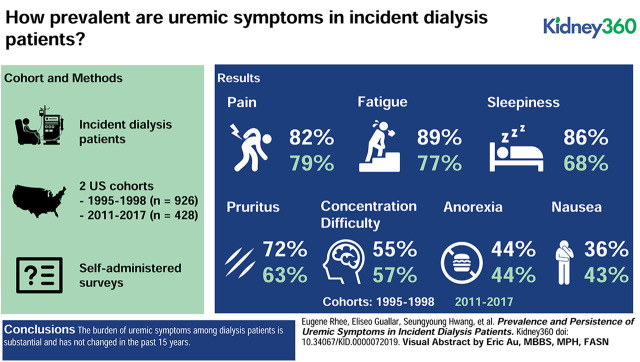
Visual Abstract
Keywords: Dialysis, Quality of Life, Renal Dialysis, Symptoms, Uremia
Abstract
Background
Uremic symptoms are major contributors to the poor quality of life among patients on dialysis, but whether their prevalence or intensity has changed over time is unknown.
Methods
We examined responses to validated questionnaires in two incident dialysis cohort studies, the Choices for Health Outcomes in Caring for ESRD (CHOICE) study (N=926, 1995–1998) and the Longitudinal United States/Canada Incident Dialysis (LUCID) study (N=428, 2011–2017). We determined the prevalence and severity of uremic symptoms—anorexia, nausea/vomiting, pruritus, sleepiness, difficulty concentrating, fatigue, and pain—in both cohorts.
Results
In CHOICE and LUCID, respectively, mean age of the participants was 58 and 60 years, 53% and 60% were male, and 28% and 32% were black. In both cohorts, 54% of the participants had diabetes. Median time from dialysis initiation to the symptoms questionnaires was 45 days for CHOICE and 77 days for LUCID. Uremic symptom prevalence in CHOICE did not change from baseline to 1-year follow-up and was similar across CHOICE and LUCID. Baseline symptom prevalence in CHOICE and LUCID was as follows: anorexia (44%, 44%, respectively), nausea/vomiting (36%, 43%), pruritus (72%, 63%), sleepiness (86%, 68%), difficulty concentrating (55%, 57%), fatigue (89%, 77%), and pain (82%, 79%). In both cohorts, >80% of patients had three or more symptoms and >50% had five or more symptoms. The correlation between individual symptoms was low (ρ<0.5 for all comparisons). In CHOICE, no clinical or laboratory parameter was strongly associated with multiple symptoms.
Conclusions
The burden of uremic symptoms among patients on dialysis is substantial and has not changed in the past 15 years. Improving quality of life will require identification of the factors that underlie the pathogenesis of uremic symptoms and better ways of removing the toxins that are responsible.
Introduction
Over 1 million people will start dialysis for ESKD in the next decade (1). Although dialysis prevents death from kidney failure over the short term, many patients continue to suffer from uremic symptoms including anorexia, nausea, pruritus, fatigue, excessive daytime sleepiness, difficulty concentrating, and pain (2). Uremic symptoms are a major contributor to the poor health-related quality of life experienced by patients on dialysis, which is often a greater concern than survival for these patients. Indeed, when asked about the possibility of improvement in quality of life or survival by switching to intensive hemodialysis, 94% would consider it for improving energy, 57% for improving sleep, but only 19% would consider it for improving survival at 3 years (3). Further, “the best ways to manage symptoms in people receiving or nearing dialysis, including poor energy and nausea” was identified as a top research priority in a survey of patients on dialysis and the providers caring for them (4,5).
Despite this significant interest from both patients and providers, relatively few studies have rigorously examined uremic symptoms in large, longitudinal ESKD cohorts, leaving several notable gaps in the existing literature. First, prevalence studies have often focused on individual symptoms rather than a broad range of symptoms (6). Second, how uremic symptoms change over time after dialysis initiation has not been examined. Third, no studies have addressed whether the burden of uremic symptoms experienced by patients on dialysis has changed over the past two decades, as might be expected given the numerous changes in clinical practice over that time.
To address these gaps, we analyzed data from two prospective cohort studies of patients who had recently initiated dialysis, the Choices for Health Outcomes in Caring for ESRD (CHOICE) study, which enrolled patients from 1995 to 1998, and the Longitudinal United States/Canada Incident Dialysis (LUCID) study, which enrolled patients from 2011 to 2017. We compared symptom burden both at study entry and at 1 year after enrollment in CHOICE, as well as symptom burden at study entry across both cohorts, which span >20 years of ESKD care. Longitudinal follow-up in CHOICE further enabled an exploratory assessment of the association between symptom burden and longitudinal outcomes. Taken together, our study outlines the prevalence and persistence of uremic symptoms among patients on dialysis and emphasizes the urgent need for an improved understanding of underlying mechanisms (7).
Materials and Methods
We analyzed data from two prospective, incident dialysis cohort studies, the CHOICE study (1995–1998) and the LUCID (2011–2017) study.
CHOICE Study
From October 1995 to June 1998, 1041 participants (767 on hemodialysis and 274 on peritoneal dialysis) were enrolled from 19 states in the United States, a median of 45 days after initiation of dialysis (95% within 3.5 months). Eligibility criteria were initiation of maintenance dialysis therapy in the preceding 3 months, ability to provide informed consent, age >18 years, and ability to speak English or Spanish. Participants were followed for all-cause mortality through December 31, 2008 and for cardiovascular mortality through December 31, 2004. The subset of 926 individuals who filled out the CHOICE Health Experience Questionnaire at study entry was included in this analysis. The Johns Hopkins Medicine Institutional Review Board (Baltimore, MD) and the Dialysis Clinic, Inc. (DCI) Institutional Review Board approved the study, which adhered to the Declaration of Helsinki, and participants provided written informed consent.
LUCID Study
From May 2011 to December 2017, 823 participants (808 on hemodialysis, 15 on peritoneal dialysis) were enrolled in three centers (New England, Washington, Indiana) a median of 77 days after initiation of dialysis (95% within 6.0 months); although the original intent was to include participants from both the United States and Canada in a single cohort, Canadian participants were ultimately enrolled in a separate study. Eligibility criteria were initiation of maintenance dialysis, ability to provide informed consent, and age >18 years. The subset of 428 individuals who filled out a Kidney Disease Quality of Life (KDQOL) survey at study entry was included in this analysis. The Massachusetts General Hospital Institutional Review Board (Boston, MA) approved the study, which adhered to the Declaration of Helsinki, and participants provided written informed consent.
Uremic Symptoms (CHOICE and LUCID)
Symptom prevalence and intensity were assessed by patient responses to self-administered questionnaires conducted at study entry. The CHOICE Health Experience Questionnaire (8) and KDQOL instrument (9) were used in CHOICE and LUCID, respectively. Both studies use similar questions to assess uremic responses, assessing symptoms during the 4 weeks preceding the survey. The responses to seven questions (Supplemental Table 1) were used to assess anorexia, nausea, pruritus, sleepiness, difficulty concentrating, fatigue, and pain. These responses were recorded on a Likert scale and scored in a standardized manner (10). The generated numeric scores have a range of 0–100, with a higher score representing better health (lower severity of symptoms). For exploratory analyses in CHOICE, an overall uremic symptom score was calculated for each study participant as an average of scores for all seven uremic symptoms weighted equally (U-score).
Longitudinal Outcomes (CHOICE)
As an exploratory analysis in CHOICE, we also considered secondary outcomes of all-cause mortality, cardiovascular mortality, and first cardiovascular event. Mortality was adjudicated using information from clinic report, hospital records, the National Death Index, Centers for Medicare and Medicaid Services death notification forms, and Social Security records, as previously described (11). We defined first atherosclerotic cardiovascular event (fatal or nonfatal) as an event due to myocardial infarction, cardiac revascularization procedure, stroke, carotid endarterectomy, extremity gangrene or peripheral revascularization procedure, limb amputation, or abdominal aortic aneurysm repair that occurred after enrollment in the study (11).
Other Covariates
In CHOICE, we collected data on participants’ age, sex, race, residual kidney function (self-reported ability to produce more than one cup of urine daily) and body mass index (BMI). We adjudicated baseline comorbidities including prevalent cardiovascular disease by abstraction of dialysis unit records, hospital discharge summaries, medication lists, consultation notes, diagnostic imaging, and cardiac imaging reports and scoring of the Index of Coexistent Disease by two trained nurses. In CHOICE, we obtained routine laboratory data including serum albumin, creatinine, Kt/Vurea, and phosphate from medical records. In LUCID, we collected data on participants’ age, sex, race, residual kidney function (self-reported ability to produce any urine), BMI, and comorbidities by patient interview and abstraction of dialysis unit records.
Statistical Analyses
We described baseline characteristics of participants in CHOICE and LUCID using means and proportions, as appropriate. We determined the distribution of the uremic symptoms (present versus absent) for each symptom at baseline in both cohorts and compared correlation between symptom scores for individual symptoms using Spearman correlation coefficients. In CHOICE, we further examined the symptoms at year 1 for participants alive at that time point (N=585) and with available data. We examined the cross-sectional association between baseline characteristics and individual symptom scores using univariate linear regression models. In exploratory analysis, we modeled the U-score as a continuous variable to examine associations with longitudinal outcomes. We used Cox proportional hazards regression to evaluate the association between U-score at baseline and outcomes (any-cause death, cardiac death, first cardiovascular event) during follow-up in the CHOICE study. We sequentially adjusted for potential confounders including demographics (age, sex, race), comorbidities (Index of Coexistent Disease severity score, cause of ESKD, BMI [categorized as <18, 18–25, and >25 kg/m2], residual kidney function (self-reported urine volume of more than one cup of urine daily), diabetes, dialysis modality), and predialysis laboratory tests (albumin, urea, Kt/Vurea, creatinine, calcium, phosphate, potassium, glucose, and cholesterol). Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Statistical significance was defined as P<0.05 using two-tailed tests.
Results
Baseline Characteristics of the Study Sample
Baseline characteristics of study participants are shown in Table 1. The CHOICE and LUCID studies included 926 and 428 patients who had recently initiated dialysis who filled out symptom surveys, respectively, with similar distributions of age (mean, 58 years versus 60 years), race (28% black versus 32% black), gender (53% male versus 60% male), diabetes status (54% versus 54%), and self-reported residual kidney function (81% versus 89%). In CHOICE, 75% of participants were on hemodialysis and 25% were on peritoneal dialysis. By contrast, almost all participants in LUCID were on hemodialysis (97%). A comparison of individuals who did or did not fill out a symptom survey in CHOICE and LUCID are shown in Supplemental Tables 2 and 3, respectively. Individuals who did not fill out a survey were more likely to be male and be on peritoneal dialysis in CHOICE, and more likely to be black in LUCID. Questionnaires were completed at a median of 45 days from dialysis initiation (25th to 75th percentiles, 26–73 days) in CHOICE and 77 days from dialysis initiation (25th to 75th percentiles, 47–121 days) in LUCID.
Table 1.
Baseline characteristics of CHOICE and LUCID participants
| Characteristic | CHOICE | LUCID |
| Sample size | 926 | 428 |
| Demographics | ||
| Age, yr | 58±15 | 60±15 |
| Male sex | 491 (53%) | 258 (60%) |
| Black race | 257 (28%) | 137 (32%) |
| Clinical characteristics | ||
| Diabetes | 498 (54%) | 231 (54%) |
| ICED score | 1.9±0.8 | — |
| Residual kidney function | 749 (81%) | 379 (89%) |
| BMI, kg/m2 | 27.1±6.7 | 28.9±7.7 |
| Dialysis characteristics | ||
| Dialysis modality, hemodialysis | 697 (75%) | 413 (97%) |
| Cause of ESKD, diabetes | 423 (46%) | 155 (36%) |
| Predialysis laboratory tests | ||
| BUN, mg/dl | 56.9±16.3 | — |
| Kt/Vurea | 1.3±0.4 | — |
| Albumin, g/dl | 3.6±0.4 | — |
| Creatinine, mg/dl | 7.3±2.5 | — |
| Calcium, mg/dl | 9.1±0.7 | — |
| Phosphate, mg/dl | 5.2±1.3 | — |
| Potassium, mEq/L | 4.5±0.6 | — |
| Glucose, mg/dl | 167±86 | — |
| Cholesterol, mg/dl | 190±48 | — |
Values for categoric variables are given as count (%); values for continuous variables, as mean±SD. CHOICE, Choices for Health Outcomes in Caring for ESRD; LUCID, Longitudinal United States/Canada Incident Dialysis; ICED, Index of Coexistent Disease; BMI, body mass index.
Prevalence and Severity of Uremic Symptoms in CHOICE and LUCID
Uremic symptom prevalence and severity in CHOICE was high and did not change substantially from baseline to year 1 follow-up (Table 2). Fatigue was the most common (89% at baseline and 89% at year 1) and nausea/vomiting was the least common symptom (36% at baseline and 40% at year 1). Participants on peritoneal dialysis (Supplemental Table 2) were more likely than those on hemodialysis to have nausea/vomiting (38% versus 31%, P=0.05) and less likely to have anorexia (42% versus 51%, P=0.04). No significant differences in symptom prevalence were observed for other symptoms between individuals on peritoneal dialysis versus hemodialysis (Supplemental Table 4).
Table 2.
Prevalence of uremic symptoms in CHOICE and LUCID
| Symptom | CHOICE | LUCID | |
| Baseline (%)(N=926) | Year 1 (%)(N=585) | Baseline (%)(N=428) | |
| Fatigue | 89 | 89 | 77 |
| Anorexia | 44 | 43 | 44 |
| Pruritus | 72 | 74 | 63 |
| Sleepiness | 86 | 87 | 68 |
| Nausea/vomiting | 36 | 40 | 43 |
| Difficulty concentrating | 55 | 60 | 57 |
| Pain | 82 | 85 | 79 |
CHOICE, Choices for Health Outcomes in Caring for ESRD; LUCID, Longitudinal United States/Canada Incident Dialysis.
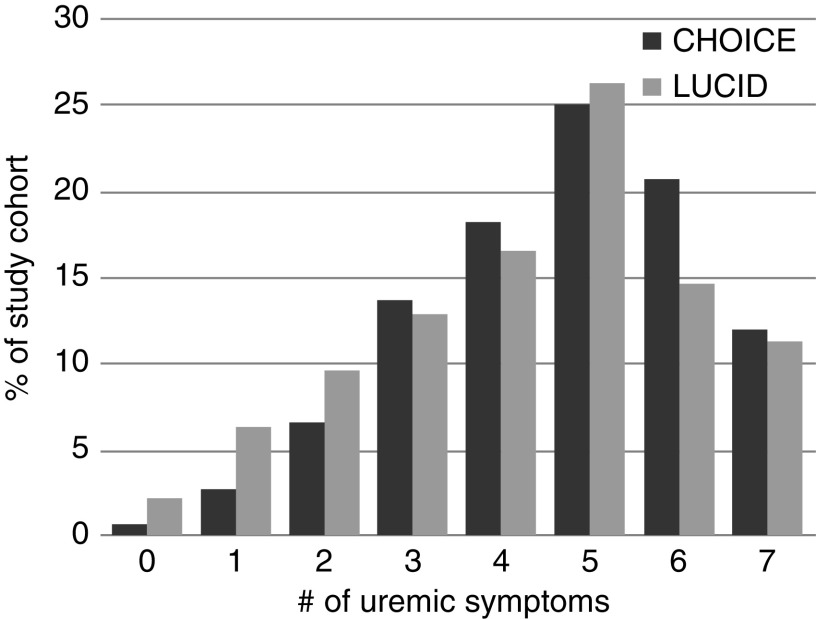
The average symptom prevalence was similar between LUCID and CHOICE despite the approximately 15–20 year difference in study enrollments (Table 2). Baseline values in CHOICE and LUCID were fatigue (89% and 77%, respectively), anorexia (44% and 44%), pruritus (72% and 63%), sleepiness (86% and 68%), nausea/vomiting (36% and 43%), difficulty concentrating (55% and 57%), and pain (82% and 79%). In both cohorts, >80% of patients had three or more uremic symptoms and >50% had five or more uremic symptoms (Figure 1).
Figure 1.
Many individuals in CHOICE and LUCID have multiple uremic symptoms. The y axis shows the percentage of each study cohort (N=926 in CHOICE and N=428 in LUCID) with the corresponding number (#) of uremic symptoms shown on the x axis. CHOICE, Choices for Health Outcomes in Caring for ESRD; LUCID, Longitudinal United States/Canada Incident Dialysis.
Characteristics Associated with Uremic Symptom Burden in CHOICE
The correlation between individual symptoms was low in CHOICE (correlation coefficient <0.4 for all comparisons) (Table 3). Similar findings were observed in LUCID (Supplemental Table 5). In CHOICE, increased age was associated with lower symptom scores for anorexia, sleepiness, nausea/vomiting, and pain; male sex was associated with lower symptom scores for anorexia, nausea/vomiting, difficulty concentrating, and pain (Table 4). Several clinical characteristics and laboratory parameters had nominally significant associations with symptoms, although several were likely indicative of reverse causation (e.g., less anorexia associated with higher BUN, albumin, phosphate, and potassium). Considering the multiple comparisons, only a handful of associations were significant at a more stringent P<0.001 threshold: increased age with less sleepiness and nausea/vomiting, male sex with less nausea/vomiting, and higher BUN with less anorexia. No association with any uremic symptom score was observed for diabetes status, BMI <25 versus ≥25 kg/m2, ESKD cause (diabetes versus other), creatinine, calcium, or glucose.
Table 3.
Spearman correlation between individual symptoms in CHOICE
| Symptoms | Fatigue | Anorexia | Pruritus | Sleepiness | Nausea/Vomiting | Difficulty Concentrating | Pain |
| Fatigue | 1 | ||||||
| Anorexia | 0.26 (<0.001) | 1 | |||||
| Pruritus | 0.20 (<0.001) | 0.20 (<0.001) | 1 | ||||
| Sleepiness | 0.23 (<0.001) | 0.24 (<0.001) | 0.18 (<0.001) | 1 | |||
| Nausea/vomiting | 0.18 (<0.001) | 0.38 (<0.001) | 0.22 (<0.001) | 0.14 (<0.001) | 1 | ||
| Difficulty concentrating | 0.31 (<0.001) | 0.22 (<0.001) | 0.23 (<0.001) | 0.27 (<0.001) | 0.18 (<0.001) | 1 | |
| Pain | 0.29 (<0.001) | 0.24 (<0.001) | 0.21 (<0.001) | 0.23 (<0.001) | 0.26 (<0.001) | 0.21 (<0.001) | 1 |
CHOICE, Choices for Health Outcomes in Caring for ESRD. Values are Spearman correlation (P value).
Table 4.
Predictors of uremic symptoms in the CHOICE study
| Predictor | β (SEM) | ||||||
| Fatigue | Anorexia | Pruritus | Sleepiness | Nausea/Vomiting | Difficulty Concentrating | Pain | |
| Age, per 10 yr older | −0.77 (0.65) | −1.46 (0.64)a | −1.19 (0.75) | −3.51 (0.69)b | −3.20 (0.58)b | −1.08 (0.58) | −1.93 (0.63)c |
| Sex, male versus female | −2.70 (1.94) | −5.55 (1.89)c | −4.05 (2.23) | −1.20 (2.09) | −6.32 (1.73)b | −5.44 (1.72)c | −3.70 (1.86)a |
| Race, black versus nonblack | −1.86 (2.16) | 4.25 (2.13)a | 0.39 (2.51) | 3.69 (2.34) | 4.37 (1.95)a | 2.18 (1.93) | 2.29 (2.08) |
| Diabetes, yes versus no | 0.31 (1.94) | 1.56 (1.90) | −1.77 (2.24) | −2.17 (2.09) | 2.44 (1.75) | 0.63 (1.73) | 2.80 (1.87) |
| ICED score, <3 versus 3 | 2.42 (2.12) | 0.68 (2.09) | −0.25 (2.45) | −1.12 (2.30) | −0.21 (1.92) | −0.86 (1.90) | 5.87 (2.04)c |
| Residual kidney function, yes versus no | −4.31 (2.60) | −6.89 (2.55)c | −5.57 (3.01) | −0.68 (2.81) | −1.46 (2.34) | −4.49 (2.33) | −5.08 (2.51)a |
| BMI, ≥25 versus <25 kg/m2 | 3.89 (2.02) | 0.36 (1.98) | 1.01 (2.31) | 0.35 (2.19) | 0.16 (1.81) | 1.68 (1.79) | 3.22 (1.94) |
| Dialysis modality, HD versus PD | 1.79 (2.23) | −3.88 (2.19) | 2.15 (2.58) | 0.61 (2.41) | 3.67 (2.01) | −0.05 (1.99) | 5.44 (2.15)a |
| Cause of ESKD, diabetes versus other | 0.30 (1.95) | 0.97 (1.91) | −2.63 (2.24) | −2.24 (2.10) | 3.21 (1.75) | 1.23 (1.74) | 1.84 (1.88) |
| BUN, per 10 mg/dL | −1.75 (0.72)a | −2.41 (0.73)b | 0.57 (0.85) | 1.28 (0.81) | −0.14 (0.69) | 0.78 (0.66) | 0.17 (0.72) |
| Kt/Vurea, per 0.2 unit | −0.63 (0.57) | −0.32 (0.56) | 0.10 (0.65) | −1.64 (0.62)c | −0.75 (0.53) | −0.53 (0.51) | −0.79 (0.56) |
| Albumin, per 0.5 g/dL | −2.77 (1.29)a | −4.09 (1.27)c | −1.92 (1.51) | 0.25 (1.40) | −1.74 (1.17) | 0.22 (1.16) | −0.47 (1.25) |
| Creatinine, per 1 mg/dL | −0.15 (0.38) | −0.54 (0.38) | −0.24 (0.45) | 0.78 (0.41) | 0.05 (0.35) | −0.14 (0.35) | −0.30 (0.37) |
| Calcium, per 1 mg/dL | 2.67 (1.36) | −0.36 (1.34) | 1.28 (1.58) | 1.27 (1.47) | 0.70 (1.23) | 1.02 (1.22) | 1.80 (1.31) |
| Phosphate, per 1 mg/dL | 0.10 (0.73) | −2.09 (0.72)c | 0.28 (0.85) | 1.90 (0.79)a | 0.92 (0.66) | 1.12 (0.66) | 1.56 (0.70)a |
| Potassium, per 1 mEq/L | 0.66 (1.64) | −4.40 (1.61)c | −0.75 (1.91) | 0.42 (1.78) | −1.05 (1.48) | 1.54 (1.48) | 0.03 (1.59) |
| Glucose, per 10 mg/dL | −0.02 (0.11) | 0.12 (0.11) | 0.09 (0.13) | 0.05 (0.12) | 0.11 (0.10) | 0.09 (0.10) | 0.13 (0.11) |
| Cholesterol, per 10 mg/dL | 0.23 (0.22) | 0.18 (0.22) | −0.23 (0.26) | 0.07 (0.24) | 0.36 (0.21) | 0.41 (0.20)a | 0.08 (0.21) |
+β denotes increased symptoms and −β denotes decreased symptoms per 1-unit increase in the predictor. CHOICE, Choices for Health Outcomes in Caring for ESRD; ICED, Index of Coexistent Disease; BMI, body mass index; HD, hemodialysis; PD, peritoneal dialysis.
P<0.05.
P<0.001.
P<0.01.
Association of Uremic Symptoms and Longitudinal Outcomes
In CHOICE, there were 580 deaths (282 cardiac deaths) and 516 cardiovascular events during follow-up. Baseline characteristics of individuals alive or dead at 1-year follow-up are shown in Supplemental Table 6, demonstrating higher baseline BMI among survivors. In exploratory analysis, a higher baseline U-score was associated with any-cause death, cardiac death, and first cardiovascular event in age-, sex-, and race-adjusted models (Supplemental Table 7). In fully adjusted models, each ten-point-lower U-score (more symptoms) was associated with a 5% higher risk of death (hazard ratio [HR], 1.05; 95% CI, 1.01 to 1.10), 6% higher risk of cardiac death (HR, 1.06; 95% CI, 0.99 to 1.12), and 5% higher risk of first cardiovascular event (HR, 1.05; 95% CI, 1.00 to 1.10).
Discussion
Our study has two principal findings. First, uremic symptoms are very common among patients with ESKD who have recently initiated dialysis. Very few (<3%) of the patients had no symptoms and >80% of the patients experienced three or more uremic symptoms. Second, the prevalence of uremic symptoms at or close to initiation of dialysis has been remarkably consistent over the past 15 or more years, despite many other changes in patient demographics and/or care processes during this time.
In a systematic review of the literature, Murtagh et al. (6) compiled 60 distinct studies of ESKD that reported on symptom prevalence. Most of these prior studies examined one or two symptoms at a time, and the few studies that looked more broadly across multiple symptoms were relatively small (12–16). A recent exception used both focus groups and surveys to demonstrate high symptom prevalence in 119 individuals with ESKD (17). In our study, we simultaneously assessed the prevalence of key uremic symptoms—fatigue, anorexia, pruritus, sleepiness, nausea, difficulty concentrating, and pain—in a total of 1354 patients, representing the largest sample examined to date. The simultaneous assessment of numerous symptoms is valuable, because it most effectively demonstrates the truly grim burden of symptoms experienced by patients on dialysis. We found that all symptoms were common in CHOICE and LUCID, with individual frequencies of 36%–89%; these values are consistent with the mean prevalence values summarized by Murtagh et al (6). Further, the majority of patients experienced three or more symptoms, and more than a half of patients experienced five or more symptoms. However, the correlation between individual symptoms was relatively low, suggesting that underlying mechanisms may differ, further reinforcing the value of examining multiple symptoms simultaneously.
Whereas prior studies have examined symptom prevalence, ours is the first to assess whether symptom prevalence has improved in the modern era. In the interval between recruitment for the CHOICE (1995–1998) and LUCID (2011– 2017) studies, several changes have occurred in hemodialysis care, including more widespread use of biocompatible and high-flux membranes, lowering of hemoglobin targets, the development of activated vitamin D analogues and calcimimetics, a focus on “fistula first” for dialysis access, and near universal attainment of Kt/Vurea targets. In parallel, numerous changes have occurred in the treatment of common comorbidities in patients on dialysis, including the management of hypertension, diabetes, and cardiovascular disease. Yet, we found only small differences in any individual symptom between CHOICE and LUCID. Because these analyses focused on baseline-symptom assessments among patients on incident dialysis, it is possible they did not reflect the full benefit of hemodialysis over a longer period of time on relieving uremic symptoms. However, we found that there was no difference in symptoms among participants in the CHOICE study from baseline to 1-year follow-up. This is consistent with a prior study that also showed no change in symptoms in a cohort of 97 patients surveyed twice over the course of 1 year (18). Taken together, these findings show that minimal progress has been made over the past 20 years in relieving symptom burden in ESKD, and that progress in this area is unlikely to be achieved by minor adjustments in current practice parameters.
The pathogenesis of uremic symptoms remains poorly characterized (19). Clearly, other factors such as anemia, depression, gastroparesis, and medications can contribute to symptom burden. Nevertheless, the observation that uremic symptoms improve markedly or resolve completely after kidney transplantation has led to the long-standing view that uremic symptoms are due, at least in part, to retained uremic toxins (20–24). Based on a given toxin’s physical properties (size, protein binding, volume of distribution, etc.) and origin (endogenous, diet, microbiome, etc.), one could theoretically design more specific treatment approaches, including both dialytic and nondialytic therapies (7). The traditional bench-to-bedside paradigm has involved demonstrating solute toxicity in vitro or in animal models followed by development of targeted assays and clinical validation. This approach, in conjunction with traditional analytical techniques, has identified approximately 150 uremic solutes over the last >40 years (25), but many are not toxic whereas others have shown mixed associations in clinical studies (26). Although the progress in identifying toxins responsible for uremic symptoms has been relatively slow, it is possible that emerging high-throughput approaches in well phenotyped dialysis cohorts may accelerate this process. As an example, Kurella Tamura et al. (27) recently used liquid chromatography, mass spectrometry–based metabolite profiling to identify 4-hydroxyphenylacetate, phenylacetylglutamine, hippurate, and prolyl-hydroxyproline as novel markers of cognitive impairment in participants of the Frequent Hemodialysis Network trial.
Our study has several strengths. We examined two multicenter ESKD cohorts, with >1300 patients in aggregate. CHOICE has longitudinal follow-up with extensive phenotyping, whereas LUCID is more reflective of contemporary practice in the United States. To assess symptoms, we used patient responses to similar questions on the Health Experience Questionnaire in CHOICE and the KDQOL in LUCID, respectively. Both instruments have been validated, and the KDQOL has emerged as the most widely used tool for health-related quality of life assessment in the nephrology literature. In fact, the KDQOL is administered annually to patients on dialysis in the United States to meet the requirements of the Center for Medicare and Medicaid Services for incorporating assessment of health-related quality of life into ESKD care (28).
Several limitations also warrant mention. Although questionnaires permit accrual of large sample numbers and comparison across data sets, they may not be ideal for assessment of some symptoms. For example, compared to an extensive neurocognitive battery, the KDQOL has been shown to have limited sensitivity and specificity for identifying worse executive function and memory (29). In addition, we did not consider all potential uremic symptoms related to ESKD, e.g., restless legs, or all questions that could be related to a given symptom. However, this is also a limitation of the symptom score generated from the KDQOL, which includes some but not all questions related to fatigue and lack of energy. To address this limitation of existing instruments, rigorous studies are needed to develop validated tools for specific uremic symptoms with gold-standard methods that objectively assess symptom severity. For example, severity of pruritus can be quantified using actigraphy (30,31), and fatigue can be assessed using the Borg rating of perceived exertion scale in response to a standardized, 5-minute slow-paced treadmill walk (0.67 m/second; 1.5 mph) at 0% grade, as was done in the Baltimore Longitudinal Study of Aging (32,33). Further, selection bias in those who chose to answer baseline symptom questionnaires, survival bias in those who were available to answer the year 1 questionnaire in CHOICE, and the vintage of CHOICE may all limit the generalizability of our findings. Finally, although symptoms are known manifestations of uremia, they are also known manifestations of other clinical conditions such as anemia, hyperphosphatemia, and depression, further complicating the exploratory examination of uremic symptoms and longitudinal outcomes.
In conclusion, uremic symptoms are common, persistent, and associated with poor outcomes, but their cause remains unknown. In addition, the identification of toxins responsible for uremic symptoms remains an important priority, with the goal to develop more specific treatment approaches and to improve quality of life, and perhaps long-term outcomes, in ESKD.
Disclosures
E. Guallar reports grants from the National Institutes of Health (NIH) during the conduct of the study. S. Moe reports personal fees from Amgen, personal fees from Ardelyx, grants from Chugai, grants from Keryx/Akebia, grants from the NIH, and grants from Veterans Administration, outside the submitted work. T. Shafi reports grants from the NIH during the conduct of the study and personal fees from Siemens outside the submitted work. R. Thadhani reports personal fees from Fresenius Medical Care during the conduct of the study. M. Tonelli reports an honorarium from B Braun that was donated to charity and grants from the Canadian Institutes of Health Research during the conduct of the study. J. Himmelfarb, S. Hwang, N. Kim, N. Powe, and E. Rhee have nothing to disclose.
Funding
This study was supported by a Government of Canada, Canadian Institutes of Health Research industry partnership (industry partner Abbott Laboratories) program peer-reviewed grant to M. Tonelli (grant MOP-84258) and Office of Extramural Research, NIH grant R01 NR017399 (to E. Rhee and T. Shafi).
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0000072019/-/DCSupplemental.
Symptom questions. Download Supplemental Table 1, PDF file, 93 KB (93KB, pdf)
Baseline characteristics of symptom survey responders and non-responders in CHOICE. Download Supplemental Table 2, PDF file, 93 KB (93KB, pdf)
Baseline characteristics of symptom survey responders and non-resonders in LUCID. Download Supplemental Table 3, PDF file, 93 KB (93KB, pdf)
Prevalence of uremic symptoms across HD/PD in CHOICE. Download Supplemental Table 4, PDF file, 93 KB (93KB, pdf)
The Spearman correlation between individual symptoms in LUCID. Download Supplemental Table 5, PDF file, 93 KB (93KB, pdf)
Baseline characteristics of individuals alive or not alive at 1-year follow-up in CHOICE. Download Supplemental Table 6, PDF file, 93 KB (93KB, pdf)
Association of uremic symptom score with outcomes in the CHOICE study. Download Supplemental Table 7, PDF file, 93 KB (93KB, pdf)
Author Contributions
E. Rhee, M. Tonelli, S. Moe, J. Himmelfarb, R. Thadhani, N. Powe, and T. Shafi conceptualized the study; E. Rhee and T. Shafi wrote the original draft of the manuscript; E. Rhee, E. Guallar, and T. Shafi provided supervision; E. Guallar, S. Hwang, N. Kim, and T. Shafi provided formal analysis; M. Tonelli, S. Moe, J. Himmelfarb, and R. Thadhani were responsible for funding acquisition; M. Tonelli, S. Moe, J. Himmelfarb, R. Thadhani, and N. Powe were responsible for project administration; and all authors reviewed and edited the manuscript.
References
- 1.Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, Chen JT, Cope E, Gipson D, He K, Herman W, Heung M, Hirth RA, Jacobsen SS, Kalantar-Zadeh K, Kovesdy CP, Leichtman AB, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, O’Hare AM, Pisoni R, Plattner B, Port FK, Rao P, Rhee CM, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, Eggers PW, Agodoa LY, Abbott KC: US renal data system 2014 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 66[Suppl 1]: S1–S305, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabrera VJ, Hansson J, Kliger AS, Finkelstein FO: Symptom management of the patient with CKD: The role of dialysis. Clin J Am Soc Nephrol 12: 687–693, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramkumar N, Beddhu S, Eggers P, Pappas LM, Cheung AK: Patient preferences for in-center intense hemodialysis. Hemodial Int 9: 281–295, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Manns B, Hemmelgarn B, Lillie E, Dip SC, Cyr A, Gladish M, Large C, Silverman H, Toth B, Wolfs W, Laupacis A: Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol 9: 1813–1821, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nissenson AR: Improving outcomes for ESRD patients: Shifting the quality paradigm. Clin J Am Soc Nephrol 9: 430–434, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murtagh FE, Addington-Hall J, Higginson IJ: The prevalence of symptoms in end-stage renal disease: A systematic review. Adv Chronic Kidney Dis 14: 82–99, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Flythe JE, Hilliard T, Lumby E, Castillo G, Orazi J, Abdel-Rahman EM, Pai AB, Rivara MB, St Peter WL, Weisbord SD, Wilkie CM, Mehrotra R; Kidney Health Initiative Prioritizing Symptoms of ESRD Patients for Developing Therapeutic Interventions Stakeholder Meeting Participants: Fostering innovation in symptom management among hemodialysis patients: Paths forward for insomnia, muscle cramps, and fatigue. Clin J Am Soc Nephrol 14: 150–160, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu AW, Fink NE, Cagney KA, Bass EB, Rubin HR, Meyer KB, Sadler JH, Powe NR: Developing a health-related quality-of-life measure for end-stage renal disease: The CHOICE Health Experience Questionnaire. Am J Kidney Dis 37: 11–21, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB: Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res 3: 329–338, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Wu AW, Fink NE, Marsh-Manzi JV, Meyer KB, Finkelstein FO, Chapman MM, Powe NR: Changes in quality of life during hemodialysis and peritoneal dialysis treatment: Generic and disease specific measures. J Am Soc Nephrol 15: 743–753, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Longenecker JC, Klag MJ, Marcovina SM, Liu YM, Jaar BG, Powe NR, Fink NE, Levey AS, Coresh J: High lipoprotein(a) levels and small apolipoprotein(a) size prospectively predict cardiovascular events in dialysis patients. J Am Soc Nephrol 16: 1794–1802, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Virga G, Mastrosimone S, Amici G, Munaretto G, Gastaldon F, Bonadonna A: Symptoms in hemodialysis patients and their relationship with biochemical and demographic parameters. Int J Artif Organs 21: 788–793, 1998 [PubMed] [Google Scholar]
- 13.Weisbord SD, Carmody SS, Bruns FJ, Rotondi AJ, Cohen LM, Zeidel ML, Arnold RM: Symptom burden, quality of life, advance care planning and the potential value of palliative care in severely ill haemodialysis patients. Nephrol Dial Transplant 18: 1345–1352, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Kimmel PL, Emont SL, Newmann JM, Danko H, Moss AH: ESRD patient quality of life: Symptoms, spiritual beliefs, psychosocial factors, and ethnicity. Am J Kidney Dis 42: 713–721, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Curtin RB, Bultman DC, Thomas-Hawkins C, Walters BA, Schatell D: Hemodialysis patients’ symptom experiences: Effects on physical and mental functioning. Nephrol Nurs J 29: 562, 567–574; discussion 575, 598, 2002 [PubMed] [Google Scholar]
- 16.Barrett BJ, Vavasour HM, Major A, Parfrey PS: Clinical and psychological correlates of somatic symptoms in patients on dialysis. Nephron 55: 10–15, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Flythe JE, Hilliard T, Castillo G, Ikeler K, Orazi J, Abdel-Rahman E, Pai AB, Rivara MB, St Peter WL, Weisbord SD, Wilkie C, Mehrotra R: Symptom prioritization among adults receiving in-center hemodialysis: A mixed methods study. Clin J Am Soc Nephrol 13: 735–745, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parfrey PS, Vavasour HM, Henry S, Bullock M, Gault MH: Clinical features and severity of nonspecific symptoms in dialysis patients. Nephron 50: 121–128, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Meyer TW, Hostetter TH: Uremia. N Engl J Med 357: 1316–1325, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Depner TA: Uremic toxicity: Urea and beyond. Semin Dial 14: 246–251, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Ozcan H, Yucel A, Avşar UZ, Cankaya E, Yucel N, Gözübüyük H, Eren F, Keles M, Aydınlı B: Kidney transplantation is superior to hemodialysis and peritoneal dialysis in terms of cognitive function, anxiety, and depression symptoms in chronic kidney disease. Transplant Proc 47: 1348–1351, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Azar SA, Hatefi R, Talebi M: Evaluation of effect of renal transplantation in treatment of restless legs syndrome. Transplant Proc 39: 1132–1133, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Molnar MZ, Novak M, Ambrus C, Szeifert L, Kovacs A, Pap J, Remport A, Mucsi I: Restless Legs Syndrome in patients after renal transplantation. Am J Kidney Dis 45: 388–396, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Kovacs AZ, Molnar MZ, Szeifert L, Ambrus C, Molnar-Varga M, Szentkiralyi A, Mucsi I, Novak M: Sleep disorders, depressive symptoms and health-related quality of life–a cross-sectional comparison between kidney transplant recipients and waitlisted patients on maintenance dialysis. Nephrol Dial Transplant 26: 1058–1065, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A; European Uremic Toxin Work Group: Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 23: 1258–1270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shafi T, Meyer TW, Hostetter TH, Melamed ML, Parekh RS, Hwang S, Banerjee T, Coresh J, Powe NR: Free levels of selected organic solutes and cardiovascular morbidity and mortality in hemodialysis patients: Results from the Retained Organic Solutes and Clinical Outcomes (ROSCO) investigators. PLoS One 10: e0126048, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurella Tamura M, Chertow GM, Depner TA, Nissenson AR, Schiller B, Mehta RL, Liu S, Sirich TL; FHN Study: Metabolic profiling of impaired cognitive function in patients receiving dialysis. J Am Soc Nephrol 27: 3780–3787, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen SS, Al Mawed S, Unruh M: Health-related quality of life in end-stage renal disease patients: How often should we ask and what do we do with the answer? Blood Purif 41: 218–224, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Sorensen EP, Sarnak MJ, Tighiouart H, Scott T, Giang LM, Kirkpatrick B, Lou K, Weiner DE: The kidney disease quality of life cognitive function subscale and cognitive performance in maintenance hemodialysis patients. Am J Kidney Dis 60: 417–426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith MP, Ly K, Thibodeaux Q, Weerasinghe T, Wu JJ, Yosipovitch G, Bhutani T, Liao W: Emerging methods to objectively assess pruritus in atopic dermatitis. Dermatol Ther (Heidelb) 9: 407–420, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalinska-Bienias A, Piotrowski T, Kowalczyk E, Lesniewska A, Kaminska M, Jagielski P, Kowalewski C, Wozniak K: Actigraphy-measured nocturnal wrist movements and assessment of sleep quality in patients with bullous pemphigoid: A pilot case-control study. Clin Exp Dermatol 44: 759–765, 2019 [DOI] [PubMed] [Google Scholar]
- 32.Simonsick EM, Glynn NW, Jerome GJ, Shardell M, Schrack JA, Ferrucci L: Fatigued, but not frail: Perceived fatigability as a marker of impending decline in mobility-intact older adults. J Am Geriatr Soc 64: 1287–1292, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borg GA: Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Symptom questions. Download Supplemental Table 1, PDF file, 93 KB (93KB, pdf)
Baseline characteristics of symptom survey responders and non-responders in CHOICE. Download Supplemental Table 2, PDF file, 93 KB (93KB, pdf)
Baseline characteristics of symptom survey responders and non-resonders in LUCID. Download Supplemental Table 3, PDF file, 93 KB (93KB, pdf)
Prevalence of uremic symptoms across HD/PD in CHOICE. Download Supplemental Table 4, PDF file, 93 KB (93KB, pdf)
The Spearman correlation between individual symptoms in LUCID. Download Supplemental Table 5, PDF file, 93 KB (93KB, pdf)
Baseline characteristics of individuals alive or not alive at 1-year follow-up in CHOICE. Download Supplemental Table 6, PDF file, 93 KB (93KB, pdf)
Association of uremic symptom score with outcomes in the CHOICE study. Download Supplemental Table 7, PDF file, 93 KB (93KB, pdf)