Abstract
After setbacks related to serious adverse events 20 years ago, gene therapy is now coming back to the central stage worldwide. In the past few years, gene therapy has shown astonishing efficacy against genetic diseases and cancers. In history, China carried out the world’s second gene therapy clinical trial in 1991 for hemophilia B and approved the world’s first gene therapy product—Gendicine—in 2003. In recent years, numerous efforts have been made on gene editing. Here, we reviewed the past of gene therapy in China and highlighted recent advances. We also discussed the regulations and future perspectives of gene therapy in China.
Subject terms: Gene delivery, Gene therapy, Haematological diseases, Cancer
Introduction
The concept of gene therapy was proposed in the early 1970s [1]. The original concept was to introduce a normal gene to replace a mutant gene—which is called ‘gene addition’ according to today’s nomenclature and is still one of the main strategies we are highly relying on though more alternatives are available today such as gene editing and base editing. Gene addition was a major challenge during the early stages of gene therapy. Initially, there was no efficient tool to deliver foreign genes to human cells until the invention of viral vectors in the 1980s [2–4]. Then, there were several notorious events in the history of gene therapy. In 1999, an 18-year-old teenager died from a severe inflammatory response to the used adenoviral vector [5]. Subsequently, the treatment of X-linked severe combined immunodeficiency (SCID-X1) with a gammaretroviral vector in 2000 led to T-cell leukemia in nearly half of patients [6–8]. These setbacks announced gene therapy went into a ‘dark age,’ but also gave precious lessons regarding immune responses and insertional mutagenesis.
In the past few years, gene therapy has come back to the central stage worldwide [9]. With its astonishing efficacy against genetic diseases and cancers, gene therapy has finally hit the way to realize what it was expected to do since half a century ago. The first landmark is the approval of Glybera in Europe in 2012, which is the first gene therapy product in the Western countries and the first gene therapy targeting genetic diseases around the world. Though Glylbera was not commercially successful, it opens the door of gene therapy. In the following years, several gene therapy products flooded into the market. Strimvelis was approved in Europe in 2016, and CAR-T therapies (Kymriah and Yescarta) and Luxturna were approved by the US Food and Drug Administration (FDA) in 2017, respectively [10]. The approved gene therapy products from worldwide were summarized in Table 1.
Table 1.
The list of approved gene therapy products worldwide.
| Number | Company/institute | Product | Clinical trial | Country | Year | Indication/disease |
|---|---|---|---|---|---|---|
| 1 | Shenzhen SiBiono GeneTech | Gendicine (recombinant human p53 oncolytic adenovirus) | Commercial market | China | 2003 | Head and neck cancer |
| 2 | Shanghai Sunway Biotech | Oncorine (Recombinant Human Adenovirus Type 5 Injection) | Commercial market | China | 2005 | Head and neck and esophagus cancer, Nasopharyngeal cancer, etc. |
| 3 | UniQure | Glybera (AAV1) | Withdrawn | EU | 2012 | LPLD |
| 4 | Amgen | Imlygic (talimogene laherparepvec, T-Vec) | Commercial market | USA | 2015 | Melanoma |
| 5 | GlaxoSmithKline | Strimvelis (autologous CD34+ cells transduced with ADA) | Commercial market | EU | 2016 | ADA-SCID |
| 6 | Novartis | Kymriah (tisagenlecleucel) | Commercial market | Switzerland/USA | 2017 | B-ALL |
| 7 | Gilead/Kite Pharma | Yescarta (axicabtagene ciloleucel) | Commercial market | USA | 2017 | DLBCL |
| 8 | Roche/Spark Therapeutics | Luxturna (voretigene neparvovec-rzyl) | Commercial market | USA | 2017 | Biallelic RPE65 mutation-associated retinal dystrophy |
| 9 | Novartis/AveXis | Zolgensma (onasemnogene abeparvovec-xioi) | Commercial market | Switzerland/USA | 2019 | SMA |
| 10 | Bluebird bio | Zynteglo (autologous CD34+ cells encoding βA-T87Q-globin gene) | Delayed commercial | EU | 2019 | TDT |
| 11 | BioMarin Pharmaceutical | Valoctocogene roxaparvovec (valrox) | Waiting for commercial | USA | 2019-2020 | Hemophilia A |
EU European Union, LPLD lipoprotein lipase deficiency, AAV1 adeno-associated virus type-1, ADA-SCID Adenosine deaminase deficiency-severe combined immune deficiency, B-ALL B-cell acute lymphoblastic leukemia, DLBCL diffuse large B-cell lymphoma, SMA spinal muscular atrophy, TDT transfusion-dependent β-thalassemia.
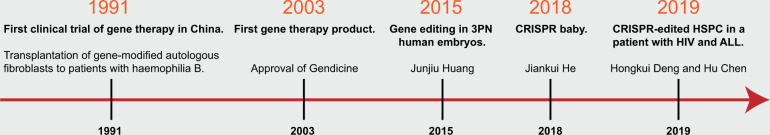
China embraced gene therapy at a very early time. The first clinical trial of gene therapy in China dates back to 1991—just 1 year after the first-ever gene therapy performed at the National Institutes of Health (NIH) in 1990 [11, 12]. In addition, China approved the world’s first gene therapy product in 2003—Gendicine—which is an oncolytic adenovirus equipped with p53 for the treatment of advanced head and neck cancer. The first CRISPR clinical trial was also in China [13]. One of the biggest scandals in life science—CRISPR baby—was also launched in China [14]. The major events of gene therapy in China are highlighted in Fig. 1. The gene therapy in China shared international commons, but also with her own characteristics (Fig. 1). Here, we will review the history and present of gene therapy in China and give a perspective of future gene therapy in China.
Fig. 1. Milestones of gene therapy in China.
HSPC hematopoietic stem and progenitor cells; ALL acute lymphocytic leukemia.
Gene therapy in China in the twentieth century
In 1991, Lu et al. conducted a clinical trial for gene therapy of hemophilia B (HB), which is the second human gene therapy trial in the world—just 1 year after the ADA-SCID trial in the USA and the first human gene therapy trial for hemophilia [12]. Hemophilia A (HA) and HB are serious X-linked bleeding diseases that mostly affect males. HA and HB are caused by a defect or decrease of factor VIII (FVIII) or factor IX (FIX) in the blood, respectively [15]. The authors used a retroviral vector to deliver hFIX cDNA to autologous skin fibroblasts and then embedded the modified cells in collagen before subcutaneously injected to two patients from the same family [12]. The retroviral vector contained a full-length long terminal repeat (LTR) and CMV promoter, both of which are strong enhancers and are against the present safety criteria. However, the therapy turned out to be safe at least for the first few years and no treatment-related adverse effects (AEs) were observed [16]. Notably, the first 3 years after gene therapy is the time point for adverse events to occur in SCID-X1 clinical trials, which are attributed to activation of oncogene by the strong enhancer in the retroviral LTR [6, 7]. Due to the relative safety of the protocol, another two patients were recruited in a new trial using a slightly differently designed retroviral vector. In all four patients, though the symptoms were alleviated, the concentration of hFIX could only reach to at best 5% of normal value (3–4 μg/mL) while the efficiency for curing the disease required more than 10% of normal value [17]. And the therapeutic levels of FIX activity (FIX:C) was sustained for 420 days, but one patient was treated again because of the decline of FIX:C [16].
Gene therapy in China in the twenty-first century
Hemophilia
The prevalence of hemophilia in China is 3.6 per 100,000 and the prevalence among males is 5.5 per 100,000, which is lower than the commonly reported worldwide prevalence—10 per 100,000 [15, 18]. However, considering the large population of China, this is one of the most influential genetic diseases affecting a large number of families. Gene therapy of hemophilia is progressing rapidly in the twenty-first century. A number of reports have shown significant clinical benefits of adeno-associated virus (AAV)-mediated gene therapy for hemophilia [19–22]. In China, however, no further clinical trial of hemophilia has even been conducted since 1991 until in recent years. Gene therapy for HB with AAV vector (NCT04135300) using single-dose intravenous infusion of BBM-H901 is recruiting three participants, which was sponsored by the Institute of Haematology & Blood Diseases Hospital in Tianjin last year. In addition, the lentiviral FVIII gene-modified HSPC transplantation for HA trial (NCT03217032) has been registered by Shenzhen Geno-Immune Medical Institute in 2017, but the clinical trial is still under the non-recruiting status till now (Table 2). Nowadays, more efforts have been re-focused on basic studies.
Table 2.
Ongoing clinical trials of gene therapy in China.
| Number | Company/Institute | Product | Route of administration | Target | Indication/disease | Clinical trial | Trial ID | Initiate year | Status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Shenzhen Geno-immune Medical Institute, Shenzhen | YUVA-GT-F801 (lentiviral gene-modified autologous stem cells) | Intravenous infusion | FVIII | Hemophilia A | Phase I | NCT03217032 | 2017 | Not yet recruiting |
| 2 | Institute of Hematology & Blood Diseases Hospital, Tianjin | BBM-H901 (AAV) | Single-dose intravenous injection | FIX | Hemophilia B | Phase I | NCT04135300 | 2019 | Recruiting |
| 3 | Nanfang Hospital, Guangzhou | Autologous CD34+ cells genetically modified (lentiviral vector) | Intravenous infusion/Auto-HSCT | HBB | β-thalassemia major | Phase I/II | NCT03276455 | 2017 | Not yet recruiting |
| 4 | Shenzhen Geno-immune Medical Institute, Shenzhen | Gene-modified autologous stem cells (lentiviral Vector) | Intravenous infusion | HBB | β-thalassemia | Phase I/II | NCT03351829 | 2017 | Not yet recruiting |
| 5 | Shanghai Bioray laboratory Inc,Shanghai | γ-globin reactivated autologous hematopoietic stem cells (CRISPR/Cas9 gene editing system) | Intravenous infusion/Auto-HSCT | NA | β-thalassemia major | Phase I/II | NCT04205435 | 2019 | Not yet recruiting |
| 6 | Sichuan University | PD-1 Knockout T Cells | Intravenous infusion | PD-1 | Metastatic non-small cell lung cancer | Phase I | NCT02793856 | 2016 | Active, not recruiting |
| 7 | Hangzhou Cancer Hospital | PD-1 Knockout T Cells | Intravenous infusion | PD-1 | Esophageal cancer | Phase I | NCT03081715 | 2017 | Completed |
| 8 | Huazhong University of Science and Technology | rAAV2-ND4 | Intravitreal Injection (IVT injection) | ND4 (complex I) | LHON | NA | NCT01267422 | 2010 | Completed |
| 9 | Huazhong University of Science and Technology | rAAV2-ND4 | IVT injection | ND4 (complex I) | LHON | Phase II/III | NCT03153293 | 2017 | Active, not recruiting |
| 10 | Huazhong University of Science and Technology | rAAV2-ND4 | IVT injection | ND4 (complex I) | Acute LHON | NA | NCT03428178 | 2018 | Recruiting |
| 11 | OrienGene Biotechnology Ltd. | OrienX010 (recombinant human GM-CSF HSV-1) | Tumor site local injections | NA | Melanoma | Phase I | NCT03048253 | 2017 | Unknown |
| 12 | Wuhan Binhui Biotechnology Co., Ltd. | OH2 Oncolytic Virus (HSV-2 strain HG52) | Intratumorally injection | NA | Malignant solid tumors (gastrointestinal cancers, head and neck cancers, soft tissue sarcomas) | Phase I | NCT03866525 | 2019 | Recruiting |
| 13 | Wuhan Binhui Biotechnology Co., Ltd. | OH2 Oncolytic Virus (HSV-2 strain HG52) | Intratumorally injection | NA | Malignant solid tumors (melanoma) | Phase I/II | NCT04386967 | 2020 | Recruiting |
| 14 | Shenzhen Geno-Immune Medical Institute | TYF-IL2RG gene-modified autologous stem cells | Intravenous infusion | IL2RG | SCID-X1 | Phase I/II | NCT03217617 | 2017 | Recruiting |
| 15 | Children’s Hospital of Chongqing Medical University | Lentiviral vector transduced bone marrow stem cells | Intravenous infusion | IL2RG | SCID-X1 | NA | NCT04286815 | 2020 | Recruiting |
| 16 | Shenzhen Geno-Immune Medical Institute | Gene-modified autologous stem cells (lentiviral vector) | Intravenous infusion | FANCA | Fanconi anemia | Phase I/II | NCT03351868 | 2017 | Recruiting |
| 17 | Shenzhen Geno-Immune Medical Institute | TYF-ADA gene-modified autologous stem cells (lentiviral vector) | Intravenous infusion | ADA | ADA-SCID | NA | NCT03645460 | 2018 | Recruiting |
| 18 | Shenzhen Geno-Immune Medical Institute | Lentiviral TYF-CGD-modified autologous stem cells | Intravenous infusion | CYBB/NCF1 | CGD | Phase I/II | NCT03645486 | 2018 | Recruiting |
| 19 | Shenzhen Geno-Immune Medical Institute | Lentiviral vector TYF-ARSA | Intracerebral injection | ARSA | MLD | Phase I/II | NCT03725670 | 2018 | Recruiting |
| 20 | Shenzhen Geno-Immune Medical Institute | Lentiviral vector carrying ABCD1 gene | Intracerebral injection | ABCD1 | X-ALD | Phase I/II | NCT03727555 | 2018 | Recruiting |
FVIII factor VIII, FIX factor IX, HSCT hematopoietic stem cell transplantation, HBB hemoglobin subunit beta, NA not available, PD-1 programmed death-1, ND4 NADH dehydrogenase, subunit 4, LHON Leber’s hereditary optic neuropathy, HSV Herpes simplex virus, SCID-X1 X-linked severe combined immunodeficiency, FANCA Fanconi anemia complementation group A, ADA adenosine deaminase, ADA-SCID adenosine deaminase severe combined immune deficiency, CYBB X-linked gp91phox gene, NCF1 gene coding for p47phox, CGD chronic granulomatous disease, ARSA Arylsulfatase A, MLD metachromatic leukodystrophy, ABCD1 ATP-binding cassette transporter, X-ALD X-linked adrenoleukodystrophy.
Guan et al. identified a novel hemophilia-causing mutation FIX Y371D mutation in the FIX gene and generated a mice model carrying this particular mutation [23]. In addition, the authors performed hydrodynamic tail vein (HTV) injection of naked Cas9-sgRNA plasmids and donor DNA into FIX Y371D mice and showed a sign of alleviation of hemostasis [23]. In contrast, the adenoviral vectors system (AdvCas9) which used to deliver Cas9 components in this study, resulted in a higher corrective efficiency but no therapeutic effects likely due to severe hepatic toxicity. However, a recent study using hAdV-5/Cas9 delivery of CRISPR machinery showed signs of phenotypic correction in a murine model of juvenile HB suggesting that more advanced adenoviral vectors could facilitate hemophilia gene therapy [24]. In a similar study, detectable gene correction (>1%) in FIX alleles of hepatocytes was observed in 62.5% of treated HB mice after HTV injection of naked Cas9-sgRNA plasmid plus donor DNA ameliorating the coagulation deficiency, however, the delivery strategy should be significantly improved before it could be considered for clinical applications [25]. Recently, our group indicated that functional hematopoietic stem and progenitor cells (HSPCs) could be generated from genome-edited iPSCs via in vivo differentiation, and combination of iPSC-derived HSPCs and CRISPR may serve a potential route for platelet-targeted gene therapy of HA [26]. Interestingly, Li et al. reported using mesenchymal stem cells (MSCs) as cell vectors in which an hFIX gene was inserted site specifically at AAVS1 locus via ZFNs-induced homologous recombination, and showed expression of hFIX in the recipient mice [27].
β-thalassemia
Mutations that reduce or abolish the expression of the β-globin chain of hemoglobin cause β-thalassemia [28]. In a health condition, balanced production of α- and β-globin chains of hemoglobin forms adult hemoglobin [29]. However, in β-thalassemia patients, the excessive α-globin chains precipitate around the membrane of red blood cell (RBC) precursors leading to the destruction of the erythroid precursors and ineffective erythropoiesis. Currently, more than 200 mutations were identified affecting the human β-globin gene. In China, β654 thalassemia caused by C to T substitution at nucleotide 654 of intron 2 in the β-globin gene is the most common form and represents approximately one-fifth of total β-thalassemia cases [29]. β-thalassemia is the most prevalent in the southern part of China including Guangxi, Guangdong, and Hainan provinces. Particularly, in Guangxi 20–31.5% of the population are carriers of thalassemia gene [30].
Gene therapy of β-thalassemia has achieved tremendous success from clinical trials in both USA and Europe regarding safety and efficacy [31, 32]. Surprisingly, β-thalassemia gene therapy is still in an infant stage in China. No clinical data have been released publically regarding the efficacy of β-thalassemia gene therapy (Table 2)—even the number of preclinical studies is very limited. Tian et al. tested the feasibility of using AAV2 to deliver β-globin for β-thalassemia gene therapy in mouse model [33]. As the AAV2 is non-insertional, the efficacy is unlikely to last long. Interestingly, Fanyi Zeng et al. performed a proof-of-concept study using iPSCs, which were transduced with human wild-type β-globin for the treatment of β-thalassemia [29]. The author concluded that 10% or more expression of the exogenous normal β-globin gene was capable of reducing the degree of anemia in their β-thalassemia mouse model [29]. Recently, Wang et al. reactivated the expression of γ-globin using RNPs of Cas9 and base editor to modify the hemoglobin subunit gamma (HBG) promoter region mimicking the effect of naturally occurring Δ13 bp allele in patients with hereditary persistence of fetal hemoglobin [34].
Clinical trial for gene editing in somatic cells
Somatic gene editing has demonstrated its potential in vivo by treating genetic diseases, infectious diseases, and acquired diseases. A clinical trial of gene editing started with ZFN which has firstly been applied for ex vivo gene therapy of HIV inspired by the ‘Berlin patient.’ Trials with ZFN have now been extended to hemophilia, Fabry disease, MPS II, and globin dystrophies.
In 2018, two phase 1/2 clinical programs have begun in the USA and Europe to study the safety and efficacy of CRISPR modified autologous hematopoietic stem cells (HSC; CTX101) in both β-thalassemia (NCT03655678) and sickle cell patients (NCT03745287). In 2019, another clinical gene editing program (NCT03872479) has been approved by the US FDA for in vivo treatment of an inherited severe retinal dystrophy—Leber congenital amaurosis type 10 (LCA10) using EDIT-101 which is an AAV-carried CRISPR therapeutic. Notably, EDIT-101 is the first in vivo CRISPR trial.
In China, all gene-editing trials have been based on CRISPR directed ex vivo strategy so far. Lu et al. from Sichuan University is the first to use CRISPR in a clinical trial where he used CRISPR to knockout PD-1 on autologous T cells with a goal to cure non-small cell lung cancer (NCT02793856). From the recently released data, 12 patients received autologous PD-1 edited T-cell infusion and the implantation of PD-1 negative T cells appeared to be safe because no dose-limiting toxicity (DLT) and grade 3–5 AEs were found during the median follow-up of 47.1 weeks [35]. Another trial led by Dr. Shixiu Wu from Hangzhou Cancer Hospital utilized the same strategy but to treat patients with advanced esophageal cancers (NCT03081715). However, for this trial, the clinical results have not been published, therefore, it is difficult to evaluate its safety and efficacy. Recently, Xu et al. reported using CRISPR to knockout of C–C chemokine receptor type 5 (CCR5) receptor of HIV entry in HSPCs, which they transplanted to a patient who suffered from both HIV and acute lymphocytic leukemia [36]. Though no evidence of therapeutic benefits for HIV, the study showed first in human evidence that CRISPR was safe at least during the 19 months follow-up, which may encourage other ex vivo gene therapy using CRISPR-edited HSPCs such as β-thalassemia and sickle cell diseases [36].
Gene editing in human embryos
In 2015, Huang et al. reported the first CRISPR gene editing in human embryos. To diminish ethical pressure, they used tripronuclear (3PN) zygotes, which have one oocyte nucleus and two sperm nuclei and are associated with spontaneous abortions after implantation [37]. They found CRISPR is highly efficient cleave the endogenous β-globin gene (HBB) in human embryos with most cleavages are repaired by non-homologous end joining and only 14.3% of them are repaired by homologous recombination [37]. Importantly, this study also shows high rates of off-targeting and mosaicism of edited embryos pointing gene editing in human embryos is far from mature [37].
Yet, about 3 years later, Jiankui He, a former associate professor from Southern University of Science and Technology, performed gene editing in human embryos using CRISPR and aimed to produce babies. At the second World Summit of Human Gene Editing, he claimed his purpose was to create CRISPR HIV-free babies by knocking out CCR5 gene, which led to the birth of two baby girls in China. Furthermore, the experimental design and data presented at the summit revealed serious misconduct on both the scientific and ethical levels. His action, however, brought fierce international criticism and was condemned by gene editing community in China and other comities and government agencies, such as the Genetics Society of China, the Chinese Society for Stem Cell Research, and the National Health Commission in China [14, 38]. Changing germline cells will irreversibly change the human gene pool and is violating the current international norms and Chinese existing regulations. At last, he was sentenced to 3 years in last December because of carrying out gene editing in human embryos for reproductive purposes, which is prohibited by the state. Recently, a Russian biologist disclosed his plan to correct a deaf gene in human embryos and knockout of CCR5 like He had done, though he would wait until he got permission from regulatory agency [39]. Clearly, a broader international framework is necessary to govern human germline editing, which could lead by the World Health Organization.
Gene editing and base editing tools
Site-specific gene editing has been long dreamed in the field of basic research and drug development. The earliest generation of gene editing tools includes zinc-finger nucleases and meganucleases (or homing endonucleases) [40, 41]. Both of them are relatively small and easy to deliver, however, they are also difficult to design and acquire, therefore, calling for new gene-editing tools. Transcription activator-like effector nucleases (TALENs) adapted from a genus of plant pathogens, Xanthomonas, are a number of modular repeats making it possible for an ordinary lab to quickly assemble a pair of functional nucleases feasible [42]. However, for gene therapy applications, the main challenge comes from delivery as TALENs are comprised of ~34 highly repetitive amino-acid DNA binding domains, making them difficult to deliver by viral vectors [43].
Class II CRISPR/Cas system contains only one effector nuclease and has quickly become the most widely used gene editing technology [44]. Today, three families of class 2 CRISPR/Cas have been characterized including Cas9, Cas12a/cpf1, and Cas12b/C2c1 [45]. Among them, Cas12b is generally smaller in size which may facilitate it for delivery by AAV [45, 46]. However, the initially identified Cas12b (AacCas12b) from Alicyclobacillus acidoterrestris works optimally at 50 °C, making it not suitable for gene therapy [46]. Wei Li group identified a new Cas12b (AaCas12b) from Alicyclobacillus acidiphilus and showed nuclease activity in mammalian cells and murine embryos [47]. Using deep sequencing and whole genome sequencing, the author showed that AaCas12b may tend to induce less off-target effects compared with spCas9 and AsCas12a [47]. Almost at the same time, Zhang et al. identified and engineered a Cas12b (BhCas12b) from Bacillus hisashii and demonstrated its nuclease function in cell lines and primary T cells [48]. Interestingly, both groups reported greater specificity of Cas12b compared with spCas9 [47, 48]. To move Cas12b toward gene therapy, the efficiency, off-target effect, and immune response should be characterized in vivo.
In contrast to CRISPR, base editors (BE) changes nucleotides without causing double-strand break and is potentially safer than CRISPR in gene therapy [49]. The early versions of BEs are based on CRISPR/Cas9 of which the recognition sites are restricted by G/C-rich protospacer-adjacent motif [50, 51]. Chen et al. reported a CRISPR/cpf1 based BE by fusing rat cytosine deaminase APOBEC1 to a catalytically inactive version of Lachnospiraceae bacterium Cpf1-therefore, expanding the editable region to T-rich sequence [52]. The same group further extends efficient base editing to highly methylated regions by screening 15 different APOBEC and AID deaminases [53]. However, using APOBEC1-fused BE for gene therapy should be cautious. Yang et al. have demonstrated that cytosine BE could induce substantial off-target effects by whole genome sequencing of the two-cell mouse embryo, indicating that further evolution of this technology is mandatory to move to the clinic [54].
Delivery
Any gene editing tools or rescuing genes eventually need a vector to deliver to cells for therapeutic purposes. However, this has been and is still a challenge. From a clinical perspective, lentiviral vector, retroviral vector, adenoviral vector, AAV are the most frequently used viral vectors while lipid nanoparticles and transposons are the most widely used non-viral vectors. Lentiviral vector has been intensively used in the HSC-based gene therapy and CAR-T-cell therapy [55]. Not a single oncogenesis event has been observed for lentivirus-based gene therapy in HSC for over 10 years follow-up, however, the worry to insertional mutagenesis is remaining [28, 56, 57]. The retroviral vector is the first FDA-approved vector to be used in a clinical trial for treating a monogenetic disease—adenosine deaminase deficiency (ADA-SCID) in 1990 [58]. Notably, in all ADA-SCID clinical trials with retroviral vectors to date, not any deadly effect has ever been reported. However, when used for treating SCID-X1, several patients developed leukemia due to the activation of nearby oncogenes [6, 7]. AAV mainly exists as episomal DNA and is generally considered as a non-insertional vector, which makes it widely used in all stages of gene therapy development (Tables 1 and 2). Notably, a recent report in China showed therapeutic benefits of gene therapy of Leber’s hereditary optic neuropathy (LHON), an inherited mitochondrial disorder resulting in vision loss, using AAV2 and safety after 7 years’ follow-up [59]. Though have shown the safety and efficacy in numerous clinical trials [60, 61], the vector has recently been suspected linking to cancers [62, 63], still informing us to be cautious regarding the long-term safety of AAV.
The vector development in China has been mostly focused on non-viral vectors for which intracellular delivery is a major challenge because of multiple barriers on the route of trafficking such as endocytosis, endosomal escape, and intracellular release. Yiyun Cheng’s group reported a fluorine-modified polymer (fluoropolymers) allowed efficient delivery of various proteins intracellularly and in vivo [64]. The same group further reported a boronic acid-rich dendrimer, which is capable of escaping cell barriers with high efficiency [65]. Using this approach, they showed moderate gene editing in cell lines by delivering CRISPR-Cas9 RNP [65]. However, it is still difficult to evaluate the therapeutic potential of polymers-delivered gene editing due to a lack of evidence from primary cells and in vivo study. Recently, our group has reported an mRNA-carrying lentiviral vector (mLP) that allows us to delivery Cas9 in the form of mRNA and show therapeutic efficacy in the mice model of HSV-1 induced keratitis and also in human-derived corneas. The whole genome sequencing of edited human tissue has not found any off-target effects in the coding region indicating the safety of this technology. The mLP may also serve as a new vaccine platform to represent the native-like immunogen without being infectious, of which the potential has been shown in a recent proof-of-concept study for eliciting strong SARS-CoV-2 neutralizing activities [66].
Oncolytic virus (OVs)
Though the oncolytic virus has entered into registered clinical trials early in the 1990s worldwide, for instance, dl1520 (Onyx-015) a recombinant adenovirus selectively replicating in p53-deficient cancers was tested in Phase I in Scotland in 1996 [67]. China is the first country to approve a gene therapy product, which is an oncolytic adenovirus equipped with p53 (Gendicine) for the treatment of advanced head and neck cancers in 2003. Gendicine showed signs of clinical benefits in several clinical studies, however, it has also been controversial and has been reviewed in detail elsewhere [68]. Two years later, in 2005, another oncolytic virus Oncorine (rAd5-H101) developed by Shanghai Sunway Biotech was approved by the China Food and Drug Administration (CFDA) to treat late-stage nasopharyngeal carcinoma [69]. However, since then, clinical trials for the oncolytic virus in China have been long discontinued according to the open information in clinicaltrial.org. Not until 2019, three clinical trials have been in actively recruiting. Among them, two are phase I studies to evaluate the safety and efficacy of an attenuated HSV-2 expressing GM-CSF either as a single agent or in combination with a PD-1 antibody (NCT03866525 and NCT04386967). In contrast, a search of clinicaltrials.gov performed on December 31, 2019, ~34 clinical trials are currently recruiting patients for treatment with OVs in the USA. The discontinued clinical trials of OV in China reflect the efficacy of OV has not been fundamentally improved since Gendicine until recently.
Until 2015—the year T-VEC was approved by the FDA, the oncolytic virus is gradually coming back in China. Yan et al. reported an oncolytic virus M1, which is a strain of Getah-like alphavirus that was isolated from culicine mosquitoes collected on Hainan Island of China [70, 71]. Notably, this is first domestically identified and characterized oncolytic strain in China. Current preclinical and clinical data support the notion that the therapeutic efficacy of oncolytic virus is very limited as monotherapy and joint therapy may perform better than each drug used alone. Numerous preclinical studies are ongoing which can be divided into different categories, (1) oncolytic virus expressing immune checkpoint inhibitor [72, 73], (2) expressing immune-stimulating cytokines [74, 75], (3) combination with small molecules [71, 76], (4) combination with radiotherapy [77].
Efforts have also been made to improve the property of the virus itself which could be the direction of next-generation OV. Lv et al. showed that an oncolytic adenovirus packaged in bioengineered cell membrane nanovesicles achieved robust antiviral immune shielding and cancer targeting capabilities in vivo [78]. Interestingly, Huang et al. reported a new type of oncolytic adenovirus by embedding a synthetic gene circuit in the viral genome to obtain tumor specificity and controlled expression of different immune effectors [79].
Governance
Chinese scientists carried out basic research and clinical trials of gene therapy dating back to the late 1980s and early 1990s. The policy on gene therapy regulation was first introduced in 1993. Up to now, the evolution of regulatory policies can be roughly divided into three stages.
The first stage (the end of the twentieth century)
In 1993, the National Science and Technology Commission issued the ‘Genetic Engineering Safety Management Measures,’ but this rule does not specifically provide detailed provisions on gene therapy. In the same year, the Drug Administration of the Ministry of Health issued the ‘Quality Control Points for Clinical Research on Human Somatic Cell Therapy and Gene Therapy,’ which provided specific quality control guidelines for gene therapy and played an important role in promoting the rapid development of gene therapy. In 1999, the State Drug Administration promulgated the ‘Approval Measures for New Biological Products’ and ‘Management Standards for Drug Clinical Trials’ to include gene therapy into the regulatory scope of clinical trials for new biological products and drugs. As a result, in the field of gene therapy, China has formed two regulatory paths of technology and drug, as well as multi-supervision models carried out by the competent authorities of science and technology, health, and drug, until today. Overall, the regulatory policies at this stage are relatively loose, and the regulatory measures are relatively rough.
The second stage (the first 15 years of the twenty-first century)
At the beginning of the twenty-first century, China has formed a relatively comprehensive but still loose regulatory policy for gene therapy, involving the drug clinical trial, gene therapy quality control, assisted reproduction, ethical review, stem cells, etc. In 2003, the State Food and Drug Administration issued three regulations about human gene therapy. The Ministry of Science and Technology and the Ministry of Health also jointly issued the Ethical Guiding Principles for the Research of Human Embryonic Stem Cell. In 2003, the Ministry of health, on the basis of the Measures for the Management of Human Assisted Reproductive Technology (2001), formulated the technical specifications of human-assisted reproductive technology and the ethical principles of human-assisted reproductive technology and human sperm bank, and established the policy of prohibiting the clinical trials of gene therapy for reproductive purposes. In 2007, the Ministry of Health issued the Measures for the Ethical Review of Biomedical Research Involving Human Subjects (trial), which provided a preliminary ethical review procedure for gene therapy. In 2009, the Ministry of Health issued the administrative measures for the clinical application of medical technology, which classified gene therapy as the third type of medical technology that should be approved. In 2009, the Ministry of Health also issued the technical management specification for cord blood stem cell therapy (trial) and the technical management specification for gene chip diagnosis. In 2010, the State Food and Drug Administration issued the Guiding Principles for Ethical Review of Drug Clinical Trials. In 2015, the National Health and Family Planning Commission and the State Food and Drug Administration jointly issued regulatory measures and ethical guidelines such as the administrative measures for clinical research of stem cells (Trial), the guiding principles for quality control and preclinical research of stem cell preparations (Trial). In the same year, the state Health and Family Planning Commission canceled the administrative examination and approval of the clinical application of the third type of medical technology, and thus cell therapy and gene therapy could be approved by the medical institutions themselves.
The third stage (2016 till now)
The period is characterized by comprehensive regulatory policies coordinated with technology and industry promotion, rights protection, and risk control. After the ‘Wei Zexi incident’ on ‘biological immunotherapy’ in 2016, the National Health and Family Planning Commission immediately suspended all unapproved clinical applications of the third type of medical technology, and the regulatory policies on gene therapy tend to be strict, but biotechnology still belongs to the field of national key promotion of development.
In terms of technology and industry promotion, gene therapy has broad prospects both as a new medical technology and as a biomedical industry. In the State Council’s ‘13th Five-Year Plan’ National Strategic Emerging Industry Development Plan (Guo Fa [2016] No. 67), “13th Five-Year Plan” National Science and Technology Innovation Plan (Guo Fa [2016] No. 43), The Program of Six New Free Trade Pilot Zones (Guo Fa [2019] No. 16) and other programmatic documents related to economic and social development all have incentive policies for industrial development in the field of gene therapy.
However, China has not yet formed a comprehensive and systematic legal regulatory system for human genetic technology issues (including gene therapy). There is no direct regulation on gene therapy at the legal (narrow sense) level. The current regulatory norms are mainly technical management methods or ethical guidelines. China’s legal (broad) framework for gene therapy mainly includes administrative regulations issued by the State Council, departmental regulations, and regulatory documents issued by ministries and commissions of the State Council (Table 3).
Table 3.
Current gene therapy regulatory norms in China.
| Number | Name of norms | Legislature | Last revised time |
|---|---|---|---|
| 1 | Key Points for Quality Control of Human Somatic and Gene Therapy Clinical Research | Pharmacy Administration of the Ministry of Health | 1993 |
| 2 | Measures for the Management of Human Assisted Reproductive Technology | Ministry of Health | 2001 |
| 3 | Technical Guidelines for Human Gene Therapy Research and Formulation Quality Control | CFDA | 2003 |
| 4 | Key Points for Quality Control of Human Recombinant DNA Products | CFDA | 2003 |
| 5 | Human Monoclonal Antibody Quality Control Technology Guidelines | CFDA | 2003 |
| 6 | Good Manufacturing Practices for Pharmaceutical Products | Ministry of Health | 2010 |
| 7 | Technology Specifications of Human Assisted Reproductive | Ministry of Health | 2013 |
| 8 | Ethical Principles for Human Assisted Reproduction Technology and Human Sperm bank | Ministry of Health | 2013 |
| 9 | Ethical Guiding Principles for the Research of Human Embryonic Stem Cell | Ministry of Science and Technology & Ministry of Health | 2013 |
| 10 | Measures for the Management of Stem Cell Clinical Research (Trial) | National Health and Family Planning Commission (NHFPC) & CFDA | 2015 |
| 11 | Stem Cell Preparation Quality Control and Pre-clinical Research Guidelines (Trial) | General Office of NHFPC & General Office of CFDA | 2015 |
| 12 | Measures for the Ethical Review of Biomedical Research Involving Human Subjects | NHFPC | 2016 |
| 13 | Guiding Principles for Cell Therapy Product Research and Evaluation (Trial) | China Food and Drug Administration (CFDA) | 2017 |
| 14 | Measures for Safety Management of Biotechnology Research and Development | Ministry of Science and Technology | 2017 |
| 15 | Measures for the Administration of the Clinical Application of Medical Technologies | National Health Commission | 2018 |
| 16 | Regulation on the Prevention and Handling of Medical Disputes | State Council | 2018 |
| 17 | Regulations on the Implementation of the Drug Administration Law of the People’s Republic of China | State Council | 2019 |
| 18 | Regulations on the Management of Human Genetic Resources | State Council | 2019 |
| 19 | Drug Administration Law of the People’s Republic of China | NPC Standing Committee | 2019 |
The Ministry of Health (卫生部) and the National Health and Family Planning Commission (NHFPC, 国家卫生和计划生育委员会) in the above table are the current National Health Commission (国家卫生健康委员会), and now the CFDA mentioned in the above is reorganized as National Medical Products Administration (NMPA).
Affected by the CRISPR baby incident, China is stepping up legislation in areas such as biosecurity, genetic technology, and biomedicine. In 2019, the ‘Biosafety Law’ has passed the first review by the Standing Committee of the National People’s Congress. The goal of this law is to become a basic, systematic, comprehensive, and dominant basic law on biosafety. The Ninth Meeting of the Central Committee for Comprehensive and Deepening Reform reviewed and approved the ‘Structure of the National Science and Technology Ethics Committee,’ which is committed to promoting the establishment of a comprehensive science and technology ethics governance system with comprehensive coverage, clear guidance, standardization, orderliness, and coordination. China’s national health, science, and technology authorities are taking corresponding legislative actions, and have drafted the ‘Regulations on the Management of Clinical Application of New Biomedical Technologies (Draft for Comment)’ and ‘Regulations on the Safety Management of Biotechnology Research and Development (Draft for Comment).’ Through the improvement of the level of laws and regulations (from the original administrative regulations to administrative regulations), the goal is that a more reasonable supervision mechanism will be formed, the supervision will be strengthened, the legal responsibility will be increased, and the healthy development of biotechnology research and development, applications and related industries will be promoted.
Future perspective
Although gene therapy is on the rising in China in the past few years, there is still much to do to narrow the gap between China and Western countries. First, we think that public education is vital to the success of gene therapy. As a new category of medicine, gene therapy is deeply changing the landscape of the pharmaceutical industry, however, it is still far from mature—that is to say, there are still many unknowns to explore. When serious adverse events occur, the public attitude may directly determine the fate of gene therapy. The leukemia event in the ‘bubble boy’ gene therapy trials at the beginning of this century tells a classic lesson. Learning instead of fearing makes gene therapy flourishing today. Second, stable funding on the field is crucial. From the history of gene therapy, China started early as shown by the world’s second gene therapy trial in 1991, however, later on, the progress was slow and very limited compared with USA and Europe during the same period. Third, the current regulation of gene therapy in China is complicated and cumbersome. How to coordinate different norms and simplify the regulations may facilitate the research and development of gene therapy in China. The subjects of gene therapy are usually those in bad condition who urgently need new therapies to improve their health condition and gain a chance of life. Better regulations should help to shorten the time from research to clinical applications.
Acknowledgements
YC is supported by National Natural Science Foundation of China [31971364]; Pujiang Talent Project of Shanghai [GJ4150006]; Natural Science Foundation of Shanghai [BS4150002]; Startup funding from Shanghai Center for Systems Biomedicine, Shanghai Jiao Tong University [WF220441504].
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kang Wang, Email: kangwang@live.com.
Yujia Cai, Email: yujia.cai@sjtu.edu.cn.
References
- 1.Friedmann T, Roblin R. Gene therapy for human genetic disease? Science. 1972;175:949–55. doi: 10.1126/science.175.4025.949. [DOI] [PubMed] [Google Scholar]
- 2.Shimotohno K, Temin HM. Formation of infectious progeny virus after insertion of herpes simplex thymidine kinase gene into DNA of an avian retrovirus. Cell. 1981;26:67–77. doi: 10.1016/0092-8674(81)90034-9. [DOI] [PubMed] [Google Scholar]
- 3.Wei CM, Gibson M, Spear PG, Scolnick EM. Construction and isolation of a transmissible retrovirus containing the src gene of Harvey murine sarcoma virus and the thymidine kinase gene of herpes simplex virus type 1. J Virol. 1981;39:935–44.. doi: 10.1128/jvi.39.3.935-944.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabin CJ, Hoffmann JW, Goff SP, Weinberg RA. Adaptation of a retrovirus as a eucaryotic vector transmitting the herpes simplex virus thymidine kinase gene. Mol Cell Biol. 1982;2:426–36. doi: 10.1128/mcb.2.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–58. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–9. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 7.Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Investig. 2008;118:3132–42. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–72. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 9.Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science. 2018;359:eaan4672. doi: 10.1126/science.aan4672. [DOI] [PubMed] [Google Scholar]
- 10.Hoggatt J. Gene therapy for “Bubble Boy” disease. Cell. 2016;166:263. doi: 10.1016/j.cell.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 11.Blaese RM, Anderson WF. The ADA human gene therapy clinical protocol original covering Memo: February 23, 1990. Hum Gene Ther. 1990;1:327–9. [Google Scholar]
- 12.Lu DR, Zhou JM, Zheng B, Qiu XF, Xue JL, Wang JM, et al. Stage I clinical trial of gene therapy for hemophilia B. Sci China B. 1993;36:1342–51. [PubMed] [Google Scholar]
- 13.Cyranoski D. CRISPR gene-editing tested in a person for the first time. Nature. 2016;539:479. doi: 10.1038/nature.2016.20988. [DOI] [PubMed] [Google Scholar]
- 14.Cyranoski D, Ledford H. Genome-edited baby claim provokes international outcry. Nature. 2018;563:607–8. doi: 10.1038/d41586-018-07545-0. [DOI] [PubMed] [Google Scholar]
- 15.Berntorp E, Shapiro AD. Modern haemophilia care. Lancet. 2012;379:1447–56. doi: 10.1016/S0140-6736(11)61139-2. [DOI] [PubMed] [Google Scholar]
- 16.Qiu X, Lu D, Zhou J, Wang J, Yang J, Meng P, et al. Implantation of autologous skin fibroblast genetically modified to secrete clotting factor IX partially corrects the hemorrhagic tendencies in two hemophilia B patients. Chin Med J. 1996;109:832–9. [PubMed] [Google Scholar]
- 17.Qiu XF, Lu DR, Xue HWW, Yang JL, Meng JM, Clinical PL. trials of gene therapy in four patients with hemophilia B (in Chinese) J Fudan Univ (Nat Sci) 1996;35:341–8. [Google Scholar]
- 18.Qu Y, Nie X, Yang Z, Yin H, Pang Y, Dong P, et al. The prevalence of hemophilia in mainland China: a systematic review and meta-analysis. Southeast Asian J Trop Med Public Health. 2014;45:455–66. [PubMed] [Google Scholar]
- 19.Rangarajan S, Walsh L, Lester W, Perry D, Madan B, Laffan M, et al. AAV5-factor VIII gene transfer in severe hemophilia A. N Engl J Med. 2017;377:2519–30. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 20.George LA, Sullivan SK, Giermasz A, Rasko JEJ, Samelson-Jones BJ, Ducore J, et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med. 2017;377:2215–27. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasi KJ, Rangarajan S, Mitchell N, Lester W, Symington E, Madan B, et al. Multiyear follow-up of AAV5-hFVIII-SQ gene therapy for hemophilia A. N Engl J Med. 2020;382:29–40. doi: 10.1056/NEJMoa1908490. [DOI] [PubMed] [Google Scholar]
- 23.Guan Y, Ma Y, Li Q, Sun Z, Ma L, Wu L, et al. CRISPR/Cas9-mediated somatic correction of a novel coagulator factor IX gene mutation ameliorates hemophilia in mouse. EMBO Mol Med. 2016;8:477–88. doi: 10.15252/emmm.201506039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephens CJ, Lauron EJ, Kashentseva E, Lu ZH, Yokoyama WM, Curiel DT. Long-term correction of hemophilia B using adenoviral delivery of CRISPR/Cas9. J Control Release. 2019;298:128–41. doi: 10.1016/j.jconrel.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huai C, Jia C, Sun R, Xu P, Min T, Wang Q, et al. CRISPR/Cas9-mediated somatic and germline gene correction to restore hemostasis in hemophilia B mice. Hum Genet. 2017;136:875–83. doi: 10.1007/s00439-017-1801-z. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, Zhang G, Gu J, Shao X, Dai Y, Li J, et al. In vivo generated hematopoietic stem cells from genome edited induced pluripotent stem cells are functional in platelet-targeted gene therapy of murine hemophilia A. Haematologica. 2020;105:e175–9. doi: 10.3324/haematol.2019.219089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li SJ, Luo Y, Zhang LM, Yang W, Zhang GG. Targeted introduction and effective expression of hFIX at the AAVS1 locus in mesenchymal stem cells. Mol Med Rep. 2017;15:1313–8. doi: 10.3892/mmr.2017.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467:318–22. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang G, Shi W, Hu X, Zhang J, Gong Z, Guo X, et al. Therapeutic effects of induced pluripotent stem cells in chimeric mice with beta-thalassemia. Haematologica. 2014;99:1304–11. doi: 10.3324/haematol.2013.087916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou YJ, Xie DN. Progress in the prevention and treatment of beta-thalassemia. Chin J Fam Plan. 2015;23:P709–13. [Google Scholar]
- 31.Thompson AA, Walters MC, Kwiatkowski J, Rasko JEJ, Ribeil JA, Hongeng S, et al. Gene therapy in patients with transfusion-dependent beta-thalassemia. N Engl J Med. 2018;378:1479–93. doi: 10.1056/NEJMoa1705342. [DOI] [PubMed] [Google Scholar]
- 32.Marktel S, Scaramuzza S, Cicalese MP, Giglio F, Galimberti S, Lidonnici MR, et al. Intrabone hematopoietic stem cell gene therapy for adult and pediatric patients affected by transfusion-dependent ss-thalassemia. Nat Med. 2019;25:234–41. doi: 10.1038/s41591-018-0301-6. [DOI] [PubMed] [Google Scholar]
- 33.Tian J, Wang F, Xue JF, Zhao F, Zhong M, Song LJ, et al. Recombinant adeno-associated virus 2-mediated gene therapy for β-thalassemia. J Shanghai Jiaotong Univ (Med Sci) 2011;31:9–14. [Google Scholar]
- 34.Wang L, Li L, Ma Y, Hu H, Li Q, Yang Y, et al. Reactivation of γ-globin expression through Cas9 or base editor to treat β-hemoglobinopathies. Cell Res. 2020;30:276–8. doi: 10.1038/s41422-019-0267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Y, Xue J, Deng T, Zhou X, Yu K, Deng L, et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat Med. 2020;26:732–40. doi: 10.1038/s41591-020-0840-5. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Wang J, Liu Y, Xie L, Su B, Mou D, et al. CRISPR-edited stem cells in a patient with HIV and acute lymphocytic leukemia. N Engl J Med. 2019;381:1240–7. doi: 10.1056/NEJMoa1817426. [DOI] [PubMed] [Google Scholar]
- 37.Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, et al. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell. 2015;6:363–72. doi: 10.1007/s13238-015-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Yang H. Gene-edited babies: What went wrong and what could go wrong. PLoS Biol. 2019;17:e3000224. doi: 10.1371/journal.pbio.3000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cyranoski D. Russian ‘CRISPR-baby’ scientist has started editing genes in human eggs with goal of altering deaf gene. Nature. 2019;574:465–6. doi: 10.1038/d41586-019-03018-0. [DOI] [PubMed] [Google Scholar]
- 40.Porteus MH, Baltimore D. Chimeric nucleases stimulate gene targeting in human cells. Science. 2003;300:763. doi: 10.1126/science.1078395. [DOI] [PubMed] [Google Scholar]
- 41.Stoddard BL. Homing endonuclease structure and function. Q Rev Biophys. 2005;38:49–95. doi: 10.1017/S0033583505004063. [DOI] [PubMed] [Google Scholar]
- 42.Briggs AW, Rios X, Chari R, Yang L, Zhang F, Mali P, et al. Iterative capped assembly: rapid and scalable synthesis of repeat-module DNA such as TAL effectors from individual monomers. Nucleic Acids Res. 2012;40:e117. doi: 10.1093/nar/gks624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG, 2nd, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491:114–8. doi: 10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makarova KS, Zhang F, Koonin EV. SnapShot: Class 1 CRISPR-Cas Systems. Cell. 2017;168:946–946 e1. doi: 10.1016/j.cell.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 45.Liu L, Chen P, Wang M, Li X, Wang J, Yin M, et al. C2c1-sgRNA complex structure reveals RNA-guided DNA cleavage mechanism. Mol. Cell. 2017;65:310–22. doi: 10.1016/j.molcel.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 46.Shmakov S, Abudayyeh OO, Makarova KS, Wolf YI, Gootenberg JS, Semenova E, et al. Discovery and functional characterization of diverse Class 2 CRISPR-Cas Systems. Mol Cell. 2015;60:385–97. doi: 10.1016/j.molcel.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teng F, Cui T, Feng G, Guo L, Xu K, Gao Q, et al. Repurposing CRISPR-Cas12b for mammalian genome engineering. Cell Discov. 2018;4:63. doi: 10.1038/s41421-018-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strecker J, Jones S, Koopal B, Schmid-Burgk J, Zetsche B, Gao L, et al. Engineering of CRISPR-Cas12b for human genome editing. Nat Commun. 2019;10:212. doi: 10.1038/s41467-018-08224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533:420–4. doi: 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komor AC, Zhao KT, Packer MS, Gaudelli NM, Waterbury AL, Koblan LW, et al. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T:A base editors with higher efficiency and product purity. Sci Adv. 2017;3:eaao4774. doi: 10.1126/sciadv.aao4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, et al. Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature. 2017;551:464–71. doi: 10.1038/nature24644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Wang Y, Liu Y, Yang B, Wang X, Wei J, et al. Base editing with a Cpf1-cytidine deaminase fusion. Nat Biotechnol. 2018;36:324–7. doi: 10.1038/nbt.4102. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Li J, Wang Y, Yang B, Wei J, Wu J, et al. Efficient base editing in methylated regions with a human APOBEC3A-Cas9 fusion. Nat Biotechnol. 2018;36:946–9. doi: 10.1038/nbt.4198. [DOI] [PubMed] [Google Scholar]
- 54.Zuo E, Sun Y, Wei W, Yuan T, Ying W, Sun H, et al. Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos. Science. 2019;364:289–92. doi: 10.1126/science.aav9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naldini L, Trono D, Verma IM. Lentiviral vectors, two decades later. Science. 2016;353:1101–2. doi: 10.1126/science.aah6192. [DOI] [PubMed] [Google Scholar]
- 56.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–23. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 57.Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351–60. doi: 10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- 58.Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, et al. T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science. 1995;270:475–80. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- 59.Yuan J, Zhang Y, Liu H, Wang D, Du Y, Tian Z et al. Seven-year follow-up of gene therapy for Leber’s hereditary optic neuropathy. Ophthalmology. 2020. 10.1016/j.ophtha.2020.02.023. [DOI] [PubMed]
- 60.Wan X, Pei H, Zhao MJ, Yang S, Hu WK, He H, et al. Efficacy and safety of rAAV2-ND4 treatment for Leber’s hereditary optic neuropathy. Sci Rep. 2016;6:21587. doi: 10.1038/srep21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu HL, Yuan JJ, Zhang Y, Tian Z, Li X, Wang D et al. Factors associated with rapid improvement in visual acuity in patients with Leber’s hereditary optic neuropathy after gene therapy. Acta Ophthalmol. 2020. 10.1111/aos.14379. [DOI] [PubMed]
- 62.Valdmanis PN, Lisowski L, Kay MA. rAAV-mediated tumorigenesis: still unresolved after an AAV assault. Mol. Ther. 2012;20:2014–7. doi: 10.1038/mt.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaiser J. How safe is a popular gene therapy vector? Science. 2020;367:131. doi: 10.1126/science.367.6474.131. [DOI] [PubMed] [Google Scholar]
- 64.Lv J, He B, Yu J, Wang Y, Wang C, Zhang S, et al. Fluoropolymers for intracellular and in vivo protein delivery. Biomaterials. 2018;182:167–75. doi: 10.1016/j.biomaterials.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 65.Liu C, Wan T, Wang H, Zhang S, Ping Y, Cheng Y. A boronic acid-rich dendrimer with robust and unprecedented efficiency for cytosolic protein delivery and CRISPR-Cas9 gene editing. Sci Adv. 2019;5:eaaw8922. doi: 10.1126/sciadv.aaw8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yin D, Ling S, Tian X, Li Y, Xu Z, Jiang H, et al. A single dose SARS-CoV-2 simulating particle vaccine induces potent neutralizing activities. bioRxiv. 2020: 2020.05.14.093054.
- 67.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An Adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 68.Zhang W-W, Li L, Li D, Liu J, Li X, Li W, et al. The first approved gene therapy product for cancer Ad-p53 (Gendicine): 12 years in the clinic. Hum Gene Ther. 2018;29:160–79. doi: 10.1089/hum.2017.218. [DOI] [PubMed] [Google Scholar]
- 69.Liang M. Oncorine, the world first oncolytic virus medicine and its update in China. Curr Cancer Drug Targets. 2018;18:171–6. doi: 10.2174/1568009618666171129221503. [DOI] [PubMed] [Google Scholar]
- 70.Lin Y, Zhang H, Liang J, Li K, Zhu W, Fu L, et al. Identification and characterization of alphavirus M1 as a selective oncolytic virus targeting ZAP-defective human cancers. Proc Natl Acad Sci USA. 2014;111:E4504–12. doi: 10.1073/pnas.1408759111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang H, Li K, Lin Y, Xing F, Xiao X, Cai J, et al. Targeting VCP enhances anticancer activity of oncolytic virus M1 in hepatocellular carcinoma. Sci. Transl. Med. 2017;9:eaam7996. doi: 10.1126/scitranslmed.aam7996. [DOI] [PubMed] [Google Scholar]
- 72.Zhu Y, Hu X, Feng L, Yang Z, Zhou L, Duan X, et al. Enhanced therapeutic efficacy of a novel oncolytic herpes simplex virus Type 2 encoding an antibody against programmed cell death 1. Mol Ther Oncolytics. 2019;15:201–13. doi: 10.1016/j.omto.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu C, Wu M, Liang M, Xiong S, Dong C. A novel oncolytic virus engineered with PD-L1 scFv effectively inhibits tumor growth in a mouse model. Cell Mol Immunol. 2019;16:780–2. doi: 10.1038/s41423-019-0264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Jin J, Wu Z, Hu S, Hu H, Ning Z, et al. Stability and anti-tumor effect of oncolytic herpes simplex virus type 2. Oncotarget. 2018;9:24672–83. doi: 10.18632/oncotarget.25122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mao LJ, Ding M, Xu K, Pan J, Yu H, Yang C. Oncolytic adenovirus harboring interleukin-24 improves chemotherapy for advanced prostate cancer. J Cancer. 2018;9:4391–7. doi: 10.7150/jca.26437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meng G, Li B, Chen A, Zheng M, Xu T, Zhang H, et al. Targeting aerobic glycolysis by dichloroacetate improves Newcastle disease virus-mediated viro-immunotherapy in hepatocellular carcinoma. Br J Cancer. 2020;122:111–120. doi: 10.1038/s41416-019-0639-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao H, Zhang X, Ding Y, Qiu R, Hong Y, Chen W. Synergistic suppression effect on tumor growth of colorectal cancer by combining radiotherapy with a TRAIL-armed oncolytic Adenovirus. Technol Cancer Res Treat. 2019;18:1533033819853290. doi: 10.1177/1533033819853290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lv P, Liu X, Chen X, Liu C, Zhang Y, Chu C, et al. Genetically engineered cell membrane nanovesicles for oncolytic Adenovirus delivery: a versatile platform for cancer virotherapy. Nano Lett. 2019;19:2993–3001. doi: 10.1021/acs.nanolett.9b00145. [DOI] [PubMed] [Google Scholar]
- 79.Huang H, Liu Y, Liao W, Cao Y, Liu Q, Guo Y, et al. Oncolytic adenovirus programmed by synthetic gene circuit for cancer immunotherapy. Nat Commun. 2019;10:4801. doi: 10.1038/s41467-019-12794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]