Supplemental Digital Content is available in the text.
Keywords: Per- and poly-fluoroalkyl substances (PFAS), Bone health, Bone mineral density, Mixture, Bayesian weighted quantile sum regression
Background:
Per- and poly-fluoroalkyl substances (PFAS) are chemicals, detected in 95% of Americans, that induce osteotoxicity and modulate hormones, thereby influencing bone health. Previous studies found associations between individual PFAS and bone mineral density in adults but did not analyze their combined effects.
Objective:
To extend weighted quantile sum (WQS) regression to a Bayesian framework (Bayesian extension of the WQS regression [BWQS]) and determine the association between a mixture of serum PFAS and mineral density in lumbar spine, total, and neck femur in 499 adults from the 2013 to 2014 National Health and Nutrition Examination Survey (NHANES).
Methods:
We used BWQS to assess the combined association of eight PFAS, as a mixture, with bone mineral density in adults. As secondary analyses, we focused on vulnerable populations (men over 50 years and postmenopausal women). Analyses were adjusted for sociodemographic factors. Sensitivity analyses included bone mineral density associations with individual compounds and results from WQS regressions.
Results:
The mean age was 55 years old (SD = 1) with average spine, total, and neck femur mineral densities of 1.01 (SD = 0.01), 0.95 (SD = 0.01), and 0.78 (SD = 0.01) gm/cm2, respectively. PFAS mixture levels showed no evidence of association with mineral density (spine: β = −0.004; 95% credible interval [CrI] = −0.04, 0.04; total femur: β = 0.002; 95% CrI = −0.04, 0.05; femur neck: β = 0.005; 95%CrI = −0.03, 0.04) in the overall population. Results were also null in vulnerable populations. Findings were consistent across sensitivity analyses.
Conclusions:
We introduced a Bayesian extension of WQS and found no evidence of the association between PFAS mixture and bone mineral density.
What this study adds.
We introduced a novel Bayesian extension of weighted quantile sum (WQS) regression, reducing assumptions and limitations of the classical approach. We then applied this method to assess the combined association of several forms of serum per- and poly-fluoroalkyl substances (PFAS) as a mixture with bone mineral density in 499 US adults from the 2013 to 2014 cycle of the National Health and Nutrition Examination Survey (NHANES).
Introduction
As the population ages, low bone mineral density has emerged as a public health concern because it is related to fractures, morbidities, hospitalizations, and premature mortality.1,2 Deterioration of bone mass differs between the sexes, with a higher prevalence in women. About 10% of women over 50 years of age suffer from low bone mineral density, but only 2% of men of the same age have similar bone deterioration.3 Traditional risk factors associated with decreased bone mineral density include chronological age, family history of bone disease, suboptimal high-impact physical activity, and smoking.4 However, recent evidence suggests that environmental exposures, including air pollution, lead, cadmium, and mercury, are associated with lower bone mineral density and higher risks for osteoporosis.5–8
Per- and poly-fluoroalkyl substances (PFAS) are synthetic organic chemicals that have been used extensively in industrial processes and commercial applications since the 1950s. These chemicals are widely used and persist for long periods of time, resulting in increased levels of environmental contamination.9,10 The primary sources of human PFAS exposure include migration from food packaging and cookware, drinking water, indoor air, and house dust.11 PFAS have been detected in vivo in human tissue samples12,13 in 95% of the US population.14 PFAS are poorly metabolized and excreted slowly from the human body, with half-lives of 4–8 years.15 Previous human and animal studies showed that PFAS bioaccumulate in bones, with perfluorooctanoate (PFOA) being predominant.16,17 Due to their limited susceptibility to degradation and slow elimination by bodies, human exposure to PFAS is of increasing concern.18,19
The toxicity of PFAS to bones has been reported in human and animal studies, with high PFAS concentrations associated with adverse skeletal outcomes, suggesting that bones are target tissues for PFAS toxicity.20 In rodents, exposure to PFAS was negatively associated with bone structure and biomechanical properties.21 Bone cell cultures showed increased bone resorption activity at low, albeit environmentally relevant, PFAS concentrations in human bone marrow and peripheral blood–derived osteoclasts, through the effect of PFAS on the cytokine and clastokine profiles during cell differentiation.20 In human studies, bone PFAS concentrations and relative bone volume have been correlated, but results are still inconclusive. In a few cross-sectional studies, serum levels of a few PFAS were negatively associated with bone mineral density only in women,22,23 but findings for most compounds are null in the general US population.22 In addition, all previous studies in adult populations focused on the association between individual PFAS and bone health outcomes; however, given their ubiquity and persistence, exposure likely occurs to many PFAS simultaneously. Those studies failed to account for the correlation structure among PFAS or to consider PFAS exposure as a mixture. Statistical approaches, taking into account mixtures, estimate the overall association of all combined exposures with the health outcome, incorporating the correlation structure among exposures, thereby limiting both collinearity effects and standard-error inflation.24
Environmental health studies have applied the weighted quantile sum (WQS) regression to assess the mixture effect of multiple co-occurring exposures and to identify the driving exposures in the mixture. Briefly, the WQS regression summarizes the overall exposure to the mixture by estimating a single weighted index and accounts for the individual contribution of each component of the mixture by using weights.25,26 The WQS regression splits the dataset into two subsets, a training set (generally 40%), and a validation set (the remaining 60%). In the training set, this approach estimates the weights in an ensemble step averaging results across bootstrap samples, and in the validation subset, it estimates the coefficient mapped to the mixture, conditionally to the estimated weights.25–27 This internal random-split validation technique reduces the statistical power of the WQS regression28 and may lead to unstable estimates.29 The WQS regression also requires a priori selection of the directionality (positive or negative) of the coefficient associated with the mixture, and it conditions on the weights in the weighted index for testing for significance using the holdout validation set. As such, it does not provide diagnostics (confidence intervals and P values) about the weights of the mixture components.25,26
Here, we proposed a novel Bayesian extension of the WQS regression (BWQS) to overcome its limitations and to illustrate the method for assessing the combined association of several forms of serum PFAS as a mixture with bone mineral density in 499 US adults from the 2013 to 2014 cycle of the National Health and Nutrition Examination Survey (NHANES).3,30 We also stratified our analysis for two vulnerable populations, men over 50 years of age and postmenopausal women. This novel approach estimates diagnostic statistics for all estimated parameters, without requiring a priori selection of the directionality of the coefficient associated with the mixture and it does not perform any splitting of the original dataset, thus improving statistical power and stability of the estimates. Here we described the BWQS approach supported by examples with synthetic data.
Methods
Study population
The NHANES is an ongoing survey of the noninstitutionalized US adult population designed to assess their health and nutritional status.30 After providing informed consent, participants visited a mobile examination center for standardized physical examination and collection of biological specimens, which were used to assess exposure to environmental chemicals. All study protocols were approved by the National Center for Health Statistics research ethics review board.3,30 In our analyses, we included the 2013–2014 NHANES cycle, in which both bone mineral density and serum PFAS concentrations were measured and had not been previously studied. The 2013–2014 cycle also included four (linear and branched) PFAS isomers that were not measured in any previous NHANES cycles. For both evaluations, the selection of NHANES participants was random and designed to maintain the original NHANES characteristics, as previously described.14,31 We excluded NHANES participants with missing information about bone mineral measurements (N = 7,060) or serum concentrations of PFAS (N = 1,450) and those with missing information on covariates (smoking and physical activity) or with bilateral oophorectomy (N = 74). A total of 499 adults (≥40 years) was included in the main analysis. Secondary analyses were performed on 115 men over 50 years and 117 postmenopausal women. Postmenopausal women included women over 60 years old or women who had not had a menstrual period in the previous 12 months. We excluded from the analysis postmenopausal women using hormone replacement treatment or taking parathyroid medication (N = 45) due to their influence on the endocrine system (Figure 1).
Figure 1.
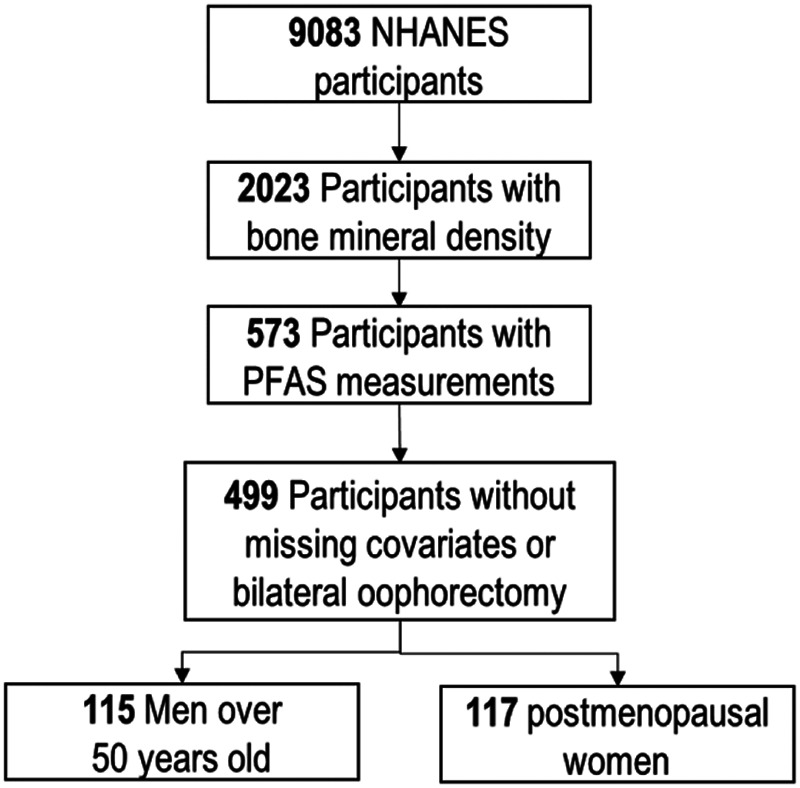
Selection of the National Health and Nutrition Examination Survey (NHANES) participants.
Bone mineral density assessment
Bone mineral density (g/cm2) was measured using dual X-ray absorptiometry (Hologic QDR 4500A fan-beam densitometers; Hologic Inc., Bedford, MA).3 Antero-posterior lumbar spine mineral density was scanned, and mean density was computed for the first through fourth lumbar vertebrae. For the total and neck femur mineral density, the left hip was routinely scanned. If a left-hip replacement or metal objects in the left leg were reported, the right hip was scanned. Participants were excluded from the femur scan if they had bilateral hip fractures, bilateral hip replacements, or pins. Participants weighing > 300 lbs (136 kg) or pregnant women (defined by self-report or positive urine pregnancy test) were ineligible for the examination. Femur neck has been proposed as the reference skeletal site for defining osteoporosis in epidemiological studies,32 whereas the total femur had been used as a benchmark for osteoporosis in the national Healthy People program.32 Each subject’s scan was reviewed in the Department of Radiology, University of California, using standard radiological techniques and NHANES protocols.3
Serum PFAS measurements
Analysis of 12 PFAS in serum was conducted at the National Center for Environmental Health in a random one-third subsample of nonfasting participants; the NHANES characteristic proportions were maintained.14,31 Briefly, serum PFAS were measured using automated solid-phase extraction coupled to isotope-dilution high-performance liquid chromatography– tandem mass spectrometry.14 The complete list of PFAS with their acronyms is included in eTable 1; http://links.lww.com/EE/A83. In our analyses, we excluded four PFAS because their concentrations were below the limit of detection for more than 60% of samples (eTable 1; http://links.lww.com/EE/A83). For the remaining PFAS, when concentrations were less than the limit of detection, a value equal to the limit of detection divided by the square root of two was used in the analyses.
Statistical methods and analyses
The Bayesian WQS (BQWS) regression
Let the values for the correlated mixture components C be scored into quantiles (qji) for the j-th component (j = 1,..,C) and the i-th (i = 1,…,N) participant. We modeled the association between the overall mixture and the outcome Y using a generalized linear model framework
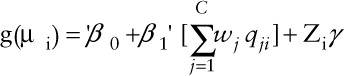 |
where µi = E(yi), β0 is the intercept, and β1 is the coefficient mapped to the 'weighted index (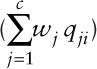 ); g(.) is link function; and γ is a vector of K coefficients mapped to the matrix of K covariates Zi. Similar to WQS regression, we modeled the weight wj for exposure to the j-th component as an arbitrary function, taking values between 0 and 1, and the sum of all mixture weights to be equal to 1.
); g(.) is link function; and γ is a vector of K coefficients mapped to the matrix of K covariates Zi. Similar to WQS regression, we modeled the weight wj for exposure to the j-th component as an arbitrary function, taking values between 0 and 1, and the sum of all mixture weights to be equal to 1.
The BWQS regression required specification of the link function, similar to the frequentist approach, and prior probability distributions on all parameters, differently from WQS regression. The general assumption for the distribution of each parameter was an uninformative prior, which is defined as a probability density function with no information about the initial distribution, mean, and SD of each parameter. Weak or uninformative priors are generally represented with Normal distributions centered in 0 and with a large variance. This implies that the estimate of the mixture-outcome association can assume all values in the domain of positive and negative real numbers, without selecting a priori the directionality of the association. Weakly and uninformative priors are recommended when we have limited, inconsistent or no information about the mixture-outcome relationship, such as in our case with PFAS mixture and bone mineral density. Informative priors can be chosen and embedded in the model when prior information about the mixture-outcome association is known. The BWQS regression computes all posterior probability distributions leveraging a Hamiltonian-Monte Carlo algorithm, which is among the most efficient algorithms for reducing correlation between sample states and thus removing instability of the estimates.33,34
The link function assumed the following forms:
(1) logit link function: yi ~ binomial (1, µi), when Y was binary;
(2) identity link function: yi~Normal(µi, σ2), with an uninformative prior for σ2~IGamma(0.1,0.1), when Y was continuous.
Priors for the coefficients were uninformative and were summarized by normal distributions with large variance [β0;β1~ Normal(0, 100); γk~Normal(0, 100) for each k = 1,...,K]. Priors for the weights were modeled as a unit-simplex in order to have non-negative weights (wj Є [0,1]) and to sum all weights to one (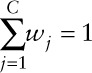 ). The natural choice was the Dirichlet distribution with the same density on each vertex for each component of the mixture; i.e., w = (w1, …, wC) ~ Dirichlet(α = [α1,...,αC]). The α parameter can be selected a priori equal to 1, when investigating the entire domain of the distribution uniformly.
). The natural choice was the Dirichlet distribution with the same density on each vertex for each component of the mixture; i.e., w = (w1, …, wC) ~ Dirichlet(α = [α1,...,αC]). The α parameter can be selected a priori equal to 1, when investigating the entire domain of the distribution uniformly.
This BWQS approach can be useful when there is no information about the mixture-outcome association because it does not select a priori the directionality of that association. The estimated coefficient mapped to the mixture identifies the association between the overall mixture and the outcome, whereas the estimated coefficients mapped to the weights identify the relative contribution of the corresponding components to the mixture. Results on simulated datasets, showing the accuracy of the estimates of BWQS, are included in the supplemental material (eFigure 8; http://links.lww.com/EE/A83-S10; http://links.lww.com/EE/A83).
Statistical Analysis
We estimated the correlations among PFAS and then performed BWQS regression in which bone mineral density (continuous)—in lumbar spine, total femur, and femur neck—was associated with mixtures of PFAS. All analyses vwere adjusted for race/ethnicity (white, black, Hispanic and other races), age (continuous), sex, physical activity (low-moderate or high), poverty-income ratio (continuous), and smoking status (never or ever smoked). All variables were selected based on previous association with the outcome.6,7,23 Priors for all coefficients were uninformative: Normal(0,100). Secondary analyses focused on two vulnerable populations—men over 50 years old and postmenopausal women.
As sensitivity analyses, we assessed the associations between the PFAS mixture and mineral density in lumbar spine, total femur, and femur neck by using the frequentist WQS regression, for the overall population. In supplemental material; http://links.lww.com/EE/A83, we provided the R2 for both BWQS and WQS regressions. We also used Bayesian linear regression to determine the association of each PFAS compound with bone mineral density in the overall population. We combined isomers from the same families (i.e., perfluorooctane sulfonate [PFOS]) to compare our results with previous findings. We also evaluated how the associations changed, including PFAS detected in at least 60% of samples, excluding women on hormonal replacement therapy and adjusting for the intake of calcium, and vitamins (K and D). We applied the BWQS regression on postmenopausal women from the 2009 to 2010 NHANES cycle. All analyses were weighted according to the NHANES weights, appropriately rescaled for the selected subsample, as described on the Centers for Disease Control and Prevention web site and in previous studies.35,36 We used R version 3.5.1 for all analyses.
Results
Study population characteristics
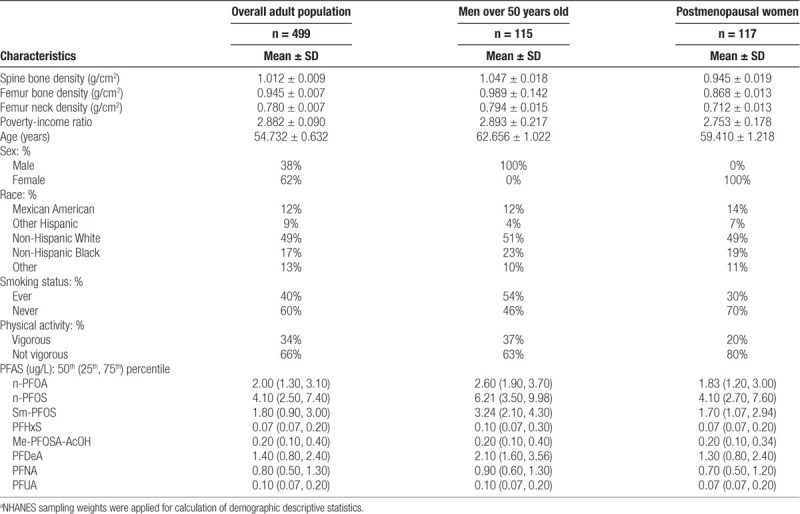
Adults included in our main analysis were 55 years old on average (SD: 0.6), mostly Caucasian (49%), never smoked (60%), reported physical activity below the optimal level (66%), and had an average poverty-income ratio of 2.9 (SD: 0.9) (Table). On average, spine and (total and neck) femur mineral densities were 1.01 (SD: 0.01), 0.95 (SD: 0.01), and 0.78 (SD: 0.01) g/cm2, respectively. Bone mineral density was different by sex, with postmenopausal women having lower density (P<0.05). In all groups, serum concentrations of the linear and branched PFAS isomers (linear-PFOA [n-PFOA], linear-perfluorooctane sulfonate [n-PFOS], and monomethyl branched isomers of PFOS [Sm-PFOS]) were higher than all other PFAS levels (perfluorohexane sulfonic acid [PFHxS], perfluorononanoic acid [PFNA], pefluorodecanoic acid, 2-(N-methyl-PFOSA) acetic acid, and perfluoroundecanoic acid) (Table). All serum PFAS concentrations were positively correlated with each other and showed similar patterns across populations (Figure 2, eFigure 1; http://links.lww.com/EE/A83).
Figure 2.
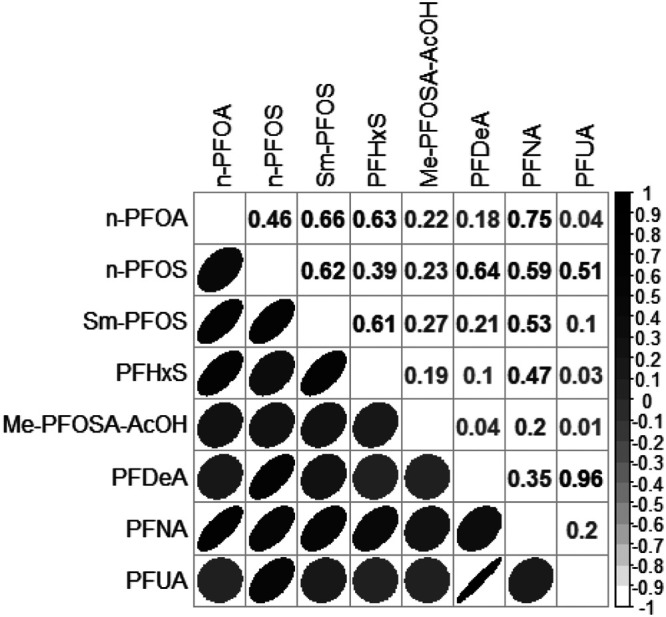
Correlation between serum PFAS in the overall adult population. Color and shape of each ellipse reflect the correlation between two compounds.
Table.
Characteristics for the overall adult population, men over 50 years old, and postmenopausal women.a
BWQS regression characteristics
We employed the BWQS regression to identify the association between the mixture of PFAS in subjects’ serum and bone mineral density in spine, total and neck femur, in all adults together and in the most vulnerable populations separately.
PFAS concentrations were categorized into quartiles, and we set the main parameters of the Hamiltonian-Monte Carlo chain to optimize the accuracy and speed of the models. In total, we set 1,000 iterations, of which 500 were burn-in and 3 were thinned. All parameters showed no autocorrelation between subsequent iterations, and had a potential scale-reduction statistic (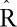 ) approximately equal to 1, thus indicating optimal convergence of the chain (eTable 2; http://links.lww.com/EE/A83). The potential scale-reduction statistic (
) approximately equal to 1, thus indicating optimal convergence of the chain (eTable 2; http://links.lww.com/EE/A83). The potential scale-reduction statistic (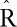 ) indicates the goodness of convergence of the Hamiltonian chain. Values approximately equal to 1 represent optimal convergence of the chain to the equilibrium distribution.
) indicates the goodness of convergence of the Hamiltonian chain. Values approximately equal to 1 represent optimal convergence of the chain to the equilibrium distribution.
Results in the overall adult population
In the overall adult population there was no evidence of association between the PFAS mixture and bone mineral density in lumbar spine (β = −0.004, 95% credible interval [CrI] = −0.04, 0.04), total femur (β = 0.002, 95% CrI = −0.04, 0.05), or femur neck (β = 0.005, 95% CrI = −0.03, 0.04). Components of the mixture contributed approximately equally to the mixture in the outcomes for the overall population (Figures 3; eTable 2; http://links.lww.com/EE/A83).
Figure 3.
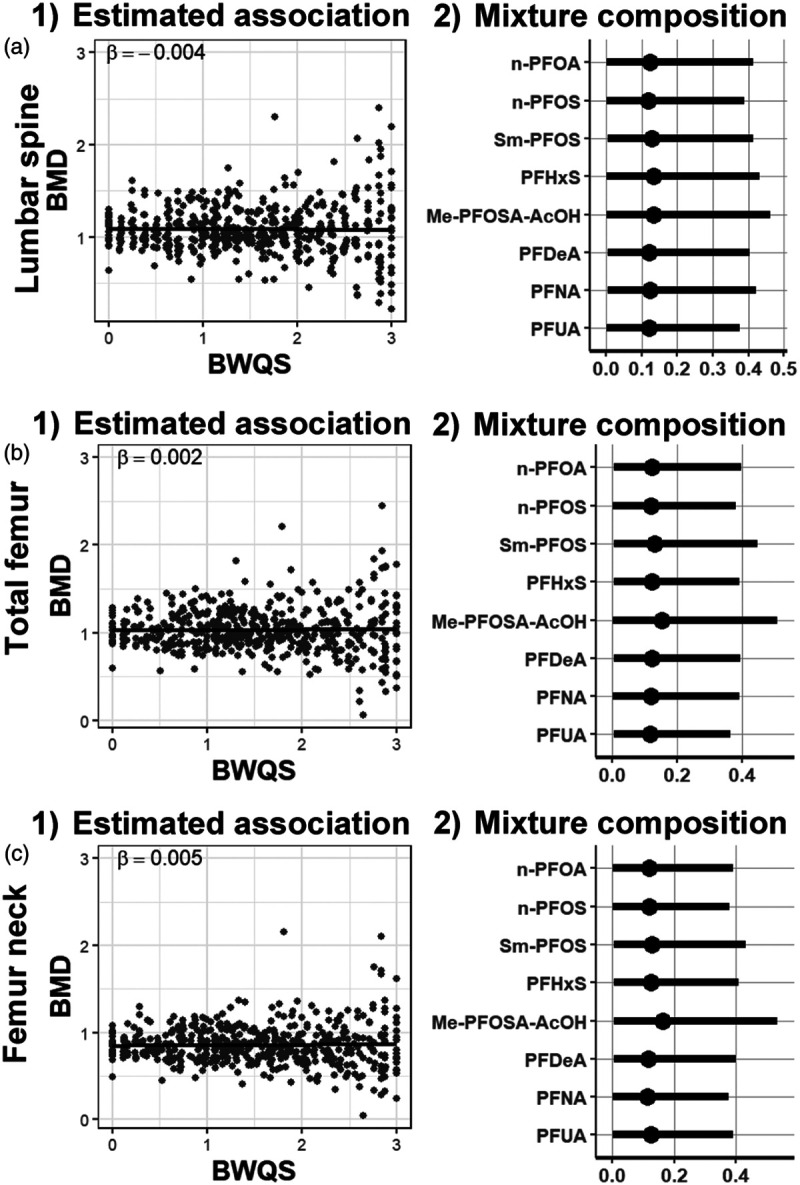
Association between PFAS mixture and bone mineral density. (1) Estimates of the association between mineral density and the PFAS mixture in the overall population and (2) mixture composition estimates: weights (percentage rescaled between 0 to 1) with 95% credible intervals for each mixture component in (A) lumbar spine, (B) total femur, and (C) femur neck. Bone mineral density (BMD) adjusted for race/ethnicity, age, sex, physical activity, poverty-income ratio, and smoking status.
Sensitivity analyses in the overall adult population
Results from sensitivity analyses using the frequentist WQS approach, assuming a negative direction between PFAS mixture and bone mineral density, were similar to those of BWQS. We found no associations between the PFAS mixture and all outcomes (lumbar spine: β = −0.01, P = 0.69; total femur: β = −0.01, P = 0.51; femur neck β = −0.004, P = 0.82) using a validation set of 70% (eFigure 2; http://links.lww.com/EE/A83, eTable 3; http://links.lww.com/EE/A83). Results from both BWQS and WQS regressions showed similar R2 (eTable 4; http://links.lww.com/EE/A83). Individual PFAS analyses showed a weak negative association among Sm-PFOS and all bones (lumbar spine: β = −0.02, 95% CrI = −0.03, −0.009; total femur: β = -0.01, 95% CrI: −0.02, −0.003; and femur neck β = −0.01, 95% CrI: −0.02, −0.004), whereas PFNA was positively associated with total femur (β = 0.04, 95% CrI: 0.01, 0.06) and neck femur (β = 0.03, 95% CrI: 0.01, 0.05) (eFigure 3; http://links.lww.com/EE/A83, eTable 5; http://links.lww.com/EE/A83). However, those associations did not persist after correcting for multiple comparisons (data not shown). We included total PFOS levels in the PFAS mixture and evaluated their role with bone mineral density. Branched PFOA levels were missing for over 80% of the samples (eTable 1; http://links.lww.com/EE/A83); therefore, we did not include the total PFOA levels in the analysis. Results were consistent with the main findings when we combined isomers (eTable 6; http://links.lww.com/EE/A83), when we included PFAS detected in at least 60% of samples (eFigure 6; http://links.lww.com/EE/A83), and when we excluded women on hormonal replacement therapy and when we adjusted for intake of calcium, and vitamins (K and D) (eFigure 7; http://links.lww.com/EE/A83). Results on postmenopausal women from 2009 to 2010 NHANES cycle showed negative directions between PFAS mixture exposure and both total and neck femur with PFOS having the strongest contribution to the mixture (eTable 7; http://links.lww.com/EE/A83).
Results in men over 50 years old and in postmenopausal women
There was no evidence of an association between the PFAS mixture and bone mineral densities in men over 50 years old (lumbar spine: β = 0.01, 95% CrI = −0.05; 0.08; total femur β = 0.01, 95% CrI = −0.04, 0.07; and femur neck β = 0.02, 95% CrI = −0.04, 0.07) or in postmenopausal women (lumbar spine β = 0.00, 95% CrI = −0.08, 0.08; total femur β = 0.02, 95% CrI: −0.04, 0.08; and femur neck β = 0.02, 95% CrI = −0.03, 0.08). The contribution of all mixture components was similar across bones and across populations (eTable 2; http://links.lww.com/EE/A83; eFigure 4; http://links.lww.com/EE/A83-S5; http://links.lww.com/EE/A83).
Discussion
We extended the WQS regression under a Bayesian framework and determined the combined association of eight PFAS with bone mineral density in lumbar spine, total, and neck femur in a survey representative US adult population in the years 2013–2014. The BWQS regression provides diagnostic statistics for all estimated parameters; it also has the advantages of inferring the estimates using the whole dataset and avoiding an a priori selection of the directionality of the coefficient associated with the mixture. The application of our novel method showed no evidence of the association between serum concentration of PFAS mixture—composed of linear and branched PFOA and PFOS isomers (n-PFOA, n-PFOS, and Sm-PFOS), PFHxS, PFNA, pefluorodecanoic acid, 2-(N-methyl-PFOSA) acetic acid, and perfluoroundecanoic acid—and bone mineral density in lumbar spine, total and neck femur. The contribution of each compound was similar across bones. Results were also consistent using the frequentist WQS and Bayesian linear regressions. Both mixture models performed similarly. We also found no evidence of an association between the PFAS mixture and bone mineral density in men over 50 years old and postmenopausal women.
Our results confirmed prior findings showing null associations between PFAS exposure and bone health in the overall adult US population.22 Indeed, serum concentrations of individual PFAS were not associated with total femur mineral density and bone fractures in the overall adult population from the 2005 to 2008 NHANES cycle.22 Previous findings showed that most significant associations between individual PFAS compounds and bone mineral density were limited to women, with higher concentrations of PFOA, PFOS, PFHxS, and PFNA associated with lower bone mineral density and with higher risk osteoporosis.22,23 However, our results on vulnerable populations could have been limited by a small number of participants (n = 115 men of 50 years and n = 117 postmenopausal women). A prior study investigated the role of PFAS mixture exposure on total-body bone mineral density in childhood by leveraging a large cohort in the Boston area.37 The authors showed that the mixture of six PFAS (PFOA, PFOS, PFSA, PFHxS, Me-PFOSA-AcOH, PFNA) impaired bone accrual in childhood.37 However, our results were not directly comparable to this study, due to differences in the outcome and age between populations, suggesting that further investigations about the role of PFAS in both elderly and childhood populations are needed.
Prior epidemiological studies support the hypothesis that PFAS are endocrine disruptors. Although the PFAS mixture showed no direct impact of bone health in our results, the mixture might affect bone mineral density via hormonal alterations. Indeed, PFAS modulate thyroid and sex hormone concentrations, which play a critical role in bone remodeling and health.38 Chronic PFAS exposure was associated with suppressed serum thyroxine and triiodothyronine (T3) levels in human studies39,40 and with altered responses to T3 in a T3-dependent cell line in vitro.41 A cross-sectional study of adult NHANES participants40 reported that serum PFHxS was positively associated with subclinical hyperthyroidism in women, which is a risk factor for decreased bone mass.42 PFAS also interfere directly with estrogen and androgen receptors, disrupting the biological effects of sex hormones and leading to reduced fecundity in women and delayed puberty in boys, both of which are associated with lower bone mineral density later in life.43,44 In studies of women’s health, PFAS were associated with decreased production of estradiol and progesterone, which are essential hormones for bone health due to their promotion of osteoblast activity. Based on this body of literature, the PFAS mixture, mediated by endocrine receptors, might affect bone health, but further studies with longitudinal design are required to address this problem.
In our analyses PFOA and PFOS, both of which were previously associated with bone mineral density in women,22,23 were analyzed using their linear and branched isomers, and we could not confirm previous results for both those compounds. In sensitivity analyses, we evaluated only the combination of PFOS isomers in the mixture, and results were consistent with the main findings. Due to the cross-sectional design of our study, we could not rule out whether it was reverse causation and bone health or hormonal alterations preceded the response to exposure.45 Indeed, Serum levels of some PFAS have been shown to change due to an altered hormonal excretion occurring in menopause.46 These PFAS changes were persistent for seven years after menopause, providing evidence that a reverse causation relationship may exist between serum PFAS levels and menopause.46 It is also possible that our results may have been limited by the smaller number of participants than other studies showing significant associations between PFAS exposure and bone mineral density.22,23 Also, participants of this study were not asked whether they attempted to prevent bone deterioration by changes in lifestyle, such as using supplements or alternative treatments or eating a healthier diet. Further studies with a longitudinal design, larger sample size and more information about lifestyle changes could help to disentangle the underlying mechanism linking PFAS exposure and bone health in older adults.
The strengths of our study include a novel statistical approach, which accounted for the correlation among co-occurring PFAS and provided information about the overall adverse associations of PFAS and bone mineral density. We used a sample that is known to represent the US population in the years 2013–2014, and we relied on PFAS concentrations and bone mineral density levels that were validated and compared across NHANES cycles.
Conclusions
This is the first study to assess the relationship between exposure to a mixture of PFAS and bone mineral density at three bone sites in adults. The novel Bayesian WQS approach identified both the overall association between PFAS mixture with bone mineral density and the contribution of each PFAS to the mixture. The serum PFAS mixture showed no association with mineral density of the lumbar spine, total and neck femur in the NHANES adult population in the years 2013–2014.
Conflict of interest statement
The authors declare that they have no conflicts of interest with regard to the content of this report.
Supplementary Material
Footnotes
Published online 30 April 2020
This research was supported by grants P30ES023515, 2U2CES026555-02, UH3OD023337-04 of the National Institute of Environmental Health Sciences of the National Institutes of Health. The authors would like to thank Dr. Christine Schubert Kabban for helpful comments.
Data are available on the Centers for Disease Control and Prevention (CDC)’s web site (National Health and Nutrition Examination Survey cycle 2013–2014). Code for the statistical method and toy examples is available in the “BWQS” R-package.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
References
- 1.Johnell O, Kanis J, Gullberg G. Mortality, morbidity, and assessment of fracture risk in male osteoporosis. Calcif Tissue Int. 2001; 69:182–184 [DOI] [PubMed] [Google Scholar]
- 2.Browner WS, Lui LY, Cummings SR. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clinical Endocrinol Metab. 2001;86:631–637 [DOI] [PubMed] [Google Scholar]
- 3.Looker AC, Melton LJ, 3rd, Harris TB, Borrud LG, Shepherd JA. Prevalence and trends in low femur bone density among older US adults: NHANES 2005-2006 compared with NHANES III. J Bone Miner Res. 2010; 25:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver CM, Gordon CM, Janz KF, et al. The national osteoporosis foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016; 27:1281–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engström A, Michaëlsson K, Suwazono Y, Wolk A, Vahter M, Akesson A. Long-term cadmium exposure and the association with bone mineral density and fractures in a population-based study among women. J Bone Miner Res. 2011; 26:486–495 [DOI] [PubMed] [Google Scholar]
- 6.Khalil N, Cauley JA, Wilson JW, et al. Relationship of blood lead levels to incident nonspine fractures and falls in older women: the study of osteoporotic fractures. J Bone Miner Res. 2008; 23:1417–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollack AZ, Mumford SL, Wactawski-Wende J, et al. Bone mineral density and blood metals in premenopausal women. Environ Res. 2013; 120:76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prada D, Zhong J, Colicino E, et al. Association of air particulate pollution with bone loss over time and bone fracture risk: analysis of data from two independent studies. Lancet Planet Health. 2017; 1:e337–e347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol.. 2006; 40:32–44 [DOI] [PubMed] [Google Scholar]
- 10.Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: past, present, and future. Environ Sci Technol. 2011; 45:7954–7961 [DOI] [PubMed] [Google Scholar]
- 11.Miralles-Marco A, Harrad S. Perfluorooctane sulfonate: a review of human exposure, biomonitoring and the environmental forensics utility of its chirality and isomer distribution. Environ Int. 2015; 77:148–159 [DOI] [PubMed] [Google Scholar]
- 12.De Silva AO, Mabury SA. Isomer distribution of perfluorocarboxylates in human blood: potential correlation to source. Environ Sci Technol. 2006; 40:2903–2909 [DOI] [PubMed] [Google Scholar]
- 13.Llorca M, Pérez F, Farré M, Agramunt S, Kogevinas M, Barceló D. Analysis of perfluoroalkyl substances in cord blood by turbulent flow chromatography coupled to tandem mass spectrometry. Sci Total Environ. 2012; 433:151–160 [DOI] [PubMed] [Google Scholar]
- 14.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ Health Perspect. 2007; 115:1596–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999-2008. Environ Sci Technol. 2011; 45:8037–8045 [DOI] [PubMed] [Google Scholar]
- 16.Pérez F, Nadal M, Navarro-Ortega A, et al. Accumulation of perfluoroalkyl substances in human tissues. Environ Int. 2013; 59:354–362 [DOI] [PubMed] [Google Scholar]
- 17.Bogdanska J, Borg D, Sundström M, et al. Tissue distribution of ³5S-labelled perfluorooctane sulfonate in adult mice after oral exposure to a low environmentally relevant dose or a high experimental dose. Toxicology. 2011; 284:54–62 [DOI] [PubMed] [Google Scholar]
- 18.Butenhoff JL, Gaylor DW, Moore JA, et al. Characterization of risk for general population exposure to perfluorooctanoate. Regul Toxicol Pharmacol.. 2004; 39:363–380 [DOI] [PubMed] [Google Scholar]
- 19.Kennedy GL, Jr, Butenhoff JL, Olsen GW, et al. The toxicology of perfluorooctanoate. Crit Rev Toxicol. 2004; 34:351–384 [DOI] [PubMed] [Google Scholar]
- 20.Koskela A, Koponen J, Lehenkari P, Viluksela M, Korkalainen M, Tuukkanen J. Perfluoroalkyl substances in human bone: concentrations in bones and effects on bone cell differentiation. Sci Rep. 2017; 7:6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thibodeaux JR, Hanson RG, Rogers JM, et al. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicol Sci. 2003; 74:369–381 [DOI] [PubMed] [Google Scholar]
- 22.Lin LY, Wen LL, Su TC, Chen PC, Lin CY. Negative association between serum perfluorooctane sulfate concentration and bone mineral density in US premenopausal women: NHANES, 2005-2008. J Clin Endocrinol Metab. 2014; 99:2173–2180 [DOI] [PubMed] [Google Scholar]
- 23.Khalil N, Chen A, Lee M, et al. Association of perfluoroalkyl substances, bone mineral density, and Osteoporosis in the U.S. Population in NHANES 2009-2010. Environ Health Perspect. 2016; 124:81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson EA, Nunez Y, Abuawad A, et al. An overview of methods to address distinct research questions on environmental mixtures: an application to persistent organic pollutants and leukocyte telomere length. Environ Health. 2019; 18:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czarnota J, Gennings C, Wheeler DC. Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk. Cancer Inform. 2015; 14suppl 2159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat. 2015; 20:100–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellavia A, James-Todd T, Williams PL. Approaches for incorporating environmental mixtures as mediators in mediation analysis. Environ Int. 2019; 123:368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steyerberg EW, Harrell FE, Jr. Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol. 2016; 69:245–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanner EM, Bornehag CG, Gennings C. Repeated holdout validation for weighted quantile sum regression. MethodsX. 2019; 6:2855–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention; National Center for Health Statistics; National Center for Health Statistics. National Health and Nutrition Examination Survey. Laboratory Procedure Manual: Creatinine. 2015–2016, Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention [Google Scholar]
- 31.Archer E, Hand GA, Blair SN. Validity of U.S. nutritional surveillance:national health and nutrition examination survey caloric energy intake data, 1971-2010. PLoS One. 2013; 8:e76632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, 3rd, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008; 42:467–475 [DOI] [PubMed] [Google Scholar]
- 33.Colicino E, Foppa Pedretti N, Busgang S, Gennings C. Per- and poly-fluoroalkyl substances and bone mineral density: results from the Bayesian weighted quantile sum regression. medRxiv. 2 November 201919010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks S, Gelman A, Jones G, Meng XL. Handbook of Markov Chain Monte Carlo. 2011, Boca Raton, FL: Chapman & Hall/CRC [Google Scholar]
- 35.Heeringa S, West BT, Berglund PA. Applied Survey Data Analysis. 20172nd ed, Boca Raton, FL: CRC Press, Taylor & Francis Group [Google Scholar]
- 36.Malin AJ, Lesseur C, Busgang SA, Curtin P, Wright RO, Sanders AP. Fluoride exposure and kidney and liver function among adolescents in the United States: NHANES, 2013-2016. Environ Int. 2019; 132:105012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cluett R, Seshasayee SM, Rokoff LB, et al. Per- and polyfluoroalkyl substance plasma concentrations and bone mineral density in midchildhood: a cross-sectional Study (Project Viva, United States). Environ Health Perspect. 2019; 127:87006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JS, Buzková P, Fink HA, et al. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med. 2010; 170:1876–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knox SS, Jackson T, Frisbee SJ, Javins B, Ducatman AM. Perfluorocarbon exposure, gender and thyroid function in the C8 Health Project. J Toxicol Sci. 2011; 36:403–410 [DOI] [PubMed] [Google Scholar]
- 40.Wen LL, Lin LY, Su TC, Chen PC, Lin CY. Association between serum perfluorinated chemicals and thyroid function in U.S. adults: the National Health and Nutrition Examination Survey 2007-2010. J Clin Endocrinol Metabolism. 2013; 98:E1456–1464 [DOI] [PubMed] [Google Scholar]
- 41.Long M, Ghisari M, Bonefeld-Jørgensen EC. Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor. Environ Sci Pollut Res Int. 2013; 20:8045–8056 [DOI] [PubMed] [Google Scholar]
- 42.El Hadidy el HM, Ghonaim M, El Gawad SSh, El Atta MA. Impact of severity, duration, and etiology of hyperthyroidism on bone turnover markers and bone mineral density in men. BMC Endocr Disord. 2011; 11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang B, Zhang R, Jin F, et al. Perfluoroalkyl substances and endometriosis-related infertility in Chinese women. Environ Int. 2017; 102:207–212 [DOI] [PubMed] [Google Scholar]
- 44.Ernst A, Brix N, Lauridsen LLB, et al. Exposure to perfluoroalkyl substances during fetal life and pubertal development in boys and girls from the danish national birth cohort. Environ Health Perspect. 2019; 127:17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor KW, Hoffman K, Thayer KA, Daniels JL. Polyfluoroalkyl chemicals and menopause among women 20-65 years of age (NHANES). Environ Health Perspect. 2014; 122:145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K. A study of reverse causation: examining the associations of perfluorooctanoic acid serum levels with two outcomes. Environ Health Perspect. 2017; 125:416–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


