Supplemental Digital Content is available in the text.
Keywords: cardiovascular diseases, phenotype, preeclampsia, pregnancy, risk
Abstract
This Swedish register-based cohort study determined the separate and joint contribution of preeclampsia and multi-fetal pregnancy on a woman’s risk of cardiovascular disease (CVD) later in life. The study included 892 425 first deliveries between 1973 and 2010 of women born 1950 until 1971, identified in the Swedish Medical Birth Register. A composite outcome of CVD was retrieved through linkage with the National Patient and Cause of Death Registers. Cox proportional hazard regression was used to assess the risk of CVD in women who had preeclampsia in a singleton or multi-fetal pregnancy, adjusting for potential confounders, and presented as adjusted hazard ratios. Compared with women who had a singleton pregnancy without preeclampsia (the referent group), women with preeclampsia in a singleton pregnancy had an increased risk of CVD (adjusted hazard ratio 1.75 [95% CI, 1.64–1.86]). Women who had a multi-fetal pregnancy without or with preeclampsia did not have an increased risk of future CVD (adjusted hazard ratios 0.94 [95% CI, 0.79–1.10] and 1.25 [95% CI, 0.83–1.86], respectively). As opposed to preeclampsia in a first singleton pregnancy, preeclampsia in a first multi-fetal pregnancy was not associated with increased risk of future CVD. This may support the theory that preeclampsia in multi-fetal pregnancies more often occurs as a result of the larger pregnancy-related burden on the maternal cardiovascular system and excessive placenta-shed inflammatory factors, rather than the woman’s underlying cardiovascular phenotype.
Preeclampsia is a pregnancy-specific hypertensive disorder that affects 2% to 8% of all pregnancies.1 Women with previous preeclampsia have a 2-fold higher risk of cardiovascular morbidity and mortality later in life.2 Additional complications to preeclampsia such as miscarriage, preterm birth, and intrauterine growth restriction synergistically increase the association to later cardiovascular disease (CVD).3–5
Preeclampsia causation is complex and multi-factorial. Preeclampsia and CVD share many common risk factors, such as hypercholesterolemia, obesity, increasing age, and diabetes mellitus.6,7 Compared with women without preeclampsia, the cardiovascular function in women who later develop preeclampsia has been found to be different before pregnancy or altered in early pregnancy. In these studies, women that later developed preeclampsia had a higher peripheral vascular resistance and a lower cardiac output compared with women with subsequent normal pregnancies.8,9 It is debated whether preeclampsia might be caused by failure of adaptation of the cardiovascular system secondary to the increased demand from the maternal system and the fetoplacental unit, leading to a state of intermittent placental hypoxia and subsequent endothelial dysfunction secondary to hypoxic debris from the placenta.8 This maladaptation could reveal an underlying deficiency in the cardiovascular system already present at the time of pregnancy, putting these women at higher risk for CVD later in life.8 Together, this reinforces the hypothesis of preeclampsia as a cardiovascular stress test during pregnancy. Because preeclampsia is an adverse pregnancy outcome with a heterogenous pathophysiology, this hypothesis could hold true for some women but perhaps not for all. Some also argue that preeclampsia in itself is an insult to the cardiovascular system during pregnancy also in women without predisposition for CVD and that the insult as such causes the increased long-term risks for CVD in these women.10
Women with multi-fetal pregnancies are at about 3 to 4 times higher risk of preeclampsia,11,12 also when adjusted for potential confounders.13 The incidence of preeclampsia among women with multi-fetal pregnancies in Scandinavia is between 10% and 18%,12–14 but these pregnancies are often excluded from preeclampsia studies.7 It is likely that preeclampsia cause in multi-fetal pregnancies differs from other preeclampsia subtypes. Multi-fetal pregnancies pose a higher load on the cardiovascular system, partly manifested by higher cardiac output and lower total vascular resistance.15 The underlying cause of preeclampsia in women with multi-fetal pregnancy could be more specific to pregnancy itself (eg, higher demand on the cardiovascular system from increased syncytiotrophoblast stress from one larger placenta or 2 placentas more rapidly stressed during pregnancy), rather than the preexisting cardiovascular risk factors described above. Data on placental histopathology from pregnancies complicated by preeclampsia show significantly lower rates of maternal and fetal vascular lesions in multi-fetal compared with singleton pregnancies, supporting the hypothesis of different mechanisms behind the disease.16 Multi-fetal pregnancy itself is not a risk factor for underlying maternal cardiovascular pathology; data show that women with multi-fetal pregnancies without preeclampsia do not experience an increased risk of later CVD compared with women with singleton pregnancies without preeclampsia.14 Theoretically, women with preeclampsia in a multi-fetal pregnancy could run a lower risk of later CVD compared with women with preeclampsia complicating a singleton pregnancy if preeclampsia in multi-fetal pregnancy is the result of the increased demand on the cardiovascular system rather than an underlying disadvantageous cardiovascular profile. To the best of our knowledge, there are no studies investigating the long-term cardiovascular outcome after a multi-fetal pregnancy complicated by preeclampsia.
The aim of this study was to further explore the long-term effects of preeclampsia by investigating if women with multi-fetal pregnancies complicated by preeclampsia have an increased risk of CVD compared with women with singleton pregnancies without preeclampsia. This would help to personalize follow-up and interventions after a pregnancy complicated by preeclampsia.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Data Sources
The study population was derived from the Swedish Medical Birth Register (MBR) and was individually linked to other national population-based registers held by the National Board of Health and Welfare (https://www.socialstyrelsen.se) and Statistics Sweden (https://www.scb.se), using the person unique identification number assigned to each citizen at birth or emigration to Sweden (Figure 1).17 The MBR includes >98% of all deliveries in Sweden since 1973. The register includes prospectively collected information on demographic data, reproductive history, and information from the pregnancy, delivery, and neonatal period using standardized prenatal, obstetric, and neonatal records.18 Diagnoses classified according to the Swedish version of the International Classification of Diseases (ICD) (ICD-8 until 1986, ICD-9 between 1987 and 1996, and ICD-10 from 1997 and onwards) for the mother and the infant at discharge from the delivery hospital are further registered.
Figure 1.
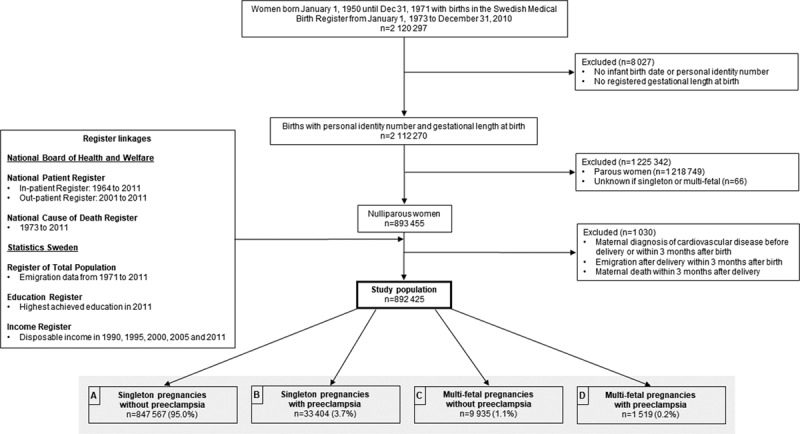
Flowchart of the study population and register linkages, with births in Sweden 1973–2010.
The Swedish Patient Register was established in 1964 (nationwide since 1987) and includes dates of hospital admission and ICD codes on in-patients. Since 2001, the register also includes information on outpatient visits in specialized health care.19 Further linkage to the Swedish Cause of Death Register20 and to the Register of Total Population for information on migration to and from Sweden.21 From the Education Register and the Income Register, information on disposable household income and the highest achieved level of education in the were included (Figure 1).
Study Population
The source population included all women born in Sweden from January 1, 1950, until December 31, 1971, with deliveries in the MBR from January 1, 1973, to December 31, 2010 (n=2 120 297; Figure 1). After exclusions, the study population consisted of 892 425 nulliparous women who were living in Sweden, with singleton or multi-fetal pregnancies, without an existing diagnosis of CVD (ischemic heart disease, heart failure, or cerebrovascular disease according to ICD diagnoses defined in Table S1 in the Data Supplement) at start of follow-up 3 months after birth (Figure 1).
Exposure Variables
The exposure was defined as a combination of maternal diagnosis of preeclampsia during the first pregnancy (yes/no) and plurality of the pregnancy (ie, singleton or multi-fetal). The key exposure category was multi-fetal pregnancies with preeclampsia (motivated by our hypothesis about normal long-term CVD risk in these women; represented as Box D in Figure 1). The reference category, representing women with normal long-term cardiovascular risk, was women with a first singleton pregnancy without preeclampsia (Box A in Figure 1). The other preeclampsia/plurality combinations were also studied, to paint a fuller picture of long-term CVD risk of this population. Women with preeclampsia complicating a singleton pregnancy (Box B, Figure 1) and women with multi-fetal pregnancies without preeclampsia (Box C, Figure 1) were also compared with the referent group of singleton pregnancies without preeclampsia. Preeclampsia was defined by corresponding ICD-8, -9, or -10 codes of preeclampsia and superimposed preeclampsia registered in the MBR (defined in Table S1 in the Data Supplement). Information on singleton or multi-fetal pregnancy was retrieved from the delivery record.
Covariates
We sought to control for key covariates that may be associated with both preeclampsia in first pregnancy and plurality, as well as long-term CVD risk. These potential confounders included maternal sociodemographic factors and health factors. Given the long study period required to track women’s development of CVD over time with sufficient statistical power, we included data from many eras of data collection. Through the years, variables have been added to the MBR, for example, smoking during pregnancy (in 1987), body mass index (in 1992), and assisted reproductive technology (in 1985). Therefore, some covariates were possible to take into account across the whole study period and others only for a restricted time of the study period.
From 1973 and Onwards
Information on date of birth, maternal age at delivery, and gestational length at delivery was retrieved from the MBR. Chronic hypertension before first birth data was retrieved from the MBR, reported as check-boxes in the standardized antenatal care record or as ICD codes coded during pregnancy (Table S1). The highest level of education for the mother was obtained from the Education Register and categorized as 9 years of schooling or less, 10 to 12 years of schooling, and 13 years or more, achieved in 2011 (for all women in the study population).
From 1987 and Onwards
Self-reported information on infertility duration and daily cigarette smoking or not in early pregnancy was retrieved from the standardized antenatal record in the MBR. The household’s disposable income in the years of 1990, 1995, 2000, 2005, and 2011 was retrieved from the Income Register. The disposable household income for all women was categorized into tertiles of the distribution of each of the year. The 2 years closest to birth was used for each woman, and the highest tertile of these was used (eg, if giving birth in 1998, the tertile of disposable income in 1995 and in 2000 was used, and the woman was categorized according to the highest tertile; for births before 1995 the disposable income in 1990 and 1995 was used).
From 1995 and Onwards
Body mass index (kg/m2) was collected from the first attendance to antenatal care, occurring before 13 weeks of gestation for 90% of women in Sweden22 and was divided according to the World Health Organization’s classification.23 The standardized antenatal record further contains information on assisted reproductive technology and concurrent diseases, kidney disease, and systemic lupus erythematosus, collected from check-boxes, diabetes mellitus, collected from check-boxes, and ICD codes and categorized as no diabetes mellitus, pregestational (type I and II) diabetes mellitus, and gestational diabetes mellitus (Table S1).
Outcome Variables
CVD event was defined as diagnosis of, or underlying cause of death due to, one of the 3 groups of diagnosis of ischemic heart disease, heart failure, or cerebrovascular disease, whichever occurred first in the case of multiple morbidities. Chronic hypertension was not part of the CVD outcomes since ICD diagnoses in the primary health care centers are not available. Diagnoses with corresponding ICD codes are defined in Table S1. To minimized capture of false-positive outcome (eg, due to a code being recorded for diagnostic testing rather than confirmed case) diagnosis of CVD was defined as the first of (1) one ICD code of CVD in the inpatient register or (2) one ICD code of CVD in the outpatient, and within 1 year one ICD code in the inpatient register (using the date from the outpatient visit) or (3) 2 codes in the outpatient register within 1 year (using the date from the first visit). Information on death due to CVD was retrieved from ICD codes in the cause of death register.
Statistical Methods
Descriptive statistics were presented with mean, medians, SD, and frequencies, comparing between our 4 exposure groups defined by preeclampsia and plurality, using the Pearson χ2 test for categorical data and the Student t test for mean of continuous variables. To maximize power and to make use of the full data, we conducted analyses in the population of women with deliveries from 1973 to 2010, 1987 to 2010, and 1995 to 2010.
Data were analyzed using time-to-event methods for the population of women giving birth 1973 to 2010 and to control for important covariates that only become available later, we also conducted time-restricted analyses among women giving birth 1987 to 2010. The confounders were chosen by a priori knowledge and in addition by availability in the registers. Some variables such as body mass index were only available from 1995 and are presented in Table S2 to give the most detailed picture possible of the study population, but there were no time-to-event analyses of the restricted population of deliveries 1995 to 2010 due to small groups.
The event of interest was the first CVD event during follow-up. Time at risk was accumulated from entry into the cohort (maternal age at 3 months after the first delivery) until CVD event or censoring (maternal age at emigration, death from other cause, or last day of follow-up, December 31, 2011). Attained age was used as the underlying time scale for survival analysis, reflecting that this is the time scale that most immediately affects risk of CVD.
The exposure was investigated as a categorical variable (multi-fetal pregnancy with preeclampsia, singleton pregnancy with preeclampsia, multi-fetal pregnancy without preeclampsia, all compared with singleton pregnancy without preeclampsia).
To investigate possible associations between the exposure and CVD incidence, we used the Kaplan-Meier curves and Cox proportional hazard models, yielding hazard ratios (HR) with 95% CIs. The proportional hazard assumption was formally assessed by using the Schoenfeld and scaled Schoenfeld residuals. Assumption testing suggested that chronic hypertension before first birth did not satisfy the assumption of proportionality.
In the main analysis of women giving birth 1973 until 2010, Kaplan-Meier curves were performed for the total population and were stratified by age at the end of follow-up (<50 years and 50 years or older), to explore the risk of right censoring. The Cox proportional hazard models were analyzed unadjusted and adjusted for maternal age at delivery (in addition to age as underlying time scale), chronic hypertension before first birth, level of education, and time period of birth. In the analysis of the restricted study population giving birth from 1987 to 2010, HRs were additionally adjusted for tertiles of disposable household income, infertility duration, and daily smoking in early pregnancy. In sensitivity analyses, analyses were conducted stratified by chronic hypertension, rather than adjusting for these in the Cox regression to account for the possible nonproportionality.
Statistical analyses were performed using the SAS software version 9.4. and STATA software version 14.
This study was approved by the Regional Ethical Review Board in Stockholm, Sweden (IRB/KI 2012/1325-31/3).
Results
Maternal Characteristics
When comparing women with multi-fetal pregnancies to women with singleton pregnancies, women with multi-fetal pregnancies were older, had a shorter mean gestational length, a larger proportion had higher household disposable income and a higher level of education than women with singleton pregnancies. The proportion of multi-fetal pregnancies over time was higher during the latter years of the study period (Tables 1 and 2).
Table 1.
Maternal and Pregnancy Characteristics According to Singleton Without and With Preeclampsia and Multi-Fetal Without and With Preeclampsia in First Deliveries Among 892 425 births 1973–2010
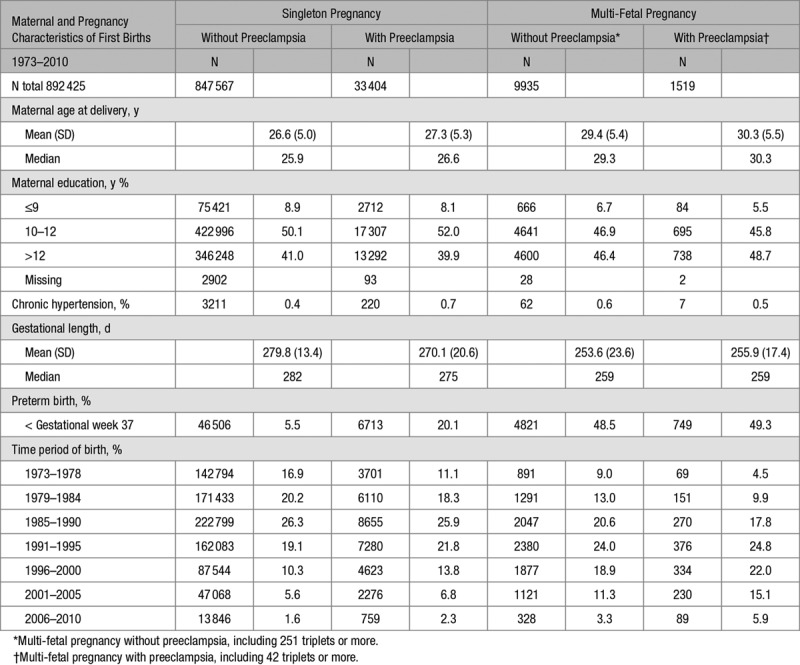
Table 2.
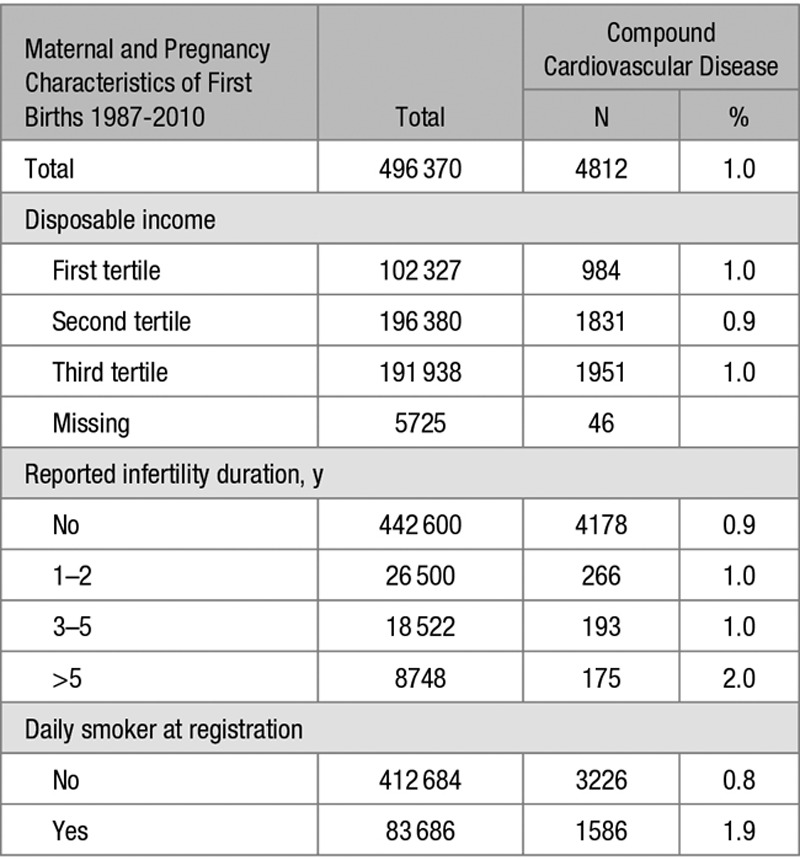
Additional Maternal and Pregnancy Characteristics According to Singleton Pregnancy Without and With Preeclampsia or Multi-Fetal Pregnancy Without and With Preeclampsia in First Deliveries in the Restricted Population of 496 370 Births 1987–2010
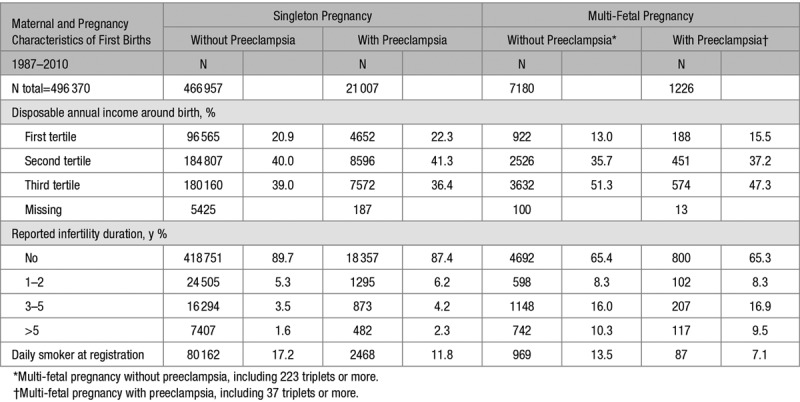
Comparing women without and with preeclampsia within plurality (ie, within singleton and within multiples), suggested that preeclampsia phenotypes did differ by plurality of the first pregnancy. Women with singleton pregnancies and preeclampsia had a shorter duration of pregnancy, a lower level of maternal education, a lower household disposable income, more often pregestational diabetes mellitus, a longer duration of infertility, and more often had infertility treatment than women with singleton pregnancies without preeclampsia. In contrast, women with preeclampsia complicating a multi-fetal pregnancy had a longer duration of pregnancy, a higher maternal education and less commonly had pregestational diabetes mellitus as compared to multi-fetal pregnancies without preeclampsia (Tables 1 and 2 and Table S2).
Shared risk factors for preeclampsia in both singleton and multi-fetal pregnancies were higher maternal age, higher body mass index, and presence of gestational diabetes mellitus. A shared protective factor was smoking.
Time to CVD
Duration of follow-up (ie, time from 3 months after giving birth to incident CVD or censoring) ranged from 9 months to 38 years and 9 months. The overall median age at the end of follow-up was 49.7 years (interquartile range 44.8–55.4) and at CVD event, the median age was 48.4 years (interquartile range 42.8–53.0). Women with multi-fetal pregnancies were older at the time of CVD diagnosis compared with women with singleton pregnancies (median 49.7 versus 48.3 years).
In total, 18 415 (2.1%) of the women in the study were diagnosed with CVD during the time of follow-up (Tables 3 and 4). In women with singleton pregnancies, CVD was reported in 17 217 (2.0%) of women without preeclampsia and 1029 (3.1%) of women with preeclampsia. In women with multi-fetal pregnancies, CVD was reported in 145 (1.5%) of women without preeclampsia and 24 (1.6%) of women with preeclampsia.
Variables associated with long-term risks of CVD were preterm birth, chronic hypertension before first birth, lower educational level, (Table 3), longer duration of infertility and lower household disposable income, smoking (Table 4), obesity, pregestational and gestational diabetes mellitus and systemic lupus erythematosus (Table S4).
Table 3.
Maternal and Pregnancy Characteristics According to Later Compound Cardiovascular Disease Among 892 425 First Deliveries 1973–2010
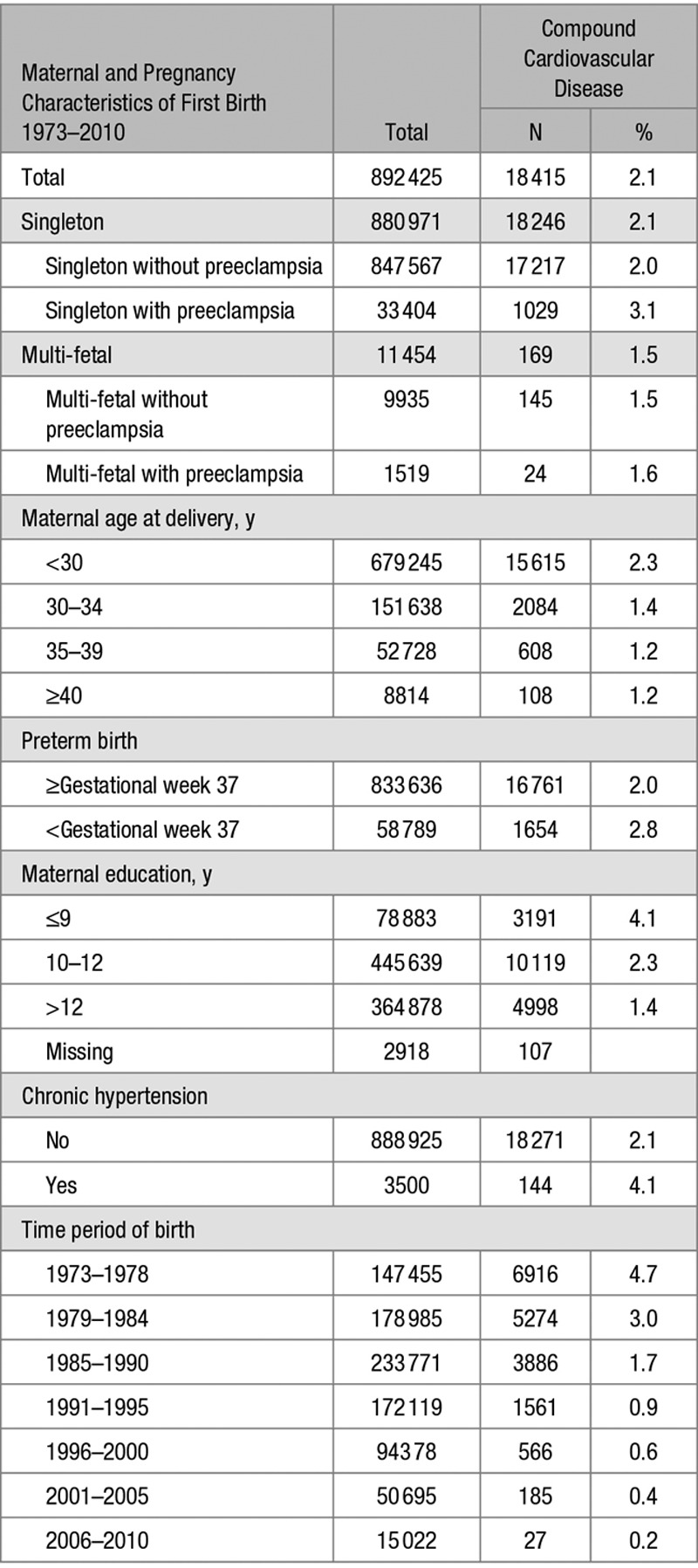
Table 4.
Additional Maternal and Pregnancy Characteristics According to Later Compound Cardiovascular Disease in the Restricted Population of 496 370 First Deliveries 1987–2010
Results from the time-to-event analyses for later CVD in births 1973 to 2010 are presented in Table 5 and Figure 2. When adjusted for maternal age at delivery, chronic hypertension before first birth, level of education and time period of birth, women with preeclampsia in a singleton pregnancy had 75% higher adjusted HR for CVD compared with women with singleton pregnancies without preeclampsia (adjusted HR 1.75 [95% CI, 1.64–1.86]). This increased risk was not observed among women with multi-fetal pregnancies without preeclampsia (adjusted HR 0.94 [95% CI, 0.79–1.10]). The hazard of CVD among women with preeclampsia in a multi-fetal pregnancy was not statistically different from the hazard among women without preeclampsia in a singleton pregnancy (adjusted HR 1.25 [95% CI, 0.83–1.86]); however, the point estimate was further from the null value of 1, and 95% CIs were wide.
Table 5.
Hazard Ratios for Later Compound Cardiovascular Disease From Maternal Age 3 Months After Delivery Among 892 425 Women With Singleton First Deliveries Without and With Preeclampsia and Multi-Fetal First Deliveries Without and With Preeclampsia 1973–2010
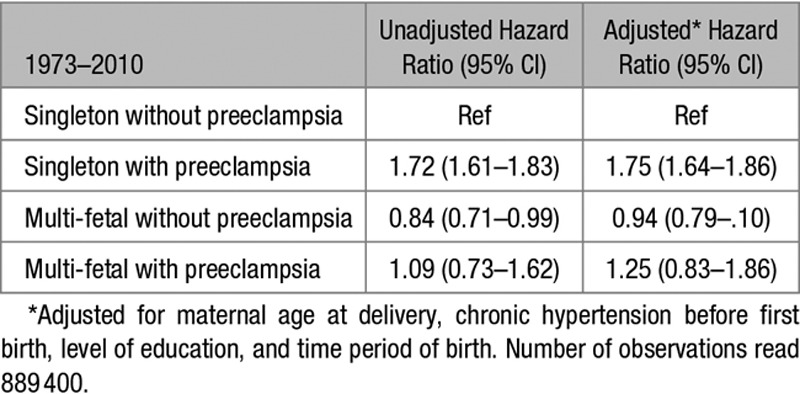
Figure 2.
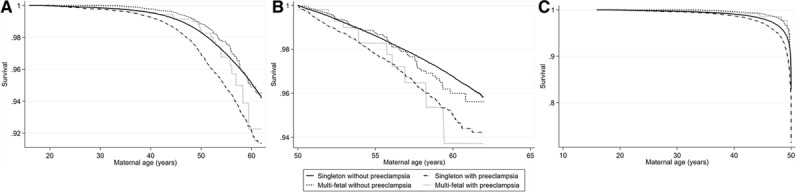
Kaplan-Meier plot for later compound cardiovascular disease. Time is presented as maternal age 3 months after delivery among. Kaplan-Meier plot for later compound cardiovascular disease from maternal age 3 months after delivery among 892 425 women with singleton first deliveries without and with preeclampsia and multi-fetal first deliveries without and with preeclampsia 1973–2010 (A) and stratified by women with age at the end of follow-up <50 y (n=457 147; B) and by women with age at the end of follow-up of 50 y or older (n=435 278; C).
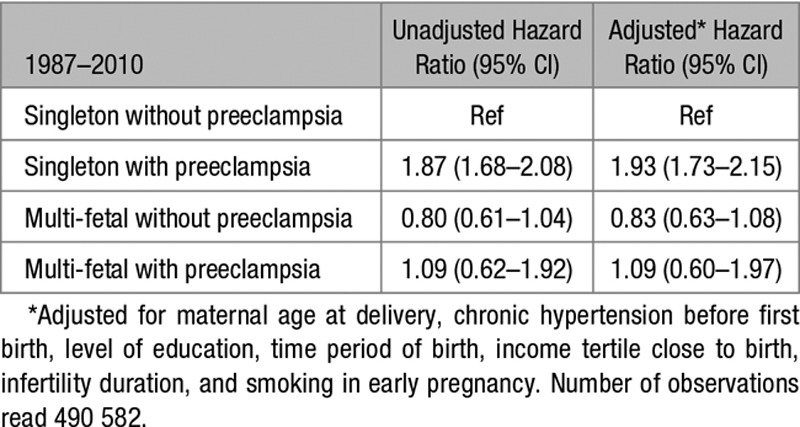
In analyses of the restricted population giving birth 1987 to 2010, additional adjustments for household disposable income tertile, infertility duration, and smoking in early pregnancy were done. The results remained similar with an increased relative risk among women with singleton pregnancies and preeclampsia but not among women with multi-fetal pregnancies without or with preeclampsia (Table 6). Additional analyses stratified by chronic hypertension gave similar results (results not shown).
Table 6.
Hazard Ratios for Later Compound Cardiovascular Disease From Maternal Age 3 Months After Delivery Among 496 370 Women With Singleton First Deliveries Without and With Preeclampsia and Multi-Fetal First Deliveries Without and With Preeclampsia in the Restricted Population of 1987–2010
To address the different lengths of follow-up, since multi-fetal pregnancies were more common in later years, we analyzed women with age at the end of follow-up <50 years and >50 years, respectively, and results remained similar (Table S5A and S5B and Figure 2B and 2C).
Discussion
Main Findings
In this large Swedish population-based cohort study of primiparous women, our results suggested that the risk of CVD after a multi-fetal pregnancy with preeclampsia was not increased compared with after a singleton pregnancy without preeclampsia.
Interpretation
Preeclampsia phenotypes appear to be different when comparing preeclampsia in singleton and multi-fetal pregnancies. Pregestational diabetes mellitus, infertility, and lower socioeconomic status are common cardiovascular and metabolic risk factors for preeclampsia and CVD; and in our study, these were confirmed risk factors for preeclampsia in singleton pregnancies. Although they were not associated with preeclampsia in multi-fetal pregnancies. This suggests that the degree to which preeclampsia is an underlying risk profile for CVD differs by plurality.
If all phenotypes of preeclampsia had a persistent harmful effect on the endothelium, the long-term CVD risk would also be present after a multi-fetal pregnancy with preeclampsia. In this cohort, women with multi-fetal pregnancies preeclampsia did not demonstrate an increased risk of CVD later in life compared with women with singleton pregnancies without preeclampsia. Furthermore, women with multi-fetal pregnancies without preeclampsia had a similar risk of later CVD as women with singleton pregnancies, supported in earlier published data.14 This implies that women with multi-fetal pregnancies have a similar risk regarding later CVD but when complicated by preeclampsia, the increased risk for later CVD is not present, supporting a different underlying pathophysiology for preeclampsia in multi-fetal pregnancy. We propose that preeclampsia in multi-fetal pregnancies might result from increased inflammatory burden as a primary placental finding disorder or secondary to placental hypoperfusion as a consequence of the increased burden of multi-fetal pregnancy on the maternal cardiovascular system, rather than an underlying cardiovascular risk profile, in line with previous published concepts.15,24–26
Recent research has emphasized the role of the cardiovascular system in the development of preeclampsia8 and emphasized that pregnancy can be seen as a window of opportunity to identify future risk of CVD among women developing preeclampsia.27 This data mostly applies for singleton pregnancies and the risk of CVD after a multi-fetal pregnancy has not been evaluated.27 The underlying hypothesis of a maladaptation of the cardiovascular system in pregnancy would be that the placenta turns hypoxic due to an insufficient delivery of oxygen secondary to an affected cardiovascular system and harmful molecules such as the antiangiogenic factor sFlt-1 (soluble FMS-like tyrosine kinase-1) enter the mothers bloodstream from the hypoxic placenta. In addition, the angiogenic PlGF (placental growth factor) is secreted in reduced amounts. Recent evidence has drawn interest to the burden of a larger placenta at term and the risk of late-onset preeclampsia in singleton pregnancies.25 The proponents of this hypothesis suggest that such placental overcrowding is paralleled by increased syncytiotrophoblast stress, an excessive antiangiogenic state, and ensuing excessive vascular inflammation. Whether the latter occurs due to a primary placental problem or secondary to maternal hypoperfusion of the placenta remains to be determined.25,27–29
Strengths and Limitations
A distinct strength of this study is the nation-wide population-based design including women giving birth over several decades and a virtually complete follow-up. The large population enabled us to study the rare exposure of multi-fetal pregnancy and preeclampsia with the outcome of CVD. The high-quality data from several Swedish national registers were merged, providing a unique possibility to define a cohort without previous diagnoses of CVD and to combine information on socioeconomic status, chronic diseases, and pregnancy data, with later diagnosis of CVD. Several important confounders were possible to consider, although some were not available for the whole study period. Analysis on the restricted population of births 1987 to 2010 was, therefore, performed with similar results.
Although the comprehensiveness of the data, the quality of the data is still a concern. Chronic hypertension could unfortunately not be included in our CVD outcomes since we cannot assess the ICD diagnosis of chronic hypertension reliably in the Patient Register, only including inpatient and outpatient diagnosis received in specialized health care and not in health centers. Thus, we would greatly underestimate the incidence of this diagnosis in our study. To improve the accuracy of the CVD diagnosis, one diagnosis in the inpatient, or one in outpatient and one in inpatient, or 2 diagnoses in the outpatient register within a year was required. The CVD diagnoses in the in-patient register have previously shown a high validity.19 Potential nondifferential misclassification of the time to CVD event by using the ICD-8 to -10 codes could have been introduced since the outpatient register was not nation-wide until 1987, the inpatient started in 2001, and multi-fetal pregnancies were more common in the latter years. This also implies that a larger proportion of multi-fetal pregnancies were followed for a shorter period of time. Furthermore, a longer follow-up time increases the possibility of developing other risk factors for CVD. These concerns were addressed by using maternal age as the time scale instead of using the start of follow-up as time zero, by adjusting for time period of birth, and for confounders as level of education that have an impact on the risk of CVD over time. Furthermore, the results persisted in the restricted analyses of births 1987 and 2010.
The highest level of education achieved in 2011 was used for all women in the study population, irrespective of year of delivery. We think that the total attained education is the optimal measure for defining maternal education as a proxy of socioeconomic status because the intention to attain higher education often exists in women before they give birth. With this definition, however, women giving birth earlier during the study period had the opportunity to attain higher education for a longer period than women giving birth closer to the end of the study period.
Despite the duration of the study period of >38 years, there is a risk of right censoring. The mean age at the end of follow-up was 50 years, and there may be special circumstances for women who are affected by CVD in early age compared with later. This was addressed by stratified analyses of women with age <50, and 50 years and older at the end of follow-up were conducted showing similar results, however, analyses were potentially hampered by lack of power (Table S5A and S5B and Figure 2B and 2C).
Earlier studies have shown a j-shaped relationship between increasing parity and higher incidence of CVD later in life. If women with multi-fetal pregnancies more rarely underwent a subsequent pregnancy than women with singleton pregnancies, the total number of pregnancies among these women will be decreased. Given that this study included a woman’s first pregnancy, any effect owing to subsequent number of pregnancies would be a mediation effect (ie, part of the causal pathway). Therefore, we did not adjust for number of subsequent pregnancies. Furthermore, parous women without previous preeclampsia could have had preeclampsia in a following pregnancy, potentially leading to an increased risk of CVD, then diluting the results. The risk of preeclampsia among nulliparous women is, however, much higher than in parous women, and the vast majority of women with preeclampsia in second pregnancy have a recurrence of the disease.30
The incidence of preeclampsia increased over the study period which is also confirmed in other populations.31 Agreement between preeclampsia diagnoses in the MBR and information in individual medical records were excellent when ICD-9 codes were used,12 and the increase is probably not due to underreporting but rather a true increase in the population.
Compared with singleton pregnancies, women with multi-fetal pregnancies have shorter median gestational length at delivery due to increased rates of spontaneous preterm birth and iatrogenic preterm birth due to higher incidence of preeclampsia, induction of labor according to practice, or other circumstances such as the individual wish of the mother or other underlying medical conditions. In Sweden, the majority of centers recommend induction of labor at 36 to 38 weeks in women with a multi-fetal pregnancy. This incidence corresponds to other published literature where numbers reported range from 41%32 to 56%.33 Generally, women with multi-fetal pregnancies, therefore, have a shorter period to develop preeclampsia than singleton pregnancies have. Preterm birth can be considered an effect of preeclampsia in women delivered preterm due to preeclampsia. Preterm birth in women without preeclampsia may, however, truncate a woman’s time at risk for developing preeclampsia (by shortening pregnancy). This would result in a reduction of person-time at risk for the exposure of preeclampsia. Preterm birth was not included in the adjusted analyses since it predominately was not considered a confounding factor.
Perspectives
Women with multi-fetal pregnancies complicated by preeclampsia did not show an increased risk of CVD compared with women without preeclampsia with singleton pregnancies. These results, and different risk factors for preeclampsia between singleton and multi-fetal pregnancies, support the hypothesis that, in multi-fetal pregnancies, preeclampsia might to a larger extent be a result of increased vascular burden by excessive inflammatory factors released from a larger and dysfunctional placenta, rather than an unfavorable cardiovascular phenotype in the mother. If so, this has important implications for long-term care of women, where women with multi-fetal pregnancies complicated by preeclampsia might not be in exaggerated risk of cardiovascular risk. It will be important for future research to further study downstream risks of both gestational hypertension and preeclampsia among multi-fetal pregnancies, such as epilepsy and dementia and longer duration of follow-up for CVDs, including chronic hypertension. This research has the potential to increase understanding of preeclampsia cause and to more individually and optimally tailor the long-term care of women.
Sources of Funding
L. Bergman was supported by the Swedish Society of Medical Research. S. Hesselman was supported by the Center for clinical research, Falun, Sweden (grant CKFUU-744551).
Disclosures
None.
Supplementary Material
Footnotes
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.120.14860.
Novelty and Significance
What Is New?
Women with multi-fetal pregnancies do not exhibit an increased risk of future cardiovascular disease compared with women with singleton pregnancies.
The underlying pathophysiology in preeclampsia in multi-fetal pregnancy is possibly caused by the increased burden of pregnancy rather than the cardiovascular phenotype of the mother.
What Is Relevant?
The increased risk of future cardiovascular disease after preeclampsia does not exist for women with multi-fetal pregnancies and our results point to that these women might not need similar follow-up regarding future cardiovascular disease as women with preeclampsia in singleton pregnancies.
When allocating resources for follow-up, more effort can be directed to women with singelton pregnancies and preeclampsia.
Summary
Women with multi-fetal pregnancies have an increased risk of preeclampsia but not an increased risk of future cardiovascular disease.
References
- 1.Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387:999–1011. doi: 10.1016/S0140-6736(15)00070-7. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 2.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1–19. doi: 10.1007/s10654-013-9762-6. doi: 10.1007/s10654-013-9762-6. [DOI] [PubMed] [Google Scholar]
- 3.Bonamy AK, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: effects of gestational age and fetal growth. Circulation. 2011;124:2839–2846. doi: 10.1161/CIRCULATIONAHA.111.034884. doi: 10.1161/CIRCULATIONAHA.111.034884. [DOI] [PubMed] [Google Scholar]
- 4.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53:944–951. doi: 10.1161/HYPERTENSIONAHA.109.130765. doi: 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 5.Parikh NI, Norberg M, Ingelsson E, Cnattingius S, Vasan RS, Domellöf M, Jansson JH, Edstedt Bonamy AK. Association of pregnancy complications and characteristics with future risk of elevated blood pressure: the västerbotten intervention program. Hypertension. 2017;69:475–483. doi: 10.1161/HYPERTENSIONAHA.116.08121. doi: 10.1161/HYPERTENSIONAHA.116.08121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magnussen EB, Vatten LJ, Lund-Nilsen TI, Salvesen KA, Davey Smith G, Romundstad PR. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335:978. doi: 10.1136/bmj.39366.416817.BE. doi: 10.1136/bmj.39366.416817.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. doi: 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 8.Melchiorre K, Sharma R, Thilaganathan B. Cardiovascular implications in preeclampsia: an overview. Circulation. 2014;130:703–714. doi: 10.1161/CIRCULATIONAHA.113.003664. doi: 10.1161/CIRCULATIONAHA.113.003664. [DOI] [PubMed] [Google Scholar]
- 9.Foo FL, Mahendru AA, Masini G, Fraser A, Cacciatore S, MacIntyre DA, McEniery CM, Wilkinson IB, Bennett PR, Lees CC. Association between prepregnancy cardiovascular function and subsequent preeclampsia or fetal growth restriction. Hypertension. 2018;72:442–450. doi: 10.1161/HYPERTENSIONAHA.118.11092. doi: 10.1161/HYPERTENSIONAHA.118.11092. [DOI] [PubMed] [Google Scholar]
- 10.Staff AC, Redman CW, Williams D, Leeson P, Moe K, Thilaganathan B, Magnus P, Steegers EA, Tsigas EZ, Ness RB, et al. Global Pregnancy Collaboration (CoLab) Pregnancy and long-term maternal cardiovascular health: progress through harmonization of research cohorts and biobanks. Hypertension. 2016;67:251–260. doi: 10.1161/HYPERTENSIONAHA.115.06357. doi: 10.1161/HYPERTENSIONAHA.115.06357. [DOI] [PubMed] [Google Scholar]
- 11.Paré E, Parry S, McElrath TF, Pucci D, Newton A, Lim KH. Clinical risk factors for preeclampsia in the 21st century. Obstet Gynecol. 2014;124:763–770. doi: 10.1097/AOG.0000000000000451. doi: 10.1097/AOG.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 12.Ros HS, Cnattingius S, Lipworth L. Comparison of risk factors for preeclampsia and gestational hypertension in a population-based cohort study. Am J Epidemiol. 1998;147:1062–1070. doi: 10.1093/oxfordjournals.aje.a009400. doi: 10.1093/oxfordjournals.aje.a009400. [DOI] [PubMed] [Google Scholar]
- 13.Laine K, Murzakanova G, Sole KB, Pay AD, Heradstveit S, Räisänen S. Prevalence and risk of pre-eclampsia and gestational hypertension in twin pregnancies: a population-based register study. BMJ Open. 2019;9:e029908. doi: 10.1136/bmjopen-2019-029908. doi: 10.1136/bmjopen-2019-029908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okby R, Shoham-Vardi I, Sergienko R, Sheiner E. Twin pregnancy: is it a risk factor for long-term cardiovascular disease? J Matern Fetal Neonatal Med. 2016;29:1626–1630. doi: 10.3109/14767058.2015.1057491. doi: 10.3109/14767058.2015.1057491. [DOI] [PubMed] [Google Scholar]
- 15.Kametas NA, McAuliffe F, Krampl E, Chambers J, Nicolaides KH. Maternal cardiac function in twin pregnancy. Obstet Gynecol. 2003;102:806–815. doi: 10.1016/s0029-7844(03)00807-x. doi: 10.1016/s0029-7844(03)00807-x. [DOI] [PubMed] [Google Scholar]
- 16.Weiner E, Feldstein O, Schreiber L, Grinstein E, Barber E, Dekalo A, Bar J, Kovo M. Placental component and pregnancy outcome in singleton versus twin pregnancies complicated by preeclampsia. Fetal Diagn Ther. 2018;44:142–148. doi: 10.1159/000479737. doi: 10.1159/000479737. [DOI] [PubMed] [Google Scholar]
- 17.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–667. doi: 10.1007/s10654-009-9350-y. doi: 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cnattingius S, Ericson A, Gunnarskog J, Källén B. A quality study of a medical birth registry. Scand J Soc Med. 1990;18:143–148. doi: 10.1177/140349489001800209. doi: 10.1177/140349489001800209. [DOI] [PubMed] [Google Scholar]
- 19.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooke HL, Talbäck M, Hörnblad J, Johansson LA, Ludvigsson JF, Druid H, Feychting M, Ljung R. The Swedish cause of death register. Eur J Epidemiol. 2017;32:765–773. doi: 10.1007/s10654-017-0316-1. doi: 10.1007/s10654-017-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludvigsson JF, Almqvist C, Bonamy AK, Ljung R, Michaëlsson K, Neovius M, Stephansson O, Ye W. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31:125–136. doi: 10.1007/s10654-016-0117-y. doi: 10.1007/s10654-016-0117-y. [DOI] [PubMed] [Google Scholar]
- 22.Høgberg U, Larsson N. Early dating by ultrasound and perinatal outcome. A cohort study. Acta Obstet Gynecol Scand. 1997;76:907–912. doi: 10.3109/00016349709034900. doi: 10.3109/00016349709034900. [DOI] [PubMed] [Google Scholar]
- 23.WHO. Obesity: preventing and managing the global epidemic. Report of a WHO cunsultation. World Health Organisation Tech Rep Ser. 2000;894:i, 1–xii. [PubMed] [Google Scholar]
- 24.Staff AC. The two-stage placental model of preeclampsia: an update. J Reprod Immunol. 2019;134–135:1–10. doi: 10.1016/j.jri.2019.07.004. doi: 10.1016/j.jri.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Redman CW, Staff AC. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am J Obstet Gynecol. 2015;213(4 Suppl):S9.e1-S11. doi: 10.1016/j.ajog.2015.08.003. doi: 10.1016/j.ajog.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Ghi T, degli Esposti D, Montaguti E, Rosticci M, Tancredi S, Youssef A, di Giovanni MV, Pilu G, Borghi C, Rizzo N. Maternal cardiac evaluation during uncomplicated twin pregnancy with emphasis on the diastolic function. Am J Obstet Gynecol. 2015;213:376.e1–376.e8. doi: 10.1016/j.ajog.2015.05.003. doi: 10.1016/j.ajog.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Thilaganathan B, Kalafat E. Cardiovascular system in preeclampsia and beyond. Hypertension. 2019;73:522–531. doi: 10.1161/HYPERTENSIONAHA.118.11191. doi: 10.1161/HYPERTENSIONAHA.118.11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kräker K, O’Driscoll JM, Schütte T, Herse F, Patey O, Golic M, Geisberger S, Verlohren S, Birukov A, Heuser A, et al. Statins reverse postpartum cardiovascular dysfunction in a rat model of preeclampsia. Hypertension. 2020;75:202–210. doi: 10.1161/HYPERTENSIONAHA.119.13219. doi: 10.1161/HYPERTENSIONAHA.119.13219. [DOI] [PubMed] [Google Scholar]
- 29.Ridder A, Giorgione V, Khalil A, Thilaganathan B. Preeclampsia: the relationship between uterine artery blood flow and trophoblast function. Int J Mol Sci. 2019;20:e3263. doi: 10.3390/ijms20133263. doi: 10.3390/ijms20133263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cnattingius S, Wikström AK, Stephansson O, Johansson K. The impact of small for gestational age births in early and late preeclamptic pregnancies for preeclampsia recurrence: a cohort study of successive pregnancies in Sweden. Paediatr Perinat Epidemiol. 2016;30:563–570. doi: 10.1111/ppe.12317. doi: 10.1111/ppe.12317. [DOI] [PubMed] [Google Scholar]
- 31.Espinoza J, and ACOG. ACOG practice bulletin no. 202 summary: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:211–214. doi: 10.1097/AOG.0000000000003019. doi: 10.1097/AOG.0000000000003019. [DOI] [PubMed] [Google Scholar]
- 32.Conde-Agudelo A, Romero R, Hassan SS, Yeo L. Transvaginal sonographic cervical length for the prediction of spontaneous preterm birth in twin pregnancies: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203:128.e1–128.12. doi: 10.1016/j.ajog.2010.02.064. doi: 10.1016/j.ajog.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1–65. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


