Abstract
Background/Aims
Crohn’s disease (CD) is a chronic inflammatory condition characterized by various abnormalities that lead to overly aggressive T cell responses. Our in vitro experiments aimed to investigate the potential use of Dental Follicle Mesenchymal Stem Cells (DF-MSCs) to suppress the exaggerated immune response in inflamed and noninflamed tissue of CD.
Materials and Methods
Dental follicle tissues were obtained from extracted third molar teeth of three healthy volunteers who had no abscess or inflammatory diseases. In total, 11 patients were included in the experiment who had been diagnosed with CD and did not receive steroid maintenance therapy for more than one month. Mononuclear cells were isolated from inflamed and noninflamed tissues of CD. Isolated cells were stimulated with anti-CD3/anti-CD28 monoclonal antibodies in the presence and absence of DF-MSCs and analyzed for lymphocytes proliferation capacity and viability, T lymphocyte subsets, CD4+IL22BP and CD4+CD25+Foxp3+ regulatory T cell (Treg) frequencies and cytokine levels.
Results
A significant downregulation of lymphocyte proliferation and CD4+IL22BP T cell ratio were found in inflamed cultures with DF-MSCs (p<0.005). Additionally, the frequency of Tregs increased with DF-MSCs (p<0.05). Proinflammatory cytokine levels (Tumor Necrosis Factor (TNF)-α and IL-6) were decreased (p<0.05), and IL-10 levels were increased (p<0.05) in the supernatant of inflamed cultures.
Conclusion
DF-MSCs reduced the inflammatory immune response, induced Tregs, and downregulated CD4+IL22BP T cell ratio in inflamed samples of CD patients, which may be exploited for significant therapeutic use.
Keywords: Mesenchymal stem cells, apoptosis, inflammatory bowel disease, immunomodulation, inflammation
INTRODUCTION
Crohn’s disease (CD) is a chronic inflammatory condition marked by remissions and exacerbations and causes a significant decrease in the patients’ quality of life. Mucosal ulceration and inflammation may most affect the distal small intestine (1). Distinguishing features include discontinuous, transmural inflammation involving the whole thickness of the bowel wall and an inflammatory response associated with lymphoid aggregates and granulomas (2). According to some hypothesis, an altered microbial flora, impaired mucosal barrier, environmental conditions, and immune dysregulation can cause inflammatory immune response (3). An increase in effector T helper (Th) subset activation (Th1/Th17) has been documented, along with reduced regulatory T cell (Treg) levels in CD patients (4). Proinflammatory cytokines levels TNF-α, IL-6, and IFN-γ increase in CD patients, but there is no clear evidence about IL-10 levels (5). Additionally, interleukin-22 (IL-22) is up regulated in the intestine of CD patients and able to increase mucosal healing. However, when uncontrolled, it can lead to intestinal pathogenesis (6). IL-22BP specifically binds to IL-22 and prevents the binding of IL-22 to membrane bound IL-22R1. The binding of IL-22 to IL-22BP is of 20- to 1000-fold higher affinity compared with its binding to the membrane bound IL-22R1(7). Careful regulation of IL-22 and IL-22BP regulate homeostasis in the intestine. A previous study demonstrated that CD4+IL22BP frequency was high in inflamed tissue of CD patients (8).
Complications of the disease include diarrhea, weight loss, lethargy, severe acute pain, and vague abdominal pain. Several first-line treatment modalities are available for induction of remission and promote mucosal healing, including steroid, anti-TNF, thiopurines, methotrexate, and infliximab. In some certain condition such as ineffective medical therapy, intestinal obstruction, malnutrition, and malignancies operative management is reserved. Because of refractory nature to therapy and common side effects of conventional and biological therapies, safe and effector novel treatment strategies are required in CD treatment (9–11). Mesenchymal stem cells (MSCs) are considered an alternative approach to the conventional therapy and surgical operation for inflammatory-based diseases with their immunomodulatory and anti-inflammatory effects.
MSCs are fibroblast-like cells that have the capacity to adhere to plastic surfaces; form colonies derived from single cells (colony forming unit-fibroblasts) and can differentiate into mature cells of mesenchymal lineages, such as osteoblasts, adipocytes, and chondrocytes (12). Its sources include bone marrow, adipose tissue, placenta, umbilical cord, dental stem cells, and connective tissue of most organs (13). Oral tissues are easily accessible as a source of MSCs. Dental follicle MSCs (DF-MSCs) have considerable self-renewal capacity, doubling time, and multidifferentiation capacity with an easy lifelong accessible source of dental stem cells. They show immune-suppressive effect compared with the other dental sources (14, 15).
The most exciting data from in vivo studies have demonstrated that MSCs could suppress inflammatory responses in an experimental colitis model. The administration of human umbilical cord blood-derived MSCs could ameliorate symptoms in mouse model through induced/activated CD4+CD25+Foxp3+ Treg with suppressive capacity on Th1 effector responses (16). However, the effect of DF-MSCs in T cell subset and cytokine levels of CD patients still remain unclear.
To provide the evidence and the reference in the field of stem cell therapeutics for CD, we examined the immunomodulatory effect of DF-MSCs on CD patients in vitro. For this purpose, we isolated peripheral mononuclear cells (MNCs) from inflamed and noninflamed intestine biopsy specimens of CD patients and culture in presence and absence of DF-MSCs. We investigated the modulatory effects of DF-MSCs on CD4+ Th and CD8+ Tc cell distribution, lymphocyte proliferation, cell viability, frequency of CD4+IL-22BP cells, and cytokine levels of culture supernatants and compared with healthy subjects.
MATERIALS AND METHODS
Study subjects
This study was approved by the Ethics Committee of Marmara University School of Medicine, Istanbul, Turkey (Protocol No: 09.2017.456). Written informed consent was obtained from all patients. We prospectively collected colonoscopic biopsy samples from 11 CD patients (F/M: 6/5, median age of 43.6±7.4 years, range of 32–59 years) between June 2015 and June 2018. The inclusion criteria were as follows: above 18 years of age, with moderate to severe CD (CD activity index >150). None of patients received steroid maintenance therapy for more than one month before enrollment. Patients were excluded for active tuberculosis, malignancy, and infection of human immunodeficiency virus, syphilis, hepatitis B virus, and hepatitis C virus. Patients underwent colonoscopy at enrollment to assess the degree of disease activity (Figure 1).
Figure 1.
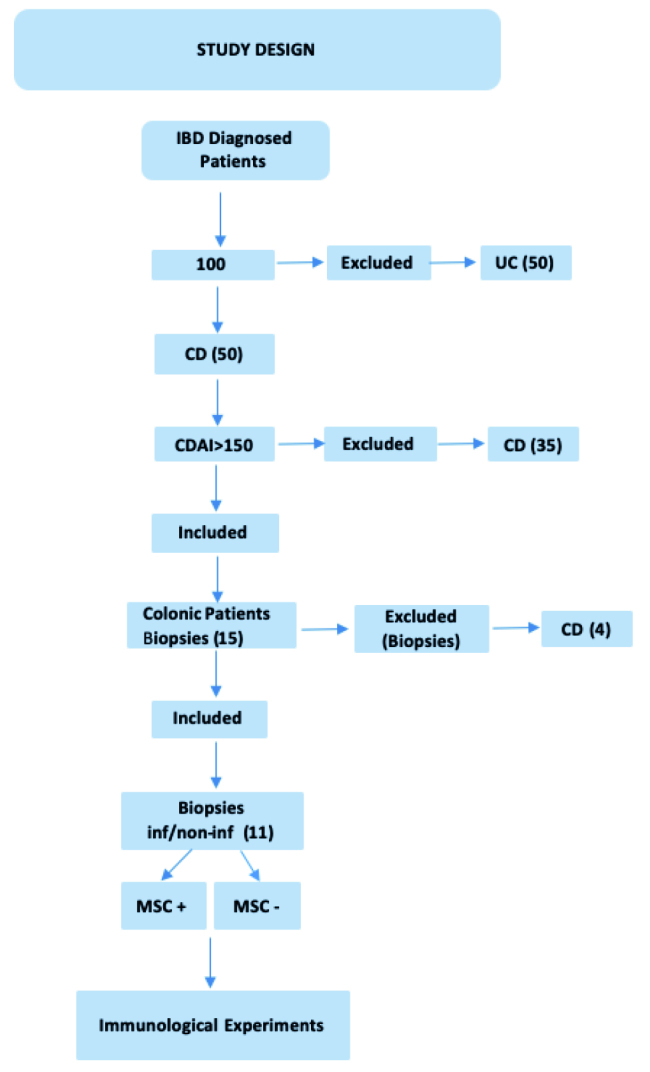
Patients’ clinical features at enrollment (inf: inflamed, non-inf: noninflamed).
Intestine tissue biopsies
Biopsies (3×3 mm) were obtained from both inflamed and noninflamed tissue of ileum during colonoscopy. Noninflamed areas were considered those where a normal vascular pattern and an intact mucosa were observed, whereas inflamed areas were those where the normal vascular pattern disappeared, and various combinations of the following lesions were evident: edema, erythema, erosions, ulcers, pseudopolyps, granular and spontaneously bleeding zones, and nodular-cobblestone appearance. Samples were transported in Dulbecco’s phosphate-buffered saline (Gibco, Grand Island, USA) containing 1% penicillin/streptomycin (P/S, Gibco, Grand Island, USA). All samples were transferred to the laboratory.
DF-MSCs isolation, characterization, and differentiation
DF-MSCs were isolated from extracted dental follicle tissues of three healthy adults (F/M: 2/1). Written informed consent was obtained from all patients. Briefly, dental follicle tissues were cut into 0.5 mm pieces and enzymatically digested with 3 mg mL−1 collagenase type I (Gibco, USA) for 45 min at 37°C. Single-cell suspensions were obtained by passing the cells through a 70-mm strainer. The obtained cell suspensions were washed for two times, then rinsed in with Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS, Gibco, Paisly, UK) and 1% P/S (referred to as complete DMEM) and cultured in 25 cm2 culture flasks. Nonadherent cells were removed by changing the cultivation medium.
Third passage DF-MSCs was used for all phenotype identifications and coculture experiments. To evaluate the expression of surface markers, DF-MSCs were detached, washed, and resuspended in phosphate-buffered saline (PBS; Gibco, Gaithersburg, MD). After fixing and blocking, the cells were incubated with FITC or PE-conjugated antihuman antibodies specific to CD34, CD45, CD14, CD28, CD25, CD73, CD90, CD146, and CD105. The mouse IgG served as isotype control. All reagents were purchased from BD Biosciences. Data were analyzed by flow cytometry (FACSCalibur; BD Biosciences, San Jose, CA) with Cell Quest software.
DF-MSCs were induced to differentiate into osteocytes, adipocytes, and chondrocytes using StemPro Osteogenesis Differentiation (Gibco, Grand Island, NY), StemPro Adipogenesis Differentiation Kit (Gibco, Grand Island, NY), and StemPro Chondrogenesis Differentiation Kit (Gibco, Grand Island, NY) according to manufacturer’s protocol. Briefly, cells (1× 105/well) were seeded in 6-well plates, and 21–28 days after seeding, the cells were replaced with differentiation mediums. The cells were grown with medium replacement twice a week. Osteogenesis was detected by alizarin red. Adipogenesis was determined by oil red O staining. Chondrogenesis was assessed by alcian blue staining. All Images of stained cells were captured using the light microscope.
Mononuclear cells isolation from inflamed and noninflamed tissue
Cell isolation was carried out following a previously described protocol (17). In brief, five endoscopic biopsies were taken from each of the inflamed and noninflamed area of the ileum and placed into PBS with P/S. All samples were incubated in Hanks’ Balanced Salt Solution (Gibco, Green Island, USA) with (Ethylenediaminetetraacetic acid) EDTA in order to remove the mucosal epithelium. The biopsies were incubated for 4 h in RPMI 1640 (RPMI medium) with collagenase type VI (Sigma, Grand Island, USA), penicillin, streptomycin, and amphotericin b (Gibco, Green Island, USA) at 37°C. The solution and remaining tissue were filtered, and the collected cellular suspension was separated by a Percoll 40%–100% gradient. The upper layer and the interphase part were collected and washed for culture.
Coculture of DF-MSCs with MNCs
In cocultures, DF-MSCs (2×104/well in a 96-well plate) were plated 48 h before the addition of 2×105 number of MNCs, to generate adherent monolayers in plates with complete culture medium. DF-MSCs and MNCs (1:10) were cocultured for three days. The cultures were stimulated using 0.5 μg mL−1 anti-CD3 (eBioscience, San Diego, CA) and 0.5 μg mL−1 CD28 (eBioscience, San Diego, CA).
CFSE assay and evaluation of lymphocyte proliferation
The proliferation of lymphocytes was evaluated by labeling PBMC with 10 μM carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen, USA). The lymphocytes were cultured unstimulated or stimulated with anti-CD3/anti-CD28 (CDmix) in the presence and absence of DF-MSCs for 72 h. Then, they were analyzed for CFSE signaling via flow cytometry (FACS Calibur).
Apoptosis analysis with annexin V/PI
After 72 h culture of lymphocytes with or without DF-MSCs, cells were collected and washed two times with 1× PBS. The pellet was resuspended in 1× binding buffer. Resuspend cells were added with 5 μl of Annexin V (PE) and 5 μl of PI and was incubated for 15 min at RT (25°C) in the dark according to manufacturer’s protocol (eBioscience). Cells were analyzed by flow cytometry.
Immunophenotyping
A panel of lymphocyte subpopulations was identified by multicolor flow cytometry. Briefly, lymphocyte which culture with or without DF-MSCs were washed in flow cytometry buffer (PBS containing 1% fetal bovine serum and 0.01% sodium azide) and treated with Fc block solution for 20 min. In order to evaluate specific lymphocyte subsets, cells were stained for 30 min with combinations of the following monoclonal human antibodies: anti-CD4 PE, anti-CD4 FITC, anti-CD8 PE, and anti-IL22BP APC (all from BD Biosciences). Immediately after staining, cells were washed, resuspended, and analyzed by flow cytometry. The instrument was checked for sensitivity and overall performance with Cytometer Setup and Tracking beads (FACSCalibur; BD Biosciences, San Jose, CA, USA) before data acquisition. Data were analyzed using CellQuest Software.
Intracellular detection of Foxp3
CD4+CD25+Foxp3+ cells were analyzed in inflamed and noninflamed cultures after 72 h. The cultures were assessed through flow cytometry using human Treg Kit according to the manufacturer’s instructions (eBioscience, USA). Briefly, cells were washed for twice with cold 1× PBS. The pellet was stained with anti-CD4 and anti-CD25 for 20 min in dark place. After incubation, the cells were washed, and the pellets were incubated with fixation/permeabilization buffer for 30 min. The cells were washed with 1× permeabilization buffer twice. The pellet was incubated with Foxp3 antibody. After incubation, cells were washed twice with 1× permeabilization buffer and centrifuged at 1500 rpm for 5 min. A minimum of 100,000 events on lymphocytes population was acquired for analysis. In the flow cytometric analysis, CD25+ cells were gated for CD4+ and Foxp3+ cells ratio.
Analysis of cytokine levels
Culture supernatants (IL-6, IL-10, IFN-γ, TNF-α) were measured simultaneously in the culture supernatants by cytokine bead array kit (BD Biosciences, USA). In total, 50 μL of culture supernatants were collected and coated with capturing antibodies. Samples were acquired in a FACSCalibur flow cytometer (BD Biosciences) and analyzed using the FCAP Array v1.0.1 software (Soft Flow Inc.). Results are expressed as picograms per milliliter.
Statistical Analysis
The statistical analysis was achieved by using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). One-way analysis of variance with Tukey’s multiple comparisons was used for multigroup comparisons, and a two-tailed unpaired Student’s t test was used for comparisons between the two groups; p<0.05 was considered statistically significant.
RESULTS
Isolation, characterization, and differentiation of DF-MSCs
The DF-MSCs were gradually formed in small colonies in three days and reached 70% confluency in the primary culture seven days after plating for the first passage. Most of the DF-MSCs exhibited fibroblast-like morphology at the P3 passage.
DF-MSCs exhibited positive staining for CD29, CD90, CD 146, CD73, and CD106, and lack the expression of CD34, CD45, CD14, CD28, and CD25.
DF-MSCs differentiated into osteocytes, adipocytes, and chondrocytes. First, the osteogenic differentiation capability was investigated with osteogenic induction medium. The DF-MSCs were stained with alizarin red, and the cells formed calcified bone nodule structures. Next, in vitro adipogenic differentiation capability was assessed by culturing the cells in adipogenic induction medium and staining with oil red O. Intracellular lipid droplets were observed in these cells. Chondrogenic differentiation capability was investigated in vitro following the culture period in chondrogenic induction medium and cell differentiation into chondrocytes was confirmed with alcian blue staining, which exhibited intracellular proteoglycans in those cells (Figure 2).
Figure 2.
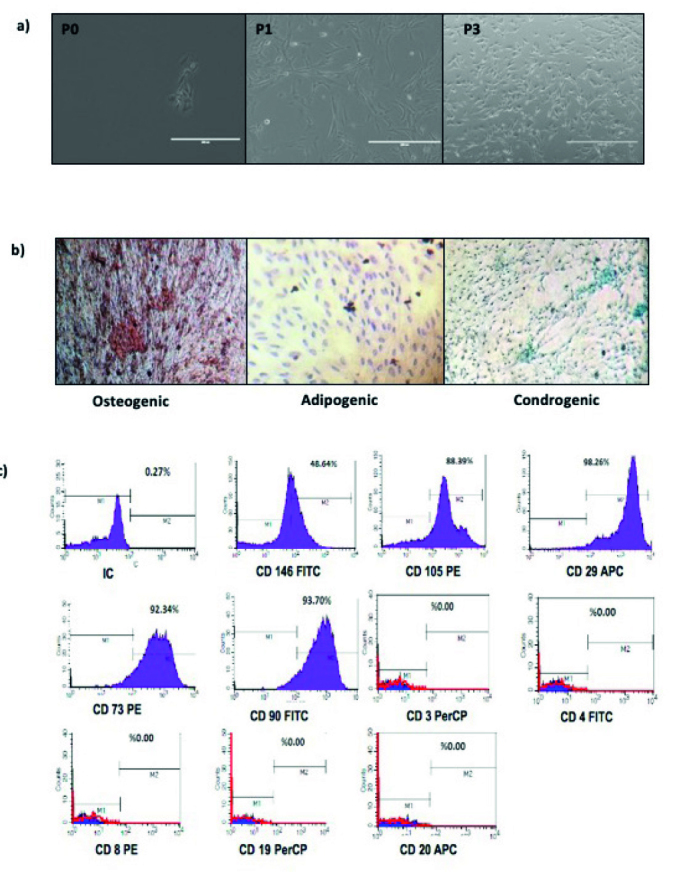
a–c. Morphological appearance, characterization, and differentiation of MSCs. (a) Morphology of DF-MSCs in P0, P1 and P3 (magnification = 10×). (b) Alizarin red staining of osteogenic induced MSCs, oil red O staining of adipogenic induced MSCs, alcian blue staining of chondrogenic induced DF-MSCs, scale bar=1000 μm. (c) Representative flow cytometry analysis of positive surface markers CD105, CD146, CD90, CD73, CD29 and negative surface marker CD25, CD28, CD14, CD45, CD34 for MSCs at the third passages.
DF-MSCs Suppressed the Proliferative Response of T Lymphocytes in Inflamed Culture
We first assessed the inhibitory effect of DF-MSCs on the proliferation of T lymphocytes of Crohn’s patients. According to our results, the proliferative response of CD lymphocytes is high in inflamed culture compared with noninflamed culture (53.77±5.25, 30.70±2.64; p<0.001, respectively). T lymphocytes proliferation capacity was decreased in the presence of DF-MSCs compared with the absence of DF-MSCs in inflamed culture (p<0.005). T cell proliferation tends to decrease in the presence of DF-MSCs in noninflamed group, but it was not statistically significant (Figure 3).
Figure 3.
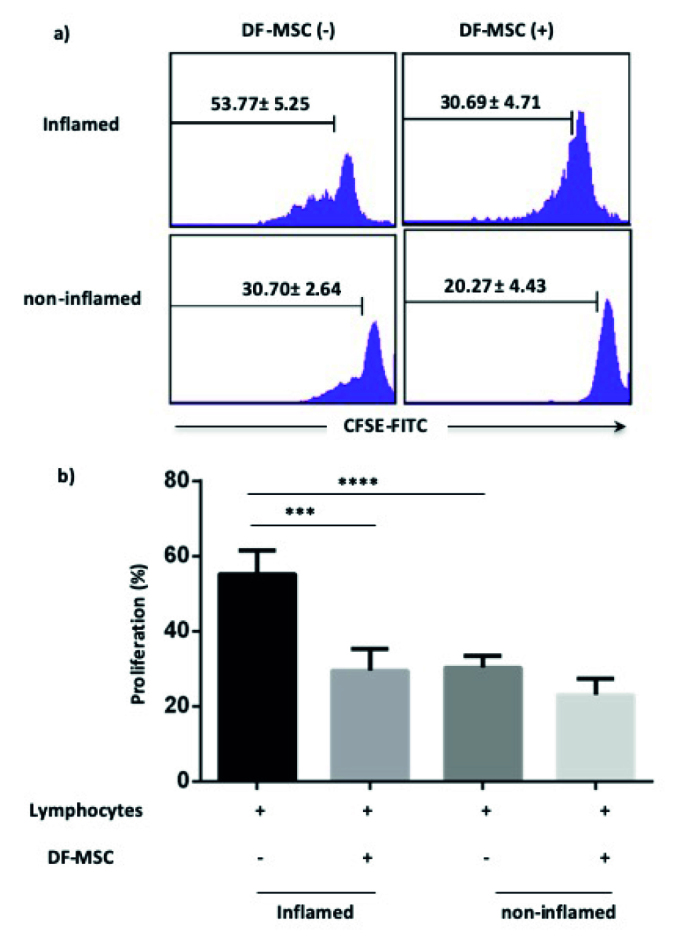
a, b. Inhibitory effect of DF-MSCs on the proliferation of lymphocytes as detected by CFSE. (a) Inhibitory effect of DF-MSCs on the proliferation of lymphocytes displayed by flow cytometry. (b) Inhibitory effect of DF-MSCs on the proliferation of lymphocytes displayed statistically. ***p<0.001, ****p<0.001. Results are shown as mean±SD.
DF-MSCs Increased the Cell Viability of Lymphocytes in Inflamed Culture
We investigate the antiapoptotic effect of DF-MSCs on the stimulated lymphocytes of patients. The cell viability of CD mix stimulated lymphocytes was significantly low in inflamed and noninflamed culture of CD patients. In the presence of DF-MSCs, T cell viability increased only in inflamed samples, and the increase was statistically significant (p<0.001; Figure 4).
Figure 4.
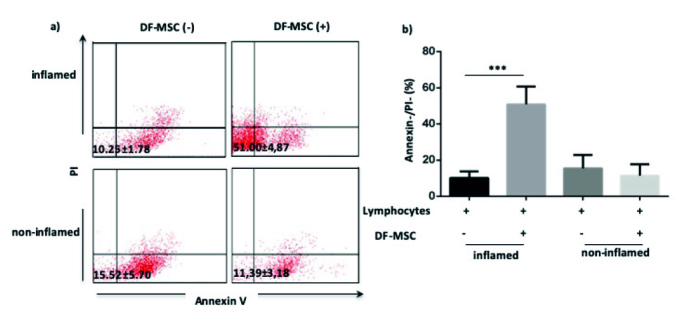
a, b. Supportive effect of DF-MSCs on the cell survival of lymphocytes as detected by Annexin (−) PI (−). (a) DF-MSCs increased T cell viability in inflamed culture of CD patients compared with noninflamed culture. (b) Supportive effect of DF-MSCs on the cell survival of lymphocytes in inflamed and noninflamed culture displayed statistically. *p<0.05, *** p<0.001. Results are shown as mean±SD.
DF-MSCs Decreased CD8+ and Increased CD4+ T Cell Subset in Inflamed Culture
To investigate the effect of DF-MSCs on CD4+ helper and CD8+ cytotoxic T cell subset, we culture isolated lymphocytes from blood and inflamed and noninflamed culture in the presence and absence of DF-MSCs. In inflamed culture, CD8+ T cell subset was high compared with noninflamed culture (23.77±2.51, 9.753±1.24, respectively). DF-MSCs decreased frequency of CD8+ T cell in inflamed culture (p<0.01; Figure 5).
Figure 5.
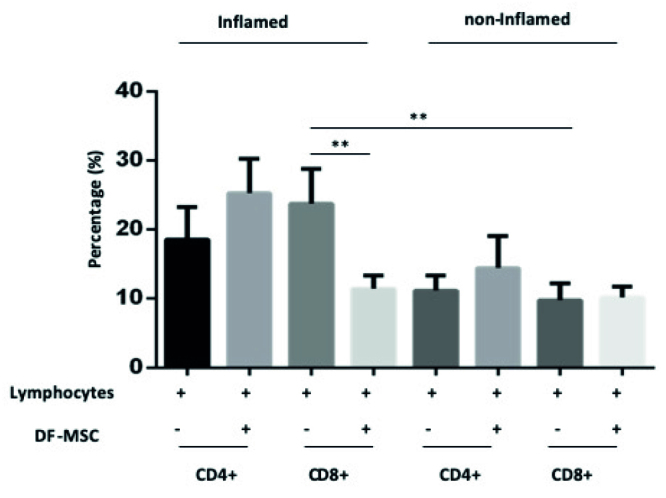
Effect of DF-MSCs on the CD4+ and CD8+ T cells subset in inflamed and noninflamed culture of CD patients. After three days of coculture, cells were stained with CD4+ and CD8+ monoclonal antibodies. In the presence of DF-MSCs, CD8+ T cell decreased in inflamed culture of CD patients compared with noninflamed culture. *p<0.05, **p<0.01, ***p<0.001. Results are shown as mean±SD.
DF-MSCs Increased CD4+IL-22BP+ T Cell Population in Inflamed Culture
To investigate the effect on DF-MSCs on CD4+IL22BP T cell population, which was isolated from inflamed and noninflamed tissue, the isolated lymphocytes were cultured in the presence and absence of DF-MSCs. According to our results, CD4+IL-22BP+ T cell ratio was high (35.69±3.24) in inflamed culture compared with noninflamed culture (9.56±2.71) in CD patients’ lymphocytes (p<0.01). In the presence of DF-MSCs, CD4+IL-22BP+ T cell frequency decreased (19.34±1.05), and the decrease was statistically significant (p<0.01) in the inflamed culture. There was no significant change in noninflamed culture of CD patients in the presence and absence of DF-MSCs (p>0.05; Figure 6).
Figure 6.
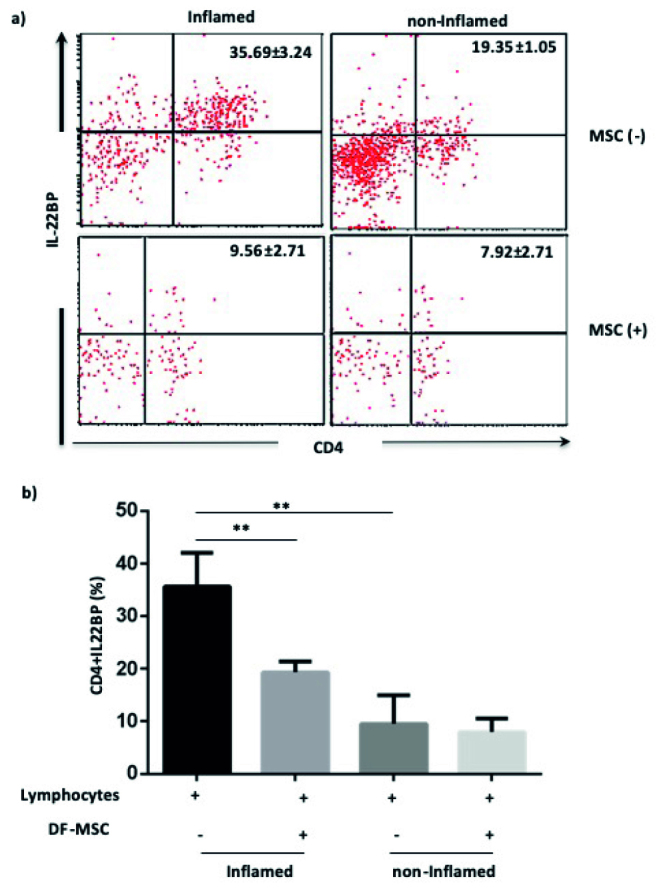
a, b. Effect of DF-MSCs on the CD4+IL22BP T cells’ inflamed and noninflamed culture of CD patients. After three days of coculture, cells were stained with CD4+IL22BP monoclonal antibodies. (a) In the presence of DF-MSCs, CD4+IL22BP cell ratio decreased in inflamed culture of CD patients compared with lymphocyte-only cultures. (b) Decreased ratio of CD4+IL22BP was shown statistically. **p<0.01. Results are shown as mean±SD.
DF-MSCs Increased CD4+Foxp3+ Regulatory T Cell in Inflamed Culture
We investigated the effect of DF-MSCs on CD4+Foxp3+ frequency. After 72 h lymphocytes culture with or without DF-MSCs, we found that DF-MSCs increased T regulatory frequency in inflamed culture compared with lymphocyte-only cultures in CD patients (DF-MSCs (+): 4.23±0.58; DF-MSCs (−): 2.05±0.49; p<0.05). There was no significant change in frequency of CD4+Foxp3+ in noninflamed culture lymphocyte (p>0.05; Figure 7).
Figure 7.
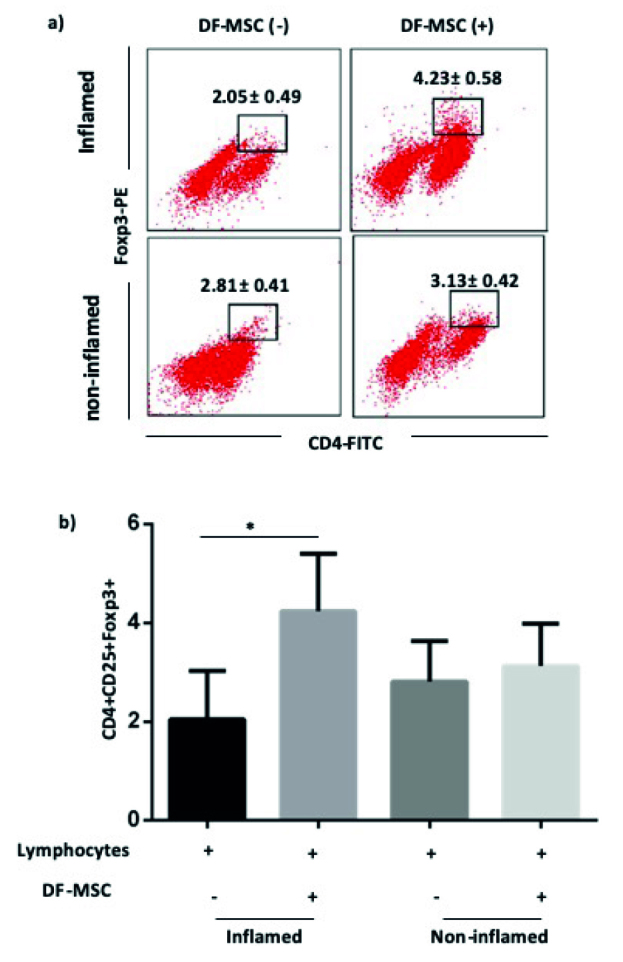
a, b. Effect of DF-MSCs on the CD4+CD25+Foxp3+ T cells in tissue samples of CD patients. After three days of coculture, cells were analyzed by gating CD25+ cells for CD4+Foxp3+ Treg ratio. (a) Dot plot analysis of CD4+CD25+Foxp3+ cells in the presence and absence of DF-MSCs in lymphocytes of inflamed and noninflamed culture of CD patients. (b) Statistical analysis of CD4+CD25+Foxp3+ cells was shown. DF-MSCs’ CD4+CD25+Foxp3+ cells increased in lymphocytes of inflamed culture of CD patients. Noninflamed cultures showed no significant change in the frequency of CD4+CD25+Foxp3+ cells in the presence and absence of DF-MSCs. *p<0.05. Results are shown as mean±SD.
DF-MSCs Reduced Inflammatory Cytokine Levels and Increased IL-10 Levels in Inflamed Culture
TNF-α level was high in supernatant of inflamed culture compared with noninflamed culture (790.6±11, 426.5±85.14, respectively) in CD patients. DF-MSCs decreased TNF-α levels in inflamed culture supernatant (415.2±73.48), whereas there was no significant change in noninflamed culture supernatant. DF-MSCs reduced high levels of IL-6 in both inflamed and noninflamed culture, and the reduction was statistically significant (p<0.05 and p<0.05, respectively). IFN-γ level was high in supernatant of inflamed culture compared with noninflamed culture (2204±297.2, 599.8±157, respectively). DF-MSCs significantly decreased the level of IFN-γ in inflamed culture (p<0.05). DF-MSCs increased IL-10 level as anti-inflammatory cytokine, and the increase was statistically significant (p<0.01; Figure 8).
Figure 8.
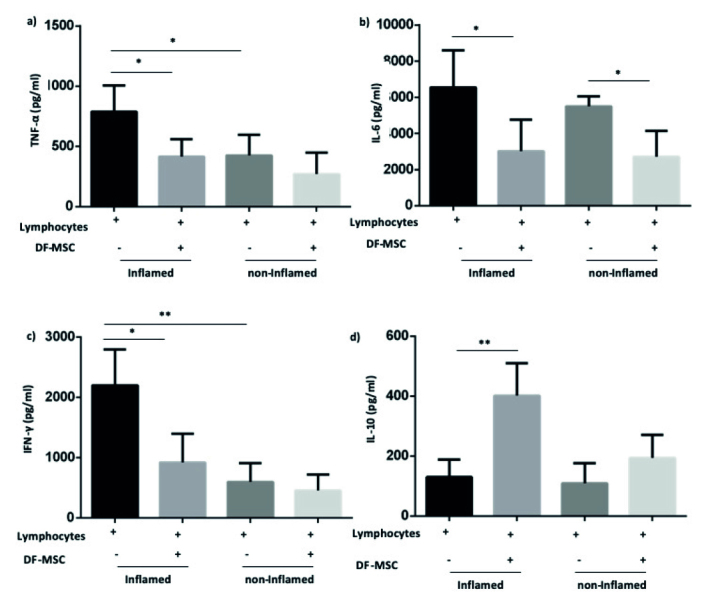
a, b. DF-MSCs modulated cytokine levels in the supernatants of lymphocyte. Isolated cells collected from patients’ inflamed and noninflamed biopsies were cultured in a 96-well plate following CDmix stimulation in the presence or absence of MSCs. The levels of cytokines in the supernatants of lymphocytes were determined using flow cytometry. DF-MSCs significantly decreased TNF-α (a), IL-6 (b), and IFN-γ (c) but significantly increase IL-10 (d) levels in inflamed culture. MSCs significantly decreased IL-6 (B) in noninflamed culture (p<0.01). IL-4 did not show significant difference in the presence of MSCs. *p<0.05, **p<0.01, Results are shown as mean±SD. DF-MSCs referred as MSCs.
DISCUSSION
In this study, we demonstrated that DF-MSCs modulated inflammatory response of T lymphocytes in inflamed culture in vitro. Results indicate that DF-MSCs can suppress proliferative response and increase the frequency of Tregs and viability of lymphocytes in inflamed cultures in the presence of DF-MSCs. Additionally, TNF-α and IL-6 levels decreased, whereas anti-inflammatory cytokine (IL-10) levels increased with DF-MSCs in the inflamed cultures. In presence of DF-MSCs, CD4+IL-22BP T lymphocytes decreased in inflamed culture.
After the evidence of MSCs’ efficacy in treating experimental colitis was convenient, in several clinical trials, MSCs were applied as a new therapeutic strategy in CD. The results invariably highlighted the feasibility and safety of their systemic and local infusion in both autologous and allogeneic settings, and efficacy seemed to be optimal when locally injected into fistula tracks (18).
However, only the patient’s refractoriness to standard therapies had been recruited and most importantly, no information on the mechanisms by which MSCs exert their action on pathogenic T cells has been available so far. We therefore investigated in vitro modifications of T lymphocytes in terms of cell viability, immunophenotyping, and cytokine profile when cultured in the presence of DF-MSCs.
CD is a Th1-driven disease, in which TNF-α, IL-6, and IFN-γ mediate the inflammatory responses in lamina propria (19). MSCs have potent immune-suppressive activity by direct cell-cell contact and production of soluble mediators that make them particularly attractive for inflammatory diseases (20). Bone marrow and adipose tissue are the main sources of MSCs; however, these sources have several disadvantages such as the invasive procedure for isolation, the small number of isolated cells, and the various proliferation and differentiation capacities related to age of the donor. For this reason, search for alternative tissue sources for MSCs has become of vital importance (21). Dental and orofacial-originated stem cells can be easily isolated and expanded in culture conditions and also have the ability for tissue repair (22). In our previous studies, we demonstrated that DF-MSCs have potent anti-inflammatory effects on CD4+ T lymphocytes by enhancing Foxp3 expressing Treg cells and can regulate both Th1 and Th2 responses, depending on the immune activation of the cells (14, 23).
Intestinal CD4+ and CD8+ T cells are crucial mediators of immune homeostasis and inflammation (24). We therefore further investigated the effects of DF-MSCs on CD4+ and CD8+ T lymphocyte subsets in tissue MNCs, which have a major role in the inflammatory response of CD. Within the intraepithelial compartment, the majority of T cells express CD8, and small proportion of the lymphocytes are CD4+ T cells (25). In tissue cultures, CD8+ T lymphocytes notably decreased, and CD4+ T cells tend to increase in the presence of DF-MSCs. The increase in the CD4+ T cell amounts may be the effect of DF-MSCs on the generation of Foxp3 expressing CD4+ T lymphocytes in the inflamed culture.
Treg cells, which can be generated by activation of CD4+ T lymphocytes, could be an extra source of cells for cellular therapies in IBD. In a study, injection of induced Tregs in a murine model of chronic colitis showed a reduction in gut inflammation response (26). Several studies demonstrated that MSCs can regulate T cell activation by suppressing proliferation of activated lymphocytes and enhance the generation of T regulatory cells by inducing the transcription factor: Foxp3 + and IL-10 (27). In a recent study, we evaluated simultaneously CD4+CD25+Foxp3+ Treg cell frequency and IL-10 levels in lymphocyte of inflamed and noninflamed cultures in the presence and absence of DF-MSCs. Foxp3 ratio increased in CD4+CD25+ T lymphocytes in the presence of DF-MSCs in inflamed culture of CD patients but no significant change observed in noninflamed culture. Regarding those results, IL-10 levels notably increased in inflamed culture supernatants of CD patients, which demonstrates that DF-MSCs enhance Treg cell frequency and anti-inflammatory cytokine secretion by T lymphocytes because of inflammatory response of MNCs.
Cytokines are crucial soluble factors that mediate interactions between immune and nonimmune cells such as epithelial stem cells and MSCs. The inflammatory process in IBD leads to corruption of the epithelial barrier and the formation of epithelial ulceration (28). In a study, it was found that IL-22 expressing CD4+ cells were increased in the inflamed mucosa of IBD patients (29). CD is generally associated with increased expression of TNF-α, IL-6, and IFN-γ in intestinal mucosa (30). In contrast, anti-TNF-α therapy and IL-6 blockade showed clinical beneficiary in CD (31, 32). In this study, we investigated the modulatory effects of DF-MSCs on cytokine profile of inflamed and noninflamed cultures. TNF-α and IFN-γ levels were high in culture supernatants of inflamed culture compared with noninflamed culture. IL-6 levels were high both in inflamed and noninflamed cultures. Interestingly, DF-MSCs downregulated the elevated levels of TNF-α and IFN-γ levels in inflamed coculture, whereas no significant change observed in cocultures of noninflamed culture. In addition, DF-MSCs reduced high levels of IL-6 in cocultures with inflamed and noninflamed culture supernatant. These data indicate that DF-MSCs can selectively regulate proinflammatory and inflammatory cytokine secretion in accordance with the levels of mediators.
IL-22BP is the inhibitor of the mucosal tissue protective cytokine IL-22. Elevated levels of IL-22BP were reported in isolated CD4+ T cells of patients with IBD, which binds to IL-22 with high affinity, and obstructs the mucosal protective effects of IL-22 in the gut (33). In this study, we evaluated the effect of DF-MSCs on IL-22BP expression in CD4+ T cells isolated from inflamed and noninflamed tissue specimens of CD patients. IL-22BP ratio was high in CD4+ T lymphocytes of inflamed culture compared with noninflamed tissue lymphocytes. DF-MSCs inhibited the expression of IL-22BP in CD4+ T lymphocytes remarkably in inflamed culture, and the reduction was similar to that in noninflamed culture without DF-MSCs. These data may be the evidence for the modulatory effect of DF-MSCs on mucosal inflammatory responses, and the inhibitory mechanism of DF-MSCs on IL-22BP can be further investigated.
According to our results, DF-MSCs display immunosuppressive effect on exaggerated inflammatory response of mucosal T cells in CD. DF-MSCs modulate significant immune response at the site of inflamed biopsy tissue. DF-MSCs suppressed T cell proliferation, increased cell viability and CD4+CD25+Foxp3+ Treg, reduced CD8+, increased CD4+ T cell population, and enhanced frequency of CD4+IL22BP in CD patients’ inflamed culture. In addition, DF-MSCs reduced the levels of TNF-α and IFN-γ and increased IL-10 level compared with noninflamed culture. Our results corroborate the hypothesis that DF-MSCs are able to transfer their effects to the exact sites of active inflammation.
Footnotes
This study was presented at the XXI. National Allergy and Clinical Immunology Congress, 25-29 October 2014, Bodrum, Turkey.
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Marmara University School of Medicine (Ethical Approval. 09.2017.456).
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – T.A., O.A., N.Z., D.G..; Design – T.A., O.A., N.Z., D.G.; Supervision – T.A., O.A.; Resource – O.A., M.B., T.A., K.G.; Materials - O.A., M.B., S.S., K.G.; Data Collection and/or Processing – N.Z., D.G., S.S., Y.D.; Analysis and/or Interpretation – T.A., N.Z., D.G., S.S., Y.D; Literature Search – T.A., O.A., D.G., N.Z.; Writing – T.A., N.Z., D.G., O.A.; Critical Reviews – T.A., O.A.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study was supported by the grant Marmara University Scientific Researches Committee (BAPKO) Project No: SAG-C-DRP-200716-0373.
REFERENCES
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–78. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgart DC, Sandborn WJ. Crohn’s disease. The Lancet. 2012;380:1590–605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid Y, Hand T. Role of the Microbiota in Immunity and inflammation. Cell. 2014;157:121–41. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gálvez J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm. 2014;2014 doi: 10.1155/2014/928461. 928461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280–8. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li LJ, Gong C, Zhao MH, Feng BS. Role of interleukin-22 in inflammatory bowel disease. World J Gastroenterol. 2014;20:18177–88. doi: 10.3748/wjg.v20.i48.18177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones BC1, Logsdon NJ, Walter MR. Structure of IL-22 bound to its high-affinity IL-22R1 chain. Structure. 2008;16:1333–44. doi: 10.1016/j.str.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelczar P, Witkowski M, Perez LG. A pathogenic role for T cell-derived IL-22BP in inflammatory bowel disease. Science. 2016;354:358–62. doi: 10.1126/science.aah5903. [DOI] [PubMed] [Google Scholar]
- 9.Sciaudone G, Di Stazio C, Limongelli P, et al. Treatment of complex perianal fistulas in Crohn’s disease: infliximab, surgery or combined approach. Can J Surg. 2010;53:299. [PMC free article] [PubMed] [Google Scholar]
- 10.Corica D, Romano C. Biological Therapy in Pediatric Inflammatory Bowel Disease: A Systematic Review. J Clin Gastroenterol. 2017;51:100–10. doi: 10.1097/MCG.0000000000000696. [DOI] [PubMed] [Google Scholar]
- 11.Lim WC, Wang Y, MacDonald JK, et al. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst Rev. 2016;7:CD008870. doi: 10.1002/14651858.CD008870.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35:e00191. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Yildırım S, Zibandeh N, Genc D, et al. The Comparison of the Immunologic Properties of Stem Cells Isolated from Human Exfoliated Deciduous Teeth, Dental Pulp, and Dental Follicles. Stem Cells Int. 2016 doi: 10.1155/2016/4682875. 4682875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Yang B, Tian J, et al. Dental Follicle Stem Cells Ameliorate Lipopolysaccharide-Induced Inflammation by Secreting TGF-β3 and TSP-1 to Elicit Macrophage M2 Polarization. Cell Physiol Biochem. 2018;51:2290–308. doi: 10.1159/000495873. [DOI] [PubMed] [Google Scholar]
- 16.Kim HS, Shin TH, Lee BC, et al. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2. Gastroenterology. 2013;145:1392–403. doi: 10.1053/j.gastro.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 17.Trapecar M, Khan S, Roan NR, et al. An Optimized and Validated Method for Isolation and Characterization of Lymphocytes from HIV+ Human Gut Biopsies. AIDS Res Hum Retroviruses. 2017;33:31–9. doi: 10.1089/aid.2017.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Squillaro T, Peluso G, Galderisi U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829–48. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Ueno A, Fort Gasia M, et al. Profiles of Lamina Propria T Helper Cell Subsets Discriminate Between Ulcerative Colitis and Crohn’s Disease. Inflamm Bowel Dis. 2016;22:1779–92. doi: 10.1097/MIB.0000000000000811. [DOI] [PubMed] [Google Scholar]
- 20.De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574–91. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 21.Flores AI, Gómez-Gómez GJ, Masedo-González Á, et al. Stem cell therapy in inflammatory bowel disease: A promising therapeutic strategy? World J Stem Cells. 2015;7:343–51. doi: 10.4252/wjsc.v7.i2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ansari S, Seagroves JT, Chen C, et al. Dental and orofacial mesenchymal stem cells in craniofacial regeneration: The prosthodontist’s point of view. J Prosthet Dent. 2017;118:455–61. doi: 10.1016/j.prosdent.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genç D, Zibandeh N, Nain E, et al. Dental follicle mesenchymal stem cells down-regulate Th2-mediated immune response in asthmatic patients mononuclear cells. Clin Exp Allergy. 2018;48:663–78. doi: 10.1111/cea.13126. [DOI] [PubMed] [Google Scholar]
- 24.Shale M, Schiering C, Powrie F. CD4(+) T-cell subsets in intestinal inflammation. Immunol Rev. 2013;252:164–82. doi: 10.1111/imr.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meresse B, Cerf-Bensussan N. Innate T cell responses in human gut. Semin Immunol. 2009;21:121–9. doi: 10.1016/j.smim.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Karlsson F, Martinez NE, Gray L, et al. Therapeutic evaluation of ex vivo-generated versus natural regulatory T-cells in a mouse model of chronic gut inflammation. Inflamm Bowel Dis. 2013;19:2282–94. doi: 10.1097/MIB.0b013e31829c32dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prevosto C, Zancolli M, Canevali P, et al. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–8. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 28.Andoh A, Yagi Y, Shioya M, et al. Mucosal cytokine network in inflammatory bowel disease. World J Gastroenterol. 2008;14:5154–61. doi: 10.3748/wjg.14.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andoh A, Zhang Z, Inatomi O, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–84. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 30.Chen ML, Sundrud MS. Cytokine networks and T cell subsets in inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:1157–67. doi: 10.1097/MIB.0000000000000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito H. Anti-interleukin-6 therapy for Crohn’s disease. Curr Pharm Des. 2003;9:295–305. doi: 10.2174/1381612033391900. [DOI] [PubMed] [Google Scholar]
- 32.Adegbola SO, Sahnan K, Warusavitarne J, et al. Anti-TNF Therapy in Crohn’s Disease. Int J Mol Sci. 2018;19:2244. doi: 10.3390/ijms19082244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelczar P, Witkowski M, Perez LG, et al. A pathogenic role for T cell-derived IL-22BP in inflammatory bowel disease. Science. 2016;354:358–62. doi: 10.1126/science.aah5903. [DOI] [PubMed] [Google Scholar]


