Abstract
The aim of this study was to investigate predictors of lymph node metastasis (LNM) in early gastric signet-ring cell carcinoma (SRCC) and determine clinicopathologic and prognostic differences of different histologic subtypes. We retrospectively analyzed 13,661 gastric cancer patients; 231 were eligible for inclusion. Data for clinical, endoscopic, and histopathologic characteristics and prognoses were collected. Patients were followed up regarding postresection survival; overall and disease-specific survival rates were estimated by the Kaplan-Meier method with a log-rank test, and prognostic factors were evaluated by Cox regression. LNM incidence in early SRCC was 16.0% (37/231) overall: 6.9% (8/116) and 25.2% (29/115) in patients with pure and mixed SRCC, respectively. Univariate and multivariate analyses revealed SM2 invasion (odds ratio [OR]=5.070, P=0.003), lymphovascular invasion (LVI) (OR=14.876, P<0.001), pathologic pattern of mixed SRCC (OR=3.226, P=0.026), ulcer presence (OR=3.340, P=0.019) and lesion size over 20 mm (OR=2.823, P=0.015) as independent risk factors for LNM. Compared with pure SRCC, the mixed subtype was associated with older age, larger lesion size, higher LVI frequency, more frequent perineural invasion, and most importantly, higher LNM incidence. Patients with pure SRCC showed significantly longer overall survival (P=0.004) and disease-specific survival (P=0.002) than mixed SRCC patients. Pathologic subtype (hazard ratio [HR]=3.682; P=0.047), age (HR=5.246; P=0.001), SM1 invasion (HR=6.192; P=0.023), SM2 invasion (HR=7.529; P=0.021) and LNM (HR=5.352; P<0.001) were independent prognostic factors. Independent risk factors for LNM in early gastric SRCC were SM2 invasion, LVI, pathologic pattern, ulcer presence and lesion size over 20 mm. Early SRCC should be further classified by the purity of the SRC component.
Key Words: early gastric carcinoma, pure signet-ring cell carcinoma, mixed signet-ring cell carcinoma, lymph node metastasis, predictors
Signet-ring cell carcinoma (SRCC) is defined as a tumor that predominantly consists of isolated or small groups of tumor cells containing intracytoplasmic mucin.1 SRCC has shown discriminative biological characteristics compared with adenocarcinoma. Therefore, pathologic reports also document partial SRCC when a mixed signet-ring cell component exists in other main histologies.2 The behavior of SRCC in gastric cancer is controversial. In advanced gastric cancer, SRCC has been characterized as a more grossly infiltrative type, with more peritoneal dissemination and a similar or worse prognosis than non-SRCCs.3–5 However, the behavior of early-stage SRCC has been reported to be potentially more favorable than4,6–8 or equivalent to9,10 that of other types of early gastric cancer (EGC) due to having a lower probability of lymph node metastasis (LNM), which indicates its suitability for less invasive surgery.11
The indications for endoscopic resection are now gradually being extended to undifferentiated gastric cancers that previously required surgical resection.12 LNM-negative patients can be curatively treated with minimally invasive endoscopic resection; hence, accurate prediction of lymph node status is of crucial importance for appropriate curative treatment management.13 Unfortunately, there have been few reports about early SRCC regarding the risk factors for LNM as well as the clinicopathologic and prognostic differences between pure and mixed SRCCs. Therefore, the aim of this research was to investigate the differences between pure and mixed SRCCs and to identify the factors that predict the successful endoscopic treatment of EGC with signet-ring cell histology.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of the Ethics Committee of the Affiliated Hospital of Qingdao University (QYFY WZLL 2019-04-04). Our study was also performed in accordance with the Declaration of Helsinki (2000) of the World Medical Association.
Patients
A total of 13,661 patients who underwent gastrectomy with lymphadenectomy for histologically proven gastric adenocarcinoma at the Affiliated Hospital of Qingdao University from June 2002 to June 2014 were reviewed retrospectively. The following EGC cases were excluded: (1) advanced-stage gastric cancer (n=12,019); (2) intestinal metaplasia or intraepithelial neoplasia (n=92); (3) metastatic gastric cancer or multiple carcinomas (n=58); (4) lymphoma (n=47); (5) gastric stump carcinoma (n=56); and (6) other life-threatening diseases (n=37). A total of 1352 EGC patients were selected for careful pathologic analysis. Ultimately, 231 early gastric SRCC patients were eligible for inclusion in this study.
Data Collection
According to the fourth edition of the Japan Gastric Cancer Association (JGCA) treatment guidelines,5 gastrectomy with lymph node dissection was performed on the enrolled patients. The specimens were serially sectioned into 3-mm-thick slices after gross examination, and 2 experienced pathologists individually examined the histologic slides retrieved and investigated each case blindly without the knowledge of clinical and endoscopic findings. For difficult cases, several specialists reached a consensus through discussion.
According to the World Health Organization (WHO) diagnostic criteria,14 pure SRCC was defined as a predominant component (>50%) of isolated carcinoma cells containing intracytoplasmic mucin, and mixed SRCC was defined as adenocarcinoma with a minor component (10% to 50%) of isolated carcinoma cells containing intracytoplasmic mucin (Fig. 1). Lymphovascular invasion (LVI) was identified immunohistochemically using the D2-40 antibody (Dako-Cytomation, Glostrup, Denmark); the S100 protein was detected to diagnose perineural invasion. The diagnostic criterion for LNM was the presence of cancerous tissue inside the lymph node capsule. In accordance with the JGCA classification, when multiple lesions were present, the tumor with the most advanced T category (or the largest lesion when the T stages were identical) was classified.15 On the basis of the Paris endoscopic classification, the macroscopic features of EGC were divided into the following 5 subtypes: type 0-I (protruded), type 0-IIa (superficial elevated), type 0-IIb (flat), type 0-IIc (superficial depressed), and type 0-III (excavated).16 The tumors were also graded as small (≤20 mm) and large (≥20 mm) to allow for the re-evaluation of endoscopic submucosal dissection (ESD) indications. Regarding invasion depth, submucosal lesions were classified into 2 groups: SM1 (≤500 μm depth of invasion) and SM2 (>500 μm depth of invasion).
FIGURE 1.

Representative pathologic images. A, Pure signet-ring cell carcinoma. B, Mixed signet-ring cell carcinoma. C, Pure signet-ring cell carcinoma. D, Mixed signet-ring cell carcinoma.
Data for the clinical features, endoscopic and pathologic characteristics of all included patients were collected, including age, sex, incidence of hypertension, heart disease and diabetes mellitus, body mass index (BMI), family history, carcinoembryonic antigen (CEA) level, lesion size, macroscopic type, depth of invasion, number of tumors, presence of ulcers, LVI, perineural invasion, and LNM.
Postoperative Follow-up
Postresection outcomes were investigated through the routine scheduled outpatient service at 3-month intervals in the first 2 years and every 6 months thereafter for clinical examination, including gastroscopy, chest x-ray, abdominal and pelvic ultrasound or computed tomography, and tumor markers. Telephone interviews by the investigators were performed to assess the general situation of each patient. Overall survival (OS) was defined from the date of the operation to the date of death or the cutoff date (August 31, 2019).
Statistical Analysis
Continuous variables were translated into categorical variables. For age, we set 60 years old as the cutoff value. According to the criteria for obesity, the enrolled patients were divided into a nonobesity group (BMI ≤28) and an obesity group (BMI >28). Statistical analyses were conducted with SPSS software (version 23.0; SPSS Inc., Chicago, IL). Differences among categorical variables associated with predictors and LNM were assessed using a χ2 test or Fisher exact test, and variables that were significant in the univariate analysis were subsequently entered into a multivariate logistic regression model for the analysis of independent risk factors for LNM. The associations between variables and LNM are described by odds ratios (ORs) and 95% confidence intervals. Survival rates was calculated by the Kaplan-Meier curve method, and the difference between the survival curves was analyzed using a log-rank test. The Cox regression model was applied to evaluate prognostic factors. P-value <0.05 (2 sided) was considered statistically significant.
The statistical methods and analyses of this study were reviewed by professor Xiaobin Zhou from the Department of Health Statistics, Qingdao University.
RESULTS
The incidence of early gastric SRCC was 17.1% (231/1352) in all EGCs. The prevalence of LNM in early SRCC was 16.0% (37/231) overall, 6.9% (8/116) in patients with pure SRCC, and 25.2% (29/115) in those with mixed SRCC.
Clinicopathologic Features of Early Gastric SRCC According to LNM
Early gastric SRCCs were more common in young (younger than 60 y old) patients than elderly patients and in males than in females (ratio: 1.48:1). Patients with CEA values that exceeded the normal level were more likely to have LNM, and this association was statistically significant (P=0.035). However, the other clinical parameters, such as age, sex, underlying diseases, and family history, were not significantly associated with LNM.
With regard to the endoscopic features, the macroscopic type and number of tumors failed to reach statistical significance. Ulcerative lesions were significantly related to LNM (P=0.011). Regarding tumor size, LNM occurred more frequently in patients with large tumors than in those with small tumors (P=0.002).
LNM was found in 37 patients (16.0%). Regarding lesion depth, 126 (54.5%) patients had tumors limited to the mucosal (M) layer, 38 had superficial submucosal (SM1) tumors (16.5%), and 67 had deep submucosal (SM2) tumors (29.0%). The percentages of LNM positivity in these 3 groups were 5.6%, 15.8%, and 35.8%, respectively (P<0.001). Among the LNM-positive cases, 78.4% (29/37) of the EGCs were mixed SRCC histologic type, while 55.7% (108/194) of the LNM-negative EGCs were pure SRCC histologic type (P<0.001). LNM was found significantly more frequently in patients with LVI (P<0.001) than in patients without invasion. However, the perineural invasion was not significantly associated with LNM in our cohort. Additional detailed clinicopathologic features of early SRCC according to LNM status are summarized in Table 1.
TABLE 1.
LNM Risk According to Clinicopathologic Parameters in Early Gastric SRCC
Risk Factors for LNM in Early SRCC by Univariate and Multivariate Analyses
The univariate analyses demonstrated that high CEA levels, tumor size (>20 mm), the presence of ulcers, histologic type, depth of invasion (SM1 and SM2), and LVI differed significantly between patients with and without LNM. On the basis of the stepwise multivariate analysis, the significant independent risk factors for LNM in early SRCC were SM2 invasion (OR=5.070, P=0.003), LVI (OR=14.876, P<0.001), the pathologic pattern of mixed SRCC (OR=3.226, P=0.026), the presence of ulcers (OR=3.340, P=0.019) and a lesion size over 20 mm (OR=2.823, P=0.015). The independent risk factors are listed in Table 2.
TABLE 2.
Multivariate Logistic Regression Analysis of LNM in Early Gastric SRCC

Comparison of the Clinicopathologic Features Between Pure SRCC and Mixed SRCC
As shown in Table 3, the following differences in clinicopathologic characteristics between pure SRCC and mixed SRCC groups were significant: (1) the LNM rate was much higher in patients with mixed SRCC (25.2%) than in those with pure SRCC (6.9%) (P<0.0001); (2) LVI was found in a significantly higher proportion of cases of mixed SRCC (20.9%) than cases of pure SRCC (6.0%) (P<0.001); (3) perineural invasion was observed more frequently in the mixed SRCC group (16.5%) than in the pure SRCC group (6.0%) (P=0.012); (4) SM1 and SM2 invasion were less frequent in the pure SRC group than in the mixed SRCC group (P=0.009); and (5) age was the only statistically significant clinical characteristic (P=0.045) in this comparison. However, the differences in sex, basic diseases, obesity, family history, CEA level, size, macroscopic type, lesion number or ulcerative findings were not statistically significant between the 2 groups.
TABLE 3.
Comparison of Clinicopathologic Parameters Between Pure and Mixed Early Gastric SRCC
Differences in LNM and OS Between Pure and Mixed Early Gastric SRCC According to Invasion Depth
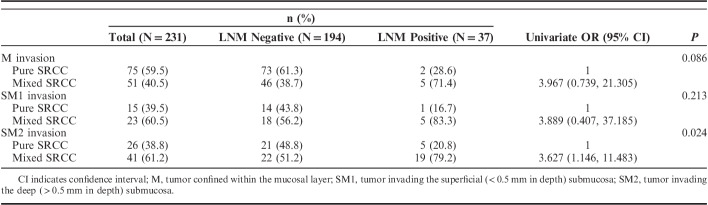
According to Table 4, LNM occurred more frequently in patients with mixed early gastric SRCC than those with pure histologic type no matter in which layer. Except for SM2 lesions (P=0.024), this difference failed to reach statistical significance in the M and SM1 groups.
TABLE 4.
LNM in Pure and Mixed Early Gastric SRCC According to Invasion Depth
With regard to the survival analyses for early gastric SRCC, patients with pure SRCC showed higher 5-year survival rates than patients with mixed histologic type in all the 3 layers. However, this association was not statistically significant in these subgroups (Table 5).
TABLE 5.
OS Among the Patients With Pure and Mixed Early Gastric SRCC According to Invasion Depth

Positive LNM Results in Early SRCC According to Indications for ESD
In further analyses, no LNM was observed in pure SRCC patients who met the expanded indications for ESD (0/26), while 8 of 90 patients beyond the expanded indications had LNM. However, in the mixed SRCC group, the LNM rate in patients with expanded indications was 5.3% (1/19) and that for patients beyond the indications was 29.2% (28/96) (Table 6).
TABLE 6.
LNM in Early Gastric SRCC According to Therapeutic Criteria

Survival Analyses of Early Gastric SRCC
At the last follow-up interview of 231 patients in the cohort, 9 (3.9%) has been lost to follow-up. The cumulative 5-year OS rate of the 231 patients with early gastric SRCC was 95.2%. Furthermore, the patients with pure SRCC showed significantly longer OS and disease-specific survival rates than patients with mixed histologic types (5-year OS: 97.4% vs. 93.0%, P=0.004; 5-year disease-specific survival: 99.1% vs. 95.6%, P=0.002) (Fig. 2). Multivariate Cox proportional regression analysis revealed that pathologic subtype (hazard ratio [HR]=3.682; P=0.047), age (HR=5.246; P=0.001), SM1 invasion (HR=6.192; P=0.023), SM2 invasion (HR=7.529; P=0.021), and LNM (HR=5.352; P<0.001) were independent prognostic factors (Table 7).
FIGURE 2.

Survival curves. A, OS of EGC patients with pure signet-ring cell carcinoma (pSRCC) and mixed signet-ring cell carcinoma (mSRCC) in months (P=0.004). B, Disease-specific survival of EGC patients with pSRCC and mSRCC in months (P=0.002).
TABLE 7.
Univariate and Multivariate Analyses of the Factors Affecting OS Among the Patients With Early Gastric SRCC

DISCUSSION
Numerous reports have identified SRCC as an independent predictor of poor prognosis due to specific characteristics such as a high incidence of LNM accompanied by a high rate of peritoneal carcinomatosis17,18 and low sensitivity to chemotherapy,19 especially during the period when the vast majority of these tumors were diagnosed at an advanced stage.
Owing to the popularization of gastroscopy, a large proportion of gastric cancers are diagnosed at an early stage, and precise histopathologic evaluations of EGC are now possible because of en bloc resection using ESD.11,20 The early detection of SRCC by an endoscopic examination and biopsy may be attributed to a high proportion of depressed lesions and easily detectable histologic features of enriched intracytoplasmic mucin with the peripherally compressed nucleus.18 Of course, for patients who have undergone ESD, meticulous pathologic examination of the resected specimen is mandatory because curability needs to be assessed based on the results of the biopsy examination. Gastrectomy with lymphadenectomy should be taken into consideration after noncurative resection.
However, it was recently demonstrated that SRCC has different clinical characteristics depending on whether the stage of SRCC is early or advanced.18,21,22 Most studies have reported that early gastric SRCC has less risk for LNM and a more favorable prognosis than other types8,20,21,23–25 Hence, SRCC is not currently identified as a predictor of poor prognosis. Technical advances in the endoscopic treatment of EGC have created unprecedented opportunities to treat EGC patients who are expected to have an extremely low probability of LNM. Therefore, the prediction of the biological behavior, especially LNM risk, of early SRCCs is an important issue in selecting the treatment modality.26 However, there are no conclusive guidelines for early gastric SRCC, which accounts for a large proportion of EGC.
We investigated the clinicopathologic factors and prognostic outcomes of early gastric SRCCs. Consistent with previous studies, early gastric SRCCs had a higher prevalence in young patients and were predominantly macroscopically depressed lesions.8 Sugihara et al27 suggested that SRCC forms more frequently in the intramucosal section than the extramucosal section of the lesion, which is in line with our results. This phenomenon was also reported in other series.20,22 The risk factors varied among relevant studies, but LVI, invasion depth and tumor size were found to be significantly related to LNM in almost every study. To further investigate the relationship between various clinicopathologic features and LNM, we performed univariate and multivariate analyses and confirmed that large tumor size, LVI, the presence of ulcers, the mixed SRCC histologic pattern and SM2 invasion depth were independent predictors of LNM.
By comparing patients’ clinical, endoscopic, and histopathologic characteristics and prognoses, we observed that compared with those with pure SRCC, those with mixed SRCC were older, had deeper invasion depth, more frequently had LVI and perineural invasion, and most importantly, had a higher incidence of LNM despite unremarkable significant differences. Furthermore, our study showed a distinctive tendency toward a difference in survival between mixed and pure SRCC patients, which is in accordance with other studies.2,22 Moreover, according to this research, the histologic type was an independent factor predicting prognosis in early gastric SRCC patients.
There is a continuous discussion concerning the indications for the endoscopic treatment of EGC. Numerous studies have reevaluated the risk of LNM in EGC following the introduction of expanded indications for ESD. A meta-analysis involving 9798 EGC patients showed that expanding the indications for ESD to include undifferentiated lesions <20 mm still requires careful investigation.28 However, many researchers recommend endoscopic resection for early gastric SRCC because it had favorable behavior compared with non-SRCC types in their studies.4,6,8,24,25 When we narrowed our cohort into a subgroup with expanded indications for ESD that had tumors <2 cm, no LVI, no ulcerations, and invasion confined to the M level, 45 cases (26 pure SRCCs and 19 mixed SRCCs) were included. The LNM incidence was 0% (0/26) for pure SRCCs and 5.3% (1/19) for mixed SRCCs. This also suggests that ESD is more feasible for pure SRCCs than for mixed SRCCs. Hence, a more accurate application of the SRCC classification could improve the ESD criteria.
However, the cellular histology of SRCC has been proven to be an important risk factor for LNM in EGC.20 Kim et al26 reported that compared with pure SRCC, the mixed SRCC subtype was associated with a higher frequency of LVI, more frequent intestinal metaplasia in the adjacent mucosa, and most importantly, a higher incidence of LNM. Imamura et al21 also stated that EGC with a mixed SRC histology exhibited more aggressive behavior than pure tubular adenocarcinoma or pure SRCC.7,26 This may be attributed to the angiogenetic process and cell proliferation in mixed-type gastric cancer. Zheng et al29 considered highly aggressive behavior, such as proliferation, apoptosis, angiogenesis, mucin secretion, and cell adhesion due to the increased expression of proteins such as Ki-67, extracellular matrix metalloproteinase inducer, and vascular endothelial growth factor, to be a possible reason for the aggressive features of mixed-type gastric cancer. Piessen et al17 also proved that mixed-type gastric cancer frequently showed cytosine-phosphate-guanine (CpG) island hypermethylation.30 Furthermore, Kim et al26 found that pure SRCCs might show a gastric phenotype, whereas mixed SRCCs could be associated with an intestinal immunophenotype.
On the basis of our findings, we propose that the presence of a pure or mixed SRCC component should be reported in daily pathologic practice, especially in cases of EGC, and such findings should be taken into consideration in the assessment of curable resection for ESD specimens. It should be noted that this was a retrospective study at a single institution, which was the major limitation. Therefore, a well-designed multicentric prospective cohort study is essential. Furthermore, we speculate that a subgroup analysis of mixed SRC histology samples categorized by the rate of minor components of isolated carcinoma cells containing mucin may reveal a significant difference in LNM frequency and survival rate. Hence, further research may be needed to investigate different subtypes more precisely in the future.
CONCLUSIONS
The independent risk factors for LNM in early SRCCs were SM2 invasion, LVI, the pathologic pattern of mixed SRCC, ulcer presence and a lesion size over 20 mm. Furthermore, compared with pure SRCC, the mixed SRCC subtype was associated with older age, larger lesion size, higher frequency of LVI, more frequent perineural invasion, and most importantly, a higher incidence of LNM and worse prognosis. Therefore, early SRCCs should be further classified by the purity of the SRCC component.
ACKNOWLEDGMENTS
The authors express their special thanks to Dr Weihua Yan (Department of Pathology, the Affiliated Hospital of Qingdao University) for her efforts of examining the histologic slides. They also thank Jie Zhu (Office of Academic Research, the Affiliated Hospital of Qingdao University) for her assistance with ethic.
Footnotes
Conflicts of Interest and Source of Funding: X.L. is currently receiving a grant (No. 81802777) from the National Natural Science Foundation of China and a grant (No. 2017M612221) from the China Postdoctoral Science Foundation. Our research was also part of Key Research and Development Plan of Shandong Province (No. 2018GSF118214). The remaining authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.WHO Classification of Tumors Editorial Board. WHO Classification of Tumours Digestive System Tumours. Lyon, France: International Agency for Research on Cancer; 2019. [Google Scholar]
- 2.Hu Q, Dekusaah R, Cao S, et al. Risk factors of lymph node metastasis in patients with early pure and mixed signet ring cell gastric carcinomas. J Cancer. 2019;10:1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942. [DOI] [PubMed] [Google Scholar]
- 4.Kunisaki C, Shimada H, Nomura M, et al. Therapeutic strategy for signet ring cell carcinoma of the stomach. Br J Surg. 2004;91:1319–1324. [DOI] [PubMed] [Google Scholar]
- 5.Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570–578. [DOI] [PubMed] [Google Scholar]
- 6.Chiu CT, Kuo CJ, Yeh TS, et al. Early signet ring cell gastric cancer. Dig Dis Sci. 2011;56:1749–1756. [DOI] [PubMed] [Google Scholar]
- 7.Huh CW, Jung DH, Kim JH, et al. Signet ring cell mixed histology may show more aggressive behavior than other histologies in early gastric cancer. J Surg Oncol. 2013;107:124–129. [DOI] [PubMed] [Google Scholar]
- 8.Hyung WJ, Noh SH, Lee JH, et al. Early gastric carcinoma with signet ring cell histology. Cancer. 2002;94:78–83. [DOI] [PubMed] [Google Scholar]
- 9.Yokota T, Kunii Y, Teshima S, et al. Signet ring cell carcinoma of the stomach: a clinicopathological comparison with the other histological types. Tohoku J Exp Med. 1998;186:121–130. [DOI] [PubMed] [Google Scholar]
- 10.Gronnier C, Messager M, Robb WB, et al. Is the negative prognostic impact of signet ring cell histology maintained in early gastric adenocarcinoma? Surgery. 2013;154:1093–1099. [DOI] [PubMed] [Google Scholar]
- 11.Fang WL, Huang KH, Lan YT, et al. The risk factors of lymph node metastasis in early gastric cancer. Pathol Oncol Res. 2015;21:941–946. [DOI] [PubMed] [Google Scholar]
- 12.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soetikno R, Kaltenbach T, Yeh R, et al. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490–4498. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe H, Jass JR, Sobin LH, et al. Histological Typing of Oesophageal and Gastric Tumours. Berlin, Germany: Springer-Verlag; 1990. [Google Scholar]
- 15.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. [DOI] [PubMed] [Google Scholar]
- 16.Participants in the Paris Workshop. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–S43. [DOI] [PubMed] [Google Scholar]
- 17.Piessen G, Messager M, Leteurtre E, et al. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009;250:878–887. [DOI] [PubMed] [Google Scholar]
- 18.Otsuji E, Yamaguchi T, Sawai K, et al. Characterization of signet ring cell carcinoma of the stomach. J Surg Oncol. 1998;67:216–220. [DOI] [PubMed] [Google Scholar]
- 19.Messager M, Lefevre JH, Pichot-Delahaye V, et al. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg. 2011;254:684–693; discussion 693. [DOI] [PubMed] [Google Scholar]
- 20.Ha TK, An JY, Youn HK, et al. Indication for endoscopic mucosal resection in early signet ring cell gastric cancer. Ann Surg Oncol. 2008;15:508–513. [DOI] [PubMed] [Google Scholar]
- 21.Imamura T, Komatsu S, Ichikawa D, et al. Early signet ring cell carcinoma of the stomach is related to favorable prognosis and low incidence of lymph node metastasis. J Surg Oncol. 2016;114:607–612. [DOI] [PubMed] [Google Scholar]
- 22.Bamboat ZM, Tang LH, Vinuela E, et al. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann Surg Oncol. 2014;21:1678–1685. [DOI] [PubMed] [Google Scholar]
- 23.Park J-M, Jang Y-J, Kim J-H, et al. Gastric cancer histology: clinicopathologic characteristics and prognostic value. J Surg Oncol. 2008;98:520–525. [DOI] [PubMed] [Google Scholar]
- 24.Maehara Y, Sakaguchi Y, Moriguchi S, et al. Signet ring cell carcinoma of the stomach. Cancer. 1992;69:1645–1650. [DOI] [PubMed] [Google Scholar]
- 25.Kim BS, Oh ST, Yook JH, et al. Signet ring cell type and other histologic types: differing clinical course and prognosis in T1 gastric cancer. Surgery. 2014;155:1030–1035. [DOI] [PubMed] [Google Scholar]
- 26.Kim YH, Park JH, Park CK, et al. Histologic purity of signet ring cell carcinoma is a favorable risk factor for lymph node metastasis in poorly cohesive, submucosa-invasive early gastric carcinoma. Gastric Cancer. 2017;20:583–590. [DOI] [PubMed] [Google Scholar]
- 27.Sugihara H, Hattori T, Fukuda M, et al. Progression of signet ring cell carcinomas in the human stomach. Cancer. 1993;71:1938–1947. [DOI] [PubMed] [Google Scholar]
- 28.Abdelfatah MM, Barakat M, Lee H, et al. The incidence of lymph node metastasis in early gastric cancer according to the expanded criteria in comparison with the absolute criteria of the Japanese gastric cancer association: a systematic review of the literature and meta-analysis. Gastrointest Endosc. 2018;87:338–347. [DOI] [PubMed] [Google Scholar]
- 29.Zheng HC, Li XH, Hara T, et al. Mixed-type gastric carcinomas exhibit more aggressive features and indicate the histogenesis of carcinomas. Virchows Arch. 2008;452:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SY, Kook MC, Kim YW, et al. Mixed-type gastric cancer and its association with high-frequency CpG island hypermethylation. Virchows Arch. 2010;456:625–633. [DOI] [PubMed] [Google Scholar]