Abstract
Background
Myriad manifestations of cardiovascular involvement have been described in patients with coronavirus disease 2019 (COVID-19), but there have been no reports of COVID-19 affecting the cardiac conduction system. The PR interval on the electrocardiogram (ECG) normally shortens with increasing heart rate (HR). The case of a patient with COVID-19 manifesting Mobitz type 1 atrioventricular (AV) block that normalized as the patient’s condition improved prompted us to investigate PR interval behavior in patients with COVID-19.
Objective
The purpose of this study was to characterize PR interval behavior in hospitalized patients with COVID-19 and to correlate that behavior with clinical outcomes.
Methods
This study was a cross-sectional cohort analysis of confirmed COVID-19 cases (March 26, 2020, to April 25, 2020). We reviewed pre–COVID-19 and COVID-19 ECGs to characterize AV conduction by calculating the PR interval to HR (PR:HR) slope. Clinical endpoints were death or need for endotracheal intubation.
Results
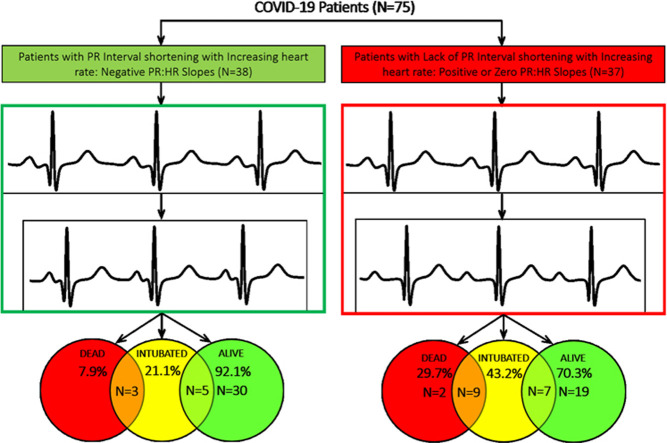
ECGs from 75 patients (246 pre–COVID-19 ECGs and 246 COVID-19 ECGs) were analyzed for PR:HR slope. Of these patients, 38 (50.7%) showed the expected PR interval shortening with increasing HR (negative PR:HR slope), whereas 37 (49.3%) showed either no change (8 with PR:HR slope = 0) or paradoxical PR interval prolongation (29 with positive PR:HR slope) with increasing HR. Patients without PR interval shortening were more likely to die (11/37 [29.7%] vs 3/38 [7.9%]; P = .019) or require endotracheal intubation (16/37 [43.2%] vs 8/38 [21.1%]; P = .05) compared to patients with PR interval shortening.
Conclusion
Half of patients with COVID-19 showed abnormal PR interval behavior (paradoxical prolongation or lack of shortening) with increasing HR. This finding was associated with increased risk of death and need for endotracheal intubation.
Keywords: Coronavirus, COVID-19, Electrocardiogram, PR Interval, SARs-CoV-2
Graphical abstract
Introduction
Coronavirus disease 2019 (COVID-19) is caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Multiple publications have described cardiac involvement in patients with COVID-19, including myocarditis, multiple infarcts, and myocardial injury with ST-segment elevation without or with nonobstructive coronary artery disease.1, 2, 3 Patients with evidence of cardiac involvement are consistently reported to have worse prognoses.4, 5, 6, 7 To date, there have been no reports on any impact of COVID-19 on the cardiac conduction system.
In healthy individuals, the PR interval shows predictable and consistent nonlinear shortening as heart rate (HR) accelerates, whether the increase in HR is achieved by exercise or by sympathomimetic drugs.8 This physiological adaptation preserves atrioventricular (AV) synchrony and maintains optimal ventricular filling during HR acceleration. Epidemiologic studies have confirmed this inverse correlation of PR interval and HR, and this relationship remains reproducible over time.9, 10, 11
We noted that many patients with COVID-19 showed abnormal PR interval behavior. This observation was first noted in a 63-year-old man with COVID-19 without previous cardiac disease. On hospital admission, the patient’s electrocardiogram (ECG) showed second-degree, Mobitz type 1 AV block at an atrial rate of 84 bpm with a narrow QRS complex, which resolved as the infection improved. This observation prompted further analysis of PR interval behavior in patients with COVID-19.
Methods
ECG analysis
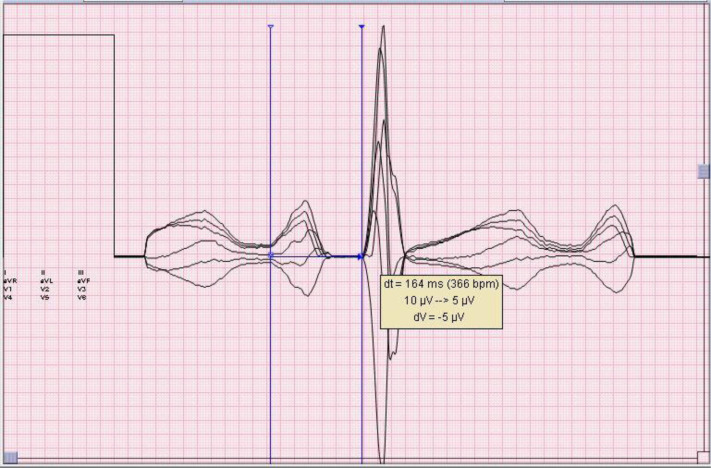
The study was determined to be exempt from review by the institutional review board in accordance with institutional policy. We reviewed current and previously recorded ECGs for all patients with COVID-19 admitted to the Jefferson Health System from March 24, 2020, to April 28, 2020. The shared electronic health record (EPIC, Verona, WI) and ECG database (MUSE GE Healthcare, Wauwatosa, WI) were used for data collection. We intentionally limited our analysis to patients with COVID-19 who had previously recorded (pre–COVID-19) ECGs in order to compare PR interval behavior during other illnesses. For study inclusion, patients were required to have at least 2 ECGs recorded before their COVID-19 hospitalization with recorded HRs that were at least 5 bpm apart and at least 2 ECGs recorded during the current hospitalization with HRs at least 5 bpm apart. Exclusion criteria were ECGs showing atrial fibrillation or atrial flutter, all pre-COVID PR intervals >200 ms, pre-COVID ECGs showing QRS duration >120 ms (reflective of pre-existing conduction system disease), and evidence of an electronic pacemaker. A maximum of 4 pre–COVID-19 and 4 COVID-19 ECGs were analyzed per patient. Pre–COVID-19 ECGs were obtained during previous illness requiring hospitalization. All ECGs were visually scanned, and PR intervals were remeasured with electronic calipers when HRs were >100 bpm or when there were obvious errors in computer-generated measurements. For manual measurements, we used the “superimposed median” format at twice paper speed and twice gain (Figure 1 ). This format is a nonlinear digital filtering technique used to remove noise by replacing each digital datapoint of the ECG tracing with the median of neighboring datapoints.12 This allowed precise measurements even with rapid HRs and baseline artifacts. PR interval measurements were recorded separately from outcomes data and laboratory values.
Figure 1.
Example of PR interval measurement. Superimposed median format used for manual PR interval measurements on electrocardiograms with heart rate >100 bpm or with overtly incorrect automated PR interval measurements. This format displays the superimposed 6 limb leads (synchronized to QRS onset) at twice paper speed and gain, and utilizes a nonlinear digital filtering technique to minimize artifact. Electronic on-screen calipers are used for precise PR interval measurement.
Clinical variables and outcome measures
The electronic health records of the patients during the current hospitalization were reviewed for the following relevant clinical variables: current age; sex; history of previous infarction; use of beta-blockers/calcium channel blockers/antiarrhythmic drugs during admission; and dates and times of recording every ECG used in the analysis. Data on total length of hospital stay; length of stay in the intensive care unit (ICU); need for endotracheal intubation; death; and several laboratory measurements (peak values of high-sensitivity [hs]-troponin T, C-reactive protein, d-dimer, ferritin, creatine phosphokinase, pro-calcitonin, pro–B-type natriuretic peptide, international normalized ratio [INR], fibrinogen, and interleukin-6) also were collected. The main clinical endpoints were death or need for endotracheal intubation.
PR interval to HR slopes
The PR interval to HR (PR:HR) slope was calculated for each set of pre–COVID-19 and COVID-19 ECGs; thus, each patient served as his or her own control. The change in slope was calculated as the mathematical difference between COVID-19 and pre–COVID-19 slopes. Based on the PR:HR slopes obtained during the COVID hospitalization, the cohort was divided into 2 groups: patients with negative PR:HR slopes (indicative of PR interval shortening with increasing HR) and patients with zero or positive PR:HR slopes (indicative of PR interval prolongation or lack of shortening with increasing HRs).
Statistical analysis
The primary analysis was an evaluation of the relationship of PR:HR slope with the primary endpoints. Continuous variables were analyzed using the 2-sided Student paired t test assuming equal variances, and the results are given as mean ± SEM. Dichotomous variables were analyzed using the Fisher exact test and are given as percentages. When a laboratory value was not provided in the patient’s electronic health record, it was assumed that the test had not been performed.
Results
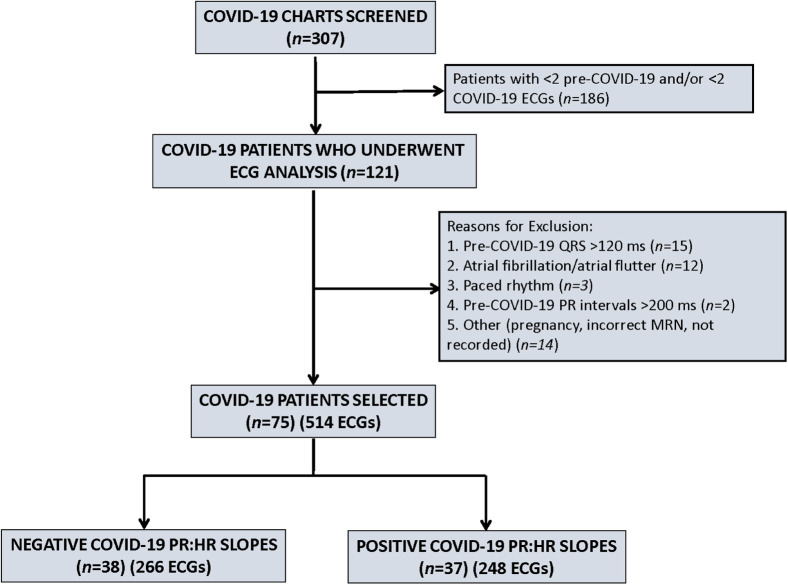
Of a total of 307 patients screened, paired ECGs for PR:HR slope analysis were obtained for 75 patients (Figure 2 ). The 3 most common reasons for noninclusion were absence of at least 2 pre–COVID-19 ECGs; absence of 2 COVID-19 ECGs; and presence of a wide QRS complex. A total of 246 pre–COVID-19 ECGs and 268 COVID-19 ECGs were included in this analysis. The mode and median values for the number of ECGs analyzed was 4. Clinical characteristics of the entire cohort and the 2 patient groups based on PR:HR slopes are given in Table 1 . The 2 groups were well matched except for a statistically significant difference in the time span for acquisition of the pre–COVID-19 ECGs compared to the COVID ECGs.
Figure 2.
Flow diagram for patient inclusion. Screening was based on availability of pre–COVID-19 and COVID-19 electrocardiograms in order to allow PR:HR slope calculation. After meeting inclusion and exclusion criteria, there were 75 patients with 514 ECGs for analysis of PR:HR slopes. COVID-19 = coronavirus 2019; HR = heart rate; MRN = medical record number.
Table 1.
Clinical characteristics of study patients grouped according to COVID-19 PR:HR slope
| Entire cohort (N = 75) | COVID-19 PR:HR slope behavior |
P value | ||
|---|---|---|---|---|
| Negative PR:HR slope (n = 38) | Positive PR:HR slope (n = 37) | |||
| Clinical variables | ||||
| Age (y) | 67.0 ± 1.6 | 67.2 ± 2.0 | 66.8 ± 2.4 | .89 |
| Female sex | 38 (50.7) | 20 (52.6) | 18 (48.6) | .82 |
| History of myocardial infarction | 13 (17.3) | 9 (23.7) | 4 (10.8) | .23 |
| Use of beta-blockers | 10 (13.3) | 5 (13.2) | 5 (13.5) | 1 |
| Use of calcium channel blockers | 10 (13.3) | 5 (13.2) | 5 (13.5) | 1 |
| Use of antiarrhythmic drugs | 3 (4) | 0 (0) | 3 (8.1) | .11 |
| Pre–COVID-19 ECGs | (N = 268) | (n = 136) | (n = 132) | |
| Time between ECGs (d) | 1285.0 ± 155.8 | 962.5 ± 162.9 | 1616.2 ± 259.0 | .035∗ |
| No. of ECGs analyzed per patient | 3.6 ± 0.1 | 3.6 ± 0.1 | 3.6 ± 0.1 | .94 |
| Pre–COVID-19 HR (bpm) | 79.6 ± 1.6 | 79.1 ± 2.3 | 80.2 ± 2.2 | .74 |
| COVID-19 ECGs | (N = 246) | (n = 130) | (n = 116) | |
| Time between ECGs (days) | 5.6 ± 0.5 | 6.3 ± 0.7 | 4.8 ± 0.6 | .14 |
| No. of ECGs analyzed per patient | 3.3 ± 0.1 | 3.4 ± 0.1 | 3.1 ± 0.1 | .12 |
| COVID-19 HR (bpm) | 87.1 ± 1.6 | 86.2 ± 2.4 | 88.1 ± 2.3 | .57 |
Values are given as mean ± SEM or n (%) unless otherwise indicated.
COVID-19 = coronavirus 2019; ECG = electrocardiogram; HR = heart rate.
Statistically significant.
For the entire cohort, a statistically significant differences was seen between mean pre–COVID and COVID HRs (79.1 ± 2.3 vs 80.2 ± 2.2; P <.001) and between mean pre–COVID-19 and COVID-19 PR:HR slopes (–0.8 vs +0.1; P <.001), paired for each patient. Analysis of pre–COVID-19 ECGs showed that 61 of 75 patients (81.3%) had negative PR:HR slopes during previous hospitalizations. Analysis of the COVID-19 ECGs of these 75 patients showed that 38 (50.7%) retained a negative slope, but 37 (49.3%) showed either PR interval prolongation or absence of shortening. Of the 38 patients whose PR intervals shortened with increasing HRs, 22 had a blunted response; that is, although they had negative COVID-19 PR:HR slopes, their COVID-19 PR intervals did not shorten to the same degree as their pre–COVID-19 PR intervals, resulting in less negative slopes compared to pre–COVID-19 slopes. Results of pre–COVID-19 and COVID-19 ECG PR:HR slope analysis are given in Table 2 .
Table 2.
Results of PR:HR slope analysis in 75 paired patients
| Negative PR:HR slope (n = 38) | Positive PR:HR slope (n = 37) | P value | |
|---|---|---|---|
| Pre–COVID-19 slope | –0.7 ± 0.2 | –0.9 ± 0.2 | .47 |
| COVID-19 slope | –0.6 ± 0.1 | +0.5 ± 0.1 | <.001∗ |
| Slope change | +0.1 ± 0.0 | +1.4 ± 0.2 | <.001∗ |
Values are given as mean ± SEM unless otherwise indicated.
COVID-19 = coronavirus 2019; HR = heart rate.
Statistically significant.
Average length of hospital stay was 11.7 ± 0.7 days (range 3–28). Median length of stay was 10.0 days. Patients who displayed a positive PR:HR slope during their COVID-19 hospitalization were more likely to meet the endpoint of death (11/37 vs 3/38; P = .019); were more likely to require endotracheal intubation (16/37 vs 8/38; P = .050); and had significantly higher peak INR measurement (Table 3 ). All other laboratory test values were not significantly different between the 2 groups, although a trend toward higher peak hs-troponin T levels was noted (P = .053).
Table 3.
Clinical outcomes and laboratory measurements of study patients grouped according to COVID-19 PR:HR slope
| Entire cohort (N = 75) | COVID-19 PR:HR slope behavior |
P value | ||
|---|---|---|---|---|
| Negative PR:HR slope (n = 38) | Positive PR:HR slope (n = 37) | |||
| Clinical endpoints | ||||
| Death | 14 (18.7) | 3 (7.9) | 11 (29.7) | .019∗ |
| Endotracheal intubation | 24 (32.0) | 8 (21.1) | 16 (43.2) | .050∗ |
| Mean length of hospital stay (days) | 11.7 ± 0.7 | 11.0 ± 0.9 | 12.4 ± 1.10 | .31 |
| Patients needing ICU stay (n) | 42 (56) | 17 (18.4) | 25 (67.6) | .06 |
| Laboratory values | ||||
| Peak hs-troponin T (ng/L) | 159.8 ± 46.4 | 64.1 ± 17.6 | 243.5 ± 83.5 | .053∗ |
| Peak C-reactive protein (μg/mL) | 21.2 ± 1.6) | 19.2 ± 2.2 | 23.2 ± 2.5 | .22 |
| Peak d-dimer (ng/mL) | 4899.7 ± 1347.3 | 3411.7 ± 1570.6 | 6387.8 ± 2184.4 | .27 |
| Peak ferritin (ng/mL) | 2158.5 ± 476.2 | 1912.9 ± 435.1 | 2356.4 ± 790.0 | .65 |
| Peak creatine phosphokinase (U/L) | 762.1 ± 194.5 | 614.0 ± 233.5 | 918.3 ± 316.1 | .44 |
| Peak pro-calcitonin (ng/mL) | 10.00 ± 6.0 | 13.13 ± 10.8 | 6.41 ± 3.7 | .58 |
| Peak pro-BNP (ng/L) | 4031.9 ± 1407.1 | 3778.4 ± 1957.8 | 4299.5 ± 2079.9 | .86 |
| Peak INR | 1.31 ± 0.0 | 1.232 ± 0.0 | 1.380 ± 0.0 | <.001∗ |
| Peak fibrinogen (mg/dL) | 698.9 ± 34.3 | 758.6 ± 45.7 | 654.5 ± 48.3 | .14 |
| Peak IL-6 (pg/mL) | 408.4 ± 206.9 | 908.8 ± 627.1 | 173.0 ± 51.7 | .10 |
Values are given as n (%) or mean ± SEM unless otherwise indicated.
BNP = B-type natriuretic peptide; COVID-19 = coronavirus 2019; HR = heart rate; hs-troponin = high-sensitivity troponin; ICU = intensive care unit; IL-6 = interleukin 6; INR = international normalized ratio.
Statistically significant.
Discussion
AV nodal conduction is expected to improve as HR increases, resulting in shortening of the PR interval with sinus rate acceleration. Sympathetic stimulation shortens the PR interval and is well described during exercise and beta-agonist exposure.13 , 14 However, there are few published data on PR interval behavior during illness. Fever has been reported to be associated with PR interval shortening, and Karjalainen and Viitasalo15 made the observation that “…even patients who demonstrated AV block during fever had shorter PR intervals than during the control period.” PR interval shortening has also been described in fever-induced Brugada syndrome.16 To date the only febrile state known to be associated with PR interval prolongation is acute rheumatic fever.17 Similar to COVID-19, rheumatic fever is known to be associated with elevations in C-reactive protein and various cytokines.18 A case report described the development of PR interval prolongation associated with bradycardia in a fatal case of swine influenza.19 A recent paper referenced a single case of heart block in a patient with COVID-19 that resolved as the patient’s condition improved.20
PR interval behavior in COVID-19
Our data demonstrate that in patients with COVID-19, the PR interval showed prolongation or absence of shortening with increasing HR in 37 of 75 patients (49.3%). Even in the remaining patients in whom the PR interval shortened, the degree of shortening was blunted in 22 of 38 patients (57.9%) compared to their PR:HR slopes on pre–COVID-19 ECGs acquired during previous hospitalizations. Average sinus rates were significantly higher on COVID-19 ECGs compared to pre–COVID-19 ECGs, consistent with a heightened sympathetic milieu. Although the temperature of each patient was not noted when the ECGs were obtained, it is likely that at least some of these patients were febrile at the time of ECG acquisition. Pre-existing conduction system disease is unlikely to be the underlying explanation for the observed PR interval behavior as most of the patients did not have previous cardiac disease and had exhibited appropriate PR interval shortening during previous illness at comparable HRs. Finally, use of AV nodal blocking medication was infrequent and comparable in the 2 groups.
Importantly, patients who showed PR interval prolongation or absence of shortening with increasing HR were more likely to meet both endpoints of death and need for endotracheal intubation. No significant association was found between PR interval slope and any other clinical variables or laboratory test results except for peak INR value, which was significantly higher in patients with positive PR:HR slopes. Although not a planned analysis, when patients who died were compared to those who did not, the only statistically significant differences were higher peak values of hs-troponin, C-reactive protein, and ferritin among the patients who died. These findings are consistent with previous publications.1 , 2
Possible pathophysiology
The underlying mechanism of cardiac involvement in COVID-19 remains speculative. Elevated antiphospholipid (anticardiolipin) antibodies and activated partial thromboplastin time have been reported in patients with COVID-19.21 , 22 Antiphospholipid antibodies, specifically anticardiolipin, are frequently associated with viral infections.23 Anticardiolipin antibodies have an established pathogenic role in rheumatologic conditions such as systemic lupus erythematosus, in which myocarditis, pericarditis, coronary arteritis, and AV conduction failure are seen. An antigen–antibody reaction affecting the cardiac conduction system has been proposed based on the finding of ribonucleoprotein antibodies in the sera of mothers with lupus whose infants exhibit congenital AV block.24 These data may provide a plausible pathophysiological explanation for our findings and may prompt assessment of PR interval behavior in patients with other viral illnesses.
Study limitations
Study limitations include those inherent to retrospective analyses of observational data. Less than 25% of screened patients met inclusion criteria, primarily because we had to limit our analysis to patients with at least 2 ECGs in order to calculate PR:HR slopes. Although this strict inclusion criterion may have selected a sicker baseline patient population, the availability of previously recorded ECGS allowed each patient to serve as his or her own control. The inclusion criteria were applied to all patients, and possible selection bias does not explain the differences in PR interval behavior seen during COVID-19 compared to that during previous illnesses. PR measurements can be prone to error, but we used the superimposed median format at twice magnification and twice paper speed along with electronic calipers to minimize such errors. Another limitation relates to the time span over which ECGs were acquired. Pre–COVID-19 ECGs were obtained over a longer period of time (mean 3.5 years) compared to COVID ECGs obtained during the index hospitalization (mean 5.5 days). Accordingly, the COVID PR:HR slopes was selected as the dichotomizing variable rather than the slope difference over time. At the time of submission, some patients still were hospitalized and their ultimate outcomes are unknown. Finally, the full complement of laboratory tests was not performed on all patients.
Conclusion
We demonstrated PR interval prolongation or absence of shortening given increasing HRs in approximately half of patients with COVID-19. Such PR interval behavior was associated with both a higher risk of death and the need for endotracheal intubation. The underlying pathophysiology remains unknown at this time. If confirmed by additional studies, paradoxical PR interval behavior with varying HRs on serial ECGs may be a simple parameter to help identify sicker patients with COVID-19.
References
- 1.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangalore S., Sharma A., Slotwiner ST-segment elevation in patients with Covid-19: a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;382 doi: 10.1056/NEJMoa2007621. :e102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Retraction: Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Retraction: Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med 2020;382:2582. [DOI] [PMC free article] [PubMed] [Retracted]
- 8.Carruthers S., McCall B., Cordell B., Wu R. Relationships between HR and PR interval during physiological and pharmacological interventions. Br J Clin Pharmacol. 1987;23:259–265. doi: 10.1111/j.1365-2125.1987.tb03043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soliman E.Z., Prineas R.J., Case L.D., Zhang Z.M., Goff D.C. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk In Communities (ARIC) study. Stroke. 2009;40:1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soliman E.Z., Rautaharju P.M. HR adjustment of PR interval in middle-aged and older adults. J Electrocardiol. 2012;45:66–69. doi: 10.1016/j.jelectrocard.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Snyder M.L., Soliman E.Z., Whitsel E.A., Gellert K.S., Heiss G. Short-term repeatability of electrocardiographic P wave indices and PR interval. J Electrocardiol. 2014;47:257–263. doi: 10.1016/j.jelectrocard.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhoi AK, Sherpa KS, Phurailatpam D, Tamang JS, Giri PK. Multidimensional approaches for noise cancellation of ECG signal. International Conference on Communication and Signal Processing, April 2–4, 2015, Adhiparasakthi Engineering College, Melmaruvathur, India. 10.13140/RG.2.1.3063.0807 [DOI]
- 13.Bay M., Vollenweider P., Marques-Vidal P., Bocchi F., Pruvot E., Schläpfer J. Clinical determinants of the PR interval duration in Swiss middle-aged adults: the CoLaus/PsyCoLaus study. Clin Cardiol. 2020;43:614–621. doi: 10.1002/clc.23356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J.U., Kim K.S., Kim J.H., Lim H.K., Lee B.H., Lee C.K. PR interval behavior during exercise stress test. Korean J Intern Med. 1995;10:137–142. doi: 10.3904/kjim.1995.10.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karjalainen J., Viitasalo M. Fever and cardiac rhythm. Arch Intern Med. 1986;146:1169–1171. [PubMed] [Google Scholar]
- 16.Mizusawa Y., Morita H., Adler A. Prognostic significance of fever-induced Brugada syndrome. Heart Rhythm. 2016;13:1515–1520. doi: 10.1016/j.hrthm.2016.03.044. [DOI] [PubMed] [Google Scholar]
- 17.Rutstein D.D., Bauer W., Dorfman A. Jones Criteria (modified) for guidance in the diagnosis of rheumatic fever: report of the committee on standards and criteria for programs of care. Circulation. 1956;13:617–620. doi: 10.1161/01.cir.13.4.617. [DOI] [PubMed] [Google Scholar]
- 18.Stollerman G.H. Rheumatic fever. Lancet. 1997;349:935–942. doi: 10.1016/S0140-6736(96)06364-7. [DOI] [PubMed] [Google Scholar]
- 19.Gokhroo R.K., Barjaty H.D., Bhawna K. Cardiac conduction system affection in a case of swine flu. J Assoc Physicians India. 2011;59:51–52. [PubMed] [Google Scholar]
- 20.Kochav S.M., Coromilas E., Nalbandian A. Cardiac arrhythmias in COVID-19 infection. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowles L, Platton SD, Yartey N, et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. Published online May 5, 2020. N Engl J Med https://doi.org/10.1056/NEJMc2013656 [DOI] [PMC free article] [PubMed]
- 23.Sène D., Piette J.C., Cacoub P. Antiphospholipid antibodies, antiphospholipid syndrome and viral infections. Rev Med Intern. 2009;30:135–141. doi: 10.1016/j.revmed.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Scott J.S., Maddison P.J., Taylor P.V., Esscher E., Scott O., Skinner R.P. Connective-tissue disease, antibodies to ribonucleoprotein, and congenital heart block. N Engl J Med. 1983;309:209–212. doi: 10.1056/NEJM198307283090403. [DOI] [PubMed] [Google Scholar]