Abstract
Introduction
Low cerebral blood flow can affect cognition in patients with high-grade asymptomatic internal carotid artery stenosis. Current clinical algorithms use stroke risk to determine which patients should undergo revascularization without considering cognitive decline. Although correlations between low-flow and cognitive impairment have been reported, it is not known whether a threshold exists below which such a correlation expresses itself. Such information would be critical in treatment decisions about whether to intervene in patients with high-grade carotid artery stenosis who are at risk for cognitive decline.
Objective
To determine how reduced blood flow correlates with lower cognitive scores.
Methods
Patients with ≥80% unilateral internal carotid artery stenosis with no history of stroke were recruited from inpatient and outpatient practices at a single, large, comprehensive stroke center. Patients underwent bilateral insonation of middle cerebral arteries with standard 2-Hz probes over the temporal windows with transcranial Doppler. Cognitive assessments were performed by an experienced neuropsychologist using a cognitive battery comprising 14 standardized tests with normative samples grouped by age. Z-scores were generated for each test and averaged to obtain a composite Z-score for each patient. Multivariable linear regression examined associations between mean flow velocity (MFV) and composite Z-score, adjusting for age, education, and depression. The Davies test was used to determine if there was a breakpoint for a non-zero difference in slope of a segmented relationship over the range of composite Z-score values.
Results
Forty-two patients with unilateral high-grade internal carotid artery stenosis without stroke were enrolled (26 males, age = 74 ± 9 years, education = 16 ± 3 years). Average composite Z-score was −0.31 SD below the age-specific normative mean (range −2.8 to +1.2 SD). In linear regression adjusted for age, education, and depression, MFV correlated with cognitive Z-score (β = 0.308, p = 0.043). A single breakpoint in the range of composite Z-scores was identified at 45 cm/s. For MFV <45 cm/s, Z-score decreased 0.05 SD per cm/s MFV (95% CI: 0.01–0.10). For MFV >45 cm/s, Z-score change was nonsignificant (95% CI: −0.07 to 0.05).
Conclusions
In high-grade, asymptomatic carotid artery stenosis, cognitive impairment correlated linearly with lower flow in the hemisphere fed by the occluded internal carotid artery, but only below a threshold of MFV = 45 cm/s. Identifying a hemodynamic threshold for cognitive decline using a simple, noninvasive method may influence revascularization decision-making in otherwise “asymptomatic” carotid disease.
Keywords: Carotid stenosis, Cognitive impairment, Cerebral hemodynamics, Transcranial Doppler
Introduction
Current algorithms for determining whether revascularization is indicated for patients with high-grade stenosis of the internal carotid artery are based solely on the risk of future stroke. Robust clinical trial evidence exists for carotid endarterectomy and carotid stenting when an index stroke or transient ischemic attack has occurred [1, 2, 3]. Because the incidence of initial stroke is quite low for patients with an asymptomatic status, particularly in the face of modern medical management, it has been difficult to show an advantage of revascularization over medical management alone [4]. A large, international, randomized, clinical trial is currently underway to test whether revascularization (either carotid endarterectomy or stenting) with intensive medical management is superior to intensive medical management alone in preventing subsequent stroke [5]. What has yet to be incorporated into management algorithms for asymptomatic carotid artery disease is cognitive impairment. Despite several lines of evidence that carotid artery disease is associated with cognitive decline, it has been difficult to quantify this relationship in a sufficiently compelling way to be adopted for clinical use.
Patients with carotid artery disease may have cognitive impairment for a variety of reasons. Clinically apparent strokes as well as silent infarction from carotid stenosis may alter cognition [6, 7]. Risk factors for atherosclerotic carotid disease − diabetes, hypertension, and the metabolic syndrome − have in themselves been associated with cognitive dysfunction [8, 9, 10]. Most pertinent to the current study, an increasing body of evidence suggests that cerebral hemodynamic impairment in patients with high-grade carotid artery stenosis may independently contribute to cognitive dysfunction [11, 12]. Among patients with “asymptomatic” carotid stenosis, this evidence carries the most weight because the confounding factors of stroke-related cognitive disturbance are not present [13, 14, 15, 16]. It is conceivable, therefore, that if hemodynamic impairment due to the carotid stenosis is a factor in cognitive decline, then improved cerebral blood flow following carotid revascularization by surgery or stenting could lead to improvements in cognition. Because carotid disease management algorithms have focused on the prevention of frank stroke rather than on cognitive outcomes, relatively little attention has been paid to the nature of the relationship between blood flow and cognition in this population.
Materials and Methods
Subjects
Patients were recruited from the Columbia Stroke Neurology in- and outpatient services, and through the Columbia Neurosonology (Doppler) laboratory. Inclusion criteria were age 18–85 years, fluent in English, able to give informed consent, ≥80% carotid stenosis, or complete occlusion by carotid Doppler, MRA, or CTA, and no stroke. A transient ischemic attack was allowed provided no acute infarct was present on brain imaging. Exclusion criteria were prior clinical stroke, diagnosis of dementia, head trauma causing loss of consciousness, substance abuse, active psychiatric disease, NYHA stage 3/4 congestive heart failure, and inadequate sonographic windows for transcranial Doppler (TCD).
Neurocognitive Testing
Patients underwent a 1-h neurocognitive battery, administered by a trained examiner under the supervision of an experienced neuropsychologist. All neurocognitive testing was audiotaped for quality assurance. The battery consisted of 14 standardized neuropsychological tests which were designed to assess left hemisphere function, right hemisphere function, and global (bilateral) function (Table 1), used previously for carotid disease research [15]. All tests had published, normative samples grouped by age. All patients were administered all 14 neurocognitive tests. The order of test administration remained constant to accommodate delays required for recall intervals and to minimize interference between memory and language tasks.
Table 1.
Neurocognitive battery
| Neurocognitive tests | Hemisphere specificity | Outcome variable |
|---|---|---|
| Trails A | Global | Time (s) |
| Trails B | Global | Time (s) |
| Digit span | Global | Digits forward + backward |
| Digit symbol | Global | Correct number of symbols |
| Boston naming | Left | Number correct |
| BDAE repetition | Left | Number correct |
| Hopkins verbal memory | Left | Total (3 trials + delayed recall) |
| COWA word fluency | Left | Total number words (F/A/S) |
| Rey figure copy | Right | Score |
| Rey figure recall | Right | Score |
| Line bisection | Right | Percent deviation |
| Target cancellation | Right | Number correct + search pattern |
| Grooved pegboard contralateral hand | Left/right | Time (s) |
| Grooved pegboard ipsilateral hand | Global | Time (s) |
Scoring
Raw test scores were transformed into Z-scores, derived from published normative samples grouped by age. Z-scores were summed and divided by the number of tests completed to calculate a composite Z-score for each patient. The generation of a composite average score obviated the need to correct for multiple comparisons within the battery.
Measurement of Blood Flow
Middle cerebral arteries were located through the temporal windows at a depth of 50–58 mm using 2-MHz probes (Terumo Trifid PMD150B; Spencer Technologies, Seattle, WA, USA) attached to a standard headframe to provide a continuous recording of cerebral blood flow velocity waveforms. Peak systolic velocity, end-diastolic velocity, and mean flow velocity (MFV) were recorded for 10 min with the patient lying supine.
Statistical Analysis
Pearson correlations and multivariable linear regression were used to look for associations between MFV and composite Z-score, adjusting for age, education, and depression, entering variables stepwise with p ≤ 0.10 (SPSS v.22). The Davies test [17] was used to determine whether there was a single breakpoint for a non-zero difference in slope of a segmented relationship between the main variables of interest: MFV and composite Z-score. Subsequently, a piecewise linear model was fitted, assuming 1 (unknown) breakpoint.
Results
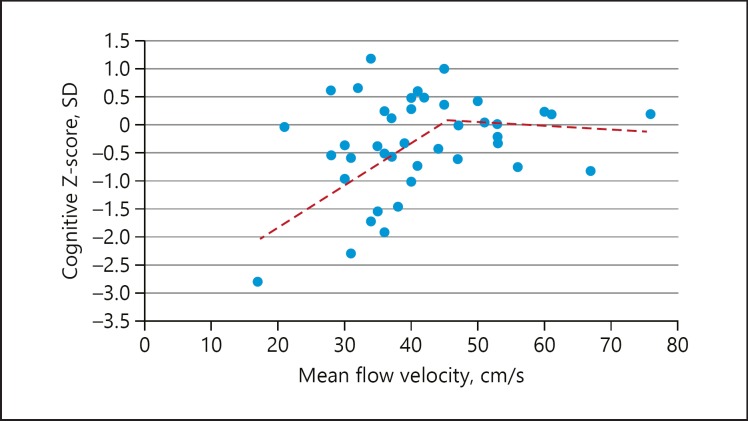
Forty-eight patients were enrolled. Of those, 3 had insufficient temporal windows for TCD insonation, and 3 additional patients were unable to undergo neurocognitive testing − 2 because of poor vision and 1 whose education was at the 2nd grade level, preventing normative scoring of cognitive data. Of the 42 remaining subjects, 26 were males; their average age was 72 years (SD = 9.0), and average MFV was 41 cm/s (range 17–76). Average composite Z-score was −0.31 SD below the normative mean (range = −2.8 to 1.2). Unadjusted correlation between MFV and composite Z-score approached but did not reach significance (R = 0.294, p = 0.059). Depression did not correlate with composite Z-score (R = −0.162, p = 0.293). Because cognitive scores were already normalized for age, age did not correlate with composite Z-score (R = 0.017, p = 0.914). Education correlated with composite Z-score (R = 0.345, p = 0.022). In the linear regression model adjusted for education, lower MFV was associated with impaired cognitive composite Z-score: (β = 0.308, p = 0.043). Figure 1 shows a scatterplot of the correlation between MFV and composite Z-score. Visual inspection suggested that the relationship between both variables might not be uniformly linear. The Davies test for non-zero difference in slope of a segmented relationship yielded a single breakpoint at 45 cm/s (p = 0.061) and demonstrated distinct linear relationships above and below the breakpoint. For MFV <45 cm/s, the Z-score decreased 0.05 SD per unit decrease in MFV (95% CI: 0.01–0.10). By contrast, for MFV >45 cm/s, the Z-score showed no significant change per unit increase in MFV (95% CI: −0.07 to 0.05).
Fig. 1.
Segmented relationship between mean flow velocity (MFV) by transcranial Doppler and composite cognitive Z-score. There is a linear correlation between MFV and cognitive Z-score below a threshold of MFV = 45 cm/s, whereas above MFV = 45 cm/s there is no change in cognition with change in MFV.
Discussion/Conclusions
Associations between cerebral blood flow and cognition have been reported in high-grade asymptomatic carotid stenosis, but prior studies have only partially quantified the nature of the cognition-hemodynamic relationship. In the current study, we used an easily attainable hemodynamic measure − MFV by TCD − to show that reduced blood flow has a linear relationship to cognitive impairment in the setting of asymptomatic high-grade carotid artery disease, but only below an identifiable flow velocity threshold. Cognitive function, as measured by a composite Z-score on a standard neurocognitive test battery, declined in a linear fashion below a threshold of 45cm/s. This threshold is in the low normal range for patients from 60 to 80 years of age [18]. In contrast, cognition remained constant when flow velocities were >45 cm/s. The relationship did not hold for every individual. As illustrated in Figure 1, 9 of the patients whose MFV was <45 cm/s had Z-scores ≥0, and 5 with MFV ≥45 cm/s had Z-scores <0. In addition, the average degree of cognitive decline was relatively mild, which has been previously reported for this population [16]. Nonetheless, our findings have implications for the management of carotid artery disease. Once the threshold is crossed, the lower the flow, the worse the cognition is likely to become, and even mild levels of cognitive impairment can affect quality of life [19].
In most studies seeking associations between hemodynamic impairment and cognitive dysfunction in carotid artery disease, only dichotomized thresholds have been used. For example, impaired vasomotor reactivity measured with a Doppler-based breath holding index has been associated with impaired cognition at a threshold <0.69 [14]. Using the breath holding method with this threshold, investigators showed that there was an increased likelihood of global cognitive deterioration measured by the modified Mini-Mental State Examination over the course of 36 months [13]. In the Randomized Evaluation of Carotid Occlusion and Neurocognition (RECON) study, it was also shown that patients with unilateral internal carotid artery occlusion and transient ischemic attack but no clinical stroke had cognitive change that occurred beyond a threshold oxygen extraction fraction asymmetry index of 1.13 [15].
Our finding of a linear correlation between blood flow and cognition below a certain threshold has implications for the management of carotid artery disease. Analogous findings for a threshold with linear decline have been reported in other cardiovascular systems. For example, in patients with aortic stenosis, asymptomatic cardiac status is preserved until a specific threshold of aortic valve stenosis is reached (<1.0 cm2 diameter or valve pressure gradient of >40 mm Hg [20]) at which point progressive, symptomatic, congestive heart failure ensues as the stenosis or pressure gradient worsens [21]. A second example is the effect of dolichoectasia in the vertebrobasilar system. Remarkably long periods of asymptomatic status may exist until a threshold of the mass effect on the brainstem is reached, at which point patients' functional status begins to decline [22]. Finally, in addition to large-vessel contributions to flow, cognitive dysfunction correlates with reduced brain blood flow in small-vessel disease [23, 24]. Affected cognitive functions overlap with those in the current study, including tests of memory and executive function. These investigators also showed a linear correlation between lower flow and cortical thinning, a marker also associated with cognitive impairment [25, 26]. This correlation was also demonstrated in patients with carotid disease [27].
In conclusion, our quantitative characterization of cerebral hemodynamic impairment in high-grade carotid stenosis reveals a segmented relationship between flow and cognitive impairment, which advances our understanding of vascular cognitive impairment in this population. Such an understanding may lead to a shift in treatment algorithms for what would otherwise be considered “asymptomatic” carotid artery stenosis, raising the issue whether patients with flow-limiting stenosis should be considered at risk for symptomatic cognitive decline, not just risk of stroke. The question of reversibility of hemodynamically induced cognitive decline is currently being tested in the Carotid Revascularization Endarterectomy versus Stenting Trial − Hemodynamics (CREST-H) [28], a substudy of the Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial (CREST-2] [5].
The public health implications are high, since 124,000 individuals over 60 years of age harbor high-grade carotid stenosis in the US alone [29]. Further investigation in a larger sample is warranted.
Statement of Ethics
All patients signed an informed consent for participation in this study, which was approved by the Columbia Institutional Review Board.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding Sources
This work was funded by a grant from the National Institute of Neurological Disorders and Stroke 1R01NS076277 (NIH) to R.S. Marshall and R.M. Lazar.
Author Contributions
R.S. Marshall: conception and design, drafting the work, interpretation, and critical revisions; M.A. Pavol: data acquisition, interpretation, and critical revisions; Y.K. Cheung: data analysis, interpretation, and critical revisions; I. Asllani: interpretation and critical revisions; R.M. Lazar: conception and design, interpretation, and critical revisions.
Data Availability
Upon request, data are available from the corresponding author.
References
- 1.Barnett HJ, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, et al. North American Symptomatic Carotid Endarterectomy Trial Collaborators Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991 Aug;325((7)):445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 2.Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, et al. CREST Investigators Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010 Jul;363((1)):11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaturvedi S, Bruno A, Feasby T, Holloway R, Benavente O, Cohen SN, et al. Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology Carotid endarterectomy—an evidence-based review: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2005 Sep;65((6)):794–801. doi: 10.1212/01.wnl.0000176036.07558.82. [DOI] [PubMed] [Google Scholar]
- 4.den Hartog AG, Achterberg S, Moll FL, Kappelle LJ, Visseren FL, van der Graaf Y, et al. SMART Study Group Asymptomatic carotid artery stenosis and the risk of ischemic stroke according to subtype in patients with clinical manifest arterial disease. Stroke. 2013 Apr;44((4)):1002–7. doi: 10.1161/STROKEAHA.111.669267. [DOI] [PubMed] [Google Scholar]
- 5.Howard VJ, Meschia JF, Lal BK, Turan TN, Roubin GS, Brown RD, Jr, et al. CREST-2 study investigators Carotid revascularization and medical management for asymptomatic carotid stenosis: protocol of the CREST-2 clinical trials. Int J Stroke. 2017 Oct;12((7)):770–8. doi: 10.1177/1747493017706238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao R. The role of carotid stenosis in vascular cognitive impairment. Eur Neurol. 2001;46((2)):63–9. doi: 10.1159/000050765. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Tang H, Sun J, Wang B, Chen S, Fu Y. Analysis of cognitive dysfunction with silent cerebral infarction: a prospective study in Chinese patients. Metab Brain Dis. 2012 Mar;27((1)):17–22. doi: 10.1007/s11011-011-9275-5. [DOI] [PubMed] [Google Scholar]
- 8.van Duinkerken E, Ryan CM. Diabetes mellitus in the young and the old: effects on cognitive functioning across the life span. Neurobiol Dis. 2020 Feb;134:104608. doi: 10.1016/j.nbd.2019.104608. [DOI] [PubMed] [Google Scholar]
- 9.Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, et al. Blood Pressure and Cognitive Decline Over 8 Years in Middle-Aged and Older Black and White Americans. Hypertension. 2019 Feb;73((2)):310–8. doi: 10.1161/HYPERTENSIONAHA.118.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reijmer YD, van den Berg E, Dekker JM, Nijpels G, Stehouwer CD, Kappelle LJ, et al. The metabolic syndrome, atherosclerosis and cognitive functioning in a non-demented population: the Hoorn Study. Atherosclerosis. 2011 Dec;219((2)):839–45. doi: 10.1016/j.atherosclerosis.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Bakker FC, Klijn CJ, Jennekens-Schinkel A, van der Tweel I, van der Grond J, van Huffelen AC, et al. Cognitive impairment is related to cerebral lactate in patients with carotid artery occlusion and ipsilateral transient ischemic attacks. Stroke. 2003 Jun;34((6)):1419–24. doi: 10.1161/01.STR.0000069725.09499.14. [DOI] [PubMed] [Google Scholar]
- 12.Marshall RS, Lazar RM. Pumps, aqueducts, and drought management: vascular physiology in vascular cognitive impairment. Stroke. 2011 Jan;42((1)):221–6. doi: 10.1161/STROKEAHA.110.595645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balestrini S, Perozzi C, Altamura C, Vernieri F, Luzzi S, Bartolini M, et al. Severe carotid stenosis and impaired cerebral hemodynamics can influence cognitive deterioration. Neurology. 2013 Jun;80((23)):2145–50. doi: 10.1212/WNL.0b013e318295d71a. [DOI] [PubMed] [Google Scholar]
- 14.Silvestrini M, Paolino I, Vernieri F, Pedone C, Baruffaldi R, Gobbi B, et al. Cerebral hemodynamics and cognitive performance in patients with asymptomatic carotid stenosis. Neurology. 2009 Mar;72((12)):1062–8. doi: 10.1212/01.wnl.0000345015.35520.52. [DOI] [PubMed] [Google Scholar]
- 15.Marshall RS, Festa JR, Cheung YK, Chen R, Pavol MA, Derdeyn CP, et al. Cerebral hemodynamics and cognitive impairment: baseline data from the RECON trial. Neurology. 2012 Jan;78((4)):250–5. doi: 10.1212/WNL.0b013e31824365d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lal BK, Dux MC, Sikdar S, Goldstein C, Khan AA, Yokemick J, et al. Asymptomatic carotid stenosis is associated with cognitive impairment. J Vasc Surg. 2017 Oct;66((4)):1083–92. doi: 10.1016/j.jvs.2017.04.038. [DOI] [PubMed] [Google Scholar]
- 17.Haybach K. Testing for a Breakpoint in Two-Phase Linear and Logistic Regression Models. Sonderforschungsbereich. 1997;386:77. [Google Scholar]
- 18.Tegeler CH, Crutchfield K, Katsnelson M, Kim J, Tang R, Passmore Griffin L, et al. Transcranial Doppler velocities in a large, healthy population. J Neuroimaging. 2013 Jul;23((3)):466–72. doi: 10.1111/j.1552-6569.2012.00711.x. [DOI] [PubMed] [Google Scholar]
- 19.Pavol MA, Sundheim K, Lazar RM, Festa JR, Marshall RS. Cognition and Quality of Life in Symptomatic Carotid Occlusion. J Stroke Cerebrovasc Dis. 2019 Aug;28((8)):2250–4. doi: 10.1016/j.jstrokecerebrovasdis.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014 Jun;63((22)):2438–88. doi: 10.1016/j.jacc.2014.02.537. [DOI] [PubMed] [Google Scholar]
- 21.Antonini-Canterin F, Pavan D, Burelli C, Cassin M, Cervesato E, Nicolosi GL. Validation of the ejection fraction-velocity ratio: a new simplified “function-corrected” index for assessing aortic stenosis severity. Am J Cardiol. 2000 Aug;86((4)):427–33. doi: 10.1016/s0002-9149(00)00959-0. [DOI] [PubMed] [Google Scholar]
- 22.Xu DS, Levitt MR, Kalani MY, Rangel-Castilla L, Mulholland CB, Abecassis IJ, et al. Dolichoectatic aneurysms of the vertebrobasilar system: clinical and radiographic factors that predict poor outcomes. J Neurosurg. 2018 Feb;128((2)):560–6. doi: 10.3171/2016.10.JNS161041. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida J, Yamashita F, Sasaki M, Yoshioka K, Fujiwara S, Kobayashi M, et al. Adverse effects of pre-existing cerebral small vessel disease on cognitive improvement after carotid endarterectomy. Int J Stroke. 2019 doi: 10.1177/1747493019874732. DOI: 1747493019874732. [DOI] [PubMed] [Google Scholar]
- 24.Alosco ML, Gunstad J, Jerskey BA, Xu X, Clark US, Hassenstab J, et al. The adverse effects of reduced cerebral perfusion on cognition and brain structure in older adults with cardiovascular disease. Brain Behav. 2013 Nov;3((6)):626–36. doi: 10.1002/brb3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weston PS, Nicholas JM, Lehmann M, Ryan NS, Liang Y, Macpherson K, et al. Presymptomatic cortical thinning in familial Alzheimer disease: A longitudinal MRI study. Neurology. 2016 Nov;87((19)):2050–7. doi: 10.1212/WNL.0000000000003322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo SW, Ahn J, Yoon U, Im K, Lee JM, Tae Kim S, et al. Cortical thinning in vascular mild cognitive impairment and vascular dementia of subcortical type. J Neuroimaging. 2010 Jan;20((1)):37–45. doi: 10.1111/j.1552-6569.2008.00293.x. [DOI] [PubMed] [Google Scholar]
- 27.Marshall RS, Asllani I, Pavol MA, Cheung YK, Lazar RM. Altered cerebral hemodyamics and cortical thinning in asymptomatic carotid artery stenosis. PLoS One. 2017 Dec;12((12)):e0189727. doi: 10.1371/journal.pone.0189727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall RS, Lazar RM, Liebeskind DS, Connolly ES, Howard G, Lal BK, et al. Carotid revascularization and medical management for asymptomatic carotid stenosis - Hemodynamics (CREST-H): study design and rationale. Int J Stroke. 2018 Dec;13((9)):985–91. doi: 10.1177/1747493018790088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Weerd M, Greving JP, Hedblad B, Lorenz MW, Mathiesen EB, O'Leary DH, et al. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta-analysis. Stroke. 2010 Jun;41((6)):1294–7. doi: 10.1161/STROKEAHA.110.581058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request, data are available from the corresponding author.