizes. FEMS Microbiol. Ecol. 23, 39–44 10.1111/j.1574-6941.1997.tb00389.x [DOI] [Google Scholar]
- 73.Yuliati L. and Yoshida H. (2008) Photocatalytic conversion of methane. Chem. Soc. Rev. 37, 1592–1602 10.1039/b710575b [DOI] [PubMed] [Google Scholar]
- 74.Hoehler T., Gunsalus R.P. and McInerney M.J. (2010) Environmental constraints that limit methanogenesis In Handbook of Hydrocarbon and Lipid Microbiology (Timmis K., ed.), pp. 635–654, Springer, Cham [Google Scholar]
- 75.Grabarse W., Mahlert F., Duin E.C., Goubeaud M., Shima S., Thauer R.K. et al. (2001) On the mechanism of biological methane formation: structural evidence for conformational changes in methyl-coenzyme M reductase upon substrate binding. J. Mol. Biol. 309, 315–330 10.1006/jmbi.2001.4647 [DOI] [PubMed] [Google Scholar]
- 76.Farber G., Keller W., Kratky C., Jaun B., Pfaltz A., Spinner C. et al. (1991) Coenzyme F430 from methanogenic bacteria: complete assignment of configuration based on an X-ray analysis of 12,13-Diepi-F430 pentamethyl ester and on NMR spectroscopy. Helv. Chim. Acta 74, 697–716 10.1002/hlca.19910740404 [DOI] [Google Scholar]
- 77.Thauer R.K. and Bonacker L.G. (1994) Biosynthesis of coenzyme F-430, a nickel porphinoid involved in methanogenesis. Biosynthesis of the tetrapyrrole pigments. Ciba Found. Symp. 180, 210–222 PMID: [DOI] [PubMed] [Google Scholar]
- 78.Buan N.R. and Metcalf W.W. (2010) Methanogenesis by Methanosarcina acetivorans involves two structurally and functionally distinct classes of heterodisulfide reductase. Mol. Microbiol. 75, 843–853 10.1111/j.1365-2958.2009.06990.x [DOI] [PubMed] [Google Scholar]
- 79.Duin E.C., Bauer C., Jaun B. and Hedderich R. (2003) Coenzyme M binds to a [4Fe-4S] cluster in the active site of heterodisulfide reductase as deduced from EPR studies with the [33S]coenzyme M-treated enzyme. FEBS Lett. 538, 81–84 10.1016/S0014-5793(03)00134-0 [DOI] [PubMed] [Google Scholar]
- 80.Duin E.C., Madadi-Kahkesh S., Hedderich R., Clay M.D. and Johnson M.K. (2002) Heterodisulfide reductase from Methanothermobacter marburgensis contains an active-site [4Fe-4S] cluster that is directly involved in mediating heterodisulfide reduction. FEBS Lett. 512, 263–268 10.1016/S0014-5793(02)02281-0 [DOI] [PubMed] [Google Scholar]
- 81.Hedderich R., Hamann N. and Bennati M. (2005) Heterodisulfide reductase from methanogenic archaea: a new catalytic role for an iron-sulfur cluster. Biol. Chem. 386, 961–970 10.1515/BC.2005.112 [DOI] [PubMed] [Google Scholar]
- 82.Simianu M., Murakami E., Brewer J.M. and Ragsdale S.W. (1998) Purification and properties of the heme- and iron-sulfur-containing heterodisulfide reductase from Methanosarcina thermophila. Biochemistry 37, 10027–10039 10.1021/bi9726483 [DOI] [PubMed] [Google Scholar]
- 83.Buckel W. and Thauer R.K. (2018) Flavin-based electron bifurcation, a new mechanism of biological energy coupling. Chem. Rev. 118, 3862–3886 10.1021/acs.chemrev.7b00707 [DOI] [PubMed] [Google Scholar]
- 84.Buckel W. and Thauer R.K (2013) Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation. Biochim. Biophys. Acta 1827, 94–113 10.1016/j.bbabio.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 85.Mitchell P. (1975) The protonmotive Q cycle: a general formulation. FEBS Lett. 59, 137–139 10.1016/0014-5793(75)80359-0 [DOI] [PubMed] [Google Scholar]
- 86.Mitchell P. (1975) Protonmotive redox mechanism of the cytochrome b-c1 complex in the respiratory chain: protonmotive ubiquinone cycle. FEBS Lett. 56, 1–6 10.1016/0014-5793(75)80098-6 [DOI] [PubMed] [Google Scholar]
- 87.Müller V., Chowdhury N.P. and Basen M. (2018) Electron bifurcation: a long-hidden energy-coupling mechanism. Annu. Rev. Microbiol. 72, 331–353 10.1146/annurev-micro-090816-093440 [DOI] [PubMed] [Google Scholar]
- 88.Garcia Costas AM G., Poudel S., Miller A.F., Schut G.J., Ledbetter R.N., Fixen K.R. et al. (2017) Defining electron bifurcation in the electron-transferring flavoprotein family. J. Bacteriol. 199, e00440-17 10.1128/JB.00440-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Costa K.C., Lie T.J., Xia Q. and Leigh J.A. (2013) VhuD facilitates electron flow from H2 or formate to heterodisulfide reductase in Methanococcus maripaludis. J. Bacteriol. 195, 5160–5165 10.1128/JB.00895-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Costa K.C., Wong P.M., Wang T., Lie T.J., Dodsworth J.A., Swanson I. et al. (2010) Protein complexing in a methanogen suggests electron bifurcation and electron delivery from formate to heterodisulfide reductase. Proc. Natl Acad. Sci. U.S.A. 107, 11050–11055 10.1073/pnas.1003653107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rouvière P.E. and Wolfe R.S. (1988) Novel biochemistry of methanogenesis. J. Biol. Chem. 263, 7913–7916 [PubMed] [Google Scholar]
- 92.Catlett J.L., Ortiz A.M. and Buan N.R. (2015) Rerouting cellular electron flux to increase the rate of biological methane production. Appl. Environ. Microbiol. 81, 6528–6537 10.1128/AEM.01162-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li Q.B., Li L.Y., Rejtar T., Lessner D.J., Karger B.L. and Ferry J.G. (2006) Electron transport in the pathway of acetate conversion to methane in the marine archaeon Methanosarcina acetivorans. J. Bacteriol. 188, 702–710 10.1128/JB.188.2.702-710.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oelgeschläger E. and Rother M. (2008) Carbon monoxide-dependent energy metabolism in anaerobic bacteria and archaea. Arch. Microbiol. 190, 257–269 10.1007/s00203-008-0382-6 [DOI] [PubMed] [Google Scholar]
- 95.Rother M., Oelgeschlager E. and Metcalf W.M. (2007) Genetic and proteomic analyses of CO utilization by Methanosarcina acetivorans. Arch. Microbiol. 188, 463–472 10.1007/s00203-007-0266-1 [DOI] [PubMed] [Google Scholar]
- 96.Laso-Pérez R., Wegener G., Knittel K., Widdel F., Harding K.J., Krukenberg V. et al. (2016) Thermophilic archaea activate butane via alkyl-coenzyme M formation. Nature 539, 396–401 10.1038/nature20152 [DOI] [PubMed] [Google Scholar]
- 97.Beinart R.A., Rotterová J., Čepička I., Gast R.J. and Edgcomb V.P. (2018) The genome of an endosymbiotic methanogen is very similar to those of its free-living relatives. Environ. Microbiol. 20, 2538–2551 10.1111/1462-2920.14279 [DOI] [PubMed] [Google Scholar]
- 98.Nitschke W. and Russell M.J. (2012) Redox bifurcations: mechanisms and importance to life now, and at its origin: a widespread means of energy conversion in biology unfolds. BioEssays 34, 106–109 10.1002/bies.201100134 [DOI] [PubMed] [Google Scholar]
- 99.Kulkarni G., Mand T.D. and Metcalf W.W. (2018) Energy conservation via hydrogen cycling in the methanogenic archaeon Methanosarcina barkeri. MBio 9, e01256-18 10.1128/mBio.01256-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mand T.D., Kulkarni G. and Metcalf W.W. (2018) Genetic, biochemical, and molecular characterization of Methanosarcina barkeri mutants lacking three distinct classes of hydrogenase. J. Bacteriol. 200, e00342-18 10.1128/JB.00342-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paul K., Nonoh J.O., Mikulski L. and Brune A. (2012) “Methanoplasmatales,” thermoplasmatales-related archaea in termite guts and other environments, are the seventh order of methanogens. Appl. Environ. Microbiol. 78, 8245–8253 10.1128/AEM.02193-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dridi B., Fardeau M.L., Ollivier B., Raoult D. and Drancourt M. (2012) Methanomassiliicoccus luminyensis gen. nov., sp nov., a methanogenic archaeon isolated from human faeces. Int. J. Syst. Evol. Microbiol. 62, 1902–1907 10.1099/ijs.0.033712-0 [DOI] [PubMed] [Google Scholar]
- 103.Sowers K.R., Baron S.F. and Ferry J.G. (1984) Methanosarcina acetivorans sp nov, an acetotrophic methane-producing bacterium isolated from marine sediments. Appl. Environ. Microbiol. 47, 971–978 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lieber D.J., Catlett J., Madayiputhiya N., Nandakumar R., Lopez M.M., Metcalf W.W. et al. (2014) A multienzyme complex channels substrates and electrons through acetyl-CoA and methane biosynthesis pathways in methanosarcina. PLoS ONE 9, e107563 10.1371/journal.pone.0107563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rotaru A.E., Shrestha P.M., Liu F., Markovaite B., Chen S., Nevin K.P. et al. (2014) Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 80, 4599–4605 10.1128/AEM.00895-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marshall C.W., Ross D.E., Fichot E.B., Norman R.S. and May H.D. (2012) Electrosynthesis of commodity chemicals by an autotrophic microbial community. Appl. Environ. Microbiol. 78, 8412–8420 10.1128/AEM.02401-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Beese-Vasbender P.F., Grote J.P., Garrelfs J., Stratmann M. and Mayrhofer K.J. (2015) Selective microbial electrosynthesis of methane by a pure culture of a marine lithoautotrophic archaeon. Bioelectrochemistry 102, 50–55 10.1016/j.bioelechem.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 108.Lienemann M., Deutzmann J.S., Milton R.D., Sahin M. and Spormann A.M. (2018) Mediator-free enzymatic electrosynthesis of formate by the Methanococcus maripaludis heterodisulfide reductase supercomplex. Bioresour. Technol. 254, 278–283 10.1016/j.biortech.2018.01.036 [DOI] [PubMed] [Google Scholar]
- 109.Deutzmann J.S. and Spormann A.M. (2017) Enhanced microbial electrosynthesis by using defined co-cultures. ISME J. 11, 704–714 10.1038/ismej.2016.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Flynn J.M., Ross D.E., Hunt K.A., Bond D.R. and Gralnick J.A. (2010) Enabling unbalanced fermentations by using engineered electrode-interfaced bacteria. MBio 1, e00190-10 10.1128/mBio.00190-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thauer R.K. (2011) Anaerobic oxidation of methane with sulfate: on the reversibility of the reactions that are catalyzed by enzymes also involved in methanogenesis from CO2. Curr. Opin. Microbiol. 14, 292–299 10.1016/j.mib.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 112.McAnulty M.J., Poosarla V.G., Li J.N., Soo V.W.C., Zhu F.Y. and Wood T.K. (2017) Metabolic engineering of Methanosarcina acetivorans for lactate production from methane. Biotechnol. Bioeng. 114, 852–861 10.1002/bit.26208 [DOI] [PubMed] [Google Scholar]
- 113.Moran J.J., House C.H., Thomas B. and Freeman K.H. (2007) Products of trace methane oxidation during nonmethyltrophic growth by Methanosarcina. J. Geophys. Res.-Biogeosci. 112, 1–17 10.1029/2006JG000268 [DOI] [Google Scholar]
- 114.Zehnder A.J.B. and Brock T.D. (1979) Methane formation and methane oxidation by methanogenic bacteria. J. Bacteriol. 137, 420–432 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yan Z. and Ferry J.G. (2018) Electron bifurcation and confurcation in methanogenesis and reverse methanogenesis. Front. Microbiol. 9, 1322 10.3389/fmicb.2018.01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang F.P., Zhang Y., Chen Y., He Y., Qi J., Hinrichs K.U. et al. (2014) Methanotrophic archaea possessing diverging methane-oxidizing and electron-transporting pathways. ISME J. 8, 1069–1078 10.1038/ismej.2013.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Timmers P.H., Welte C.U., Koehorst J.J., Plugge C.M., Jetten M.S.M. and Stams A.J.M. (2017) Reverse methanogenesis and respiration in methanotrophic archaea. Archaea 2017, 1654237 10.1155/2017/1654237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Evans P.N., Parks D.H., Chadwick G.L., Robbins S.J., Orphan V.J., Golding S.D. et al. (2015) Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics. Science 350, 434–438 10.1126/science.aac7745 [DOI] [PubMed] [Google Scholar]
- 119.Vanwonterghem I., Evans P.N., Parks D.H., Jensen P.D., Woodcroft B.J., Hugenholtz P. et al. (2016) Methylotrophic methanogenesis discovered in the archaeal phylum Verstraetearchaeota. Nat. Microbiol. 1, 16170 10.1038/nmicrobiol.2016.170 [DOI] [PubMed] [Google Scholar]
- 120.Castelle C.J. and Banfield J.F. (2018) Major new microbial groups expand diversity and alter our understanding of the tree of life. Cell 172, 1181–1197 10.1016/j.cell.2018.02.016 [DOI] [PubMed] [Google Scholar]
- 121.Thomas N.A. and Jarrell K.F. (2001) Characterization of flagellum gene families of methanogenic archaea and localization of novel flagellum accessory proteins. J. Bacteriol. 183, 7154–7164 10.1128/JB.183.24.7154-7164.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ueno Y., Yamada K., Yoshida N., Maruyama S. and Isozaki Y. (2006) Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era. Nature 440, 516–519 10.1038/nature04584 [DOI] [PubMed] [Google Scholar]
- 123.Sorokin D.Y., Makarova K.S., Abbas B., Ferrer M., Golyshin P.N., Galinski E.A. et al. (2017) Discovery of extremely halophilic, methyl-reducing euryarchaea provides insights into the evolutionary origin of methanogenesis. Nat. Microbiol. 2, 17081 10.1038/nmicrobiol.2017.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.López-García P., Eme L. and Moreira D. (2017) Symbiosis in eukaryotic evolution. J. Theor. Biol. 434, 20–33 10.1016/j.jtbi.2017.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martin W. and Müller M. (1998) The hydrogen hypothesis for the first eukaryote. Nature 392, 37–41 10.1038/32096 [DOI] [PubMed] [Google Scholar]
- 126.Costa K.C. and Leigh J.A. (2014) Metabolic versatility in methanogens. Curr. Opin. Biotechnol. 29, 70–75 10.1016/j.copbio.2014.02.012 [DOI] [PubMed] [Google Scholar]
- 127.Leigh J.A., Albers S.V., Atomi H. and Allers T. (2011) Model organisms for genetics in the domain Archaea: methanogens, halophiles, Thermococcales and Sulfolobales. FEMS Microbiol. Rev. 35, 577–608 10.1111/j.1574-6976.2011.00265.x [DOI] [PubMed] [Google Scholar]
- 128.Buan N., Kulkarni G. and Metcalf W. (2011) Genetic methods for methanosarcina species. Methods Enzymol. 494, 23–42 10.1016/B978-0-12-385112-3.00002-0 [DOI] [PubMed] [Google Scholar]
- 129.Diender M., Stams A.J. and Sousa D.Z. (2015) Pathways and bioenergetics of anaerobic carbon monoxide fermentation. Front. Microbiol. 6, 1275 10.3389/fmicb.2015.01275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ferry J.G. (2011) Fundamentals of methanogenic pathways that are key to the biomethanation of complex biomass. Curr. Opin. Biotechnol. 22, 351–357 10.1016/j.copbio.2011.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.De Vrieze J., Hennebel T., Boon N. and Verstraete W. (2012) Methanosarcina: the rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 112, 1–9 10.1016/j.biortech.2012.02.079 [DOI] [PubMed] [Google Scholar]
- 132.Lessner D.J., Lhu L., Wahal C.S. and Ferry J.G. (2010) An engineered methanogenic pathway derived from the domains Bacteria and Archaea. MBio 1, e00243-10 10.1128/mBio.00243-10 [DOI] [PMC free article] [PubMed] [Google Scholar]



