Abstract
Background
The COVD-19 global pandemic has placed a large demand on personal protective equipment for healthcare workers. N-95 respirators, required to perform aerosolizing procedures, are in short supply and have increased significantly in cost. The lack of a clear end to the pandemic requires hospitals to create a long-term, cost effective solution to the N95 shortage. We initially used previously described methods to reuse and resterilize N95 masks; however, we found they did not solve the issues related to just-in-time fit-testing and cost.
Study design
We initiated a program with the aim to reduce our dependence on N95 masks by initiating a phased program to acquire industrial style elastomeric P100 masks as a substitute for reuse and resterilization of disposable N95s. We created an allocation strategy based on availability of the masks, as well as an operational plan to fit test, educate, and disinfect the masks.
Results
Within 1 month, we were able to reduce the number of N95s needed by our network by 95%. We also found that the cost was, conservatively, 10 times less per month than purchasing disposable N95s, and the cost benefit increases the longer they are needed.
Conclusions
Establishment of an elastomeric mask program is feasible and less expensive than programs focused on reusing and disinfecting disposable N95 masks. A well thought out elastomeric distribution and disinfection program does not pose greater operational challenges than an N95 reuse and resterilization program. In addition, elastomeric masks can be stored for future surges and should be considered an essential part of all healthcare facilities’ supply of personal protective equipment. Implementation of the program has eliminated our dependence on disposable N95s to maintain normal operations during the global pandemic.
Visual Abstract
The worldwide COVID-19 crisis has brought to light the importance of having a reliable supply of respirators for our healthcare providers. The N95 shortage has led the Centers for Disease Control (CDC) to create guidelines for extending the use of N95 beyond what is conventionally recommended by the manufacturer. In addition, the CDC has also recommended that fit testing of N95s be suspended to help reduce the number of N95s normally used during this process.1 The cost of N95 masks has skyrocketed as a result of the increase in worldwide demand combined with the limited availability due to supply chain constraints. The unknown duration of the current pandemic requires healthcare organizations to create a long-term solution to disposable N95s that can be achieved in a cost effective manner and scalable across large organizations. Many innovative methods for reusing and sterilizing masks have been introduced with early success in reducing the amount of N95s that are required for an organization to source.2 , 3 We, like other healthcare networks, operationalized a mask reuse and sterilization protocol. However, implementing a sterilization and reuse program created an equally intensive operational plan and does not allow for use beyond a set number of resterilizations.4 The introduction of numerous types and brands of N95 and N95 equivalents also poses challenges related to fit testing and supply availability. For clinical efficacy, there must be consistency in the types of masks available in order to ensure that individuals have access to the masks for which they were fit tested. We implemented a widespread program to replace the majority of our N95 usage with reusable P100 elastomeric half-mask respirators (Fig. 1 ) and powered air purifier respirators (PAPRs) to help alleviate the issues with N95 reusage and resterilization.
Figure 1.
Photograph of elastomeric half mask respirator with disposable P100 filter.
Methods
Allotment process
The Allegheny Health Network is 9-hospital system comprising approximately 2,200 licensed beds, with sites in Pennsylvania and Western NY, employing 21,000 individuals. The distribution of a limited protective resource within a 9-hospital network can be a controversial and stressful process. Every new patient contact brings some risk to the individual provider, and therefore, all providers can argue that they should be given priority to the limited resource. We believed that a utilitarian approach toward distribution was best suited to weighing risks and benefits to both our staff and patients. A panel of clinical personnel was assembled, with participation from institutional leadership among nursing, anesthesia, critical care medicine, surgery, and supply chain. We prioritized clinical units and personnel based on their inherent risk to COVID-19 exposure. Greatest priority was given to those clinical staff with direct airway manipulation and those dealing with high acuity COVID-19 patients (Table 1 ).
Table 1.
Calculation of Number of Room Entries by Team Taking Care of Critically Ill COVID-19 Patients in Medical ICU
| Staff | Room entries (24-h period), n |
|
|---|---|---|
| Minimum | Maximum | |
| Nursing; assessment every 2 hours repositioning, titration of drips, hygiene | 12 | 40 |
| Nursing assistant; blood sugar repositioning, stocking of supplies | 7 | 28 |
| Respiratory therapy; assessment every 4 hours, ventilator changes, arterial blood gases | 5 | 15 |
| MICU physician team; attending, fellow, resident, intern | 8 | 18 |
| Consultant; per service attending, fellow, resident | 0 | 9 |
| Dietary | 0 | 6 |
| Environmental services | 1 | 2 |
| Physical and occupational therapy | 0 | 3 |
| X-ray | 0 | 6 |
| IV team; IV access, PICC, midline, blood cultures, blood draws | 0 | 3 |
| Pharmacy; discuss with nursing, assessment of level of sedation | 0 | 2 |
| Total | 41 | 133 |
MICU, medical intensive care unit; PICC, peripherally inserted central catheter.
Initially, emergency rooms, ICUs, and anesthesia providers were targeted for conversion of disposable N95 usage to reusable P100 half-mask respirators.1 Staffing per shift was broken down across the 9 hospitals, and a sufficient number of respirators were assigned to each unit for a 24-hour period to provide protection for physicians, physician-extenders, nursing, and respiratory therapy in the first wave of distribution. Those devices were assigned to the specific clinical units rather than providers, with the assumption that they would be turned in for cleaning and processing after shift completion. System-wide fit testing was scheduled by nursing leadership. As additional shipments were made available, that initial disbursement was supplemented to expand device availability to phase 2 and 3 caregivers. Proceduralist physicians were included based on practice exposure to the airway or lung tissue.5, 6, 7
Finally, as more devices were delivered from the manufacturer, we transitioned from a high turnover unit-assigned, shared-device model to assigning devices to specific providers to use and maintain. This transition was necessary to maintain efficiency as we used individuals from our central sterile processing to disinfect the masks. We wanted to ensure that this was sustainable when those individuals would be redeployed for elective operative case volume once it returned.
Training and education
To prepare for the launch and distribution of the masks, we engaged the director of education and the chief certified registered nurse anesthetist (CRNA) for each network hospital. Each site identified 1 to 2 site coordinators and multiple super users to perform the real time education, fit testing, and return demonstration with distribution of each mask. Super users were trained via an electronic module from the manufacturer and an in-person demonstration session.
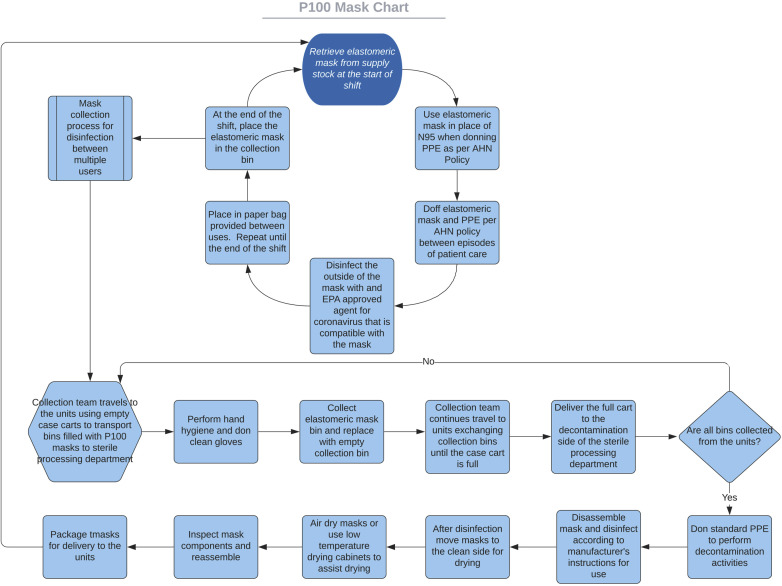
A multimodal approach to staff education was selected. This included electronic/printed directions, in-person demonstration, and video support. Super users completed 1-on-1 mask demonstrations with each staff member, to ensure user safety and create a record of training activities. We filmed a real time video demonstration of mask donning, seal checks, and doffing accessible through QR (quick response) codes to provide staff with access to directions at all times. During the time period when the supply of masks did not allow for individuals to have their own masks, we initiated a system of collection and disinfection (Fig. 2 ).
Figure 2.
Flowchart of mask disinfection and redistribution to caregivers. AHN, Allegheny Health Network; EPA, Environmental Protection Agency; PPE, personal protective equipment.
The sterile processing departments were trained in a similar fashion for disassembly, disinfection, and reassembly of the masks. A super user completed an onsite overview at each facility. The site managers signed each staff member off on their knowledge of the disinfectant solution, cleaning process, and return demonstration of mask disassembly and reassembly.
Fit testing
In the first wave of distribution, 1,962 staff members were fit tested, with 1,840 staff members passing. Staff members were required to remove make-up and report for fit testing clean shaven, as applicable. Fit testing was performed via hood and sensitivity solution testing or using quantitative methods only after the staff member successfully donned the mask and passed positive and negative seal checks. If the staff member did not initially pass the seal checks, the mask would be adjusted or a different size would be selected. If the staff member could not successfully pass the seal checks, we did not proceed to fit testing. Staff members who failed P100 fit testing were offered an N95 mask, if applicable, or a powered air purifying respirator (PAPR) to use in clinical situations.
N95 usage
We measured N95 usage in the medical intensive care unit (MICU). The MICU is an 18-bed negative pressure ICU, where the majority of COVID-19 patients were cared for. We recorded the number of individuals caring for the patients in a 24-hour period. We also measured the number of room entries per individual/day. This would correlate with the number of N95 masks that would be used without extended reuse interventions.
Room entries (individuals multiplied by number of room entries) ranged from 41 to 133 entries in 1 patient room in a 24-hour period, with a mean room entry of 87 times per patient in a 24-hour period (Table 2 ). At the beginning of the study, we had not used a policy to re-use N95s, therefore 87 N95 masks were being used per patient per 24-hour time period. Approximately 1,566 N95 masks would be used per day in the MICU. We restructured who could enter the room, to further decrease the usage of PPE. This eliminated consultants, dietary, environmental health services, and nursing assistants from entering the room. After institution of the elastomeric N-100 masks, our usage of N95 masks has decreased to 0. Staff members who failed fit testing for the elastomeric N-100 masks were allocated PAPRs, effectively reducing our disposable N95 usage to zero. After more than 1 month of use, no healthcare workers chose to return to N95 usage.
Table 2.
Phased Approach to Distributing Elastomeric Masks
| First wave, high risk, front line | Second wave, moderate risk | Third wave, at risk |
|---|---|---|
| Anesthesia CRNA, physicians, extenders | EP nurses, physicians, extenders | OR staff for at risk cases |
| ED nurses, physicians, extenders | Cath lab nurses, physicians, extenders | PACU staff for at risk cases |
| EMS, life flight nurses, physicians | TEE nurses, physicians, extenders | Preop nurses |
| Pulmonary and respiratory techs | Trauma OR nurses, physicians, extenders | Transplant surgery nurses, physicians, extenders |
| Pulmonary nurses, physicians, extenders | OR staff for moderate risk cases | SICU nurses, physicians, extenders |
| GI Nurses, physicians, extenders | PACU staff for moderate risk cases | CCU nurses, physicians, extenders |
| Critical care nurses, physicians, extenders | Ophthalmology nurses, physicians, extenders | Orthopaedic nurses, physicians, extenders |
| PACU nurses, physicians, extenders | Cardiovascular nurses, physicians, extenders | Others as deemed necessary by PPE committee |
| ENT, OMFS nurses, physicians, extenders | Dental nurses, physicians, extenders | – |
| Colorectal nurses, physicians, extenders | Hospitalist physicians, extenders | – |
| Thoracic nurses, physicians, extenders | Urology nurses, physicians, extenders | – |
| Plastics nurses, physicians, extenders | CT techs | – |
| Neurosurgery nurses, physicians, extenders (performing endo access procedures) | – | – |
| IR techs, nurses, physicians, extenders | – | – |
As masks were procured, they were distributed to the caregivers in phases as listed.
CCU, cardiac care unit; CRNA, certified registered nurse anesthetist; ED, emergency department; EMS, emergency medical services; ENT, ear, nose, and throat; EP, electrophysiology; GI, gastrointestinal; IR, interventional radiology; OMFS, oral and maxillofacial surgery; OR, operating room; PACU, post-anesthesia care unit; SICU, surgical intensive care unit; TEE, transesophageal echocardiogram.
Cost
We used a cost ratio methodology to evaluate the economics of the program. We found that 116 P100 respirator and cartridges replaced 2,088 disposable N95s/day. After more than 1 month of usage, we have found that filters have not needed to be changed more frequently than once a month. Given that the cartridges are able to be used until they are visibly soiled and/or there is difficulty with airflow, we set a time period of 1 month of use for routine replacement. Part of the education included proper replacement of the filter cartridges to prevent inadvertent soilage and contamination. We included replacement of the filter monthly in the cost analysis. In order to calculate the current savings, we used a price paid of approximately $3.00/N95, which is well below the current market rate compared to approximately $20 for an elastomeric mask and $10 per cartridge. We also reduced the usage of disposable N95 masks by 75% to take in to account the aggressive reuse and sterilization programs that would have been used had we not purchased the elastomeric. The cost of an elastomeric mask programs is, conservatively, 10 times cheaper per month and the cost benefit continues to increase the longer they are in use (Fig. 3 ).
Figure 3.
Cost ratio of disposable N95s to reusable elastomeric masks with filters. Price assumptions were made of $3.00/disposable N95, $20 for elastomeric mask and $10 for disposable filters, assuming filters were replaced monthly.
Discussion
The global impact of COVID 19 has revealed several weaknesses in our ability to secure critical PPE such as respirators. Use of disposable N95s during a global pandemic has several key limitations including supply, cost, and the inability to adequately fit test healthcare workers without using masks in the process. Resuming elective procedures requires that facilities demonstrate an adequate amount of resources including PPE. In order for hospitals to protect staff and patients in our procedural areas, the ability to provide respiratory protection is paramount. Implementation of elastomeric masks has allowed us to create an environment in which universal respiratory precautions for all intubations and other aerosolizing procedures can be adopted without any noticeable impact on our supply of disposable N95 respirators.
In addition, the advantage of implementing an elastomeric respirator program is that it does not require any additional hospital resources as compared to an N95 reuse and resterilization programs. The ability to disinfect and store these devices in healthcare has proven feasible.8 Elastomeric masks have also been found to be well tolerated for use by healthcare workers during long periods of patient care.9 In addition, the use of a standardized type of mask eliminates the need for multiple fit testings related to the different brands and styles of masks that are acquired during a pandemic.10
Conclusions
Elastomeric masks with disposable P100 filters were found to be superior to disposable N95s for protection of healthcare workers at a large academic medical center. They do not have the waste associated with fit testing disposable N95s, so there is no barrier to fit testing all front line caregivers. A return and disinfection program does not pose a greater operational challenge than previously described disposable N95 resterilization programs. The nature of the elastomerics and their ability to be disinfected allows for multiple caregivers to share the same mask. The one-time cost and storage aspects related to the program lend a significant cost benefit when compared with disposable N95s. The financial impact that many hospitals are facing related to the pandemic requires long-term cost-effective solutions, and implementation of an elastomeric respirator program will obviate many of the current issues that are being faced with disposable mask usage and allow for health systems to eliminate their dependence on a continual supply of disposable N95s. In order to resume operations, hospitals would benefit by using the time between surges to create and operationalize a reusable elastomeric mask program.
Author Contributions
Study conception and design: Chalikonda, Waltenbaugh, Servello, Diaz-Garcia
Acquisition of data: Angelelli, Dumont, Singh
Analysis and interpretation of data: Chalikonda, Angelleli, Kvasager, Servello, Singh
Drafting of manuscript: Chalikonda, Waltenbaugh, Angelelli, Dumont, Servello, Singh, Diaz-Garcia
Critical revision: Chalikonda, Waltenbaugh, Dumont, Sauber, Singh, Diaz-Garcia
Footnotes
Disclosure Information: Nothing to disclose.
References
- 1.Centers for Disease Control Strategies for Optimizing the Supply of N95 Respirators. Coronavirus Dis 2019(COVID-19) 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirators-strategy/ Available at:
- 2.Duke F.M. How to decontaminate N95 masks for reuse | RT. RT Decis Makers Respir Care. 2020 [Google Scholar]
- 3.Kenney P., Chan B.K., Kortright K. Hydrogen peroxide vapor sterilization of N95 respirators for reuse. MedRxiv. 2020 doi: 10.1101/2020.03.24.20041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman J., Pierce A., Mody J. Institution of a novel process for N95 respirator disinfection with vaporized hydrogen peroxide in the setting of the COVID-19 pandemic at a large academic medical center. J Am Coll Surg. 2020 doi: 10.1016/j.jamcollsurg.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volgenant C., Persoon I., DeRuiter Infection control in dental health care during and after the SARS-CoV-2 outbreak. Oral Dis. 2020 doi: 10.1111/odi.13408. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thornton M., Reid D., Shelley B., Steven M. Management of the airway and lung isolation for thoracic surgery during COVID-19 pandemic. Anesthesia. 2020 doi: 10.1111/anae.15112. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard B. High-risk aerosol-generating procedures in COVID-19: Respiratory protective equipment considerations. Otolaryngol Head, Neck, Surg. 2020 doi: 10.1177/0194599820927335. [DOI] [PubMed] [Google Scholar]
- 8.Bessesen M.T., Adams J.C., Radonovich L., Anderson J. Disinfection of reusable elastomeric respirators by health care workers: A feasibility study and development of standard operating procedures. Am J Infect Control. 2015;43:629–634. doi: 10.1016/j.ajic.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Roberge R.J., Coca A., Williams W.J. Reusable elastomeric air-purifying respirators: Physiologic impact on health care workers. Am J Infect Control. 2010;38:381–386. doi: 10.1016/j.ajic.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chughtai A.A., Seale H., Rawlinson W.D. Selection and use of respiratory protection by healthcare workers to protect from infectious diseases in hospital settings. Ann Work Expo Heal. 2020;64:368–377. doi: 10.1093/annweh/wxaa020. [DOI] [PubMed] [Google Scholar]