Abstract
Crude secondary attack rate (SAR) of COVID-19 in Taiwan was 0.84% using nationwide contact-tracing data till April 8, 2020. The random-effect Bayesian metaanalysis yielded 95% credible intervals of 0.42%–1.69% and 0.08%–8.32%, respectively, for estimated SAR pooling from 15 case series and for predicted SAR in the future if pandemic continues.
Keywords: COVID-19, Secondary attack rate, Meta-analysis, Pandemic, Bayesian
Pandemic of coronavirus disease 2019 (COVID-19) originated from person-to-person transmission. Epidemiological studies in several aspects, including the transmissibility of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) of COVID-19, are urgently needed for setting containment strategies. Secondary attack rate (SAR) is the probability that an infection occurs among susceptible persons within a reasonable incubation period after known contact with an infectious person in household or other close-contact environments.1 SAR can be influenced by many factors including personal hygiene habits, social behaviors, and characteristics of close-contact environments.
Up till now, there have been only few studies reporting an estimate SAR of COVID-19 with a wide range from 0.46% to 63.87%.1, 2, 3, 4 These studies used cases or cases series with relatively small sample size in a specific close-contact setting such as during meal, household or travel-related environments. Instead of a specific setting, estimation of SAR using data from multiple close-contact environments is more valuable for describing the overall burden and evaluating the effectiveness of the containment approaches.1 It is known that case reports and case series are study designs known for increased risk of bias, and meta-analysis is considered as an appropriate approach to attain a weighted average of results from such studies.5 Proportional meta-analysis has been used previously to estimate SAR of emerging infectious disease such as H1N1 Influenza A and Ebola Virus.6 , 7 Considering the heterogeneity among close contact environments of secondary cases, we used the nationwide contact tracing data from the Centers for Disease Control (CDC) in Taiwan and performed proportional meta-analyses to estimate SAR of COVID-19.8
In the world, only Taiwan Central Epidemic Command Center (CECC) announces daily report of contact tracing data regarding susceptible individuals who had close contact with imported or indigenous confirmed COVID-19 cases.8 We collected these data during January 21st, 2020 to April 8th, 2020 from Taiwan CDC.8 Only closing follow-up cases with complete surveillance more than 14 days were enrolled. A total of 3795 individuals who were closely contacted with primary confirmed cases were registered and monitored under tracing and/or quarantine. These individuals were screened for positive of SARS-CoV-2 by reverse-transcription diagnostic polymerase chain reaction tests. We calculated the crude SAR with the formula of “number of secondary confirmed cases/number of susceptible cases.” We then performed proportional meta-analyses by Bayes’ theorem to estimate the pooled SAR based on the current data and to predict SAR in the future if pandemic continues.
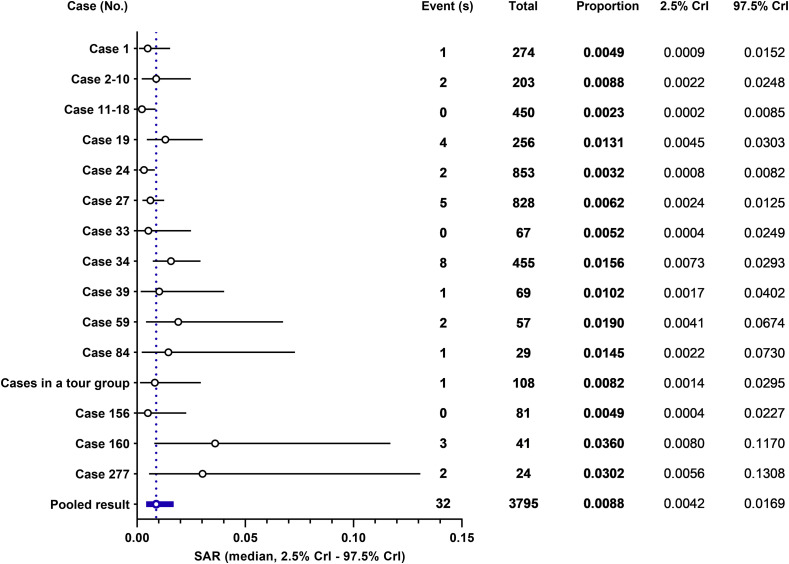
Among 3795 susceptible individuals who had close contact with primary confirmed cases, 32 cases were later confirmed to be SARS-CoV-2 positive (Table 1 ). The close contact environments included household, aircraft, school classroom, hospital, and working places. Based on these data, the value of crude SAR calculation was 0.84%. We furthermore undertook the Bayesian random effect meta-analysis by using software WinBUGS with Markov chain Monte Carlo methods, noninformative priors, and 3 chains with 150,000 simulations. By pooling the proportion of 15 case series, the median of estimated SAR was 0.88% (95% Credible Interval [CrI]: 0.42%–1.69%; Fig. 1 ). This interval denotes the possible range of SAR based on past data in Taiwan included in our analysis. One advantage of the Bayes’ theorem is to forecast the post probability based on evidence of current efforts, the estimated SAR from the announced case series, as prior.9 It can provide a predicted SAR in the future if the pandemic continues its progress and spreads to community, and yielded a predicted median SAR of 0.88% (95% CrI: 0.08%–8.32%).
Table 1.
Environments of secondary attacks.
| Primary confirmed cases with serial numbera | Number of susceptible persons with close-contact tracing | Number of confirmed cases with secondary attack | Environments for close contact |
|---|---|---|---|
| Case 1 | 274 | 1 | Aircraft |
| Case 2 to 10 | 203 | 2 | Household |
| Case 19 | 256 | 4 | Dinning and household |
| Case 24 | 853 | 2 | Household |
| Case 27 | 828 | 5 | Household and hospital |
| Case 34 | 455 | 8 | Hospital |
| Case 39 | 69 | 1 | Classroom |
| Case 59 | 57 | 2 | Classroom |
| Case 84 | 29 | 1 | Household |
| Cases in a tour groupb | 108 | 1 | Household |
| Case 160 | 41 | 3 | Working place |
| Case 277 | 24 | 2 | Working place and household |
Serial numbers of primary confirmed cases were defined by Taiwan Centers for Disease Control.
Case 55, 63, 71, 101, 111, 140, 161, and 162 joined the same tour group to Egypt and were confirmed when back to Taiwan.
Figure 1.
Results of meta-analysis for estimation of secondary attack rate (SAR) of coronavirus disease 2019 (COVID-19) using nationwide contact tracing data in Taiwan. The result from the random effect of Bayesian model is presented. This analysis included data during the period from January 21st, 2020 to April 8th, 2020 were obtained from Taiwan Centers for Disease Control. Event (s), secondary confirmed cases after close contact with primary confirmed cases; total, all contact tracing cases who exposed to primary confirmed cases; CrI, credible interval. Details of the close contact environments for each case of group are listed in Table 1.
In prior reports of COVID-19 SAR from different countries and environments, data were all collected at the early stage of COVID-19 pandemic. In United State, one study reported 12 travel-related COVID-19 cases under condition of hospital or household isolation immediately after immigration and monitoring of active symptoms was performed on 445 close contacts.3 The results showed symptomatic cases with SAR of 0.45% (95% confidence interval [CI]: 0.12%–1.6%) among all contacts, and 10.5% (95% CI: 2.9%–31.4%) among household members.3 However, such situation with a low SAR was far different from that of the community-based or country-based prevention of pandemic. In South Korea, one study reported first 30 confirmed cases as of February 17th 2020, and 13 individuals of 2370 traced contacts were infected. The overall and household SAR were 0.55% (95% CI: 0.31%–0.96%) and 7.56% (95% CI: 3.73%–14.26%), respectively.4 The low SAR reported in this study stand for the beginning of COVID-19 outbreak. There was no more available data of contact tracing after the first 30 cases because the government of South Korea shifted the policy from contamination to mitigation.4 Low SAR of COVID-19 in our results based on multiple close-contact environments with duration of three months represented different significance in epidemiology.
The value of crude SAR could be easily influenced by different contacts. However, our data regarding crude SAR still lines within the ranges calculated by our meta-analytic model. The results indicated that the containment of COVID-19 pandemic in Taiwan remained consistent in the past three months. The low SAR of COVID-19 in Taiwan may result from the effective strategic approaches implemented by CECC, including inspecting imported passengers with symptoms, monitoring quarantined individuals by government-issued cell phones, the efficient distribution of facial masks to people in need, and discouraging mass gathering.10 The high awareness and cooperation of the whole population, especially regarding hand washing and mask wearing, also plays an important role. Most importantly, the value of SAR might stay low in the future, according to the prediction by Bayes’ theorem in our study.
Nosocomial spread of SARS-CoV-2 is a critical issue in curbing the pandemic progress, not only to hospitalized patients but also to medical staffs.11 In our result, the estimate of SAR for the Case 34 yielded a median of 1.56% with 95% CrI of 0.73%–2.93% because of intra-hospital transmission. These data are valuable because this may be the first report of nosocomial COVID-19 SAR. The challenge for clinical staffs is that COVID-19 cannot be distinguished from other causes of pneumonia merely by clinical, radiologic or laboratory information.12 Besides, nosocomial transmission from asymptomatic carriers is possible.11 In 2003, SARS demonstrated a high nosocomial transmission, which gave more than 56% of attack rate among health care workers.13 Thus, the relatively very low nosocomial COVID-19 SAR in Taiwan should be because extensive training and high-degree awareness of clinical staffs learned from the experience of SARS.
In conclusion, the SAR of COVID-19 in Taiwan was low and consistent during January 21st, 2020 to April 8th, 2020. Based on the good results, Taiwan's experience is worth offering these strategies worldwide to reduce SAR of COVID-19.
Funding
TCF-MP 108-01-02 from Buddhist Tzu Chi Medical Foundation Academic Advancement, Hualien, Taiwan.
Declaration of Competing Interest
None.
References
- 1.Liu Y., Eggo R.M., Kucharski A.J. Secondary attack rate and superspreading events for SARS-CoV-2. Lancet. 2020;395:e47. doi: 10.1016/S0140-6736(20)30462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun W.W., Ling F., Pan J.R., Cai J., Miao Z.P., Liu S.L. Epidemiological characteristics of 2019 novel coronavirus family clustering in Zhejiang Province. Zhonghua Yufang Yixue Zazhi. 2020;54:E027. doi: 10.3760/cma.j.cn112150-20200227-00199. [DOI] [PubMed] [Google Scholar]
- 3.Burke R.M., Midgley C.M., Dratch A., Fenstersheib M., Haupt T., Holshue M. Active monitoring of persons exposed to patients with confirmed COVID-19 - United States, January-February 2020. MMWR Morb Mortal Wkly Rep. 2020;69:245–246. doi: 10.15585/mmwr.mm6909e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 National Emergency Response Center Epidemiology and case management team, Korea centers for disease control and prevention. coronavirus disease-19: summary of 2,370 contact investigations of the first 30 cases in the Republic of Korea. Osong Public Health Res Perspect. 2020;11:81–84. doi: 10.24171/j.phrp.2020.11.2.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glatman-Freedman A., Portelli I., Jacobs S.K., Mathew J.I., Slutzman J.E., Goldfrank L.R. Attack rates assessment of the 2009 pandemic H1N1 influenza A in children and their contacts: a systematic review and meta-analysis. PloS One. 2012;7:e50228. doi: 10.1371/journal.pone.0050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean N.E., Halloran M.E., Yang Y., Longini I.M. Transmissibility and pathogenicity of Ebola virus: a systematic review and meta-analysis of household secondary attack rate and asymptomatic infection. Clin Infect Dis. 2016;62:1277–1286. doi: 10.1093/cid/ciw114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taiwan Centers for Disease Control Daily press release of COVID-19. https://www.cdc.gov.tw/En available from:
- 9.López Puga J., Krzywinski M., Altman N. Points of significance: bayes' theorem. Nat Methods. 2015;12:277–278. doi: 10.1038/nmeth.3335. [DOI] [PubMed] [Google Scholar]
- 10.Wang C.J., Ng C.Y., Brook R.H. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. J Am Med Assoc. 2020;323:1341–1342. doi: 10.1001/jama.2020.3151. [DOI] [PubMed] [Google Scholar]
- 11.Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53(3):404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang W.H., Teng L.C., Yeh T.K., Chen Y.J., Lo W.J., Wu M.J. 2019 novel coronavirus disease (COVID-19) in Taiwan: reports of two cases from Wuhan, China. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . vol. 78. 21 March 2003. p. p82.https://www.who.int/wer/2003/en/wer7812.pdf (The weekly epidemiological record (WER)). 12. available from: [Google Scholar]