Abstract
Obesity has been linked to cognitive impairment, cognitive decline and dementia. Given that 38.5% of U.S. adults 60 years and older are obese and these numbers are rapidly increasing, strategies to decouple obesity from cognitive decline are needed. Innovative lifestyle strategies that may postpone the onset of subclinical symptoms or even arrest the transition to overt dementia in at-risk individuals are critically needed. Poor diet is central to the development of obesity and diet may affect cognition. Adherence to a Mediterranean Diet (MedDiet) is associated with reduced risk of cognitive impairment and dementia. Furthermore, weight loss through caloric restriction improves cognitive function. This paper describes the Building Research in Diet and CoGnition (BRIDGE) study, a randomized trial examining the effect of the MedDiet, with and without weight loss, on cognitive functioning in obese older adults. Obese (BMI ≥30 and ≤50 kg/m2) older adults (≥ 55 years) (n = 180) will be randomized in a 2:2:1 allocation scheme to: Typical Diet Control; MedDiet alone, without weight loss; or MedDiet lifestyle intervention to promote weight loss and weight loss maintenance. Both MedDiet intervention groups will meet for one individual session and 27 group sessions over an 8-month period. Individuals in the control group will be asked to maintain their current lifestyle. Outcomes will be assessed at baseline, 8 and 14 months. The primary outcome is cognitive functioning; secondary outcomes will include changes in body weight, diet, cardiovascular, metabolic, and inflammatory biomarkers.
Keywords: Cognition, Obesity, Weight Loss, Mediterranean Diet, Older Adults
1. Introduction
In the United States (U.S.), dementia affects approximately 10% of adults over 65 and 30% of adults over 80. (1, 2) Moreover, > 20% of older adults exhibit subclinical cognitive impairments (3) African Americans and Hispanics are more likely than non-Hispanic whites to suffer from cognitive impairment, dementia and Alzheimer’s disease (4-6) and are also disproportionately affected by obesity. (7) While the deleterious effects of obesity on cardiovascular (CVD) and metabolic disease risk are well-documented, (8) some preliminary evidence also suggests that obesity is independently linked to cognitive decline and dementia. (8, 9) Obesity may contribute independently to cognitive decline and dementia through metabolic, inflammatory and neuronal abnormalities. (10) Although, a recent systematic review questions the independence of this relationship given the methodologic limitations of the current literature, specifically failure to control for comorbidities that are known to influence cognition including CVD, type 2 diabetes, dyslipidemia, and depression. (10) Nonetheless, when these comorbidities are considered, there is modest evidence of an independent relationship between obesity and psychomotor performance and speed, visual construction, verbal memory, concept formation, and decision-making. Given the paucity of efficacious pharmacological treatments to prevent cognitive decline, (11) innovative lifestyle strategies that may postpone the onset of subclinical symptoms or even arrest the transition to overt dementia in at-risk individuals are critically needed. (12-14)
Lifestyle behaviors are central to the development and maintenance of obesity and may influence onset and progression of cognitive decline. (12) In cross-sectional and observational studies, the consumption of foods high in saturated fat and calories and physical inactivity (15, 16), increase the risk of cognitive decline. (17) Observational studies show that adhering to a Mediterranean Diet (MedDiet), characterized by high intake of vegetables, fruits, whole grains, nuts, legumes, unsaturated fatty acids, and modest alcohol consumption with meals, is associated with slower cognitive decline, improved cognitive function, and decreased risk for dementia, independent of body weight. (18) Furthermore, early evidence from the European PREDIMED intervention trial indicates that adoption and adherence to a MedDiet rich in olive oil or nuts is associated with better cognitive performance in older adults at vascular risk compared to a individuals randomized to a low-fat eating pattern. (19) Healthy dietary fats and antioxidant- and polyphenol-rich foods abundant in the MedDiet may work synergistically to improve cognitive function through improved glucose metabolism, reduced blood pressure and lipids, and lower systemic inflammation and oxidative stress. (19-21) Lifestyle interventions leading to weight loss may also improve functional capacity and cognitive function in obese individuals (22-24) through similar beneficial changes to lipid and glucose metabolism, inflammation, and oxidative stress. (25-27) Thus, we hypothesize that the combined effect of the MedDiet plus lifestyle intervention for weight loss will result in even greater improvements in metabolic, inflammatory, and other CVD risk factors, but also in cognitive functioning, compared to a MedDiet alone. However, to our knowledge, no randomized clinical trials (RCTs) in the U.S. have examined the effect of the MedDiet alone or combined with lifestyle intervention to promote weight loss on cognitive functioning in a racially and ethnically diverse sample of obese older adults.
This paper describes the design of the Building Research in Diet and CoGnition (BRIDGE) trial that will test whether a MedDiet with caloric restriction to promote weight loss (MedDiet-WL) produces better results than a MedDiet alone without caloric restriction (MedDiet-A) or Typical Diet Control condition on the primary outcomes of cognitive functioning, and secondary outcomes of weight control, dietary intake, cardiovascular, metabolic, and immune-related biomarkers within a sample of obese older primarily minority adults.
2. Methods
The BRIDGE trial was approved by the University of Illinois at Chicago (UIC) Institutional Review Board. All participants will provide informed written consent prior to study participation. The BRIDGE trial is registered at ClinicalTrials.gov (NCT03129048).
2.1. Study Design
This study is a three-arm RCT that will randomize in a 2:2:1 allocation scheme, 180 obese (BMI ≥ 30 kg/m2 and ≤ 50.0 kg/m2) older adults (55 - 85 years of age) to: 1) Typical Diet Control; 2) a MedDiet alone (MedDiet-A) lifestyle program; or 3) a MedDiet with lifestyle program to promote weight loss (MedDiet-WL). Both MedDiet lifestyle interventions are 8 months long and include one individual and 27 group sessions at community sites such as local park districts and senior centers. Following the 8-month intervention, there will be a 6-month minimal contact maintenance period. Participants randomized to the Typical Diet Control condition will not receive encouragement to change current lifestyle behaviors. Study outcomes will be assessed at baseline, 8 months, and 14 months. Figure 1 provides an overview of the study design.
Figure 1.
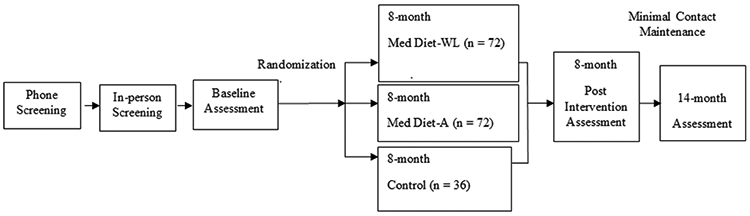
Overview of the Building Research in Diet and Cognition (BRIDGE) Trial Design
Abbreviations: MedDiet – A = Mediterranean Diet isocaloric lifestyle intervention; MedDiet-WL = Mediterranean Diet caloric restricted for intentional weight loss lifestyle intervention.
2.2. Setting and recruitment
We are partnering with the Chicago Park District (CPD) to conduct the BRIDGE trial. We will implement the trial in three waves with 60 people randomized per wave (24 participants per MedDiet intervention arm and 12 participants in the control arm) to enroll a total of 180 individuals. We plan to involve three CPD sites on the south, west, and north sides of Chicago.
Participants will be recruited through advertisements and research staff presentations at the CPD sites and City of Chicago Senior Centers located in close proximity to the CPD sites. The trial will also be advertised through paid print ads placed on Chicago public transit (i.e., buses and subway trains). We expect the full cohort to be 20% male and 80% female and approximately 50% African American, 25% Hispanic, and 25% Caucasian, which is representative of the racial/ethnic mix of older adults residing in and around the intervention site neighborhoods.
2.3. Participants
2.3.1. Phone screen for basic inclusion/exclusion criteria
Potential participants will first be screened for eligibility over the phone by research staff.
Inclusion criteria:
Men and women 55 - 85 years of age, BMI 30.0 – 50.0 kg/m2 based on self-report height and weight, willingness to: participate in measurements and intervention procedures including maintaining current dietary intake and level of physical activity; provide informed consent; and be randomized. Participants must also understand English and plan to reside in the Chicago area for the upcoming 14 months.
Exclusion criteria:
< 55 or > 85 years of age, BMI < 30.0 or > 50.0 kg/m2, significant health conditions including a diagnosis of dementia, renal disease, autoimmune disorders, immunodeficiency, severe pulmonary disease, bariatric surgery, neurological disease, schizophrenia, bipolar disorder, treatment for cancer in the previous 12 months, using Coumadin, illicit drug use, excessive alcohol intake defined as greater than 2 alcoholic drinks daily, > 450 lbs. due to the weight limitations of the body composition scanner, currently on a weight loss diet or enrolled in a formal weight loss program, or participating in memory/concentration research in the previous 12 months.
2.3.2. In-person eligibility screening visit
If a potential participant meets the initial eligibility criteria during the phone screen, he/she will be invited to an in-person eligibility screening visit at UIC. During this visit, BMI will be verified by measuring height with a fixed stadiometer and weight with an electronic scale. Individuals will then be screened using the Montreal Cognitive Assessment (MoCA), (28) a sensitive screen for cognitive impairment recommended by the National Institute of Neurological Diseases and Stroke and Canadian Stroke Network’s Neuropsychology working group. (29) Participants obtaining scores ≥19 will be included in an effort to increase the generalizability of the study and accrue participants with objectively assessed mild cognitive deficits (e.g., MoCA 19-26) who could potentially benefit most from the intervention. We will also assess current adherence to a MedDiet using a 13-item screener developed by Martinez-Gonzalez and colleagues (30) and adapted by our team for a U.S. population. Those scoring ≤6 points on the screener will qualify for study inclusion. Persons meeting the eligibility criteria for BMI, MoCA, and MedDiet adherence will be invited to enroll in the study and to undergo baseline assessments.
2.4. Interventions
2.4.1. Theoretical underpinnings
The Social Cognitive Theory (SCT) (31, 32) and Self-Determination Theory (SDT) (33, 34) were used to guide the development, intervention design, and measures selection. SCT suggests that behavior change occurs as a result of the dynamic interaction between modifications of behavior, cognition (self-efficacy, perceptions of barriers to lifestyle changes), and the environment (social support), and that modeling and reinforcement can be used to encourage change. SDT is a broad-based theory of human motivation (35) that is increasingly cited to explain how personal and intrinsic motivation leads to improved eating patterns. (36) According to SDT, an individual’s increased intrinsic motivation to improve eating patterns should positively relate to self-efficacy and the ability to overcome barriers and solicit support. Both the MedDiet-A and MedDiet-WL intervention arms will use self-monitoring food diaries, individualized goal setting, and shared problem-solving to increase mastery and goal achievement in small incremental steps to enhance self-regulatory skills related to the MedDiet interventions.
2.4.2. Intervention instructors
Instructors delivering the MedDiet-A and MedDiet-WL interventions will be certified and licensed registered dietitians referred to herein as the study RD(s). One study RD will be assigned to the MedDiet-A intervention and one study RD to the MedDiet-WL intervention. A third study RD will serve as the master intervention trainer, will perform intervention fidelity checks, and will also conduct the one-on-one in-person counseling sessions for control participants as described below. A certified physical activity instructor will lead the once weekly supervised physical activity session included in the MedDiet-WL intervention arm only.
2.4.3. Intervention instructor training
The study RDs implementing the MedDiet interventions will engage in one-on-one and group training sessions with the master intervention trainer. Topics covered during the training include: aims of the study; concept of and health benefits of a MedDiet; theoretical underpinnings of the trial including how to promote behavior change strategies consistent with the SCT and SDT models; overview of the dietary intervention (MedDiet-A or MedDiet-WL) specifically the food exchange list approach; discussion of establishing rapport with participants during the one-on-one meetings and group sessions to promote enthusiasm and retention; and instruction regarding weekly body weight assessment and dietary adoption and adherence checks using a MedDiet adherence screener. (30) The study RDs will also review and role play the group-based curriculum during the training sessions.
2.4.4. In-person session with intervention instructor
Participants in both the MedDiet-A and MedDiet-WL groups will attend a one-hour, individual, in-person session with a study RD during the two weeks prior to the start of the group-based sessions. The study RDs will provide a brief overview of the program, the benefits of consuming a MedDiet, and detailed instructions on adopting an eating pattern consistent with the MedDiet using an easy to follow MedDiet exchange list approach originally developed by Djuric and colleagues (37) with an accompanying exchange list booklet developed by our team. For the MedDiet-A group, dietary advice and corresponding MedDiet exchange lists will be given within the context of promoting weight stability. For the MedDiet-WL group, advice and exchange lists will be designed to promote a 1-2 lb. per week weight loss (an approximately 30% calorie restriction or a reduction of approximately 600 calories per day) for an end goal of a 7% weight loss from baseline body weight at 8 months that should be maintained through 14 months. Daily energy needs in both groups will be determined using the sex-specific Institute of Medicine Dietary Reference Intakes Macronutrient Report Estimated Energy Requirements (EER) equation with an activity factor representative of the participant's self-reported baseline physical activity. (38) During this meeting, the study RD will also work with the participants to tailor the MedDiet Exchange list to accommodate for existing health conditions, taste, and cultural preferences. Participants in both MedDiet groups will receive a tracking booklet and instructions on how to self-monitor daily food and beverage intake using a checklist format corresponding to their individual MedDiet exchange list recommendations. The in-person session with the study RD will incorporate behavioral change strategies consistent with the SCT and SDT theories. Thus, this training will underscore the concepts of self-efficacy and intrinsic motivation through goal setting problem solving self-monitoring and social support to enhance adherence to the dietary recommendations. (37, 39, 40)
2.4.5. Intervention diets
As described above, the MedDiet intervention groups will be advised to adopt and consume a MedDiet by utilizing an individualized dietary exchange list. The MedDiet-A and MedDiet-WL groups will be prescribed diets with similar macro- and micronutrient composition that diverge only in total calories (Table 1). Both groups will be advised to consume 6 - 10 exchanges of a variety of non-starchy fruits and vegetables; 5 - 11 exchanges of grain foods, with an emphasis on whole grain selections; 2 - 4 exchanges of low-fat dairy; 5 - 6 ounces of lean or very lean poultry, meat, or seafood; and at least one exchange of tree nuts, including almonds, per day and 1 - 2 exchanges of red wine with meals (this will be omitted and swapped for another exchange for participants who abstain from alcohol). Emphasis will be placed on consuming olive oil as the main fat, with a recommended consumption of at least 2 tablespoons daily. Consumption of fast foods, red and processed meats, fried foods, pastries, typical snack foods (e.g., chips), refined carbohydrates (e.g., white bread, pasta, and rice), sugared beverages, and high-fat dairy foods will be discouraged.
Table 1.
Building Research in Diet and Cognition (BRIDGE) Trial Dietary Recommendations for MedDiet-A and MedDiet-WL intervention arms
| Dietary Component | Recommended Consumption |
|---|---|
| Calories | MedDiet-A, Isocaloric MedDiet-WL, ~ 600 kcal/day deficit |
| Total fat | ~ 35% of total calories |
| Saturated fat | ~ 5 - 7% of total calories |
| Monounsaturated fat | ~ 20% of total calories |
| Polyunsaturated fat | ~ 8 - 10% of total calories |
| Carbohydrates | ~ 40% of total calories |
| Protein | ~ 20% of total calories |
| Alcohol | ~ 5% of total calories |
| Sodium | < 2400 mg or < 1500 for African American and hypertensive participants |
| Dietary fiber | 25 – 35 grams daily |
Abbreviations: MedDiet – A = Mediterranean Diet isocaloric lifestyle intervention; MedDiet-W = Mediterranean Diet caloric restricted for intentional weight loss lifestyle intervention.
2.4.6. Group-based interventions
The MedDiet-A (60 minutes) and MedDiet-WL (90 minutes) interventions will each include 27 in-person group sessions conducted by the study RDs. Group sessions will be approximately weekly for though month 6 and bi-weekly thereafter. Table 2 describes the session content. For sessions 1 – 19, both MedDiet-WL and MedDiet-A groups will receive the content presented in Table 2, column 1. This content focuses on making lifestyle changes and adopting and maintaining a MedDiet eating pattern. Additional content will be presented to the MedDiet-WL group during sessions 1 – 19 that focuses on weight loss through caloric restriction and engaging in physical activity at a level consistent with public health recommendations (Table 2, column 2). In sessions 20 – 27, both intervention arms will receive content pertaining to maintaining lifestyle changes consistent with a MedDiet eating pattern. The Med-WL arm will also receive content pertaining to weight loss maintenance. To increase adoption and adherence to a MedDiet pattern, we will also provide 1 ounce of almonds daily, olive oil, and $10.00 weekly to participants in both the MedDiet-A and MedDiet-WL who bring in a grocery receipts reflecting food purchases consistent with the MedDiet (e.g., fruits, vegetables, whole grains, and legumes). To help facilitate physical activity in the Med-WL arm, primarily through increasing daily steps, participants will be provided with a FitBit ™that will track steps, distance, calories burned, active minutes, and stationary time.
Table 2.
Building Research in Diet and Cognition (BRIDGE) Trial Session Content for MedDiet-A and MedDiet-WL Interventions
| MedDiet-A & MedDiet-WL | Additional MedDiet-WL Content | |
|---|---|---|
| Sessions 1-19 Making MedDiet Friendly Lifestyle Changes | ||
| 1 | Review of the intervention program. Discussion of MedDiet, MedDiet health benefits and using the MedDiet Food Exchange List Booklet. | Why MedDiet + weight loss? |
| 2 | MedDiet Food Exchange List Booklet review and self-monitoring with activity. | Review physical activity recommendations. Importance of weight and activity monitoring. |
| 3 | Meal planning and shopping guide on the MedDiet. |
Strategies to increase physical activity. |
| 4 | Eating out on the MedDiet. | Losing weight and challenges when eating out. |
| 5 | Cooking with olive oil – health benefits & tasting. | Portion control on the MedDiet (fats and oils). |
| 6 | Challenges to reducing intake of red meat and processed foods; the value of plant based protein. | Consistency: how to maintain weight loss efforts through physical activity planning |
| 7 | Increasing whole grains – health benefits, cooking demonstration & tasting. | Learning to identify fullness - "Hands on" practice during tasting. |
| 8 | Health benefits, strategies for increasing intake and purchasing fruits and vegetables. | Portion control on the MedDiet: fruits and vegetables |
| 9 | Hands-on cooking with vegetables | Portion control on the MedDiet: plating correct portions |
| 10 | Consideration of cultural and family food preferences when incorporating the MedDiet. | Weight loss when cooking for and/or eating with family members and cultural preferences; challenges to activity. |
| 11 | Choosing meals over snacks: satisfying hunger and stress eating. | Role of snacking and importance of monitoring calories with the MedDiet Exchange List Booklet. |
| 12 | Shopping on a budget and shopping tips when incorporating the MedDiet. | Shopping Do's and Don'ts when losing weight; incorporating activity into daily life. |
| 13 | Spouse/partner/family focus when changing to the MedDiet (participants will be encouraged to invite family and friends). | Challenges to weight loss and physical activity with spouses, partners, family members. |
| 14 | Utilizing leftovers, proper food storage and refrigerator organization to support healthy eating. Preparing MedDiet comfort foods. |
Revisiting fullness and hunger cues to support weight-loss. Using physical activity to stay energized and accelerate weight loss. |
| 15 | Reviewing the benefits a plant based diet and consuming less processed foods and beverages. | Discuss weight management progress. |
| 16 | Review of the MedDiet Exchange List Booklet. | Opportunities for engaging friends and family in an active lifestyle. |
| 17 | Review eating out on the MedDiet. | Review challenges to portion control. |
| 18 | Review cooking with olive oil - what works and what doesn't. | Planning meals with a focus on reducing calories; making time to be physically active. |
| 19 | Review incorporating cultural and family food preferences on the MedDiet | Review weight loss when cooking for or eating with family members, and challenges to physical activity |
| Sessions 20-27 Maintaining Lifestyle Changes | ||
| 20 | Solidifying changes and monitoring food purchasing and cooking patterns. | Solidifying weight loss strategies and activity patterns identifying "high risk" periods. |
| 21 | Budget considerations for adopting the MedDiet long-term. | The importance of continued food, activity, and weight monitoring, and enhancing self-efficacy. |
| 22 | Maintaining MedDiet motivation when cooking and eating with others. | Long-term portion control and asking for support for activity goals. |
| 23 | Staying on track during special occasions. | Maintaining physical activity throughout the year. |
| 24 | Developing a MedDiet recipe repertoire for the long-term. | Utilizing fitness and activity groups to maintain weight-loss and activity efforts. |
| 25 | Reinforcement: MedDiet tools to help you stay on track. | Using your activity monitor to meet goals and maintain your progress long-term. |
| 26 | Reflection: things that work and things to improve. | Varying activity to stay engaged in long-term physical activity. |
| 27 | Share dishes, recipes, and graduation | Share calorie cutting tips and physical activity successes |
Abbreviations: MedDiet – A = Mediterranean Diet isocaloric lifestyle intervention; MedDiet-WL = Mediterranean Diet caloric restricted for intentional weight loss lifestyle intervention.
2.4.7. Physical activity
The MedDiet-WL group will be prescribed an activity program consistent with the American College of Sports Medicine (41) and to programming prescribed in the DPP (42) and Look Ahead trials (43, 44) with an initial recommendation of 100 min/week progressing to 150 min/week within six weeks. Participants will be encouraged to be active in bouts of > 10 minutes and at a moderate-to-vigorous intensity level, i.e., walking briskly or jogging. (45) Increasing daily steps will also be encouraged with a goal of 10,000 steps per day consistent with the goals of America on the Move (http://anschutzwellness.com/community-programs/), a nonprofit initiative designed to advance “small change’” approaches to addressing obesity by modifying diet and physical activity. (46) In addition, at each group session, 30 minutes of supervised structured physical activity conducted by a certified physical activity instructor will be implemented. Sessions will be set to music and will focus on strength, flexibility, and cardio exercises. Participants in the MedDiet-A and Typical Diet Control group will not be encouraged to change their level of physical activity during the trial.
2.4.8. Monitoring participant adherence
Several methods will be implemented to optimize and assess compliance throughout the trial. First, we will monitor session attendance. Participants that screen eligible but anticipate missing more than three sessions if randomized to the interventions will be asked to defer to a later iteration. For MedDiet-A and MedDiet-WL, participants that miss more than two consecutive classes (staff not alerted prior to absence), will be called by research staff to encourage regular attendance. Second, the study RDs will call each participant during the first two weeks of the trial to assess adherence to the dietary recommendations using a MedDiet adherence screener. (32) Third, body weight will be privately assessed at each group session, and participants’ randomized to the MedDiet-WL group who lose < 1 pound per week will meet one-on-one with the study RD to identify barriers to adoption and adherence. When necessary, the study RD will revise the participant’s MedDiet exchange list and meet with the participant to review the new dietary targets. Similarly, in the MedDiet-A group, if weight changes ± 2% from baseline, the study RDs will adjust the MedDiet exchange list and review it with the participant in order to promote weight stability.
2.4.9. Intervention fidelity checks
Program fidelity will be monitored throughout the trial to ensure that the interventions are implemented as intended. During a given iteration, the master intervention trainer will attend two individual sessions per study RD and three group sessions for both the MedDiet-A and MedDiet-WL arms to assess fidelity to the program content using a checklist developed by the study team.
2.5. Typical Diet Control Condition
A study RD will meet with each participant randomized to the control group for 60 minutes in weeks 1/2 of the 14-month program. The participant will be instructed to maintain current eating and activity patterns and weight over the upcoming 14 months. No dietary or physical activity change recommendations will be provided. The control group will receive weekly health-related newsletters (e.g., dental health, flu facts, poison control, and sun safety). To assure compliance with eating, activity, and weight maintenance instructions, participant weight will be assessed in-person by an intervention instructor at around 3 months. During this visit, the instructor will also evaluate if the participant has adopted a MedDiet eating pattern using the MedDiet adherence screener. (30) Individuals who have > 2% weight loss from baseline or dietary changes consistent with adoption of a MedDiet will receive additional instruction from the study RD to maintain baseline lifestyle patterns. Like the MedDiet groups, the control participants can also receive $10 weekly for bringing in their grocery receipts for any foods purchased; this will also serve as an additional opportunity to monitor dietary intake. No other contact will be made except for the baseline, 8 and 14-month follow-up data collection visits. At the end of the 14-month trial, all MedDiet-WL intervention materials in a self-directed format and an in-person visit with a study RD will be offered to participants in the control group. The minimal contact in the control condition could pose a challenge for retention. However, we have found that an individual session following the completion of the intervention and a self-guided program is effective as a retention tool.(47, 48)
2.6. Data collection and measures
2.6.1. Procedures and training
All data collection staff will be trained by the project coordinator and the Diet and Behavior Shared Resource staff of the Cancer Center to ensure high-quality and standardized consent and assessment procedures. A protocol manual will be developed for all study measures and will include step-by step instructions for written informed consent, survey administration, anthropometric assessment, cognitive testing, body composition measurement via Dual Energy X-ray Absorptiometry (DXA), diet assessment, and biological sample procurement, processing and storage. Data will be collected at baseline, post-intervention (8 months) and end of study (14 months). Data collection will occur on the UIC campus or at the participating CPD intervention sites.
2.6.2. Measures
An overview of all study measures and time-points that each measure will be assessed is presented in Table 3.
Table 3.
Building Research in Diet and Cognition (BRIDGE) Trial Measures and Data Collection
| Measure | Baseline | 8 Months | 14 Months |
|---|---|---|---|
| Anthropometrics | |||
| Weight | x | x | x |
| Height | x | ||
| Body Composition (DXA) | x | x | x |
| Demographics | x | ||
| Framingham Risk Score | x | x | x |
| Systemic Inflammation (hsCRP) | x | x | x |
| Adipokines (Adiponectin) | x | x | x |
| Oxidative Stress (F2IsoP) | x | x | x |
| Blood Lipids | |||
| Total Cholesterol | x | x | x |
| LDL Cholesterol | x | x | x |
| HDL Cholesterol | x | x | x |
| Triglycerides | x | x | x |
| Blood Pressure | x | x | x |
| Metabolic Risk | |||
| Insulin | x | x | x |
| Glucose | x | x | x |
| Hemoglobin A1c | x | x | x |
| Diet behavior (Harvard FFQ) | x | x | x |
| Adherence to Med Diet | x | x | x |
| Physical Activity (Godin & Accelerometer) | x | x | x |
| Functional Capacity (6 min walk test) | x | x | x |
| Psychosocial Variables | |||
| Quality of Life | x | x | x |
| Self-efficacy | x | x | x |
| Social Support | x | x | x |
| Intrinsic Motivation | x | x | x |
| Depression | x | x | x |
| Cognitive Variables | |||
| Digit Span | x | x | x |
| Trail Making Test | x | x | x |
| Digit Symbol | x | x | x |
| Verbal Fluency | x | x | x |
| Stroop Color and Word Test | x | x | x |
| California Verbal Learning Test-II | x | x | x |
Abbreviations: DXA = Dual energy x-ray asborptiometry; hs-CRP = high sensitivity C-reactive protein; F2IsoP = plasma F2 isoprostane; LDL = low density lipoprotein; HDL = high density lipoprotein; FFQ = food frequency questionnaire
2.6.2.1. Cognitive protocol.
We have selected a 60-minute neuropsychological protocol in keeping with standards for the description and study of cognition associated with vascular risk developed by the National Institute of Neurological Disorders and Stroke and the Stroke-Canadian Stroke Network’s Neuropsychology working group. (29) Alternate forms of test measures will be used where possible to guard against practice effects from repeat testing. The cognitive protocol is comprised of the following and will be administered by trained psychometrists overseen by a licensed Clinical Neuropsychologist (Lamar):
Digit span test.
This test from the Wechsler Adult Memory Scale – III (WAIS-III) (49) assesses auditory attention (Forward) and working memory in the form of mental manipulation (Backward and Sequencing). Participants repeat digits of increasing length, first in the forward direction as they have heard the numbers, and then are required to either hold the sequence in mind while re-ordering the information into the reverse order (Backward) or while identifying either numbers or letters and re-ordering the information accordingly. Aspects of this task have been shown to rely not only on working memory associated with the prefrontal cortex, but also spatial memory and sequencing associated with the parietal lobe. (50, 51)
Trail making test.(52)
This test measures visuomotor attention and visual search (Part A) as well as working memory in the form of shifting mental set between two distinct categories of information (Part B). Participants draw lines to connect consecutively numbered circles in Part A and then to connect consecutively numbered or lettered circles by alternating between sequences (1-A-2-B-3-C, etc.) in Part B.
Digital symbol.
This test (also part of the WAIS-III) (49) assesses attention and information processing speed by requiring participants to use a key to match numbers and symbols under time constraints.
Verbal fluency. (53)
Verbal flexibility, establishing and maintaining mental set are measured by asking participants to rapidly generate words to a particular letter (F-A-S or C-F-L) or category (animals) in 60 seconds. This task also requires semantic knowledge and language functioning.
Stroop color and word test. (54)
Assessing sustained attention and cognitive interference inhibition, participants are asked to read a list of color words printed in black ink (attention), followed by X’s printed in color, and finally color words printed in incongruent colors (cognitive interference inhibition).
California Verbal Learning Test – II (CVLT-II) (55)
This test measures auditory verbal list learning and short as well as long delay memory and recognition. A 16-item list is repeated across 5 learning trials and then followed by a distracter list, prior to short free and cued recall; after a 20-minute delay, free and cued recall are again tested followed by recognition memory to allow for distinctions between encoding and retrieval performance. Forced choice will also be assessed as a measure of effort.
2.6.2.2. Blood pressure and anthropomorphic measures.
Blood pressure.
Blood pressure will be assessed in duplicate using the Omron HEM-907 (Lake Forest, IL) electronic blood pressure monitor with the participant comfortably seated and taking into consideration proper cuff size selection for the participant. (56) The participant will sit quietly for 5 minutes prior to the first assessment. Between the first and second reading, the participant will again sit quietly for 5 minutes prior to the second measurement.
Height (baseline only).
Participant height will be assessed in duplicate to the nearest 0.5 cm using a fixed stadiometer (seca, Birmingham, UK).
Body weight.
Participant body weight will be measured in duplicate to the nearest 0.1 kg using a digital scale (Tanita, Arlington Heights, IL). Body mass index (BMI) will be calculated as weight (kg) divided by height (m2).
Body composition.
We will assess body composition using the General Electric Lunar iDXA machine (GE Healthcare, US). Total body fat (grams), total body fat percentage, regional fat mass (trunk & extremities in grams), estimated visceral fat area (grams), total lean mass (grams), and regional lean mass (trunk & extremities in grams) will be extracted from the raw data output file and used for analysis.
2.6.2.3. Dietary intake.
Dietary intake will be estimated using the Harvard Food Frequency Questionnaire (HFFQ). (57) The HFFQ is a semi-quantitative, 131 food and beverage item questionnaire that includes responses on serving sizes and intake frequency. (58) This survey has been validated against food diaries, (59) and blood based biomarkers representative of actual nutrient intake.(60) The HFFQ will be interviewer-administered by trained members of our research team. The survey takes approximately 30 - 40 minutes to complete. Completed surveys will be processed by the Channing Lab at Harvard University.
2.6.2.4. Calculating adherence to a Mediterranean Diet pattern.
Data from the Harvard FFQ will be used to calculate a MedDiet adherence score. We will calculate adherence using the Alternate MedDiet score (aMED). (61) The aMED score is based on intake of 9 dietary items including vegetables (excludes potatoes), legumes, fruits, nuts, whole grains, red and processed meats, fish, alcohol, and the ratio of monounsaturated to saturated fats. The score ranges from 0 – 9 points; higher scores reflect greater adherence. Intake above the median is awarded 1 point each for vegetables (excluding potatoes), legumes, fruits, nuts, whole grains, fish and the ratio of monounsaturated fat, and 1 point is awarded for intake below the median for red and processed meats and for alcohol intake between 5-15 grams/day.
2.6.2.5. Physical activity, self-report and objectively measured.
Self-report physical activity.
The Godin-Shepard Leisure Time Physical Activity Questionnaire will be used to measure self-reported leisure time physical activity. (62) This questionnaire allows for the ranking of individuals from lowest to highest level of leisure time physical activity and has been validated in healthy adults and those at risk for cognitive impairment. (63)
Accelerometry.
To objectively measure participant physical activity, we will use the ActiGraph wGT3X triaxial accelerometer. (64) Participants will be asked to wear the accelerometer on their non-dominant wrist for 7 days. Accelerometry data will be downloaded and analyzed using the ActiLife program provided by the manufacturer. We selected the wrist-worn model for participant ease and comfort, which may translate to higher compliance with our 7-day protocol. Furthermore, previous reports have cited moderate agreement between the wrist-worn and hip-worn Actigraph models. (65)
2.6.2.6. Functional activity capacity.
To assess functional activity capacity, we will use the Six-Minute Walk Test, (66) which employs a standard protocol to determine exercise tolerance. The test measures the distance that a subject can walk on a hard fat surface in a period of 6 minutes. (66)
2.6.2.7. Blood-based biomarkers
Fasting venous blood will be processed for serum and plasma at each time-point, according to standard protocols, and immediately sent for analysis to a commercial lab (Quest Diagnostics, Wood Dale, IL), or stored at −80°C until analysis.
Blood lipids.
Total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, and triglycerides will be measured by spectrophotometry at Quest Diagnostics (Wood Dale, IL).
Markers of metabolic risk.
Insulin, glucose, and HbA1c will be measured from serum via immunoassay (insulin), spectrophotometry (glucose), or immunoturbidity (HbA1c) by Quest Diagnostics (Wood Dale, IL).
Systemic inflammation.
Serum levels of high sensitivity C-reactive protein will be assessed with an enzyme linked immunosorbant assay (ELISA) (R &D systems, Minneapolis, MN).
Adipokines.
Adiponectin modulates the body’s sensitivity to insulin, is involved in glucose and fatty acid metabolism, and has potent anti-inflammatory effects. (67) Serum levels of adiponectin will be assessed via ELISA (R & D Systems, Minneapolis, MN).
Oxidative stress.
F2-isoprostanes (F2_IsoPs) are considered the best available in vivo biomarkers of oxidative stress, are chemically stable, detectable in all normal tissues and biological fluids, and increase substantially with increased oxidative stress. (68) The current gold standard for measuring F2_IsoPs in biological fluids, gas chromatography mass spectrometry (GC-MS) will be utilized to assess F2-IsoPs in plasma. (68)
2.6.2.8. Psychosocial measures.
Perceived quality of life will be assessed using the PROMIS© Global Health Scale version 1.2. (69) Concepts of the SCT, self-efficacy and social support will be evaluated via Perceived Competence Scale for Healthy Eating (70) and the Medical Outcomes Study Social Support Survey. (71) Intrinsic motivation, a concept of the SDT, will be assessed using the Treatment Self-regulation Questionnaire (72) which examines autonomous and intrinsic motivation and controlled and extrinsic motivation for weight management. Depression will be evaluated using the Center for Epidemiologic Studies Depression Scale (CES-D). (73)
2.6.2.9. Cardiovascular disease risk assessment.
To assess cardiovascular disease risk, the Framingham Risk Score will be calculated. (74) The Framingham Risk Score is a sex-specific algorithm used to estimate an individual's 10-year cardiovascular disease risk. Variable used to calculate this score include age, sex, total cholesterol, HDL cholesterol, systolic blood pressure, current smoker (yes/no), and currently on medication to treat hypertension (yes/no).
2.6.2.10. Socio-demographics.
Socio-demographic variables including race/ethnicity, date of birth, marital status, educational attainment, occupation, and family household income will be obtained.
2.7. Randomization
Shortly before the intervention classes are scheduled to begin, participants who have completed the baseline visit will be contacted to confirm that they still wish to participate in the study. The data manager, who has no contact with participants, will then use the Research Electronic Data Capture (REDCap) randomization module to randomly assign participants to one of the three study conditions: Typical Diet Control, MedDiet-A, or MedDiet-WL. Randomization will be stratified by program cohort (3 iterations of 60 participants each will be conducted) for age (55-69 or 70-85 years) and level of cognitive function (MoCA scores – 19-26 or 27-30). Stratification prior to random assignment will ensure approximately equal distribution of these variables between the three treatment arms. Stratified block randomization sequences will be created in SAS and uploaded into the REDCap randomization module. To increase our power to detect differences between MedDiet-A and MedDiet-WL, we will use a 2:2:1 allocation ratio: 72 of the 180 participants will be assigned to each MedDiet group, with the remaining 36 individuals assigned to the control group. Due to the nature of the intervention, it will not be possible for participants and research staff to be blinded to the randomization assignment.
2.8. Power and sample size considerations.
The primary effects of interest are the treatment group differences in the composite score of executive function, memory, and attention post-intervention (i.e., at 8 months), focusing on the contrasts outlined in the analysis section below. We calculated the power to detect group differences in cognitive function using the results of the ENCORE trial(75) as a guide. The power estimates are based on the following specifications: 1) separate general linear models for executive function, attention, and memory; 2) pre-treatment cognitive function, age and years of education as covariates in the model; 3) an initial sample of 180 participants assuming 20% attrition using the intention to treat principle for analysis; 4) alpha of 0.05; and 5) an R2 of 0.73 and 0.47 between the covariates and executive function and memory (these values are taken directly from the ENCORE trial).(75)
The proposed design will provide adequate power to detect clinically meaningful effects. Based on our prior estimates, the proposed sample will yield a power of about 0.80 to detect a treatment effect of 0.32 standard deviations between treatment groups and control, and 0.38 standard deviations between the two treatment groups for executive function. Power for our attention composite score will be very similar to that of executive function, as these measures overlapped substantially in ENCORE. (75) Similarly, our sample will yield a power of 0.80 to detect a treatment effect of about 0.35 standard deviations between both MedDiet groups and control, and 0.44 standard deviations between the two active treatment groups for memory. In the previous RCT of the DASH diet, moderate effect sizes of d=0.56 on a composite score of executive function, memory, and attention in the combined DASH and weight management group (compared to usual care) were observed, and an effect size of d=0.30 when the DASH +WL group was compared to DASH alone. We note that these power estimates are likely to be conservative in that we have not accounted for the additional power that is obtained with methods for missing data. We have achieved similar effect sizes in our prior work, so that these effects are feasible for this trial. With respect to secondary endpoints, such as systemic inflammation, oxidative stress, and body composition, we will have power of 0.80 to detect at least a 0.40 standard deviation difference between the active treatment groups (MedDiet-A and MedDiet-WL) and control, and a 0.46 standard deviation difference between the two active treatment groups. Assuming a 0.2 correlation between changes in weight and improvements in neurocognition, which were observed in ENCORE, (75) we would need to observe an average 6.2-lb weight loss difference between participants in both MedDiet treatment groups and the control group, and a 7.1-lb weight loss difference between participants in the MedDiet-WL and MedDiet-A groups to have 80% power to detect treatment mediation for changes in body composition. (76) In the ethnically diverse ENCORE trial, an average weight loss difference of 11 lbs. across both treatment groups compared with controls was observed, and an 18-lb weight loss difference between the combined and diet-alone groups. Among a sample of African-American women in our ORBIT trial, women in the intervention lost 3.0 kg, compared to a 0.2 kg gain in the control group, and the adjusted difference between groups was; 3.27 kg (p < 0.001). This translates to 7.2 pounds(47) suggesting that these estimates are realistic.
2.9. Data management
Anthropometric data, blood pressure, 6-minute walk data, and psychosocial measures will be entered directly in REDCap, a secure web-based portal for building surveys and maintaining research-related data. Cognitive measures will be collected and scored on paper forms, and scores will be entered in REDCap. REDCap includes a number of features for ensuring data quality, such as data type validation (integer, date, etc.) and range checks.
Diet, blood, body composition, and accelerometer data will be processed as described in the Measures section above, and results will be received in electronic form.
2.10. Data analytic plan
The effect of treatments on the primary endpoint of the composite score for attention, executive function, and memory will be examined using the generalized linear model in the R software package (http://r-project.org). We will adapt the approach recommended by O’Brien, (77) constructing a weighted composite variable to represent each of our cognitive outcomes. The weights of the composite will be derived using confirmatory factor analysis in the Mplus software. (78)
Primary analyses will be carried out using Mplus. Components of the Attention composite will include the Digit Span Forward, Trail Making Test Part A, and the Digit Symbol test; our Executive function composite will include the Verbal Fluency Test, Digit Span Backward, Trail Making Test Part B, and Stroop Color-Word Interference, and the memory composite will include the total learning, delayed free recall and recognition discriminability from the CVLT-II. The composite approach has advantages over testing multiple individual endpoints, including increased reliability, enhanced precision of the treatment estimate, improved power, and minimization of Type I Error. (79-81) The 8-month composite will serve as the response variable. Predictor variables will be specified a priori, with treatment group as the predictor of interest and age, education, and the pre-treatment level of each composite as adjustment covariables. A priori comparisons of group differences will be evaluated within the model by way of two orthogonal contrasts: 1) MedDiet-WL and MedDiet-A versus Typical Diet Control and 2) MedDiet-WL versus MedDiet-A. Analyses will follow the intention-to-treat principle using Full Information Maximum Likelihood available in Mplus to account for missing data. Mediational analyses examining the relationship between CVD/metabolic risk factors, body composition, systemic inflammation, oxidative stress, and improvements in cognitive function will proceed using procedures outlined by Kenny and MacKinnon. (82, 83) Formal tests of mediation will be conducted using the indirect effect approach with bootstrapped confidence intervals in Mplus Version 7.3.1. We will also conduct analyses of potential treatment moderators, including baseline age, race/ethnicity, body weight, SES, and cognition.
3. Discussion
t By 2040, 81 million older adults worldwide will be living with some form of dementia.(84) Obesity, which is also highly prevalent among older adults in the U.S. (35.4 % in adults aged 60 and older) (7) is considered to be an important contributor to deficits in multiple cognitive domains. (24, 85, 86) Although, there is some controversy regarding an independent association between excess adiposity and cognitive functioning given many of the existing studies have failed to account for the effect of obesity-related comorbidities (i.e., CVD, depression) that also can adversely influence cognition. (10) In the studies that have controlled for these factors, obesity is moderately independently associated with deficits in several cognitive domains including psychomotor performance and speed, visual construction, verbal memory, concept formation, and decision-making. (10)The BRIDGE trial is designed to account for these comorbid factors including CVD risk factors and depression, which was recommended by Prickett and colleagues, (10) to allow for a more methodologically sound assessment of the association between obesity and cognition. .
Recent studies also indicate that variations in dietary practices are predictive of cognitive decline, dementia, and Alzheimer's disease. (21, 87) Fortunately, diet and body weight are both amenable to intervention. Independently, weight loss (22) and adherence to a MedDiet, (88) have been associated with reduced risk of cognitive decline and dementia. Although the MedLey study reported that a six month MedDiet intervention did not produce a beneficial effect on cognitive functioning among healthy older adults compared to a control group, (89) the combined impact of a MedDiet with weight loss on cognitive functioning and age-related decline has not been explored in an RCT and may have a more profound effect than either intervention alone. To our knowledge, the BRIDGE trial is the first RCT in the U.S. to investigate if a MedDiet lifestyle intervention promoting weight loss produces better results than an isocaloric MedDiet lifestyle intervention or Typical Diet Control condition on the primary outcomes of cognitive functioning in a racially and ethnically diverse sample of obese older adults. We are enrolling a largely African American and Hispanic sample given the higher prevalence of obesity and the growing body of evidence suggesting that cognitive decline and dementia may disproportionately affect minority groups in the US. (90-93)
In the BRIDGE trial, we are utilizing the MedDiet exchange list developed by Djuric and colleagues (37) to promote adoption and adherence to an isocaloric or calorie restricted MedDiet in the two treatment arms of the study. Exchange lists provide an easy-to-follow format for establishing new dietary patterns and allow a high degree of individual tailoring. (37) An exchange list classifies target foods and beverages into several categories with associated portion sizes. Individuals are assigned daily consumption goals in a given category, and they are allowed to choose from a large variety of foods within each category that are consistent with their taste and cultural preferences, as well as tolerance to a given food (e.g., in the low-fat dairy group, individuals with lactose intolerance can choose a food that does not promote gastrointestinal upset). This allows for maximal flexibility in food choice and encourages variety, which can enhance adherence and maintenance. (37)
Several mechanisms linked to a poor diet and excess adiposity including metabolic and cardiovascular perturbations, chronic inflammation, and oxidative stress are hypothesized to be pathophysiologic contributors to cognitive decline. (17, 21, 22, 86) Specifically, these factors may have an adverse effect on brain glucose metabolism, (94) neurotransmission, (95) brain structure, (96) and neuronal functioning and integrity (97) that are important to cognition. Within the context of the BRIDGE trial, the investigation of several metabolic and inflammatory mediators may provide evidence regarding the role of these systemic factors in cognitive health. Independently, intentional weight loss and adherence to an isocaloric MedDiet improve blood lipid profiles, (20, 98) lower blood pressure, (20, 99) reduce systemic inflammation (27, 100) and oxidative stress, (101, 102) and improve glucose metabolism, (99, 103). The combined effect of the MedDiet + WL has demonstrated superior effects on systemic inflammation compared to either intervention alone when tested in a group of men with metabolic syndrome. (104), but the inclusion of blood biomarkers may provide lurther insight into the underlying biological mechanisms of the MedDiet-A and MedDiet+WL for preventing and slowing the progression of age-related cognitive decline.
In summary, given the alarming statistics related to both dementia and obesity in older adults and the aging US population, the MedDiet-WL intervention of the BRIDGE trial could be an important approach to prevent and slow age-related cognitive decline if proven efficacious. It’s important to acknowledge that the European PREDIMED investigators have recently launched the PREDIMED PLUS trial, which is testing the effect of an isocaloric versus calorie restricted MedDiet lifestyle program on the primary outcomes of cardiovascular disease endpoints and weight loss and secondary outcomes related to cognition in a Spanish population of older adults with metabolic syndrome. (105) Given the necessity for advancing our understanding of age-related cognitive decline and the need to develop effective strategies to prevent and slow cognitive decline and progression to dementia, (106) our BRIDGE trial and the PREDIMED PLUS trial will contribute significantly to our understanding of the influence of diet and weight control on these processes as well the prevention and progression of chronic diseases that adversely affect older adults.
Acknowledgements
This study is funded by the National Heart Lung and Blood Institute of the National Institutes of Health (R01HL129153).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests.
None to disclose.
References
- 1.Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM, et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl 5):S4–9. PubMed PMID: 10854354. Epub 2000/06/15. eng. [PubMed] [Google Scholar]
- 2.Robertson D, Rockwood K, Stolee P. The prevalence of cognitive impairment in an elderly Canadian population. Acta Psychiatr Scand. 1989. October;80(4):303–9. PubMed PMID: 2589085. Epub 1989/10/01. eng. [DOI] [PubMed] [Google Scholar]
- 3.Yaffe K, Ackerson L, Hoang TD, Go AS, Maguire MG, Ying GS, et al. Retinopathy and cognitive impairment in adults with CKD. Am J Kidney Dis. 2013. February;61(2):219–27. PubMed PMID: 23206534. Pubmed Central PMCID: Pmc4030670. Epub 2012/12/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shadlen MF, Siscovick D, Fitzpatrick AL, Dulberg C, Kuller LH, Jackson S. Education, cognitive test scores, and black-white differences in dementia risk. J Am Geriatr Soc. 2006. June;54(6):898–905. PubMed PMID: 16776783. Epub 2006/06/17. eng. [DOI] [PubMed] [Google Scholar]
- 5.Mehta KM, Simonsick EM, Rooks R, Newman AB, Pope SK, Rubin SM, et al. Black and white differences in cognitive function test scores: what explains the difference? J Am Geriatr Soc. 2004. December;52(12):2120–7. PubMed PMID: 15571554. Pubmed Central PMCID: Pmc2939725. Epub 2004/12/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rovner BW, Casten RJ, Harris LF. Cultural diversity and views on Alzheimer disease in older African Americans. Alzheimer Dis Assoc Disord. 2013. Apr-Jun;27(2):133–7. PubMed PMID: 22828323. Pubmed Central PMCID: Pmc3492535. Epub 2012/07/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. Jama. 2014;311(8):806–14. PubMed PMID: 24570244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007. Jan-Feb;48(1):57–61. PubMed PMID: 17145283. Epub 2006/12/06. eng. [DOI] [PubMed] [Google Scholar]
- 9.Naderali EK, Ratcliffe SH, Dale MC. Obesity and Alzheimer's disease: a link between body weight and cognitive function in old age. Am J Alzheimers Dis Other Demen. 2009 Dec-2010 Jan;24(6):445–9. PubMed PMID: 19801534. Epub 2009/10/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prickett C, Brennan L, Stolwyk R. Examining the relationship between obesity and cognitive function: a systematic literature review. Obesity research & clinical practice. 2015. Mar-Apr;9(2):93–113. PubMed PMID: 25890426. [DOI] [PubMed] [Google Scholar]
- 11.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013. May 7;80(19):1778–83. PubMed PMID: 23390181. Pubmed Central PMCID: Pmc3719424. Epub 2013/02/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, et al. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. 2013. July;24(4):479–89. PubMed PMID: 23680940. Epub 2013/05/18. eng. [DOI] [PubMed] [Google Scholar]
- 13.Valls-Pedret C, Ros E. Commentary: Mediterranean diet and cognitive outcomes: epidemiological evidence suggestive, randomized trials needed. Epidemiology. 2013. July;24(4):503–6. PubMed PMID: 23732733. Epub 2013/06/05. eng. [DOI] [PubMed] [Google Scholar]
- 14.Rolland Y, Abellan van Kan G, Vellas B. Healthy brain aging: role of exercise and physical activity. Clin Geriatr Med. 2010. February;26(1):75–87. PubMed PMID: 20176294. Epub 2010/02/24. eng. [DOI] [PubMed] [Google Scholar]
- 15.Lovden M, Xu W, Wang HX. Lifestyle change and the prevention of cognitive decline and dementia: what is the evidence? Curr Opin Psychiatry. 2013 May;26(3):239–43. PubMed PMID: 23493129. Epub 2013/03/16. eng. [DOI] [PubMed] [Google Scholar]
- 16.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014. August;13(8):788–94. PubMed PMID: 25030513. Epub 2014/07/18. eng. [DOI] [PubMed] [Google Scholar]
- 17.Ramassamy C, Belkacemi A. Nutrition and Alzheimer's disease: is there any connection? Curr Alzheimer Res. 2011. August;8(5):443–4. PubMed PMID: 21679155. Epub 2011/06/18. eng. [DOI] [PubMed] [Google Scholar]
- 18.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009. February;66(2):216–25. PubMed PMID: 19204158. Pubmed Central PMCID: Pmc2653223. Epub 2009/02/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvado J, San Julian B, et al. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. 2013. December;84(12):1318–25. PubMed PMID: 23670794. Epub 2013/05/15. eng. [DOI] [PubMed] [Google Scholar]
- 20.Chiva-Blanch G, Badimon L, Estruch R. Latest evidence of the effects of the Mediterranean diet in prevention of cardiovascular disease. Curr Atheroscler Rep. 2014. October;16(10):446. PubMed PMID: 25115436. Epub 2014/08/15. eng. [DOI] [PubMed] [Google Scholar]
- 21.Alles B, Samieri C, Feart C, Jutand MA, Laurin D, Barberger-Gateau P. Dietary patterns: a novel approach to examine the link between nutrition and cognitive function in older individuals. Nutr Res Rev. 2012. December;25(2):207–22. PubMed PMID: 22874455. Epub 2012/08/10. eng. [DOI] [PubMed] [Google Scholar]
- 22.Siervo M, Arnold R, Wells JC, Tagliabue A, Colantuoni A, Albanese E, et al. Intentional weight loss in overweight and obese individuals and cognitive function: a systematic review and meta-analysis. Obes Rev. 2011. November;12(11):968–83. PubMed PMID: 21762426. Epub 2011/07/19. eng. [DOI] [PubMed] [Google Scholar]
- 23.Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci USA. 2009. January 27;106(4):1255–60. PubMed PMID: 19171901. Pubmed Central PMCID: Pmc2633586. Epub 2009/01/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunstad J, Strain G, Devlin MJ, Wing R, Cohen RA, Paul RH, et al. Improved memory function 12 weeks after bariatric surgery. Surg Obes Relat Dis. 2011. Jul-Aug;7(4):465–72. PubMed PMID: 21145295. Pubmed Central PMCID: Pmc3117085. Epub 2010/12/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lofgren IE, Herron KL, West KL, Zern TL, Patalay M, Koo SI, et al. Carbohydrate intake is correlated with biomarkers for coronary heart disease in a population of overweight premenopausal women. J Nutr Biochem. 2005. April;16(4):245–50. PubMed PMID: 15808329. Epub 2005/04/06. eng. [DOI] [PubMed] [Google Scholar]
- 26.Logue J, Sattar N. Tackling obesity in adult primary care. Practitioner. 2010. June;254(1730):31–2, 4, 3. PubMed PMID: 20669822. Epub 2010/07/31. eng. [PubMed] [Google Scholar]
- 27.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003 Apr 9;289(14):1799–804. PubMed PMID: 12684358. Epub 2003/04/10. eng. [DOI] [PubMed] [Google Scholar]
- 28.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005. April;53(4):695–9. PubMed PMID: 15817019. Epub 2005/04/09. Eng. [DOI] [PubMed] [Google Scholar]
- 29.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006. September;37(9):2220–41. PubMed PMID: 16917086. Epub 2006/08/19. eng. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Gonzalez MA, Garcia-Arellano A, Toledo E, Salas-Salvado J, Buil-Cosiales P, Corella D, et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PloS one. 2012;7(8):e43134. PubMed PMID: 22905215. Pubmed Central PMCID: Pmc3419206. Epub 2012/08/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bandura A Self-efficacy mechanism in human agency. Am Psychol. 1982;37(2):122–47. [Google Scholar]
- 32.Bandura A Human agency in social cognitive theory. Am Psychol. 1989. September;44(9):1175–84. PubMed PMID: 2782727. Epub 1989/09/01. eng. [DOI] [PubMed] [Google Scholar]
- 33.EL D RM R. The empirical exploration of intrinsic motivational processes. L B, editor. New York: Academic Press; 1980. [Google Scholar]
- 34.EL D RM R. A motivational approach to self: Integration in personality In: Dienstbier R, editor. Perspectives on motivation. 38 Lincoln, Nebraska: University of Nebraska Press; 1991. [Google Scholar]
- 35.Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000. January;55(1):68–78. PubMed PMID: 11392867. Epub 2001/06/08. eng. [DOI] [PubMed] [Google Scholar]
- 36.Deci EL, Ryan RM. Intrinsic motivation and self-determination in human behavior. New York: Plenum; 1985. [Google Scholar]
- 37.Djuric Z, Vanloon G, Radakovich K, Dilaura NM, Heilbrun LK, Sen A. Design of a Mediterranean exchange list diet implemented by telephone counseling. J Am Diet Assoc. 2008. December;108(12):2059–65. PubMed PMID: 19027409. Pubmed Central PMCID: Pmc2610261. Epub 2008/11/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acides, Cholesterol, Protein, and Amino Acids Panel on Macronutrients, Panel on the Definition of Dietary Fiber, Subcommittee on Upper Reference Levels of Nutrients, Subcommittee on Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes - Food and Nutrition Board. Washington, D.C.2002. [Google Scholar]
- 39.Sidahmed E, Cornellier ML, Ren J, Askew LM, Li Y, Talaat N, et al. Development of exchange lists for Mediterranean and Healthy Eating diets: implementation in an intervention trial. J Hum Nutr Diet. 2014. October;27(5):413–25. PubMed PMID: 24112099. Pubmed Central PMCID: Pmc3961569. Epub 2013/10/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.A. B. Social foundations of thought and action : a social cognitive theory. Englewood Cliffs, N.J.: Prentice-Hall; 1986. 617 p. [Google Scholar]
- 41.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009. February;41(2):459–71. PubMed PMID: 19127177. Epub 2009/01/08. eng. [DOI] [PubMed] [Google Scholar]
- 42.The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes care. 2002. December;25(12):2165–71. PubMed PMID: 12453955. Pubmed Central PMCID: Pmc1282458. Epub 2002/11/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Controlled clinical trials. 2003. October;24(5):610–28. PubMed PMID: 14500058. Epub 2003/09/23. eng. [DOI] [PubMed] [Google Scholar]
- 44.Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring). 2006. May;14(5):737–52. PubMed PMID: 16855180. Pubmed Central PMCID: Pmc2613279. Epub 2006/07/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jakicic JM. The role of physical activity in prevention and treatment of body weight gain in adults. J Nutr. 2002. December;132(12):3826s–9s. PubMed PMID: 12468633. Epub 2002/12/07. eng. [DOI] [PubMed] [Google Scholar]
- 46.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003. February 7;299(5608):853–5. PubMed PMID: 12574618. Pubmed Central PMCID: 12574618 Epub 2003/02/08. eng. [DOI] [PubMed] [Google Scholar]
- 47.Fitzgibbon ML, Stolley MR, Schiffer L, Sharp LK, Singh V, Dyer A. Obesity reduction black intervention trial (ORBIT): 18-month results. Obesity (Silver Spring). 2010. December;18(12):2317–25. PubMed PMID: 20300081. Pubmed Central PMCID: Pmc3775663. Epub 2010/03/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stolley MR, Fitzgibbon ML, Schiffer L, Sharp LK, Singh V, Van Horn L, et al. Obesity reduction black intervention trial (ORBIT): six-month results. Obesity (Silver Spring). 2009. January;17(1):100–6. PubMed PMID: 18997671. Pubmed Central PMCID: Pmc3888450. Epub 2008/11/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wechsler D Wechsler Wechsler Memory Scale 3ed. San Antonio, TX: Psychological Corporation; 1997c. [Google Scholar]
- 50.Lamar M, Price CC, Libon DJ, Penney DL, Kaplan E, Grossman M, et al. Alterations in working memory as a function of leukoaraiosis in dementia. Neuropsychologia. 2007. January 28;45(2):245–54. PubMed PMID: 16950457. Pubmed Central PMCID: 2911013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamar M, Catani M, Price CC, Heilman KM, Libon DJ. The impact of region-specific leukoaraiosis on working memory deficits in dementia. Neuropsychologia. 2008. August;46(10):2597–601. PubMed PMID: 18501390. [DOI] [PubMed] [Google Scholar]
- 52.Reitan R, Wolfson D. The Halstead–Reitan Neuropsycholgical Test Battery: Therapy and clinical interpretation. Tucson, AZ: Neuropsychological Press; 1985. [Google Scholar]
- 53.Lezak M, Howieson D, Bigler E, D T. Neuropsychological Assessment. 5 ed. New York, NY: Oxford University Press; 2012. 1200 p. [Google Scholar]
- 54.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643–62. [Google Scholar]
- 55.Delis DC, Kramer JH, Kaplan E, Ober BA. Manual for the California Verbal Learning Test, (CVLT-II). San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 56.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005. February 08;111(5):697–716. PubMed PMID: 15699287. [DOI] [PubMed] [Google Scholar]
- 57.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985. July;122(1):51–65. PubMed PMID: 4014201. Epub 1985/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 58.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992. May 15;135(10):1114–26; discussion 27-36. PubMed PMID: 1632423. Epub 1992/05/15. eng. [DOI] [PubMed] [Google Scholar]
- 59.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and Validity of an Expanded Self-Administered Semiquantitative Food Frequency Questionnaire among Male Health Professionals. Am J Epidemiol. 1992;135(10):1114–26. [DOI] [PubMed] [Google Scholar]
- 60.Michaud DS, Giovannucci EL, Ascherio A, Rimm EB, Forman MR, Sampson L, et al. Associations of plasma carotenoid concentrations and dietary intake of specific carotenoids in samples of two prospective cohort studies using a new carotenoid database. Cancer Epidemiol Biomarkers Prev. 1998. April;7(4):283–90. PubMed PMID: 9568782. Epub 1998/05/06. eng. [PubMed] [Google Scholar]
- 61.Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005. July;82(1):163–73. PubMed PMID: 16002815. Epub 2005/07/09. eng. [DOI] [PubMed] [Google Scholar]
- 62.G G. The Godin-Shephard Leisure-Time Physical Activity Questionnaire. Health Fit J Can 2011;4(1). [Google Scholar]
- 63.Amireault S, Godin G, Lacombe J, Sabiston CM. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: a systematic review. BMC Med Res Methodol. 2015;15:60. PubMed PMID: 26264621. Pubmed Central PMCID: Pmc4542103. Epub 2015/08/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santos-Lozano A, Marin PJ, Torres-Luque G, Ruiz JR, Lucia A, Garatachea N. Technical variability of the GT3X accelerometer. Med Eng Phys. 2012. July;34(6):787–90. PubMed PMID: 22417978. Epub 2012/03/16. eng. [DOI] [PubMed] [Google Scholar]
- 65.Kamada M, Shiroma EJ, Harris TB, Lee IM. Comparison of physical activity assessed using hip- and wrist-worn accelerometers. Gait & posture. 2016. February;44:23–8. PubMed PMID: 27004628. Pubmed Central PMCID: 4806562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.ATS statement: guidelines for the six-minute walk test. American journal of respiratory and critical care medicine. 2002. July 1;166(1):111–7. PubMed PMID: 12091180. Epub 2002/07/02. eng. [DOI] [PubMed] [Google Scholar]
- 67.Teixeira AL, Diniz BS, Campos AC, Miranda AS, Rocha NP, Talib LL, et al. Decreased levels of circulating adiponectin in mild cognitive impairment and Alzheimer's disease. Neuromolecular medicine. 2013. March;15(1):115–21. PubMed PMID: 23055000. Epub 2012/10/12. eng. [DOI] [PubMed] [Google Scholar]
- 68.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000. February 15;28(4):505–13. PubMed PMID: 10719231. Epub 2000/03/17. eng. [DOI] [PubMed] [Google Scholar]
- 69.Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, et al. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care. 2007. May;45(5 Suppl 1):S22–31. PubMed PMID: 17443115. Epub 2007/04/20. Eng. [DOI] [PubMed] [Google Scholar]
- 70.Williams GC, Freedman ZR, Deci EL. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes care. 1998. October;21(10):1644–51. PubMed PMTD: 9773724. Epub 1998/10/17. Eng. [DOI] [PubMed] [Google Scholar]
- 71.Moser A, Stuck AE, Silliman RA, Ganz PA, Clough-Gorr KM. The eight-item modified Medical Outcomes Study Social Support Survey: psychometric evaluation showed excellent performance. J Clin Epidemiol. 2012. October;65(10):1107–16. PubMed PMTD: 22818947. Pubmed Central PMCTD: Pmc4119888. Epub 2012/07/24. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carey KB, Neal DJ, Collins SE. A psychometric analysis of the self-regulation questionnaire. Addict Behav. 2004. February;29(2):253–60. PubMed PMTD: 14732414. Epub 2004/01/21. eng. [DOI] [PubMed] [Google Scholar]
- 73.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997. June;12(2):277–87. PubMed PMTD: 9189988. Epub 1997/06/01. Eng. [DOI] [PubMed] [Google Scholar]
- 74.D'Agostino RB Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008. February 12;117(6):743–53. PubMed PMTD: 18212285. Epub 2008/01/24. eng. [DOI] [PubMed] [Google Scholar]
- 75.Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med. 2010. January 25;170(2):126–35. PubMed PMTD: 20101007. Pubmed Central PMCTD: Pmc3633078. Epub 2010/01/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007. March;18(3):233–9. PubMed PMID: 17444920. Pubmed Central PMCID: Pmc2843527. Epub 2007/04/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O'Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. 1984. December;40(4):1079–87. PubMed PMID: 6534410. Epub 1984/12/01. eng. [PubMed] [Google Scholar]
- 78.Mplus Statistical Analysis With Latent Variables User’s Guide 6ed. Los Angeles, CA. : Muthén & Muthén; 1998–2010. [Google Scholar]
- 79.Comelli M, Klersy C. Different methods to analyze clinical experiments with multiple endpoints: a comparison of real data. J Biopharm Stat. 1996. May;6(2):115–25. PubMed PMID: 8732908. Epub 1996/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 80.LaVange LM, Durham TA, Koch GG. Randomization-based nonparametric methods for the analysis of multicentre trials. Stat Methods Med Res. 2005. June;14(3):281–301. PubMed PMID: 15969304. Epub 2005/06/23. eng. [DOI] [PubMed] [Google Scholar]
- 81.Lehmacher W, Wassmer G, Reitmeir P. Procedures for two-sample comparisons with multiple endpoints controlling the experimentwise error rate. Biometrics. 1991. June;47(2):511–21. PubMed PMID: 1912258. Epub 1991/06/01. eng. [PubMed] [Google Scholar]
- 82.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986. December;51(6):1173–82. PubMed PMID: 3806354. Epub 1986/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 83.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002. March;7(1):83–104. PubMed PMID: 11928892. Pubmed Central PMCID: Pmc2819363. Epub 2002/04/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005. December 17;366(9503):2112–7. PubMed PMID: 16360788. Pubmed Central PMCID: Pmc2850264. Epub 2005/12/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cserjesi R, Molnar D, Luminet O, Lenard L. Is there any relationship between obesity and mental flexibility in children? Appetite. 2007. November;49(3):675–8. PubMed PMID: 17543417. Epub 2007/06/05. eng. [DOI] [PubMed] [Google Scholar]
- 86.Gunstad J, Lhotsky A, Wendell CR, Ferrucci L, Zonderman AB. Longitudinal examination of obesity and cognitive function: results from the Baltimore longitudinal study of aging. Neuroepidemiology. 2010;34(4):222–9. PubMed PMID: 20299802. Pubmed Central PMCID: Pmc2883839. Epub 2010/03/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Plassman BL, Williams JW Jr., Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010. August 3;153(3):182–93. PubMed PMID: 20547887. Epub 2010/06/16. eng. [DOI] [PubMed] [Google Scholar]
- 88.McMillan L, Owen L, Kras M, Scholey A. Behavioural effects of a 10-day Mediterranean diet. Results from a pilot study evaluating mood and cognitive performance. Appetite. 2011. February;56(1):143–7. PubMed PMID: 21115083. Epub 2010/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 89.Knight A, Haines J, Stals A, Li D, Uyttendaele M, Knight A, et al. A systematic review of human norovirus survival reveals a greater persistence of human norovirus RT-qPCR signals compared to those of cultivable surrogate viruses. Int J Food Microbiol. 2016. January 4;216:40–9. PubMed PMID: 26398283. Epub 2015/09/24. Eng. [DOI] [PubMed] [Google Scholar]
- 90.2010 Alzheimer's disease facts and figures. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2010. March;6(2):158–94. PubMed PMID: 20298981. Epub 2010/03/20. eng. [DOI] [PubMed] [Google Scholar]
- 91.Anderson N, Bulatao R, Cohen B. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life National Research Council (US) Panel on Race E, and Health in Later Life., editor. Washington (DC): National Academy of Sciences.; 2004. [PubMed] [Google Scholar]
- 92.Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EH, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999. June;14(6):481–93. PubMed PMID: 10398359. Epub 1999/07/09. eng. [PubMed] [Google Scholar]
- 93.Sachs-Ericsson N, Blazer DG. Racial differences in cognitive decline in a sample of community-dwelling older adults: the mediating role of education and literacy. Am J Geriatr Psychiatry. 2005. November;13(11):968–75. PubMed PMID: 16286440. Epub 2005/11/16. eng. [DOI] [PubMed] [Google Scholar]
- 94.Volkow ND, Wang GJ, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N, et al. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity (Silver Spring). 2009. January;17(1):60–5. PubMed PMID: 18948965. Pubmed Central PMCID: 2681079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Castanon N, Luheshi G, Laye S. Role of neuroinflammation in the emotional and cognitive alterations displayed by animal models of obesity. Frontiers in neuroscience. 2015;9:229. PubMed PMID: 26190966. Pubmed Central PMCID: 4490252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC neurology. 2005. December 02;5:23. PubMed PMID: 16321166. Pubmed Central PMCID: 1325254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stanek KM, Grieve SM, Brickman AM, Korgaonkar MS, Paul RH, Cohen RA, et al. Obesity is associated with reduced white matter integrity in otherwise healthy adults. Obesity (Silver Spring). 2011. March;19(3):500–4. PubMed PMID: 21183934. [DOI] [PubMed] [Google Scholar]
- 98.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992. August;56(2):320–8. PubMed PMID: 1386186. Epub 1992/08/01. eng. [DOI] [PubMed] [Google Scholar]
- 99.Blumenthal JA, Sherwood A, Gullette EC, Babyak M, Waugh R, Georgiades A, et al. Exercise and weight loss reduce blood pressure in men and women with mild hypertension: effects on cardiovascular, metabolic, and hemodynamic functioning. Arch Intern Med. 2000. July 10;160(13):1947–58. PubMed PMID: 10888969. Epub 2000/07/11. eng. [DOI] [PubMed] [Google Scholar]
- 100.Estruch R Anti-inflammatory effects of the Mediterranean diet: the experience of the PREDIMED study. Proc Nutr Soc. 2010. August;69(3):333–40. PubMed PMID: 20515519. Epub 2010/06/03. eng. [DOI] [PubMed] [Google Scholar]
- 101.Chae JS, Paik JK, Kang R, Kim M, Choi Y, Lee SH, et al. Mild weight loss reduces inflammatory cytokines, leukocyte count, and oxidative stress in overweight and moderately obese participants treated for 3 years with dietary modification. Nutr Res. 2013;33(3):195–203. PubMed PMID: 23507225. Epub 2013/03/20. eng. [DOI] [PubMed] [Google Scholar]
- 102.Fito M, Guxens M, Corella D, Saez G, Estruch R, de la Torre R, et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch Intern Med. 2007. June 11;167(11):1195–203. PubMed PMID: 17563030. Epub 2007/06/15. eng. [DOI] [PubMed] [Google Scholar]
- 103.Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ruiz-Gutierrez V, Covas MI, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006. July 4;145(1):1–11. PubMed PMID: 16818923. Epub 2006/07/05. eng. [DOI] [PubMed] [Google Scholar]
- 104.Richard C, Couture P, Desroches S, Lamarche B. Effect of the Mediterranean diet with and without weight loss on markers of inflammation in men with metabolic syndrome. Obesity (Silver Spring). 2013. January;21(1):51–7. PubMed PMID: 23505168. Epub 2013/03/19. eng. [DOI] [PubMed] [Google Scholar]
- 105.Effect of an intensive lifestyle intervention program with an energy-restricted mediterranean diet, increased physical activity and behavioral treatment on the primary prevention of cardiovascular dieseases [pdf]. Predimed-Plus; [cited 2016]. Research Project]. Available from: http://predimedplus.com/uk/public/Protocolo_PREDIMED_PLUS_eng_03062015.pdf.
- 106.Yaffe K, Hoang T. Nonpharmacologic Treatment and Prevention Strategies for Dementia. Continuum (Minneap Minn). 2013;19(2, Dementia):372–81. PubMed PMID: 00132979-201304000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]


