Supplemental Digital Content is available in the text.
Key Words: chronic kidney disease, quality of care, elderly population, end-stage kidney disease
Background:
The quality of care received by a growing number of older patients with chronic kidney disease (CKD) has not been adequately examined.
Objective:
The objective of this study was to assess the quality of CKD care among older patients and to clarify its association with the incidence of end-stage renal disease (ESRD).
Research Design:
This was a population-based cohort study.
Subjects:
Older (65 y and above) CKD patients diagnosed between October 2010 and September 2014 from the National Database of Health Insurance Claims of Japan.
Measures:
A composite quality score (QS) of 3 quality measures for CKD care during the 6 months after CKD diagnosis was computed. The validated quality measures included urine testing for proteinuria, nutritional guidance, and nonsteroidal anti-inflammatory drugs avoidance. To assess the association between the QS and ESRD incidence, we used instrumental variable analysis after stratification for the history of diabetes.
Results:
Among the 890,773 older CKD patients, 2.9% progressed to ESRD (incidence rate of 12.5 per thousand person-years). In total, 59.9% underwent urine testing, 4.5% received nutritional guidance, and 91.2% avoided regular use of nonsteroidal anti-inflammatory drugs. An instrumental variable analysis revealed that a higher QS was associated with—lower ESRD incidence in patients diagnosed with diabetes (hazard ratio: 0.25, 95% confidence interval: 0.24–0.27 for each point higher score) but not in patients without a diagnosis of diabetes (hazard ratio: 0.99, 95% confidence interval: 0.92–1.05).
Conclusion:
Among older CKD patients, quality of CKD care varied between patients, and better quality of CKD care was associated with a lower ESRD incidence in patients with diabetes but not in nondiabetic patients.
Chronic kidney disease (CKD) is common1,2 and is a known risk factor for mortality and cardiovascular disease.3,4 Patients with late-stage CKD not only have a higher probability of developing the end-stage renal disease (ESRD) but also experience a lower quality of life and higher medical costs.5,6 Medical costs for treating 300,000 ESRD patients in Japan have reached 1.5 billion USD annually, accounting for 4% of the total medical expenditure.7 Improving the quality of care (QoC) for CKD to prevent the development of ESRD is, therefore, a clinically and socially important issue.8
The number of CKD patients in aging societies is increasing.9 Because older CKD patients are more likely to exhibit multiple comorbidities,10 including both age-related and disease-related kidney dysfunction, managing these patients requires extra caution.9,11–14 To improve the QoC for CKD and outcomes in older patients, it is important to evaluate QoC using real-world health data.15 We developed a set of measures for QoC in CKD using the Delphi method,16 which is applicable to routinely collected data such as medical claims data and hospital data.
To quantify and evaluate the multifaceted CKD QoC, we investigated 3 indicators16 and their association with ESRD in a national health care claims database in Japan,17 where all older adults are completely covered by a universal health care system.
METHODS
Data Source
We extracted all claims data for patients aged 65 years and above with CKD-related diagnosis codes entered between October 2010 and September 2014, from the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB). The NDB was established by the Japanese government to monitor health care claims, costs, evaluate medical utilization, and conduct clinical research and is the only claims database that can be used to assess real-world health care in a country where 100% of its older population is covered by a universal health care system.17,18 The NDB database contains data for all diagnoses and all medical practices covered by universal health insurance, but specific items that can be extracted for each research purpose are limited. For this study, only data related to 3 indicators were available, and the available data did not include laboratory values, such as estimated glomerular filtration rate (eGFR) or hemoglobin levels.
Study Cohort and Participants
All older patients (aged 65 y and above) with new CKD diagnosis and CKD-related codes during the selection period (October 2010–September 2014) were identified. The CKD-related diagnosis codes comprised International Classification of Diseases, 10th Revision (ICD-10) codes for CKD, tubule-interstitial nephritis, chronic glomerular nephritis, diabetic nephropathy, hypertensive nephrosclerosis, and polycystic kidney disease (Supplemental Digital Content 1, Table 1, http://links.lww.com/MLR/B958). We excluded patients with existing CKD-related codes or who had received dialysis therapy 6 months before the selection period. We excluded patients with lupus nephritis according to the ICD-10 codes (M320-321, 329), as they represent a population with special care needs. We also excluded patients visiting small-volume facilities (housing <10 patients/mo) during the baseline period, because the facility-level quality score (QS) for such institutions is unstable. The selection process is shown in Supplemental Digital Content 2, Figure 1 (http://links.lww.com/MLR/B959). For each patient, the baseline period was defined within the first 6 months after a new diagnosis of CKD. After the baseline period, patients were followed until March 2015.
Definition of Quality Score and Covariates
Our main exposure variable of the QS for CKD care was the sum of the scores of 3 quality indicators (QIs). Each QI was defined as a binary variable defining whether or not the recommended CKD care was observed. We previously developed a set of 11 QIs for CKD patients using the Delphi method (modified RAND appropriateness method)16 and validated these indicators using a claims database in Taiwan.19 Herein, we selected 3 QIs: urine test, nutritional guidance, and nonsteroidal anti-inflammatory drugs (NSAIDs) avoidance identifiable by procedure codes in the claims data. NSAIDs avoidance was defined as evidence of prescription of NSAIDs for <14 days/mo. We selected 3 indicators from the original 11 indicators studied because they were retrievable from the Japanese claims database without the need for additional information (such as laboratory data of eGFR, urine protein and hemoglobin level) and are recommended in a wide CKD population including older patients.20,21 We show applicability and measurability of the original 11 indicators in Supplemental Digital Content 8, Table 7, (http://links.lww.com/MLR/B965). All 3 indicators are recommended in clinical guidelines and are indicative of multifaceted CKD care (Supplemental Digital Content 9, Fig. 2, http://links.lww.com/MLR/B966). Because we aimed to assess the overall multifaceted QoC for CKD, we used the sum of the scores of the 3 indicators rather than that of each indicator separately.
Outcome Measures
Our main outcome was ESRD incidence, defined as the first appearance of a claim for maintenance dialysis therapy, which comprised procedure codes for both hemodialysis and peritoneal dialysis.
Instrumental Variable Analysis
In the instrumental variable (IV) analysis, we used the sum of 3 indicators as our main exposure. Because indicators were not independent, it was difficult to assess each indicator separately due to violation of exclusion restriction assumption (ie, no pathway between instrument and outcome except through exposure).22 We used the facility-level QS at the facility where the patient was diagnosed with CKD, as the instrument. The facility-level QS was defined as the sum of the facility-level QIs of urine test, nutritional guidance, and NSAIDs avoidance. The facility-level QI was calculated as the proportion of patients receiving the recommended care within the facility over the baseline period. The facility-level QS served as a surrogate for provider preference, which is often used as an instrument in clinical research.23,24
To determine the validity of the IV analysis, we assessed endogeneity using Hausman and Durbin-Wu-Hausman tests. Three assumptions were examined for the IV analysis (Supplemental Digital Content 3, Table 2, http://links.lww.com/MLR/B960). First, we examined the association between IV and exposure variables. The facility-level QS was strongly associated with the QS each patient received (F-statistics>10, Supplemental Digital Content 4, Table 3, http://links.lww.com/MLR/B961). Second, we examined differences in the measured patient characteristics among IV categories divided by the median IV value. We found that the covariate balance had improved, except for a history of diabetes (Supplemental Digital Content 5, Table 4, http://links.lww.com/MLR/B962). Third, we examined the absence of a direct effect of IV on outcomes aside from the exposure variable (assumption of exclusion restriction). Examining the effect of a specific treatment, instrument-related concomitant treatment would violate the assumption of exclusion restriction. Therefore, we focused on the effects of the total QoC, which encompasses several aspects of CKD care.
Statistical Analyses
Patient characteristics and QSs were described using numbers and proportions for categorical variables. Incidence rates of ESRD and 95% confidence intervals (CIs) were described as incident counts and incidence rates.
The association between the QS and incidence of ESRD was examined for all patients and in the diabetes subgroups. We analyzed the diabetes subgroups because clinical practices and ESRD incidence differ among diabetes and nondiabetes groups. We used a nonparametric survival model using a Weibull distribution and estimated the hazard ratio (HR)25,26 with and without adjustment for measured confounders of age, sex, cause of CKD (chronic glomerular nephritis, hypertension), facility volume, and year of CKD diagnosis.
In the IV analysis, we applied a 2-stage residual inclusion approach.25 In the first stage, we used a linear regression model with the patient-level QS as a dependent variable and the facility-level QS and also measured confounders as independent variables. Using this first-stage model, we estimated the residual for each patient. In the second stage, we applied a nonparametric survival model using a Weibull distribution with the incidence of ESRD as a dependent variable and the QS, residual from the first-stage model, and measured confounders as independent variables. HRs and their 95% CIs were estimated using a bootstrap approach with 200 replications.27
Subgroup and Sensitivity Analyses
To examine the robustness of the results from the IV analysis, we performed subgroup and sensitivity analyses. To examine the effects of age on the association between the QS and ESRD, subgroup analysis was stratified by age group (<75 and ≥75 y) (Supplemental Digital Content 6, Table 5, http://links.lww.com/MLR/B963). Next, to treat for differences between facilities in the IV analysis, we used a within-facility variation of the QS as the instrument (Supplemental Digital Content 7, Table 6, http://links.lww.com/MLR/B964).
All analyses were performed using Stata (StataCorp, College Station, TX), version 14.0. All tests were 2-sided with P-values<0.05 considered statistically significant.
Additional Analysis
To verify whether the 3 indicators could be applied as a measure of the overall multifaceted QoC for CKD, we added analysis using the external data, including claims and laboratory data. We obtained external data from a large health insurer in Japan (the Health Insurance Association for Architecture and Civil Engineering companies). In the external data, we assessed 6 QIs (additional 3 indicators: CKD screening, CKD diagnosis, and biguanide avoidance) and examined associations between the 3 indicators score and the 6 indicators score (Supplemental Digital Content 8, Fig. 2, http://links.lww.com/MLR/B965).
RESULTS
Patient Characteristics
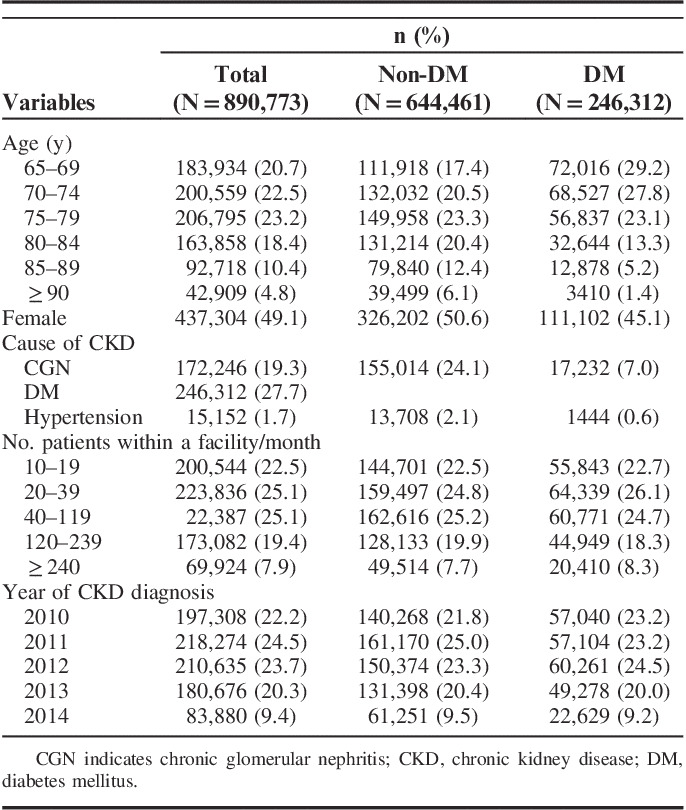
We identified 1,350,068 patients aged 65 years and above with CKD diagnosed between October 2010 and September 2014. We excluded 2544 (0.2%) patients with lupus nephritis and 456,751 (33.8%) patients who visited small-volume facilities. We ultimately analyzed 890,773 patients (Supplemental Digital Content 2, Fig. 1, http://links.lww.com/MLR/B959). Of these, 506,280 (56.8%) were aged older than 75 years and 246,312 (27.7%) had been diagnosed with diabetes mellitus (Table 1).
TABLE 1.
Patient Characteristics Overall and According to Diabetes Status
Quality of Chronic Kidney Disease Care
During the 6-month baseline period after CKD diagnosis, variations were noted in QSs and the proportion of achievement for each QI. Overall, 59.9% of patients underwent a urine test, 4.5% received nutritional guidance, and 91.2% avoided regular use of NSAIDs. Regarding achievement of indicators, 2.8% of patients received the recommended care for all 3 QIs, 53.6% received care for 2 QIs, 40.2% received care for 1 QI, and 3.4% did not receive care for any QI (Table 2). Diabetes patients tended to receive higher QSs than nondiabetes patients (Table 2). Older age was associated with a lower proportion of patients who received urine testing and nutritional guidance (Fig. 1).
TABLE 2.
Quality Score Overall and According to Diabetes Status
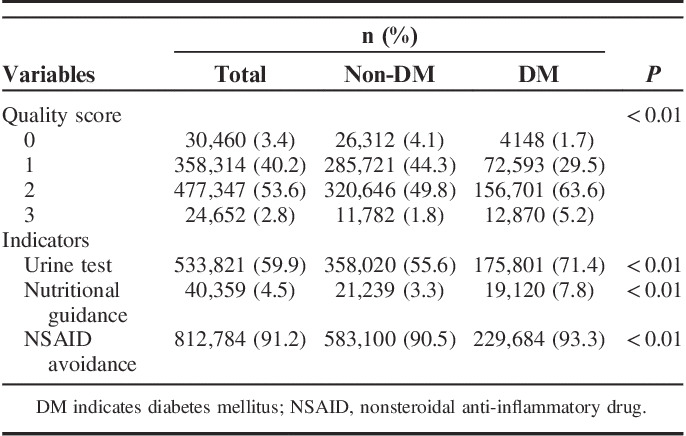
FIGURE 1.
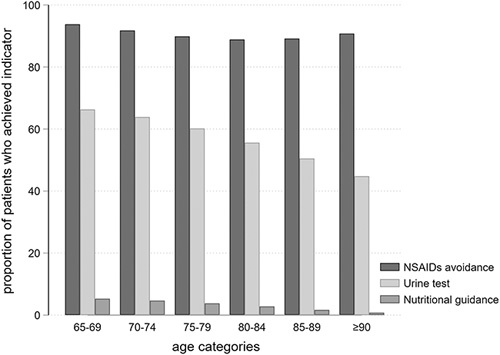
Quality indicators stratified according to age categories. The proportions of patients who achieved each indicator according to different age categories are indicated. NSAIDs indicates nonsteroidal anti-inflammatory drugs.
Incidence of End-Stage Renal Disease
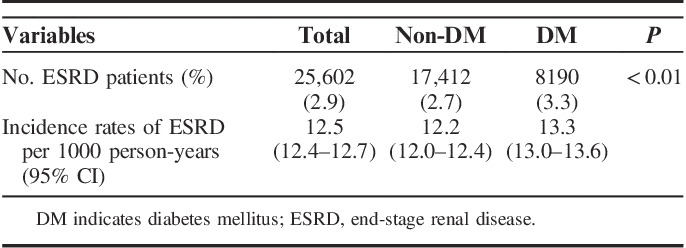
The median follow-up duration was 2.1 years (interquartile range: 1.3–3.3 y). During that period, 25,602 (2.9%) older CKD patients progressed to ESRD, resulting in an incidence rate of 12.5 (95% CI: 12.4–12.7) per 1000 person-years. Diabetes patients had a higher incidence rate of ESRD than nondiabetes patients (P<0.01; Table 3).
TABLE 3.
Incidence Rates of ESRD Overall and According to Diabetes Status
Association Between Quality Score and Incidence of End-Stage Renal Disease
In the multivariate nonparametric survival models with IV, after adjusting for unmeasured confounders, a higher QS was associated with a lower incidence of ESRD (Table 4). In the analysis stratified by the presence of diabetes, a higher QoC was associated with a lower incidence of ESRD in diabetes patients only.
TABLE 4.
Association of Quality Score and Incidence of End-Stage Renal Disease Overall and According to Diabetes Status
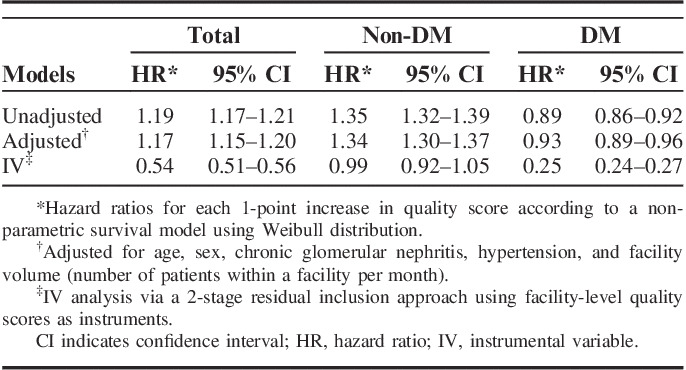
In the multivariate nonparametric survival models without IV, which might have been affected by unmeasured confounders, a higher QS was associated with a higher incidence of ESRD.
Subgroup and Sensitivity Analyses
Subgroup analysis examining the effects of age on the association of the QS with the incidence of ESRD revealed consistent results between age groups (<75 and ≥75 y) (Supplemental Digital Content 6, Table 5, http://links.lww.com/MLR/B963).
A sensitivity analysis using a within-facility variation of the QS as the instrument showed a similar trend of associations but wider CIs compared with the original analysis (Supplemental Digital Content 7, Table 6, http://links.lww.com/MLR/B964).
Additional Analysis
From the external data, the 3 indicators’ score was strongly associated with the 6 indicators’ score (correlation coefficient 0.82, P<0.01) (Supplemental Digital Content 8, Fig. 2, http://links.lww.com/MLR/B965).
DISCUSSION
Our assessment of data from older CKD patients obtained from a national claims database in the aging Japanese population revealed that the QoC for CKD, defined by 3 metrics (urine test, nutritional guidance, and NSAIDs avoidance), varied among patients and was associated with the ESRD outcomes. The proportion of patients receiving the recommended CKD care was 59.9% for the urine test, 4.5% for nutritional guidance, and 91.2% for NSAIDs avoidance. Older age was associated with lower QoC for CKD. Furthermore, nondiabetes patients tended to receive inferior QoC than diabetes patients. An IV analysis adjusted for both measured and unmeasured confounders showed that higher QS was associated with a lower incidence of ESRD among diabetes patients but not in nondiabetes patients. These results suggested that the QoC for CKD among older Japanese adults could be improved further, and such an improvement in QoC might help reduce ESRD rates at least among older patients with diabetes.
With the growing availability of large amounts of health data, it is not surprising that these ‘big data’ are being used to guide improvements in the quality of health care. Our study applied QIs to a huge nationwide claims database to measure the QoC among older CKD patients. To the best of our knowledge, this is the first study to comprehensively examine the real-world QoC for older CKD patients and their association with renal outcomes in Japan. The proportion of older CKD patients is increasing in today’s aging society.9 Older patients often have multiple morbidities and decreased biological function than younger patients; thus, we often observe marked differences in practice approaches between these age groups.28 Evidence from Japan, which is currently the most rapidly aging country, will help other aging countries address the needs of this growing population. We used 3 QIs for recommended CKD care: a urine test, nutritional guidance, and NSAIDs avoidance. CKD care encompass multiple factors, including “assessing the severity of CKD by a test,” “lifestyle guidance,” and “removing risk factors.” We selected 3 indicators from the original 11 indicators16 mainly owing to applicability in older population and data availability in this study. We feel that a composite score based on those 3 QIs is a good composite surrogate for clinical assessment of the multifaceted QoC for CKD. To show the validity of these 3 indicators as a measure of the overall QoC for CKD, we have added an analysis of external data (Supplemental Digital Content 8, Fig. 2, http://links.lww.com/MLR/B965). We assessed 6 indicators (additional 3 indicators: CKD screening, CKD diagnosis, and biguanide avoidance) and found that the 3 indicators’ score strongly associated with that of the 6 indicators (correlation coefficient 0.82), suggesting that the 3 indicators’ score could be used as a measure of overall quality of CKD care. Further, according to our preestablished research aims, we focused on the assessment of overall QoC for CKD rather than on a specific form of CKD care. Because each CKD approach is not independent, we assessed associations between the summed scores and outcomes. The higher multifaceted QoC for CKD might play a role in appropriate medical monitoring to provide timely intervention for renal protection. This study also reports on the variability of QoC for CKD across patients. The results of lower QoC in older patients may suggest lost opportunities for receiving appropriate care owing to an older age. In future studies, we need to identify high-risk patients who should be prioritized for improving QoC for CKD in the older population.
Because CKD practice and ESRD incidence differed among diabetes and nondiabetes subgroups, we assessed the association between the composite score and ESRD outcomes by the subgroups (diabetes and nondiabetes). We assessed the recommended care for renal protection but not diabetes-specific care. Obviously, it would be difficult to divide care for patients with both CKD and diabetes. Several reasons may explain why a higher QS was associated with a lower incidence of ESRD in diabetes patients but not in nondiabetes patients. First, diabetes patients may more likely to benefit significantly from medical care than nondiabetes patients. Our QS included a urine test, nutritional guidance, and NSAIDs avoidance. Although we were unable to obtain the results of urine tests, diabetes patients would be more likely than those without diabetes to have clinically meaningful proteinuria. Thus, undergoing a urine test may be associated with more intensive medical treatment in diabetes patients than in those without diabetes. Such “intensive medical treatment” after a urine test may include visiting a physician more often, controlling one’s blood pressure more carefully, and more strictly avoiding other risk factors for ESRD progression. Nutritional guidance may also be stricter for diabetes patients than for nondiabetes patients. While clinical guidelines support nutritional guidance for diabetes patients, no such evidence-supported guidance is available for nondiabetes patients.29,30 Second, we only examined the QS during the 6-month baseline period after CKD diagnosis. Because nondiabetes patients had a lower incidence of ESRD than diabetes patients, we may need to consider the QoC over a longer period.
In the setting of an observational study (especially one utilizing a health care claims database), the effects of unmeasured confounders should be considered. The claims database in this study do not contain laboratory data. Therefore, we were unable to measure strong indicators of the severity of CKD defined by eGFR and proteinuria laboratory data at a minimum. Patients with greater CKD severity are likely to receive more intensive medical care than others, leading to high QS, but are nevertheless at greater risk of CKD progression (confounding by indication). This powerful confounding factor might reverse the associations between the quality of CKD care and renal outcomes. To overcome this confounding effect, we conducted IV analyses adjusting for both measured and unmeasured confounders. The findings of the Durbin-Wu-Hausman endogeneity test were significant, indicating that the usual regression models presented residual confounding due to unmeasured factors. These results support the rationale behind our performing the IV analyses. Of note, we obtained different results for the multivariate models and IV analysis, suggesting that we should beware of unmeasured confounders when analyzing similar databases.
When interpreting the results from the IV analysis, we should consider the population to which the results apply. Although the usual regression model estimates the average effect among eligible patients, IV analysis estimates the local average treatment effect31 among compliers whose exposure variable is influenced by the instrument. In this study, compliers were borderline patients whose facility-level QS influenced the patient-level QS. The difference in the associations between usual regression and IV model may derive from population differences to which the results apply.
Several limitations to the present study warrant mention. First, we selected CKD patients defined by the CKD-related diagnosis code from claims data. Because we analyzed a claims database, we were unable to include patients with undiagnosed CKD in our evaluation. Second, the validity of some assumptions for the IV analysis could not be determined completely from the data. However, we tried to examine those assumptions by partial tests and discussed theories to support those assumptions. Because “no unmeasured confounders” of the necessary assumption for the usual regression model was unacceptable in our case, we opted to perform an IV analysis. The presence of confounding between IVs and outcomes cannot be determined directly from the data. However, we conducted our analyses stratified by the presence of diabetes and adjusted for the measured patient and facility characteristics. We also found that the covariate balance improved in the IV analysis compared with regression analysis. We also found a similar direction of association even if we used within-facility variation as the instrument, to treat for the differences between facilities (Supplemental Digital Content 7, Table 6, http://links.lww.com/MLR/B964). These results support the validity of our IV analysis.32 Third, we only analyzed a Japanese claims database; thus, our results may not be generalizable to other countries. However, we detected similar associations between the QS and ESRD among diabetes patients in Taiwan.19 Fourth, death during the follow-up period was treated as a target for censorship in this study; death can be a competing risk for ESRD. Although the mortality rate was expected to increase with age, we found consistent results among age groups (Supplemental Digital Content 6, Table 5, http://links.lww.com/MLR/B963). Therefore, the effect of competing risk due to death may have been limited. Fifth, it is difficult to demonstrate that there is no association between the IV (facility-level QS) and unmeasured confounders. Japanese patients do not decide which facility to go to based on the QoC of the facility because there is no publicly available information about the quality of the facility. However, medical professionals may be aware of the reputation of medical facilities and may influence a patient’s choice on referral. This may lead to differences in patient characteristics between facilities, and it may violate the exclusion restriction assumption. To address this issue, we conducted a sensitivity analysis using the within-facility variation of the QS as an IV and found similar associations to the original analysis (Supplemental Digital Content 7, Table 6, http://links.lww.com/MLR/B964). Finally, the Japanese national claims database has facility IDs, but do not physician IDs. We could assess variations in QoC between facilities but not among physicians. For example, patients who see nephrologist may receive higher QoC. However, we adjusted for those unmeasured differences between physicians using IV analysis.
In conclusion, we detected variations in the QoC among older CKD patients in Japan, and report that higher QS for CKD care is associated with a lower incidence rate of ESRD in patients with diabetes but not in patients without diabetes. Our results point to a significant need for further improvement in the quality of CKD care for older patients in this population. Applying QIs to a claims database may assist physicians and policy makers to improve QoC and patient outcomes. Further studies are required to assess the impact of applying those quality measures in the health system.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.lww-medicalcare.com.
Footnotes
Members of BiDAME (Big Data Analysis of Medical care for the Older in Kyoto): Funakoshi T., Goto Y., Goto E., Hanaki N., Hiragi S., Iwao T., Kawakami K., Kondo N., Kunisawa S., Mori Y., Nakatsui M., Neff Y., Ohtera S., Okamoto K., Otsubo T., Saito H., Saito Y., Sakai M., Sato I., Seto K., Takahashi Y., Yamashita K., Yoshida S., in Kyoto University Hospital/Kyoto University Graduate School of Medicine, School of Public Health, Kyoto, Japan.
Supported by a Ministry of Health, Labor and Welfare Grant-in-Aid for Scientific Research (Research on Policy Planning and Evaluation) (H27-policy-strategy-013).
The authors declare no conflict of interest.
Contributor Information
Collaborators: and on behalf of BiDANE: Big Data Analysis of Medical Care for the Older in Kyoto
REFERENCES
- 1.Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–822. [DOI] [PubMed] [Google Scholar]
- 2.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. [DOI] [PubMed] [Google Scholar]
- 3.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 5.Perlman RL, Finkelstein FO, Liu L, et al. Quality of life in chronic kidney disease (CKD): a cross-sectional analysis in the Renal Research Institute-CKD study. Am J Kidney Dis. 2005;45:658–666. [DOI] [PubMed] [Google Scholar]
- 6.St Peter WL, Schoolwerth AC, McGowan T, et al. Chronic kidney disease: issues and establishing programs and clinics for improved patient outcomes. Am J Kidney Dis. 2003;41:903–924. [DOI] [PubMed] [Google Scholar]
- 7.Travers K, Martin A, Khankhel Z, et al. Burden and management of chronic kidney disease in Japan: systematic review of the literature. Int J Nephrol Renovasc Dis. 2013;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silver SA, Bell CM, Chertow GM, et al. Effectiveness of quality improvement strategies for the management of CKD: a meta-analysis. Clin J Am Soc Nephrol. 2017;12:1601–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens LA, Coresh J, Levey AS. CKD in the elderly—old questions and new challenges: World Kidney Day 2008. Am J Kidney Dis. 2008;51:353–357. [DOI] [PubMed] [Google Scholar]
- 10.Stevens LA, Li S, Wang C, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2010;55:S23–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown MA, Collett GK, Josland EA, et al. CKD in elderly patients managed without dialysis: survival, symptoms, and quality of life. Clin J Am Soc Nephrol. 2015;10:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glassock RJ, Winearls C. CKD in the elderly. Am J Kidney Dis. 2008;52:803; author reply 803–804. [DOI] [PubMed] [Google Scholar]
- 13.Munikrishnappa D. Chronic kidney disease (CKD) in the elderly—a geriatrician’s perspective. Aging Male. 2007;10:113–137. [DOI] [PubMed] [Google Scholar]
- 14.Patel SS. The elderly with CKD: proceed with care. Adv Chronic Kidney Dis. 2016;23:6–7. [DOI] [PubMed] [Google Scholar]
- 15.Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence—what is it and what can it tell us? N Engl J Med. 2016;375:2293–2297. [DOI] [PubMed] [Google Scholar]
- 16.Fukuma S, Shimizu S, Niihata K, et al. Development of quality indicators for care of chronic kidney disease in the primary care setting using electronic health data: a RAND-modified Delphi method. Clin Exp Nephrol. 2017;21:247–256. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama T, Imanaka Y, Okuno Y, et al. Analysis of the evidence-practice gap to facilitate proper medical care for the elderly: investigation, using databases, of utilization measures for National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB). Environ Health Prev Med. 2017;22:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikegami N, Yoo BK, Hashimoto H, et al. Japanese universal health coverage: evolution, achievements, and challenges. Lancet. 2011;378:1106–1115. [DOI] [PubMed] [Google Scholar]
- 19.Wu HY, Fukuma S, Shimizu S, et al. Effects of higher quality of care on initiation of long-term dialysis in patients with CKD and diabetes. Am J Kidney Dis. 2017;70:666–674. [DOI] [PubMed] [Google Scholar]
- 20.Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 21.Japanese Society of Nephrology. Evidence-based clinical practice guideline for CKD 2013. Clin Exp Nephrol. 2014;18:346–423. [Google Scholar]
- 22.Garabedian LF, Chu P, Toh S, et al. Potential bias of instrumental variable analyses for observational comparative effectiveness research. Ann Intern Med. 2014;161:131–138. [DOI] [PubMed] [Google Scholar]
- 23.Ertefaie A, Small DS, Flory JH, et al. A tutorial on the use of instrumental variables in pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26:357–367. [DOI] [PubMed] [Google Scholar]
- 24.Brookhart MA, Wang PS, Solomon DH, et al. Evaluating short-term drug effects using a physician-specific prescribing preference as an instrumental variable. Epidemiology. 2006;17:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terza JV, Baqsu A, Rathouz PJ. Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ. 2008;27:531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terza JV, Bradford WD, Dismuke CE. The use of linear instrumental variables methods in health services research and health economics: a cautionary note. Health Serv Res. 2008;43:1102–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med. 2000;19:1141–1164. [DOI] [PubMed] [Google Scholar]
- 28.Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294:716–724. [DOI] [PubMed] [Google Scholar]
- 29.Academy of Nutrition and Dietetics Evidence Analysis Library. Chronic kidney disease (CKD) guideline (2010); 2015. Available at: www.andeal.org/topic.cfm?menu=5303&cat=3927 Accessed December 11, 2017.
- 30.Beto JA, Schury KA, Bansal VK. Strategies to promote adherence to nutritional advice in patients with chronic kidney disease: a narrative review and commentary. Int J Nephrol Renovasc Dis. 2016;9:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swanson SA, Hernan MA. Commentary: how to report instrumental variable analyses (suggestions welcome). Epidemiology. 2013;24:370–374. [DOI] [PubMed] [Google Scholar]
- 32.Brookhart MA, Schneeweiss S. Preference-based instrumental variable methods for the estimation of treatment effects: assessing validity and interpreting results. Int J Biostat. 2007;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.lww-medicalcare.com.


