Supplemental Digital Content is available in the text.
Keywords: Breast cancer, Pesticide, Herbicide, Fumigant, Fungicide, Agricultural Health Study
Background:
Evidence from epidemiologic and laboratory studies relating pesticides to breast cancer risk is inconsistent. Women engaging in agricultural work or living in agricultural areas may experience appreciable exposures to a wide range of pesticides, including herbicides, fumigants, and fungicides.
Methods:
We examined exposure to herbicides, fumigants, and fungicides in relation to breast cancer risk among farmers’ wives with no prior history of breast cancer in the Agricultural Health Study. Women provided information on pesticide use, demographics, and reproductive history at enrollment (1993–1997) and at a 5-year follow-up interview. We used Cox proportional hazards regression to estimate associations (hazard ratios [HRs] and 95% confidence intervals [CIs]) between the women’s and their husbands’ self-reported use of individual pesticides and incident breast cancer risk.
Results:
Out of 30,594 women, 38% reported using herbicides, fumigants, or fungicides and 1,081 were diagnosed with breast cancer during a median 15.3 years of follow-up. We found elevated risk in relation to women’s ever use of the fungicide benomyl (HR = 1.6; 95% CI = 0.9, 2.7) and the herbicide 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) (HR = 1.6; 95% CI = 0.8, 3.1) and to their husbands’ use of the herbicide 2-(2,4,5-trichlorophenoxy) propionic acid (2,4,5-TP) (HR = 1.5; 95% CI = 0.9, 2.7). We observed few other chemical associations and little evidence of differential risk by tumor estrogen receptor status or linear exposure-response relationships.
Conclusion:
We did not observe clear excesses between use of specific pesticides and breast cancer risk across exposure metrics, although we did observe elevated risk associated with women’s use of benomyl and 2,4,5-T and husbands’ use of 2,4,5-TP.
What this study adds
The detailed pesticide usage information for women and their husbands collected prospectively allowed us to examine possible risks from many individual pesticides, several of which have not been evaluated in epidemiologic studies before. The Agricultural Health Study also has extensive information on potential confounders and modifiers, including time-varying menopausal status and potential concomitant pesticide exposures. With 15 years of follow-up and over 1,000 breast cancer cases, this study represents one of the largest resources, in terms of sample size and scope of exposures, to assess relationships between pesticides and breast cancer.
Introduction
Breast cancer is the most common cancer and leading cause of cancer-related death among women globally and in the United States.1 An estimated 1.7 million cases are diagnosed annually worldwide, over 250,000 of which are in the United States.2 Although certain demographic and reproductive characteristics are established risk factors, a substantial portion of breast cancer risk remains unexplained.3,4 The uncertain relationship between breast cancer and environmental exposures has been identified as a research priority by the Interagency Breast Cancer and Environmental Research Coordinating Committee.5,6 Given the importance of hormonally mediated risk factors (and estrogen’s growth promoting and carcinogenic activity) in relation to breast cancer risk, endocrine-disrupting chemicals are hypothesized to be particularly relevant environmental exposures.7
Pesticides, which include insecticides, herbicides, fungicides, and fumigants, are widely used in residential and agricultural settings. Exposure may occur directly when mixing or applying the chemicals or indirectly via. pesticide residues, spray drift, contaminated water, or other take-home exposures that occur from living with a person who applies pesticides.8 Because organochlorine insecticides, including dichlorodiphenyltrichloroethane (DDT), are known endocrine disruptors, research investigating breast cancer and pesticides has emphasized these chemicals.9 However, other pesticides, including some herbicides, fumigants, and fungicides, also exhibit estrogenic activity and some induce mammary tumors in vitro and in animal models.10–13
Epidemiologic evidence linking pesticides to breast cancer has been mixed, with most studies relying on nonspecific exposure indicators. The exposures in these studies do not identify specific chemicals or pesticides, thereby introducing possible exposure misclassification and confounding. Some studies have shown increased risk of breast cancer in association with reported exposure to agricultural pesticides generally or to farming experience, though others did not find increased risk.14–19 Neither residential proximity to agricultural pesticide use nor self-reported exposure to residential or agricultural pesticides during childhood and adolescence was associated with breast cancer.20–22
Few individual pesticides have been evaluated in association with breast cancer risk prospectively. An earlier study of pesticide use and breast cancer risk among wives of farmers in the Agricultural Health Study (AHS) found suggestive associations with several different pesticides, but it was limited by a relatively short follow-up period and modest number of cases (n = 309).23 The present study adds to this previous investigation with an additional 10 years of follow-up and 772 additional incident cases. The AHS is one of few studies with the size and exposure information to examine numerous individual pesticides in relation to breast cancer risk. The risk estimates presented here for several herbicides, fungicides, and fumigants add to a previous article’s findings related to insecticides and breast cancer in this cohort.24
Methods
Study population
The AHS is a large prospective cohort of farmers and their spouses in Iowa and North Carolina.25 In brief, 52,394 private pesticide applicators enrolled between 1993 and 1997 by completing a questionnaire on farm exposures and health while attending mandatory certification sessions for applying restricted-use pesticides. Private applicators who indicated that they were married were invited to take two questionnaires home for their spouses to complete: a “Spouse” questionnaire, providing information about their farm exposures and general health, and a “Female and Family Health” questionnaire providing reproductive health history. We restricted our study population to female wives of pesticide applicators, due to the small number of male spouses (n = 219). Of the 32,126 wives (an estimated 75% of those eligible) enrolled in the cohort, 19,578 (61% of those enrolled) completed both questionnaires. In addition, 23,676 of the wives (74%) completed a 5-year follow-up telephone interview. Further detail defining the analytic population has been reported and is provided here (Figure e1; http://links.lww.com/EE/A89, which shows a flow chart of selection into the study).24
Exposure ascertainment
Pesticide exposure information was obtained at enrollment and at the 5-year follow-up interview (questionnaires are available at https://www.aghealth.nih.gov/collaboration/questionnaires.html). At enrollment, farmers and their spouses were asked about ever use of 50 specific pesticides, including 18 herbicides, four fumigants, and six fungicides. In the 5-year follow-up interview, they were asked to report the pesticides used on specific crops and animals in the previous growing season and how often (number of hours per day, number of days) during that growing season they used the pesticide. They were also asked detailed questions about their use of personal protective equipment and practices when handling pesticides. Questions on demographic, lifestyle, health, and reproductive factors were included in questionnaires at both time points. At enrollment, farmers additionally reported on duration, frequency, and decade of first use of specific pesticides. We used information about the farmers’ lifetime pesticide use to assess potential “indirect” pesticide exposure among the farmers’ wives and examined the risk associated with cumulative potential lifetime exposure to each pesticide from the husband’s use among the 13,500 wives who reported no prior personal pesticide use at enrollment.
Pesticide-specific exposures were estimated as the average number of days per year that each pesticide was used × the number of years the pesticide was used since marriage. For wives missing data on the year of marriage (38%), we assigned the later of the years that either spouse was 20 years old as the year of marriage. Exposure to each pesticide was assumed to begin at the later of the husband’s reported decade of first use of that pesticide or the year of marriage. Exposure to each pesticide ceased at the earliest of the husband’s self-reported last use of the pesticide, breast cancer diagnosis, censoring date, or end of follow-up.
Outcome ascertainment
Incident breast cancer cases were ascertained through population-based cancer registries in Iowa and North Carolina, using International Classification of Diseases for Oncology (3rd edition) codes C50.0–C50.9. Vital status was ascertained through state death registries and the National Death Index. The median duration of follow-up from enrollment was 15.3 years (through 2010 in North Carolina and 2011 in Iowa).
Statistical analysis
We conducted analyses using methods previously reported for insecticide exposures and breast cancer risk in this cohort.24 Briefly, we used Cox proportional hazards regression with age as the time scale and left truncation at enrollment (or the 5-year follow-up interview, where appropriate) to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Two primary exposure metrics were investigated for individual pesticides: (1) ever use of the pesticides by the women as reported at enrollment; and (2) cumulative potential exposure from the husband’s use reported from enrollment and 5-year follow-up.
Participants with a breast cancer diagnosis before enrollment (n = 478) or, for analyses of risk since the first follow-up, before the 5-year follow-up interview (n=310) were excluded. The outcome of interest was first primary invasive breast cancer, with censoring at the time of any in situ breast cancer diagnosis. Accrual of person-time ended at the breast cancer diagnosis, movement out of state, death, or end of follow-up, whichever came first. The proportional hazards assumption was evaluated for each exposure by the P value associated with a time-varying interaction term, exposure × age.
We evaluated 26 pesticides (18 herbicides, three fumigants, and five fungicides) that were included in the enrollment questionnaires and reported by at least five cases for any exposure metric. One fungicide (ziram) and one fumigant (ethylene dibromide) were omitted because they did not meet this threshold. Aluminum phosphide, a fumigant, is presented for analyses of husbands’ use only. Pesticide-specific quantiles of exposure were determined using cut points from the exposure distribution among noncases, with a minimum of 20 exposed cases per quantile; pesticides with fewer than 20 exposed cases were treated as any/none.
Of the 32,126 women enrolled in the study, we excluded those with prevalent breast cancer at enrollment (n = 478), living outside of North Carolina or Iowa at enrollment (n = 113), and missing all data on pesticide use (n = 948), for an analytic cohort of 30,594 women. For analyses of ever/never use by the women, we examined risk associated with participants’ ever use, reported at enrollment, of each pesticide among the analytic cohort. We attempted to further examine breast cancer risk associated with women’s use of pesticides in quantitative exposure-response analyses based on reported exposure at the 5-year follow-up; however, the number of women reporting exposure at follow-up did not permit such analyses.
Wives who reported first pesticide use at the 5-year interview or whose pesticide use at the 5-year interview could not be determined were censored at the midpoint between their date of enrollment and their 5-year interview (or the imputed date of the 5-year interview if they did not complete the interview). We used multiple imputation (n = 5) to estimate use of individual pesticides at 5 years among the 37% of farmers who did not complete the 5-year interview.26
All analyses were adjusted for time-varying menopausal status, race (white, other), state (Iowa, North Carolina), and combined parity/age at first birth (one birth before age 30; two or more births, with the first before age 30; nulliparous or all births after age 30). We also adjusted for all other pesticides (including insecticides) found to be associated with breast cancer in the present analysis, defined as a demographics-adjusted HR ≥1.50 or ≤0.67 and a minimum of five exposed cases. The set of adjustment pesticides was the same for all analyses within each exposure metric but varied between exposure metrics. Other potential confounders (body mass index, age at menarche, family history of breast cancer, physical activity, cigarette smoking, alcohol consumption, education, usual daily sun exposure, and nonfarm employment) were not included in the final models because they did not materially change risk estimates (i.e., <10% change). Missing data for covariates were imputed using IVEware (University of Michigan, Ann Arbor, MI). Risk estimates that incorporated imputed data were similar to those that included only observed data, so we present risk estimates based on models incorporating imputed covariate data.
We conducted sensitivity analyses examining breast cancer risk associated with the relative extent of direct exposure to each pesticide by modeling exposure as women’s reported use at enrollment only, the 5-year follow-up interview only, both enrollment and the follow-up interview, or neither. We also completed subanalyses assessing indirect exposure among only the women whose husbands completed the 5-year follow-up interview by excluding imputed pesticide exposure data.
We also conducted sensitivity analyses evaluating stratum-specific risk estimates for state of residence, tumor receptor status, and time-varying menopausal status at diagnosis. We used joint proportional hazards models defining cases by their estrogen receptor (ER) and progesterone receptor (PR) status.27 We stratified menopausal analyses using a product interaction term between pesticide exposure and time-varying menopausal status. When age at menopause was unknown, we substituted the cohort median value (52 years) as a proxy for individual age at menopause. Given that some pesticides are hormonally active, timing of menopause may be on the causal pathway between pesticides and breast cancer.28 Further, pesticide use has been associated with modest delays in menopause in this cohort.29 Therefore, we also examined associations between pesticide use and breast cancer risk unadjusted for menopause.
The institutional review boards of participating institutions approved the study, including the use of implied informed consent for enrollment. We performed all analyses using SAS (version 9.4; SAS Institute Inc., Cary, NC). All tests were two-sided with α = 0.05. Analyses were based on AHS data releases P1REL0906.00, P1REL201209.00, and P2REL201209.00.
Results
There were 1,081 incident cases of breast cancer diagnosed in this cohort of 30,594 women. Thirty-eight percent of women reported ever using an herbicide, fungicide, or fumigant. Most (98%) women were white, 68% lived in Iowa, and at enrollment, they had a median age of 46 years and nearly half (46%) were premenopausal (Table 1). Characteristics of women who never used pesticides were similar to those of the full cohort, although this subgroup was more likely to be from North Carolina. Both ER and PR status were known for 83% of cases.
Table 1.
Demographic characteristics of farmers’ wives in the Agricultural Health Study
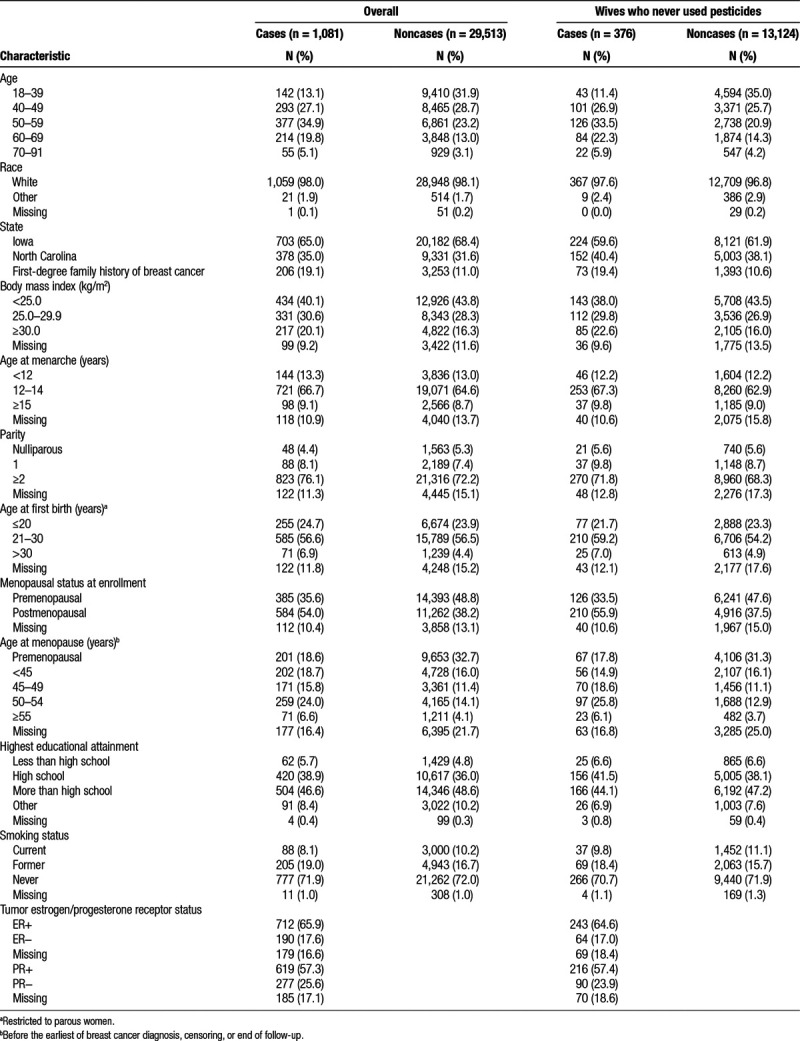
Risk of breast cancer was not associated with ever using any of the queried herbicides (HR = 0.9; 95% CI = 0.8, 1.0), fungicides (HR = 0.9; 95% CI = 0.6, 1.2), or fumigants (HR = 0.9; 95% CI = 0.5, 1.4) queried at study enrollment (Table 2). We observed nonsignificantly increased breast cancer risk associated with ever using the herbicide 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) (HR = 1.6; 95% CI = 0.8, 3.1) and the fungicide benomyl (HR = 1.6; 95% CI = 0.9, 2.7). Risk was nonsignificantly reduced in relation to ever use of butylate (HR = 0.4; 95% CI = 0.2, 1.0). Associations were generally similar between fully adjusted models (including use of other pesticides) and those adjusted for demographic/reproductive factors only.
Table 2.
Associations between the wives’ ever use of individual herbicides, fumigants, and fungicides at enrollment and risk of breast cancer among farmers’ wives in the Agricultural Health Study (n = 30,594)
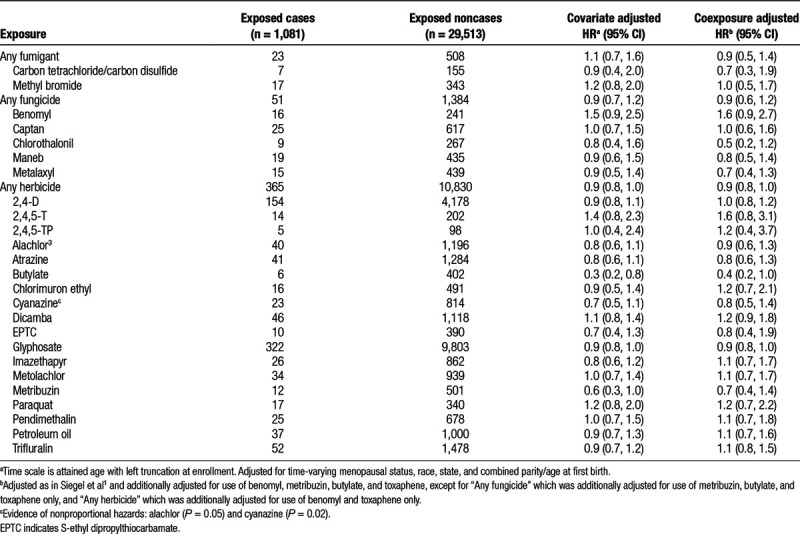
When considering possible indirect exposure via. husband’s reported use of pesticides among women who did not apply pesticides themselves (n = 13,500), ever use of 2-(2,4,5-trichlorophenoxy) propionic acid (2,4,5-TP) was associated with a nonsignificantly elevated breast cancer risk (HR = 1.5; 95% CI = 0.9, 2.7) (Table 3). Similarly, we observed nonsignificant increases in risk associated with husbands’ use of 2,4,5-T below (HR = 1.5; 95% CI = 0.7, 2.9) and above (HR = 1.3; 95% CI = 0.8, 2.0) the median, with limited evidence of a monotonic exposure-response trend (Ptrend = 0.08). Ever use of metribuzin by the husbands was associated with nonsignificantly elevated risk of breast cancer (HR = 1.3; 95% CI = 0.9, 1.9), whereas any use of trifluralin was associated with a significant reduction in breast cancer risk (HR = 0.7; 95% CI = 0.5, 0.9) with a significant, but nonmonotonic, exposure-response trend across quartiles of exposure (Ptrend = 0.01). Results did not change in sensitivity analyses excluding imputed 5-year pesticide use data for the husbands (data not shown).
Table 3.
Associations between the husbands’ use of individual herbicides, fumigants, and fungicides and risk of breast cancer among farmers’ wives who never used pesticides in the Agricultural Health Study (n = 13,500)
Because women reported limited use of pesticides at the 5-year follow-up interview, analyses of relative extent of direct exposure to each pesticide were restricted to five herbicides. The association between 2,4-dichlorophenoxyacetic acid (2,4-D) and breast cancer risk was apparent among women who reported use at both enrollment and the 5-year follow-up (HR = 1.6; 95% CI = 1.0, 2.5), but not among women who reported use at only enrollment (HR = 1.1; 95% CI = 0.8, 1.4) or follow-up (HR = 0.6; 95% CI = 0.3, 1.4) only (Table e1; http://links.lww.com/EE/A89, which reports on relative extent of use for a subset of chemicals). Recent use of trifluralin reported at follow-up significantly increased risk (HR = 1.7; 95% CI = 1.0, 2.8), though there were not enough cases exposed at both enrollment and follow-up to estimate this association.
State-specific associations did not differ in direction, though risk estimates tended to be higher in North Carolina (Tables e2 and e5; http://links.lww.com/EE/A89, which report state-stratified associations for women’s and husbands’ use, respectively). Specifically, we observed elevated risk in North Carolina, but not in Iowa, associated with women’s use of 2,4-D (North Carolina HR = 1.4; 95% CI = 0.9, 2.1; Iowa HR = 0.9; 95% CI = 0.7, 1.1) and dicamba (North Carolina HR = 2.8; 95% CI = 1.2, 6.4; Iowa HR = 1.1; 95% CI = 0.8, 1.7). Similarly, husbands’ use of 2,4,5-TP was associated with nonsignificantly increased risk in North Carolina (HR = 2.6; 95% CI = 0.9, 7.7), but the HR was considerably lower in Iowa (HR = 1.3; 95% CI = 0.7, 2.6).
We did not detect patterns in associations between pesticide use and breast cancer risk according to ER tumor status (Tables e3 and e6; http://links.lww.com/EE/A89, which report tumor receptor-stratified associations for women’s and husbands’ use, respectively), though these analyses were limited by the low incidence of ER− tumors. We did, however, observe nonsignificantly increased risk associated with women’s use of chlorimuron ethyl among those with ER− tumors (ER+ HR = 0.9; 95% CI = 0.4, 1.9; ER− HR = 2.3; 95% CI = 0.9, 5.7; Pinteraction = 0.10). Conversely, husbands’ use of metribuzin was associated with elevated risk in ER+ tumors (ER+ HR = 1.6; 95% CI = 1.1, 2.5), but ER− tumors were not (ER− HR = 0.8; 95% CI = 0.3, 1.8; Pinteraction = 0.10). Risk estimates were similar when we examined associations by joint ER+/PR+ status rather than by ER+ status alone (data not shown).
Despite the low incidence of exposed premenopausal breast cancer cases, we did observe increased risk of premenopausal breast cancer associated with women’s use of alachlor (premenopausal HR = 2.2; 95% CI = 1.4, 2.3; postmenopausal HR = 0.7; 95% CI = 0.4, 1.1; Pinteraction = 0.003) and petroleum oil (premenopausal HR = 2.1; 95% CI = 1.0, 4.3; postmenopausal HR = 0.9; 95% CI = 0.6, 1.4; Pinteraction = 0.05) (Table S4; http://links.lww.com/EE/A89). In analyses of indirect exposure from husbands’ use of pesticides, we observed nonsignificant heterogeneity by menopausal status for metribuzin (premenopausal HR = 0.8; 95% CI = 0.4, 1.7; postmenopausal HR = 1.5; 95% CI = 1.0, 2.2; Pinteraction = 0.12) and metolachlor (premenopausal HR = 0.6; 95% CI = 0.3, 1.1; postmenopausal HR = 1.1; 95% CI = 0.8, 1.5; Pinteraction = 0.09) (Table e7; http://links.lww.com/EE/A89, which presents associations stratified by menopausal status at diagnosis). Husband’s use of butylate increased risk for postmenopausal cancer (HR = 1.5; 95% CI = 1.0, 2.2); however, we did not estimate an association for premenopausal cancer due to insufficient exposed cases. In sensitivity analyses unadjusted for menopausal status, associations between pesticide use and breast cancer were similar to overall results (not shown).
Discussion
Overall, we did not observe any clear associations between pesticide use and breast cancer risk in this prospective cohort of farmers’ spouses in North Carolina and Iowa. Associations were also inconsistent between exposure from women’s personal direct use and indirect exposure from husbands’ use of a given chemical in various subanalyses. Women’s use of the fungicide benomyl and the herbicide 2,4,5-T was nonsignificantly associated with increased risk of breast cancer. Husbands’ use of the herbicide 2,4,5-TP, which is structurally similar to 2,4,5-T, demonstrated a nonsignificant elevation in risk. Husbands’ use of the herbicide metribuzin was also associated with modest increases in risk which were more pronounced among postmenopausal and ER+ cases. Risks associated with alachlor, petroleum oil, metolachlor, and metribuzin differed by menopausal status.
The present study adds 10 years of follow-up and 772 new cases to a previous analysis of breast cancer in this cohort, allowing us to estimate associations for additional herbicides, fungicides, and fumigants not explored in the previous study.23 Although the increase in breast cancer risk associated with the fungicide captan in the initial study was not replicated in our follow-up analysis, we did observe associations with the phenoxy herbicides 2,4,5-T and 2,4,5-TP, as noted earlier. Increased risk was associated with the husbands’ use of 2,4,5-T in the previous study, but with the women’s use of 2,4,5-T in the present study. Both studies found elevated risk associated with husbands’ use of 2,4,5-TP, though results were attenuated and nonsignificant in the present study. Production of both of these phenoxy herbicides ceased in 1979 because of contamination by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a known endocrine disrupter and human carcinogen.30–34 Excess breast cancer was observed among women occupationally exposed to TCDD via. phenoxy herbicides and separately among women with higher serum TCDD levels after the 1976 chemical explosion in Seveso, Italy.35–37 We cannot rule out the possibility that the observed risks associated with 2,4,5-T and 2,4,5-TP in the present analyses are due, to some extent, to TCDD contamination.
Because most epidemiologic investigations of pesticide exposure have been occupational studies that did not include women, epidemiologic research on specific nonpersistent pesticides and breast cancer is limited—though the persistent pesticide DDT has been investigated.38,39 Available studies emphasize the hormonally active organochlorine insecticides, though a number of herbicides, fungicides, and fumigants also exhibit endocrine-disrupting properties.5,10 Atrazine, a widely used herbicide with estrogenic activity, induces mammary tumors in some rodents, though the mechanisms by which atrazine induces mammary tumors in rats do not operate in humans, and results are inconsistent.12,40–42 We did not observe an increase in risk associated with atrazine use, though it was associated with a slightly later age at menopause in this cohort and an ecologic study in Kentucky found higher breast cancer incidence in areas with greater potential atrazine exposure.29,43 Women’s use of alachlor was significantly associated with increased risk of premenopausal breast cancer in our study. Alachlor suppresses apoptosis in ER+ breast tumor cells in vitro, though we did not observe heterogeneity of the relationship by tumor estrogen receptor status.44 Similarly, our observed increase in risk associated with women’s use of benomyl, a carbamate fungicide, is interesting because of in vitro evidence of benomyl’s estrogenic activity.45,46
We did not observe consistent differences between states in stratified analyses, although there were a small number of suggestive differences between Iowa and North Carolina. The number of exposed cases in stratified analyses tended to be small in general, limiting our ability to explore this further. The reasons for these differences can be better addressed with data collected later in the AHS on women’s detailed use of personal protective equipment, though that is beyond the scope of this study.
This study has several limitations. We could not analyze exposure-response relationships for several individual chemicals included in our ever/never analyses because of insufficient exposed cases. At enrollment, spouses were asked only about ever use of specific pesticides, and at follow-up, few women reported using pesticides. As a number of the pesticides included here have not been evaluated in epidemiologic studies before, these are considered exploratory analyses. Moreover, because of the number of statistical tests performed, some associations may have occurred by chance. To address the potential for spurious associations, we looked for consistency across multiple exposure metrics (i.e., women’s use and husbands’ use) and in sensitivity analyses. Although participants were asked to report on pesticide usage during their lifetime, data collection occurred during their adulthood and recall of early life exposures may be inferior to that of more recent pesticide use. As such, our analyses may not adequately account for early life pesticide exposure, including the time between menarche and first birth, which may be a particularly important temporal window for breast cancer risk.47 In addition, we could not quantify cumulative exposures for the women’s use because pre-enrollment exposure was ascertained only as ever/never use. Self-reported pesticide use is potentially subject to exposure misclassification, though prior work in the cohort demonstrated high reliability of self-reported pesticide use.48 Still, there is the possibility for misclassification, though previous analyses of the AHS indicate that this type of misclassification is likely nondifferential with respect to breast cancer diagnosis and would have attenuated effect estimates.48,49
Evaluating associations between pesticides and breast cancer risk in this large prospective cohort had several advantages over existing studies, few of which have evaluated individual pesticides and breast cancer. The large sample size and detailed pesticide usage information for women and their husbands collected prospectively allowed us to examine possible risks from many individual pesticides. The Agricultural Health Study also has extensive information on potential confounders and modifiers, including time-varying menopausal status and potential concomitant pesticide exposures. With 15 years of follow-up, more than 1,000 breast cancer cases, and information on 50 pesticides, this study represents one of the largest resources, in terms of sample size and scope of exposures, to assess relationships between pesticides and breast cancer.
The suggestive links between some pesticides and breast cancer observed here indicate that more research on associations between pesticides and breast cancer is warranted, particularly in the context of the widespread use of many of these chemicals and uncertainty about etiologically relevant timing of exposure.
ACKNOWLEDGMENTS
We thank C. Sima (Memorial Sloan Kettering Cancer Center) for statistical assistance.
Conflicts of interest statement
The authors declare that they have no conflicts of interest with regard to the content of this report.
Supplementary Material
Footnotes
Published online 27 May 2020
These authors are co-senior authors.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of the article.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
This research was supported by National Institutes of Health (NIH) grant R03CA137824 and by the Intramural Research Program of the NIH (National Cancer Institute [NCI; Z01CP010119] and the National Institute of Environmental Health Sciences/NIH [Z01-ES049030]).
The data and computer code are not available for replication because the data are not publicly available.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017; 67:7–30 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386 [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global cancer in women: burden and trends. Cancer Epidemiol Biomarkers Prev. 2017; 26:444–457 [DOI] [PubMed] [Google Scholar]
- 4.Brinton LA, Gaudet MM, Gierach GL. Thun MJ, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D. Breast cancer. In: Cancer Epidemiology and Prevention. 20184th ed, New York, NY: Oxford University Press [Google Scholar]
- 5.Rodgers KM, Udesky JO, Rudel RA, Brody JG. Environmental chemicals and breast cancer: an updated review of epidemiological literature informed by biological mechanisms. Environ Res. 2018; 160:152–182 [DOI] [PubMed] [Google Scholar]
- 6.IBCERCC (Interagency Breast Cancer and Environmental Research Coordinating Committee) Breast Cancer and the Environment, Prioritizing Prevention. 2013. Available at: https://www.niehs.nih.gov/about/assets/docs/breast_cancer_and_the_environment_prioritizing_prevention_508.pdf. [Google Scholar]
- 7.Rojas K, Stuckey A. Breast cancer epidemiology and risk factors. Clin Obstet Gynecol. 2016; 59:651–672 [DOI] [PubMed] [Google Scholar]
- 8.Deziel NC, Friesen MC, Hoppin JA, Hines CJ, Thomas K, Freeman LE. A review of nonoccupational pathways for pesticide exposure in women living in agricultural areas. Environ Health Perspect. 2015; 123:515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker ME, Lathe R. The promiscuous estrogen receptor: evolution of physiological estrogens and response to phytochemicals and endocrine disruptors. J Steroid Biochem Mol Biol. 2018; 184:29–37 [DOI] [PubMed] [Google Scholar]
- 10.McKinlay R, Plant JA, Bell JN, Voulvoulis N. Endocrine disrupting pesticides: implications for risk assessment. Environ Int. 2008; 34:168–183 [DOI] [PubMed] [Google Scholar]
- 11.Morinaga H, Yanase T, Nomura M, et al. A benzimidazole fungicide, benomyl, and its metabolite, carbendazim, induce aromatase activity in a human ovarian granulose-like tumor cell line (KGN). Endocrinology. 2004; 145:1860–1869 [DOI] [PubMed] [Google Scholar]
- 12.Rudel RA, Attfield KR, Schifano JN, Brody JG. Chemicals causing mammary gland tumors in animals signal new directions for epidemiology, chemicals testing, and risk assessment for breast cancer prevention. Cancer. 2007; 10912 suppl2635–2666 [DOI] [PubMed] [Google Scholar]
- 13.Thongprakaisang S, Thiantanawat A, Rangkadilok N, Suriyo T, Satayavivad J. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem Toxicol. 2013; 59:129–136 [DOI] [PubMed] [Google Scholar]
- 14.Duell EJ, Millikan RC, Savitz DA, et al. A population-based case-control study of farming and breast cancer in North Carolina. Epidemiology. 2000; 11:523–531 [DOI] [PubMed] [Google Scholar]
- 15.Salerno C, Carcagnì A, Sacco S, et al. An Italian population-based case-control study on the association between farming and cancer: are pesticides a plausible risk factor? Arch Environ Occup Health. 2016; 71:147–156 [DOI] [PubMed] [Google Scholar]
- 16.Brophy JT, Keith MM, Watterson A, et al. Breast cancer risk in relation to occupations with exposure to carcinogens and endocrine disruptors: a Canadian case–control study. Environmental Health. 2012; 11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teitelbaum SL, Gammon MD, Britton JA, Neugut AI, Levin B, Stellman SD. Reported residential pesticide use and breast cancer risk on Long Island, New York. Am J Epidemiol. 2007; 165:643–651 [DOI] [PubMed] [Google Scholar]
- 18.Blair A, Dosemeci M, Heineman EF. Cancer and other causes of death among male and female farmers from twenty-three states. Am J Ind Med. 1993; 23:729–742 [DOI] [PubMed] [Google Scholar]
- 19.Xu X, Dailey AB, Talbott EO, Ilacqua VA, Kearney G, Asal NR. Associations of serum concentrations of organochlorine pesticides with breast cancer and prostate cancer in U.S. adults. Environ Health Perspect. 2010; 118:60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds P, Hurley SE, Goldberg DE, et al. ; California Teachers Study Residential proximity to agricultural pesticide use and incidence of breast cancer in the California Teachers Study cohort. Environ Res. 2004; 96:206–218 [DOI] [PubMed] [Google Scholar]
- 21.Reynolds P, Hurley SE, Gunier RB, Yerabati S, Quach T, Hertz A. Residential proximity to agricultural pesticide use and incidence of breast cancer in California, 1988-1997. Environ Health Perspect. 2005; 113:993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niehoff NM, Nichols HB, White AJ, Parks CG, D’Aloisio AA, Sandler DP. Childhood and adolescent pesticide exposure and breast cancer risk. Epidemiology. 2016; 27:326–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engel LS, Hill DA, Hoppin JA, et al. Pesticide use and breast cancer risk among farmers’ wives in the agricultural health study. Am J Epidemiol. 2005; 161:121–135 [DOI] [PubMed] [Google Scholar]
- 24.Engel LS, Werder E, Satagopan J, et al. Insecticide use and breast cancer risk among farmers’ wives in the Agricultural Health Study. Environ Health Perspect. 2017; 125:097002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alavanja MC, Sandler DP, McMaster SB, et al. The Agricultural Health Study. Environ Health Perspect. 1996; 104:362–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heltshe SL, Lubin JH, Koutros S, et al. Using multiple imputation to assign pesticide use for non-responders in the follow-up questionnaire in the Agricultural Health Study. J Expo Sci Environ Epidemiol. 2012; 22:409–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue X, Kim MY, Gaudet MM, et al. A comparison of the polytomous logistic regression and joint cox proportional hazards models for evaluating multiple disease subtypes in prospective cohort studies. Cancer Epidemiol Biomarkers Prev. 2013; 22:275–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collaborative Group on Hormonal Factors in Breast Cancer Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012; 13:1141–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farr SL, Cai J, Savitz DA, Sandler DP, Hoppin JA, Cooper GS. Pesticide exposure and timing of menopause: the Agricultural Health Study. Am J Epidemiol. 2006; 163:731–742 [DOI] [PubMed] [Google Scholar]
- 30.All uses of 2,4,5-T and silvex pesticides banned. Chem Eng News. 1985; 63:6. doi: 10.1021/cen-v063n012.p006a [Google Scholar]
- 31.Lilienfeld DE, Gallo MA. 2,4-D, 2,4,5-T, and 2,3,7,8-TCDD: an overview. Epidemiol Rev. 1989; 11:28–58 [DOI] [PubMed] [Google Scholar]
- 32.National Academies of Sciences Engineering and Medicine Veterans and Agent Orange: Update 11 (2018). 2018, Washington, DC: The National Academies Press; [PubMed] [Google Scholar]
- 33.Steenland K, Bertazzi P, Baccarelli A, Kogevinas M. Dioxin revisited: developments since the 1997 IARC classification of dioxin as a human carcinogen. Environ Health Perspect. 2004; 112:1265–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kogevinas M. Human health effects of dioxins: cancer, reproductive and endocrine system effects. Hum Reprod Update. 2001; 7:331–339 [DOI] [PubMed] [Google Scholar]
- 35.Manz A, Berger J, Dwyer JH, Flesch-Janys D, Nagel S, Waltsgott H. Cancer mortality among workers in chemical plant contaminated with dioxin. Lancet. 1991; 338:959–964 [DOI] [PubMed] [Google Scholar]
- 36.Warner M, Eskenazi B, Mocarelli P, et al. Serum dioxin concentrations and breast cancer risk in the Seveso Women’s Health Study. Environ Health Perspect. 2002; 110:625–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warner M, Mocarelli P, Samuels S, Needham L, Brambilla P, Eskenazi B. Dioxin exposure and cancer risk in the Seveso Women’s Health Study. Environ Health Perspect. 2011; 119:1700–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ingber SZ, Buser MC, Pohl HR, Abadin HG, Murray HE, Scinicariello F. DDT/DDE and breast cancer: a meta-analysis. Regul Toxicol Pharmacol. 2013; 67:421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.International Agency for Research on Cancer DDT, lindane, and 2,4,-D. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2018; 113, Lyon, France: IARC [Google Scholar]
- 40.Alavanja MC, Ross MK, Bonner MR. Increased cancer burden among pesticide applicators and others due to pesticide exposure. CA Cancer J Clin. 2013; 63:120–142 [DOI] [PubMed] [Google Scholar]
- 41.Gammon DW, Aldous CN, Carr WC, Jr, Sanborn JR, Pfeifer KF. A risk assessment of atrazine use in California: human health and ecological aspects. Pest Manag Sci. 2005; 61:331–355 [DOI] [PubMed] [Google Scholar]
- 42.Simpkins JW, Swenberg JA, Weiss N, et al. Atrazine and breast cancer: a framework assessment of the toxicological and epidemiological evidence. Toxicol Sci. 2011; 123:441–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kettles MK, Browning SR, Prince TS, Horstman SW. Triazine herbicide exposure and breast cancer incidence: an ecologic study of Kentucky counties. Environ Health Perspect. 1997; 105:1222–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burow ME, Tang Y, Collins-Burow BM, et al. Effects of environmental estrogens on tumor necrosis factor alpha-mediated apoptosis in MCF-7 cells. Carcinogenesis. 1999; 20:2057–2061 [DOI] [PubMed] [Google Scholar]
- 45.Kawaratani Y, Matsuoka T, Hirata Y, Fukata N, Nagaoka Y, Uesato S. Influence of the carbamate fungicide benomyl on the gene expression and activity of aromatase in the human breast carcinoma cell line MCF-7. Environ Toxicol Pharmacol. 2015; 39:292–299 [DOI] [PubMed] [Google Scholar]
- 46.Lemaire G, Mnif W, Mauvais P, Balaguer P, Rahmani R. Activation of α- and β-estrogen receptors by persistent pesticides in reporter cell lines. Life Sci. 2006; 79:1160–1169 [DOI] [PubMed] [Google Scholar]
- 47.Fenton SE, Birnbaum LS. Timing of environmental exposures as a critical element in breast cancer risk. J Clin Endocrinol Metab. 2015; 100:3245–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blair A, Stewart P, Lubin JH, Forastiere F. Methodological issues regarding confounding and exposure misclassification in epidemiological studies of occupational exposures. Am J Ind Med. 2007; 50:199–207 [DOI] [PubMed] [Google Scholar]
- 49.Blair A, Thomas K, Coble J, et al. Impact of pesticide exposure misclassification on estimates of relative risks in the Agricultural Health Study. Occup Environ Med. 2011; 68:537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


