Abstract
Governments all over the world are struggling with the regulatory status of gene-edited organisms. Are they regulated? Should they be regulated? In the present paper, the main focus is on the regulatory status of gene-edited organisms within the European regulatory framework. A stepwise analysis is performed that comes to the conclusion that gene-edited agricultural products that carry edits that can also occur naturally by mating and/or natural recombination are not a genetically modified organism. On the question whether they should be regulated, it is argued that it is difficult to require regulatory oversight that would go beyond what we now require for conventional products that can carry the same types of alterations. A regulatory approach is pleaded for that abides to fundamental principles of law making, and which allows for gene editing to develop responsibly.
Keywords: gene editing, GMO, legal status, regulatory framework
Introduction
Gene editing presents yet another tool in the continuum of plant breeding innovations. In contrast with ‘classical genetic modification’ which in most cases involves the introduction of sequences that are ‘foreign’ to the organisms' gene pool, gene editing is mostly about creating additional genetic variation within an existing gene pool in a very precise and directed manner. It offers great opportunities, but also creates regulatory challenges. Is it regulated? Should it be regulated? And if so, how? Governments all over the world are struggling with these questions.
It is not for the first time that governments struggle with regulatory challenges in the face of new technological developments. The current EU (European Union) genetically modified organism (GMO) regulatory framework was developed at the end of the 1980s following the development of recombinant DNA (deoxyribose nucleic acid) technology. This technology created novel possibilities to alter the genetic composition of crops and experience with the technology was still limited. This prompted the European authorities to take a precautionary approach and consequently a regulatory framework was set up that required elaborate risk assessments and government authorization prior to the deliberate release and marketing of such GMOs. This was the birth of EC (European Community) Directive 90/220/EEC (European Economic Community).1 In 2001, this Directive was replaced by EU Directive 2001/18/EC,2 which has an almost identical scope.
In the same period, many other jurisdictions in the world also set up their GMO regulatory frameworks. The United States of America (USA) did not develop specific GMO legislation, but instead chose to regulate such organisms under existing legislation governing plant pests, plant protection and food safety. Canada set up a specific regulatory framework governing ‘plants with novel traits’ (PNTs), in which it is not the use of certain technology that triggers the legislation, but the introduction of a ‘novel’ trait.
In the present paper, I will mainly focus on the place of gene-edited organisms in the EU regulatory framework, but briefly discuss North and South America as well.
The EU GMO regulatory framework
‘…has been altered in a way…’
In Europe, there is already a decade of discussion on whether gene-edited organisms are covered by EU Directive 2001/18/EC. The answer to that question is not a matter of opinion. There is only one correct legal interpretation of the scope of the Directive. To determine what the actual scope is, I will perform the following stepwise analysis:
What does the actual wording of the Directive say?
If the analysis of the actual wording is not conclusive, what other clues does the Directive offer for a correct interpretation?
Is the emerging interpretation consistent with relevant other regulatory frameworks?
Step 1 Article 2(2) of EU Directive 2001/18/EC defines GMO as follows:
“genetically modified organism (GMO) means an organism, with the exception of human beings, in which the genetic material has been altered in a way that does not occur naturally by mating and/or natural recombination”
I have indicated in bold the part of the definition that is open for interpretation. It is often portrayed that the EU has a process-based GMO legislation, meaning that most people interpret these words to refer only to the technique used. If that would be the case, then genome-edited organisms would indeed be covered by this definition. But from the actual wording, one cannot conclude whether this applies to the technique used, to the end product, or to both. So, in order to draw relevant conclusions, we have to take Step 2 and look for other clues in the Directive. We find those clues in Annex IA part I of the Directive which lists techniques of genetic modification. This annex is formulated as follows:
“Techniques of genetic modification referred to in Article 2(2)(a) are inter alia:
(1) recombinant nucleic acid techniques involving the formation of new combinations of genetic material by the insertion of nucleic acid molecules produced by whatever means outside an organism, into any virus, bacterial plasmid or other vector system and their incorporation into a host organism in which they do not naturally occur but in which they are capable of continued propagation;
(2) techniques involving the direct introduction into an organism of heritable material prepared outside the organism including micro-injection, macro-injection and micro-encapsulation;
(3) cell fusion (including protoplast fusion) or hybridisation techniques where live cells with new combinations of heritable genetic material are formed through the fusion of two or more cells by means of methods that do not occur naturally.”
Again, I have indicated the important parts in bold. Perhaps surprisingly, this part of the annex does not just list a number of techniques. No, it articulates that genetic modification, which is subject to the provisions of the Directive, is only achieved when the application of these techniques leads to a particular result. This is most apparent under (1) and (3), where both in case of the use of recombinant nucleic acid techniques and in case of cell fusion the use of these techniques only leads to genetic modification if ‘a new combination of (heritable) genetic material’ is formed. For recombinant nucleic acid techniques, it is additionally added that they should be introduced ‘into a host organism in which they do not naturally occur’. This is where the notion comes from that GMOs are mostly organisms that contain ‘foreign’ genetic material. But in (1) and (3), clearly both the use of a certain technique AND the characteristics of the end product are referred to in a cumulative way: process AND product.
Note that the annex uses the words ‘inter alia’ meaning that it does not provide a limitative list. In other words, the annex does not exclude techniques of genetic modification that are not listed. So also the technique of gene editing using CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9 could be covered, on the condition that it leads to genetic modification.
And yet another important note: do not mix up the technical concept of ‘genetic alteration’ and the legal concept of ‘genetic modification’. There is a difference between the two.
The Directive should be internally consistent. It cannot say opposite things in different places. This is why the actual wording used in Annex IA Part I directs us to conclude that the phrase ‘has been altered in a way that does not occur naturally’ in the GMO definition should be read as referring to both the technique AND the end product.
But is this legal interpretation consistent with other relevant legal instruments (Step 3 of the analysis)? For that we turn to the most relevant international GMO treaty that the EU has signed up to and ratified: the Cartagena Protocol on Biosafety.3 This protocol does not use the term ‘genetically modified organisms,’ but speaks about ‘living modified organism’ (LMO). This, however, boils down to the same thing. The protocol provides the following definition of LMO:
“LMO: any living organism that possesses a novel combination of genetic material developed through modern biotechnology”
This definition clearly refers to the technique (‘modern biotechnology’) and the end product (‘an organism that possesses a novel combination of genetic material’) in a cumulative way. Our formulated interpretation of the EU GMO definition is therefore consistent with this important international treaty. Additionally, EU Commissioner Tonio Borg, has also confirmed that the EU GMO definition refers to both the technique and the end product in response to a European parliamentary question.4
The first main conclusion is that the EU GMO definition is not purely process-based but refers to the technique used and the characteristics of the end product in a cumulative way.
‘…a new combination…’
Now we know that the GMO definition also refers to characteristics of the end product, we still have not answered the question of whether gene-edited organisms are subject to the EU Directive. We have to dig even deeper. We have to determine what characteristics of the end product make an organism qualify as a GMO. As already indicated above, the organism should possess a new/novel combination of genetic material. But what actually constitutes ‘new’ or ‘novel’ in this context? The answer to that question is for a large part already enclosed within the GMO definition itself. As the phrase ‘has been altered in a way that does not occur naturally’ refers to both the technique and the end product, the end product should be an organism that has a genetic composition that ‘does not occur naturally by mating and/or natural recombination’. There is also logic to this as the Directive has been set up to capture man-made organisms with genetic compositions that cannot or are extremely unlikely to spontaneously arise in classical breeding, in nature or in a field.
It is important to also point out that the definition uses the words ‘does not occur’, and not ‘did not occur’ or ‘has not occurred’. This means that the definition also refers to possible future events. In this way, the Directive prevents that possible future organisms arising from conventional cross-breeding would be captured by the GMO definition.
New or novel also means that the new genetic composition should be distinguishable on the DNA level from other organisms (non-GMOs) in a meaningful manner. The same product should not be regulated in two different ways. And, the GMO should be technically distinguishable from the non-GMO. Otherwise, the Directive would not be enforceable.
The second main conclusion is that the EU GMO definition only refers to organisms in which the genetic material is altered beyond what does occur naturally by mating and/or natural recombination.
Are gene-edited organisms within the scope of EU Directive 2001/18/EC?
Gene editing can be achieved using different techniques. Oligo-directed mutagenesis can be used to generate single-nucleotide alterations. The same can also be achieved using site-directed nuclease (SDN) technology, such as the revolutionary CRISPR/Cas9 system. This latter system can also be used to generate small deletions, larger deletions including complete gene deletions, small nucleotide additions and also for exchanging complete alleles. Table 1 lists many concrete examples of such edits.
Table 1. Examples of gene edits in organisms.
 |
The major question now is whether such edits are beyond what does occur naturally by mating and/or natural recombination and whether such edits can be distinguished on the DNA level from alterations that can occur spontaneously in nature or can be the result of classical cross-breeding. The answer to these questions for the examples given in Table 1 is NO. Such alterations can and do occur spontaneously in nature and during classical cross-breeding processes. And technically, one can also not make any distinction between man-made edits and spontaneous alterations. The use of edit technology to introduce sequences that are foreign to the organisms' gene pool on the other hand clearly leads to the formation of a GMO.
The third conclusion is that organisms that carry edits such as presented in Table 1 do not fall within the legal EU GMO definition. They do not fulfill the end product criterion.
The European mutagenesis exemption
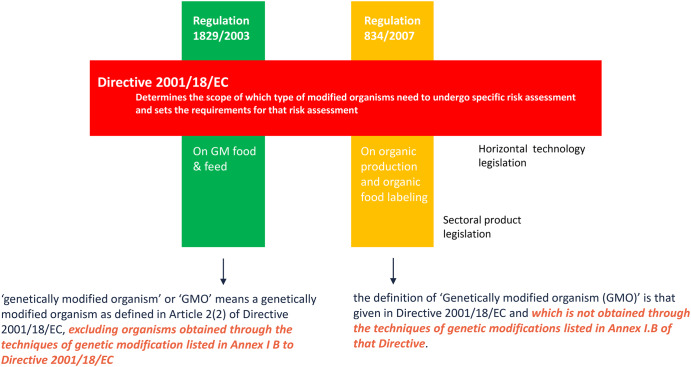
The scope of EU Directive 2001/18/EC is not just determined by the GMO definition. Article 3 and Annex IB of the Directive provide a number of exemptions. This means that organisms which have been obtained following the application of certain techniques of genetic modification are exempted from the scope. This applies to organisms which have been obtained using ‘mutagenesis’ and ‘cell fusion’. In other words, and there is absolute consensus about this: the application of these techniques of genetic modification does not result in organisms subject to the provisions of the Directive. There are, however, different views on whether such organisms de facto fall within the scope of the GMO definition. When looking at the actual text I note that these exemptions are not part of the GMO definition itself. I therefore have to conclude that the definition of a GMO is wider than the scope of the Directive. There is a difference between the organisms that fall within the legal GMO definition and the organisms that are subject to the provisions of the Directive. The exemptions narrow the scope of the Directive. This is confirmed by the way vertical product legislation, such as EU Regulation 1829/2003 (known as the ‘GM food & feed Regulation’) and the EU organic production Regulation 834/2007, refer to GMOs. They do not just refer to the GMO definition of article 2(2) of EU Directive 2001/18/EC, but additionally refer to the exemptions to narrow the scope. If they would have only referred to the GMO definition, organisms resulting from induced mutagenesis would have been subject to the GMO provisions of these Regulations. But these vertical product legislations are not allowed to overrule the horizontal technology legislation (see Figure 1).
Figure 1.
The interplay between the horizontal technology legislation and the sectoral product legislation in the EU regulatory framework.
EU Directive 2001/18/EC and the Cartagena Protocol on Biosafety use different legal mechanisms to come to the same scope. The EU Directive uses a wider definition of GMO, but narrows its scope through exemptions, where the Cartagena Protocol uses a more narrow definition of LMO.
In the discussion on the legal status of gene-edited organisms, many EU member states have been focusing on the mutagenesis exemption. This is somehow understandable, because in technical terms gene editing is a form of mutagenesis. But, by doing so, they have ignored the real issue, namely whether gene editing leads to the formation of a GMO in the first place.
How can one then explain the difference between gene-edited organisms that are not GMOs and organisms obtained by classical mutagenesis that apparently have to be exempted from the scope of the Directive? The only logical and consistent explanation is that classical mutagenesis, such as the use of radiation, leads to the formation of a new combination of genetic material that does not, or is at least extremely unlikely to spontaneously arise in nature or by cross-breeding. The deliberate use of radiation on a plant, indeed, leads to the formation of hundreds to thousands of mutations in one go, and the regulator apparently judged that even though each individual mutation can arise spontaneously in nature, this is beyond what ‘does occur naturally by mating and/or natural recombination’. Gene editing, on the other hand, leads to the formation of a single or very small number of mutations that would be far more likely to occur naturally.
Prejudicial questions at the Court of Justice of the EU
The debate about the legal status of gene-edited crops has led to two court cases in Europe. One at the court in Braunschweig in Germany where NGOs challenged a decision by the German authorities that a GMO permit was not necessary for a particular type of gene-edited crop. The second court case was started by an alliance of different organizations in France against the authorities with the goal to prevent these authorities also placing gene-edited crops outside of the scope of the GMO legislation. In the French case, the French Conseil d'Etat decided to forward four prejudicial questions to the Court of Justice of the EU (CJEU).5 This is of great importance as the CJEU is the highest European body that can lawfully rule on how the scope of EU Directive 2001/18/EC should be interpreted. I am not going to discuss these four questions in detail here. I will only make the remark that the prejudicial questions are too much focused on the mutagenesis exemption and fail to address the real issue, namely the question whether gene editing leads to organisms that are covered by the GMO definition in the first place.
Gene-edited organisms and regulatory frameworks in North and South America
In the USA, the USDA Animal and Plant Health Inspection Service (APHIS) has proposed alterations to its regulatory framework that would result in certain gene-edited crops not being a regulated article under their Plants Pest regulatory framework (see Table 2).6 In particular, plants containing a single base-pair substitution, a deletion of any size or the introduction of naturally occurring nucleic acid sequences would not be a regulated article. The Environmental Protection Agency (EPA) has not formulated proposals to change its regulatory approach, meaning that gene-edited crops would remain a regulated article if the alteration results in the introduction of a plant-incorporated protectant. The US Food & Drug Agency has launched a proposal to treat gene-edited animals as an animal drug.7 This results in the rather inconsistent situation that for instance, a gene-edited food crop with a single-nucleotide change is not a regulated article, while a gene-edited livestock animal with a single-nucleotide change is a fully regulated article. Both will be consumed by humans in the same way. It is important to note that the USDA-APHIS and FDA (US Food & Drug Administration) proposed rules are not final yet.
Table 2. The regulatory status of gene-edited organisms under proposed US legislation.
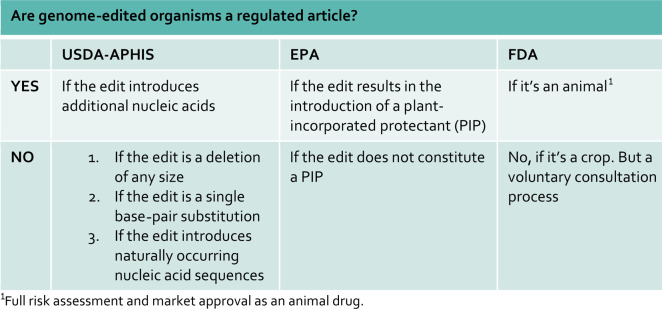 |
In Canada, gene-edited crops are regulated in the same way as any other crop: if the gene-edited crop is considered to possess a ‘novel trait’, then it will be regulated article that needs to go through a risk assessment and market approval process.
In Argentina, alterations to the regulatory framework have been implemented that allow for a case-by-case determination whether a gene-edited crop will have to go through a GMO risk assessment and market approval process. In practice, decisions have already been taken that crops with a small deletion do not have to go through this process.
Should gene-edited organisms be regulated?
The question about the legal status of gene-edited organisms under current regulatory frameworks is of course completely different from whether gene-edited crops should be subject to regulatory oversight. The main regulatory trigger for subjecting organisms to regulatory oversight is safety. But do gene-edited organisms present a hazard or risk in a way that would necessitate regulatory oversight? To answer that question we have to compare them with the products of conventional breeding and with GMOs.
GMOs were placed under regulatory oversight for two reasons: (1) recombinant nucleic acid technology made alterations to genomes possible way beyond what could be achieved before and (2) there was still little experience with the technique. Gene editing has been applied for little more than a decade now and we have seen an enormous acceleration since 2012 when the CRISPR/Cas9 nuclease technology was proven to also be functional in eukaryotic cells. Experience with these specific technologies is still limited, but in contrast with classical recombinant DNA technology, we do already have lots of experience with most of the genetic alterations that are being generated by gene editing. Single-nucleotide substitutions, deletions, and frame-shift mutations, these types of alterations already happen spontaneously in nature. They contribute to the genetic variation within species that we exploit in plant breeding. Also, classical induced mutagenesis using chemical mutagens or ionizing radiation results in the same types of genetic alterations. The main difference between these conventional techniques and gene editing is that the latter is no longer a blind approach, but a knowledge-based, directed approach.
In practice, we have been handling conventionally bred crops carrying spontaneous mutations and induced mutations through the following stepwise process:
Plant breeders in a first step generate lots of different individuals, all carrying their own genetic variations.
From these pools they select individuals that show interesting, desired properties.
These individuals are then further characterized. Among others field trials are performed over multiple years.
A final genotype is chosen to become a new variety.
That variety is then subjected to plant variety registration testing.
The variety is added to the common catalog of plant varieties and placed on the market.
Food crops that have gone through this process are generally recognized as safe. There is a history of safe use with this process and its end products. Governments have never required pre-market safety assessments and for good reasons. Even though there are some exceptional examples of plant varieties that had negative health impacts and had to be removed from the market. The most well-known example is a celery variety that produced too high amounts of psoralen toxin that caused serious skin irritation [1].
Even though pre-market safety assessments are not required for such conventional crops, there are some general overarching food law principles that help to prevent unsafe foods being placed on the market.8 On top of that there are also legal obligations to remove foods from the market if they are shown to present unacceptable health risks. Operators introducing food crops onto the market that cause damage to protected species and natural habitats, damage to water, or land damage are liable under EU Directive 2004/35 on environmental liability. In other words, even in the absence of pre-market risk assessment requirements, there are some general overarching safety principles that should direct operators to think twice before introducing a food crop onto the market that presents true health or environmental risks. But these safeguards are, of course, of a very general and indirect nature.
Is this then enough to guarantee safety for gene-edited crops and can we trust that crops that go through this process will be safe in the same way as conventional crops? It should be considered that gene editing is a much less blind approach than conventional cross-breeding or induced mutagenesis breeding. In terms of uncertainties or relative risks, there are also no reasons to place gene-edited crops that have alterations to their genomes that can also occur in nature, in a higher risk class than conventionally cross-bred crops. From a scientific point of view, it would not be defensible to punish plant breeders for knowing much better what they do.
Conclusion
The stepwise analysis of the scope of the EU GMO regulatory framework that I have presented has led to the conclusion that gene-edited crops with genetic alterations that can also occur in nature are not subject to the provisions of this EU legislation. They are outside of the scope of the EU GMO definition. They are not outside the scope of the legislation for the reason that they would fall under one of its exemptions. If the CJEU comes to that same conclusion, then undoubtedly a debate will follow on whether gene-edited crops should be subject to regulatory oversight. I have shown that it is difficult to present and defend an argument that such gene-edited crops should be heavily regulated and be subject to regulatory oversight that is more stringent than currently applies to conventional crops. Therefore, I also believe that Europe — or anyone else for that matter — should think twice before altering its regulatory framework.
If alterations were to be considered, such alterations in my opinion are only acceptable if they contribute to a regulatory framework that is science-based, proportionate, non-discriminatory, provides true legal certainty, and is enforceable.9 The proposed alterations to the US regulatory framework show that it is not straightforward to comply with all of these basic legal principles. I call upon all actors worldwide to work hard to respect them and allow for gene editing to develop responsibly.
Summary
The EU GMO definition should be read as referring to both the techniques used and the characteristics of the end product in a cumulative way.
Most gene-edited agricultural products are not subject to the provisions of EU Directive 2001/18/EC, because they are not GMOs.
It is difficult to present and defend an argument that such gene-edited crops should be heavily regulated and be subject to regulatory oversight that is more stringent than currently applies to conventional crops.
If alterations to legislation were to be considered, such alterations are only acceptable if they contribute to a regulatory framework that is science-based, proportionate, non-discriminatory, provides true legal certainty, and is enforceable.
Acknowledgements
The contents of the present paper would not have been possible without having had discussions with many different persons. The author particularly wishes to thank Piet van der Meer and Henk Schouten for sharing their views.
Abbreviations
- CJEU
Court of Justice of the European Union
- CRISPR
clustered regularly interspaced short palindromic repeats
- DNA
deoxyribose nucleic acid
- EC
European Community
- EPA
US Environmental Protection Agency
- EU
European Union
- FDA
US Food & Drug Administration
- GMO
genetically modified organism
- HDR
homology directed repair
- LMO
living modified organism
- NHEJ
non-homologous end joining
- PNT
plant with novel trait
- SDN
site-directed nuclease
- USDA-APHIS
United States Department of Agriculture—Animal and Plant Health Inspection Service
Footnotes
Council Directive of 23 April 1990 on the deliberate release into the environment of GMOs.
Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of GMOs and repealing Council Directive 90/220/EEC.
The Cartagena Protocol on Biosafety to the Convention on Biological Diversity.
Answer of Commissioner Tonio Borg of 17 October 2014 to a parliamentary question (ref. no. E-006525-14) posed by Jan Huitema, Peter van Dalen and Bas Belder.
Federal Register/Vol. 82 No. 12 Thursday, 19 January 2017/Proposed Rules [Docket No. APHIS 2015-0057].
FDA Guidance for Industry #187, Regulation of Intentionally Altered Genomic DNA in Animals, Draft Guidance.
For instance Regulation (EC) No. 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety.
The principles of proportionality and non-discrimination are general ethical and law making principles that are present in, for instance, the UN Declaration on Human Rights, the World Trade Organization's Agreement on the Application of Sanitary and Phytosanitary Measures (SPS Agreement). Scientific organizations and Academies from all over the world have pleaded for a science-based approach and alerted to the importance of enforceability of legislation.
Author Contribution
R.C. is the sole author of the present paper and fully responsible for its contents.
Funding
The work of the author is funded through an institutional grant agreement with the Flanders government.
Competing Interests
The Author declares that there are no competing interests associated with the manuscript.
Reference
- 1.Blair R. and Regenstein J.M. (2015) Genetic Modification and Food Quality; A Down-to-Earth Analysis, Wiley-Blackwell [Google Scholar]