Abstract
There has been increasing interest in understanding the role of the human microbiome in skin diseases. Microbiome studies are being utilized in skin cancer research in numerous ways. Commensal bacteria are being studied as a potential tool to judge the biggest environmental risk of skin cancer, ultraviolet (UV) radiation. Owing to the recognized link of skin microbes in the process of inflammation, there have been theories linking commensal bacteria to skin cancer. Viral metagenomics has also provided insight into virus linked forms of skin cancers. Speculations can be drawn for skin microbiome that in a manner similar to gut microbiome, they can be involved in chemoprevention of skin cancer. Nonetheless, there are definitely huge gaps in our knowledge of the relationship of microbiome and skin cancers, especially in relation to chemoprevention. The utilization of microbiome in skin cancer research seems to be a promising field and may help yield novel skin cancer prevention and treatment options. This review focuses on recent utilization of the microbiome in skin cancer research, and it explores the potential of utilizing the microbiome in prevention, earlier diagnosis, and treatment of skin cancers.
Keywords: skin microbiome, immune system, chemoprevention
Introduction
Each year, more new cases of skin cancer are diagnosed than the combined incidence of breast, lung, prostate, and colon cancer (1). In the United States, about 5 million people are treated for skin cancer annually (2). Non-melanoma skin cancer (NMSC), the most common cancer in the United States, includes basal cell skin cancer (BCC), squamous cell skin cancer (SCC), and a number of less common skin cancers. The main risk factors for basal cell and squamous cell carcinomas are UV light exposure, having light-colored skin, age, and immunosuppressive status (3). The cancer that forms from melanocytes, melanoma, is the most dangerous form of skin cancer, as it is the most aggressive and most resistant to treatment. Though it is less common than other skin cancers, melanoma is estimated to account for 9730 deaths in 2017 (1). The annual cost for treating skin cancers in the United States is estimated to be $8.1 billion, with $4.8 billion for NMSC and $3.3 billion for melanoma (2).
The human skin microbiome consists of all the bacteria, archaea, fungi, and viruses that reside on human skin (4). It is estimated that the microbes that live on humans outnumber human somatic and germ cells by ten-fold (5). Years of research from dermatologists have shown that microbes influence the normal courses of many skin diseases (6). Germ-free studies in animals have displayed the crucial role of normal flora in immunological and physiological functions (7). Just as microbes can alter immunological and physiological functions, the human body can alter the microbiome. For example, a recent study demonstrated that the skin microbiome of cachectic cancer patients is less diverse than that of healthy participants, portraying an effect of cancer and cachexia on human skin bacterial communities (8).
Traditional culture-based methods of isolating microbes are mostly being replaced by contemporary metagenomics. Metagenomics, introduced in 1998, allows microbial genome studies in natural conditions; it has revolutionized human microbial research and has revealed more about the diversity and complexities that make up the microbiome (9). Unlike traditional culture-based methods of isolating microbes, contemporary human microbiome research limits bias by sampling all genes from all members of the sampled communities (10). Whole genome sequencing provides the entire DNA sequence of an organism’s genome at a single time (4).
Microbial studies have been motivated by inquiries regarding the potentially beneficial role of normal flora and the pathogenic microbes that cause cutaneous and systemic diseases (4). Studying the relationship between microbiome and disease may provide new perspective about human evolution. More specifically, by studying how environment may affect human “micro-evolution,” human health and predisposition to disease may be better understood (11). This review discusses both recent and potential utilization of the human microbiome in skin cancer research, and outlines the potential of utilizing the microbiome in prevention, earlier diagnosis, and treatment of skin cancers.
Microbiome, Immunity, Inflammation and Skin Cancer
Inflammatory responses play decisive roles at different stages of tumor development and also affect immune surveillance and responses to therapy (12). Immune cells that infiltrate tumors engage in an extensive and dynamic crosstalk with cancer cells. It has been found that microbial exposure plays a critical role in cancer immunobiology by preventing establishment of chronic inflammation in its early stages (12). Recently, a third subset of T-helper (Th) cells, Th17 cells, and inflammatory cytokine, interleukin-23 (IL-23), have been elucidated to play centre-stage in tumor-associated inflammation (13). On the other hand, regulatory T cells (T-regs) have been found to suppress the inflammation induced by Th17/IL-23 axis (14). Continuous microbial exposure strengthens this regulatory network, that in turn suppresses cancer-promoting inflammation. In inflammatory diseases of skin like psoriasis and atopic dermatitis, Th17 cell induced inflammation and T-reg cell induced immune tolerance is observed (12). Continuous signalling via toll-like receptors (TLRs) that recognize pathogen associated molecular patterns (PAMPs) as well as damage-associated molecular patterns (DAMPs) is critical for cell integrity, tissue repair and recovery from injury but unregulated activation of TLRs leads to inflammatory responses that can ultimately contribute to carcinogenesis (15).
The skin is the body’s largest organ and acts as a physical barrier between invading pathogens and the human body. Microbes, keratinized skin cells and immune cells work together to maintain physical and immune barriers of skin under homeostatic healthy conditions (16, 17). Keratinocytes and melanocytes, the main skin cell types involved in melanoma and non-melanoma skin cancers express TLRs that induce inflammatory responses against invading pathogens (15). Nevertheless, uncontrolled activation of TLRs leads to chronic inflammation that may give rise to skin cancer. Interestingly, melanoma and basal cell carcinoma have been found to be successfully treated by TLR agonists targeting TLR 7, 8, and 9 (15).
Studies on how the skin microbiome affects/induces cancer remains in its infancy. However, microbiome in inflammatory diseases have been suggested to be associated with skin cancers. Psoriasis and acne, chronic inflammatory diseases involving Th17 immune stimulation, are some of these diseases (18–20). It can be said that unregulated inflammation in addition to microbiome dysbiosis may increase the risk of carcinogenesis. UV radiation, a well-established carcinogen, has also been suggested to cause inflammation that may lead to skin cancer. Inflammatory changes upon UV exposure include erythema (sunburn) and production of inflammatory mediators (21). UV-induced inflammation and damage leads to changes in cutaneous and systemic immunity that induces activation of suppressor T cells and lowers cell-mediated immunity (21). These impairments of immune system reduce the capacity of the body to reject skin cancers rather they promote carcinogenesis. Although UV induced inflammation has been linked to skin cancer but its effect on skin microbiome has not been much explored. Disruption of normal skin microflora may also contribute to inflammatory mechanisms that lead to induction of carcinogenesis but further studies are needed to prove the claim.
Numerous studies have suggested a possible role of bacterial microbiota in skin carcinogenesis. In 1976, Sacksteder demonstrated a reduced rate of skin cancer in germ-free rats (22). More recent studies have demonstrated a similar trend in mice lacking receptors or adaptor molecules for pro-inflammatory bacteria microorganism-associated molecular patterns (MAMP) (23–25). While there are no published studies on how microbes directly influence either the onset or propagation of skin cancer specifically, there has been research on how certain microbial components heighten immune-surveillance, providing anti-tumor activity, in the bladder (26) and colon (27). Scientists hope future microbial studies will lead to potential cancer prevention strategies with prebiotics, probiotics, and microbiota transplants to reduce microbially induced activation of genotoxicity and activation of inflammatory, proliferative and anti-apoptotic pathways (28). The introduction of microbes has actually been utilized for certain cancer treatments for a while. For example, Mycobacterium bovis has been used to treat superficial bladder cancer since 1976 (29).
There have been a lot of theories about the relationship between external microbial environments and carcinogenesis (30). Studies have shown that workers that are heavily exposed to environmental microbes (such as farmers, fishermen, waste incinerator workers, etc.) seem to have lower cancer rates (31). Similarly, a study conducted in various regions throughout the world revealed a rather linear relationship between gross national product (GNP) and cancer incidence. One explanation was that the more simple living conditions in lower GNP areas provide greater exposure to environmental micro-organisms, and thus may have anti-neoplastic potential (32). Because adequate exposure to a great variety of microbes seems to be critical for development of a normal, functioning immune system and cancerous growths, the study of potential deleterious effects of antibiotics and immunosuppressive drugs on micro-biome and skin cancer is of great interest as well (12).
Studies have shown that tissue injury and chronic inflammation increase the risk for cancer (30). For example, 2% of burn scars undergo malignant transformation, and SCC is the most common form of cancer that develops (33). The skin microbiome is vital in inflammation modulation and in immune system functioning, and there has been speculation of a relationship between commensal species on inflammation and thus eventual skin malignancy (30). Yuping et al. demonstrated how commensal skin bacteria reduce inflammation during wound healing by regulating TLR3-dependent inflammation, displaying how microflora can modulate particular cutaneous inflammatory responses (34). The way micro-biota may directly influence the immune systems may be an area of active investigation in studying the influence of microbiota on skin cancer (30). Further research in microbiome and skin cancer may provide insight into novel skin cancer therapy utilizing micro-biota.
Gut Microbiome, Skin Microbiome and Skin Cancer
Many associations of gut microflora have been found to be with gastrointestinal cancer (18). The most prominent is association of Helicobacter pylori with gastric adenocarcinoma and gastric-mucosa associated lymphoid tissue lymphoma (18) (Table 1). Moreover, the bacterium Campylobacter jejuni and Salmonella typhi have also been associated with small intestine lymphoma and gall bladder cancer respectively (35, 36) (Table 1). In all these cases, generally chronic inflammation at the tumor site induces carcinogenesis. Th17 mediated inflammation is usually been found to be associated with all such malignancies. Interestingly, other reports support that gut microflora play protective roles as well against cancer. Helicobacter pylori has been found to reduce the risk of esophageal adenocarcinoma, esophageal squamous cell carcinoma and pancreatic cancer (37, 38). Similarly, several species of Lactobacillus have also been found to play preventive roles against colorectal cancer (39) (Table 1). Immune response against cancer cells and activation of anti-inflammatory T-regs has been suggested to be involved in such protections. Moreover, activation of Th17 and TLRs by bacteria may decrease the risk of cancer by improving immune surveillance of cancer cells (18). This suggests that a balanced microbiome is necessary for a robust immune response. Therefore, dysbiosis may lead to induction of mechanisms involved in carcinogenesis.
Table 1.
Association of skin/gut microbiome with various types of cancer
| Gut microbiome | Association with the type of cancer |
|---|---|
| Carcinogenic effect | |
| Helicobacter pylori | Gastric adenocarcinoma, gastric-mucosa associated lymphoid tissue lymphoma (18) |
| Campylobacter jejuni | Small intestine lymphoma (35) |
| Salmonella typhi | Gall bladder cancer (36) |
| Protective effect | |
| Helicobacter pylori | Esophageal adenocarcinoma, esophageal squamous cell carcinoma and pancreatic cancer (37, 38) |
| Lactobacillus | Colorectal Cancer (39) |
| Skin microbiome | Association with the type of cancer |
| Carcinogenic effect | |
| Staphyloccocus aureus | Cutaneous T cell lymphoma (43, 44) |
| Chlamydophila pneumonia | Cutaneous T cell lymphoma (43) |
| Borrelia burgdorferi | Cutaneous T cell lymphoma (43) |
| Bacterial infection in denuded skin or burns | Marjolin’s ulcer (squamous cell carcinoma) (18, 33) |
Gut microbiome, in addition to having protective or carcinogenic effects against gastrointestinal tract, has been found to have an association with cancers of other organs including skin (40). Gut-skin connection of several skin diseases like acne vulgaris, atopic dermatitis, psoriasis and icthyosis vulgaris has been suggested to be because of a link between gut and skin microbiome (40). Inflammation and barrier defects in the gut appear to have a correlation with the diseases of the skin. For instance, small intestinal bacterial overgrowth is found to increase the risk of acne vulgaris by increasing immune response to some species of gut bacteria. Interestingly, oral probiotics have been found to be beneficial in treating acne (41). Moreover, Lactobacillus paracasei has also been found to induce beneficial immunomodulatory effects on the skin (42). This indicates that gut microbiome modulates immune function in the skin and can have a critical role in development/prevention of skin cancer.
It is believed that as altering regulation of Th17, T-reg and innate pathways by gut microbiome promotes malignancies of digestive tract, in a similar manner, skin microbiome may have carcinogenic effects in the skin. Cutaneous T cell lymphoma has been linked to colonization of skin by Staphyloccocus aureus (43, 44). Chlamydophila pneumonia and Borrelia burgdorferi have also been found to be associated with cutaneous T cell lymphoma (43) (Table 1). Moreover, Marjolin’s ulcer, a squamous cell carcinoma, has been associated with inflammation caused by bacterial infection in denuded skin or burns (18, 33) (Table 1). These data indicate that skin microbiome may promote skin carcinogenesis by inducing chronic inflammation directly or indirectly by association with an auto-inflammatory disease state. Microbiome may lead to inflammation or may be associated with inflammatory mechanisms that ultimately give rise to cancer. It can be exemplified from increase in squamous cell carcinoma in hiradenditis suppuritiva patients (18). Nevertheless, beneficial microflora can also lead to reduction in inflammation by inducing T-regs and activating anti-cancer immuno-surveillance as is done by gut microbes.
Viral Infections and Skin Cancer
Just as bacterial flora is present on normal skin and mucosa, viral flora is also present (45). Viral metagenomics has been a powerful tool in investigating unrecognized etiology of viruses and identifying novel viral species (46). The last 20–30 years have brought forth a lot of data in the association between human cancers and viruses, and more than 20% of cancers worldwide have been linked to viruses as their etiological agents. While most of the viruses present on normal skin are considered resident symbiotic organisms, some of them are linked with skin cancers (47).
Merkel cell carcinoma (MCC) is a rare, highly aggressive primary neuroendocrine carcinoma of the skin that usually affects elderly Caucasians on sun-exposed regions of the face, neck, and head (47). In 2008, a newly discovered human polyomavirus, Merkel cell polyomavirus (MCPyV), was found to contribute to the development of the majority of MCC, making MCPyV the first polyomavirus found to be associated with a human cancer (47). Research shows that inhibiting production of MCPyV proteins causes MCPyV-infected carcinoma cells to die, but does not have any impact on malignant Merkel cells that are not infected by this MCPyV (48). About 80% of MCC have this virus clonally integrated into the cancerous cells. Because MCPyV infection is nearly present in all healthy subjects, it is thought that MCPyV is a persistent resident of skin microbiome. Immunosuppressed patients are more prone to develop MCC, indicating that the immune system modulates the disease progression (49). However, the steps and co-factors required for this skin cancer to develop are unknown (48). Current research focuses on strategies to target the virus by inhibiting viral-induced carcinogenesis or by preventing infection (50).
Human papillomaviruses (HPV) are DNA viruses that are abundant among human populations, and there are 155 known HPV types (51). The majority of HPV infect the skin of normal and immunocompromised individuals. In non-immunocompromised individuals, it seems that HPV establishes a latent infection of the skin, most likely as normal flora in hair follicles (51). HPV can be transmitted directly or indirectly from warts and condylomata to human hosts (52). Evidences have suggested that genus Beta Human papillomaviruses (Beta-HPV) play an important role in the development of SCC of the skin in humans (53). Although further investigations in the mechanistic association of Beta-HPV and SCC are needed, this research suggests potential reduction of such skin cancers through prevention or treatment of Beta-HPV infection (53). Ma et al. recently conducted a large-scale survey of HPV using a shotgun sequencing approach (54). The study mapped HPV infections among various body sites in a cohort of 103 healthy human subjects, displaying some site specificity and co-occurrence or exclusion. The non-random organization of HPV types described in the study indicates interaction among microbes, either competitive or facilitative. Further research may study how these interactions of viral flora affect the development of skin cancer. For example, some non-oncogenic HPV types may reduce the risk of cancer by eliminating oncogenic viral infections with cross immunity or viral interference. On the other hand, certain HPV types might increase the risk of cancer by sustaining a supportive cellular environment, favoring a persistent infection in a co-infection with oncogenic HPV (54). In addition, although statistical precision was limited, one population based case-control study suggests that the association of HPV and SCC seems to be even greater with those who have a history of glucocorticoid use, bringing into question the complex relationship between immunosuppressive drugs, viral flora, and skin cancer (53).
Research should focus in further clarifying the role of these viruses in skin cancer in order to develop specific therapies. Some vaccines in the past have been developed to target virus-associated cancers. For instance, a hepatitis B vaccine has demonstrated a reduction in hepatocellular carcinoma in Taiwanese infants (55). However, it is unlikely that such an expensive mass immunization program for a vaccine to target a polyomavirus like MCPyV would be created considering the small number of MCCs, which usually occur late in life (45). Another option for future therapeutic intervention may be the silencing of specific viral proteins, like E6/E7 proteins of HPVs and T antigens of polyomaviruses, which are respectively essential in the maintenance of tumor (45).
UV Radiation and Microbiome
UV is a non-ionizing radiation from sunlight (56, 57), and it is valuable in many ways, especially in its role in vitamin D synthesis (58). However, UV radiation is also considered the main environmental risk factor for non-melanoma skin cancers (59). In addition, although melanoma is strongly dependent on genetics (as seen with the allelic variances in melanocortin 1 receptor), experiments studying the UV-associated induction of benign nevi and UV-induced mutation patterns suggest an important role of UV radiation as a risk for melanoma as well (60). Global radiation fear makes it necessary to develop a simple bio-dosimetry to predict the risk of radiation (61).
Various UV-mediated biological markers have been identified, including DNA damage responses, transcription factor inductions (62), and cytokine regulations in skin cells (63). Utilizing these biomarkers within skin cells in order to predict skin cancer is challenging. Patients in healthy conditions are normally unwilling to provide skin samples (61). Skin microbiome seems to be a more accessible realm for UV damage identification and skin cancer prediction.
Most skin commensal bacteria are found in the superficial layers of the epidermis, and would thus have as much UV radiation exposure as skin keratinocytes (61). Utilizing skin commensals to detect UV radiation risks is much easier than collecting live human tissue. Analyzing skin commensal bacteria in response to sun radiation also produces a response profile over time. The bacterial species can conveniently be collected and studied over set periods instead of providing a snapshot from a single tissue. Wang, et al. demonstrated the effects of UV-B exposure on the bacterial species Propionibacterium acnes (P. acnes) collected from facial skin (61). P. acnes is an important commensal species on the skin flora, and it makes up about half the total skin microbiome (64). The bacterium can produce porphyrins such as coproporphyrin III and uroporphyrin III (65). UV-B exposure in this study resulted in a dose-dependent decrease of porphyrins, suggesting the responsiveness of facial bacteria to UV radiation. In untanned Caucasian humans, minimal erythema is induced on un-irradiated skin by a dose of 20–70 mJ/cm2 UV-B, and a decrease of porphyrins in facial bacteria can be detected at doses less than 20 mJ/cm2. This indicates that P. acnes may respond to UV-B before significant skin damage is detected. In addition to porphyrin production, the effects of UV-B radiation were compared to the effect of γ radiation on P. acnes. Though both types of radiation resulted in a reduction of porphyrin production, each form of radiation generated different signatures of protein oxidation/de-oxidation. Additional work in establishing a UV-B-specific oxidative/de-oxidative signature according to changes in time and dose-dependent studies may be used to help create a pre-symptomatic diagnosis of skin cancer. This also opens the platform for investigation on the effects of antioxidants contained in skin products and intrinsic factors, such as age, on oxidative/de-oxidative signatures. Overall, studying bacterial response to UV, by using non-invasive bacterial sampling, may serve as a detection tool of environmental and accidental radiation and serve as a biomarker for skin cancers (61).
Microbiome and Chemoprevention
Fruits and vegetables have been told time and again to play protective roles in prevention of various diseases including cancer (66, 67). In other words, diet plays an important role in modulating the composition of our gut microbiota and is critical in respect to chemoprevention. Therefore, a well-balanced diet may be a key factor in maintaining normal microbiome concerning good health and overall well-being and preventing microbial imbalance that increases the risk of pathogenic infections and cancer. Our commensal bacteria may reduce cancer risk by metabolizing dietary factors into bioactive food components which may influence tumor microenvironment (66, 67). Therefore, evaluating the effect of diet on microbial balance, microbial gene expression and metabolite production is an active area of research.
Dietary fibers are one of the most extensively studied dietary factors in chemoprevention. These are plant edible parts that are not digested and absorbed in small intestine but are up-taken by the resident microbiota in large intestine after partial or complete fermentation (67). Polysaccharides (starch, cellulose, hemicellulose, pectins, gums etc.), oligosaccharides and lignins constitute fiber. The current trend of changing diets from high-fiber in-take to processed foods has increased cancer incidence considerably (66). These dietary fibers interact with gut microbiota and various metabolites are synthesized. Enterococcus casseliflavus is described to be involved in the hydrolysis of sugar moieties, such as in quercetin-3-glucoside, releasing the aglycone quercetin, with acetate, lactate, formate, and ethanol production (67). Eubacterium ramulus, Eubacterium oxidoreducens, Flavonif ractor plautii, as well as several Clostridium strains have been associated with the fermentation of the aglycone quercetin, leading to the formation of taxifolin, 3,4-dihydroxyphenyl-acetic acid, acetate, and butyrate (67, 68–70).
Butyrate is a short chain fatty acid (SCFA) which functions as an energy source and controls epigenetic functions of epithelial cells and has tumor suppressive properties (66). It has been recently demonstrated that dietary fibers change the composition of our gut microbiome more than any other dietary factor. In a recent study, it has been found that fiber protects against colorectal cancer in a gnotobiotic mice (in which the microbiota are known) in a butyrate dependent manner (71). The high fiber diet was found to be effective against colorectal cancer in mice having butyrate producing-bacterium but not in those lacking butyrate producer. Interestingly, mice that lacked butyrate producing bacteria but were kept on diet having butyrate had lower tumor burden than other groups. This suggests that fiber-microbiota chemopreventive effect is because of butyrate. In normal colonic epithelial cells, butyrate is the primary energy source and is almost wholly metabolized in the mitochondria. In cancerous colonic cells, although glucose is the main source of energy due to Warburg effect, butyrate is also transported but is not metabolized in the mitochondria and accumulates in the nucleus and functions as histone deacetylase (HDAC) inhibitor for epigenetically controlling the genes involved in cell proliferation and apoptosis (66, 71). This suggests that probiotic (butyrate producing bacteria) and/or prebiotic (fermentable fiber) may be administered for increasing endogenous HDAC inhibition and reducing tumorigenesis.
Gut microbe derived metabolites can have broader bioavailability and effects other than gut. There is a wealth of chemopreventive data on polyphenols that include flavonoids, anthrocyanins, lignins and phenolic acids (66). Resveratrol can be converted to trans-resveratrol metabolites like dihydro-resveratrol by gut microbiota and has been found to have chemo-preventive effects (72). Ellagic acid, a polyphenol, present in berries and nuts has anti-oxidant properties which contribute to chemoprevention (73). Colonic microbiota metabolize ellagic acid into urolithins which have anti-cancer effects (74). Daidzein is a soy based isoflavone metabolized by gut microbiota into equol (75–77), which is suggested to promote the population of sulphate-reducing bacteria and reduce the population of Clostridium coccoides and Eubacterium rectale (78). Studies have linked Equol to reduced breast and prostate cancer risks. Glucosinolates, present in broccoli and cabbage, are converted to isothiocyanates such as sulphoraphane (butyrate-like HDAC inhibitor) by bacteria-derived thioglucosidases (77). Certain gut microbiota such as Lactobacilli and Bifidobacteria can conjugate linoleic acid, an omega-6 polyunsaturated fatty acid (PUFA) and reduce its levels which is important for reduced inflammation as linoleic acid is a precursor of arachidonic acid that gives rise to prostaglandins and inflammation (76). Bacteria conjugated linoleic acid isomers have also been found to have anti-inflammatory and anti-cancer roles (Figure 1).
Figure 1.
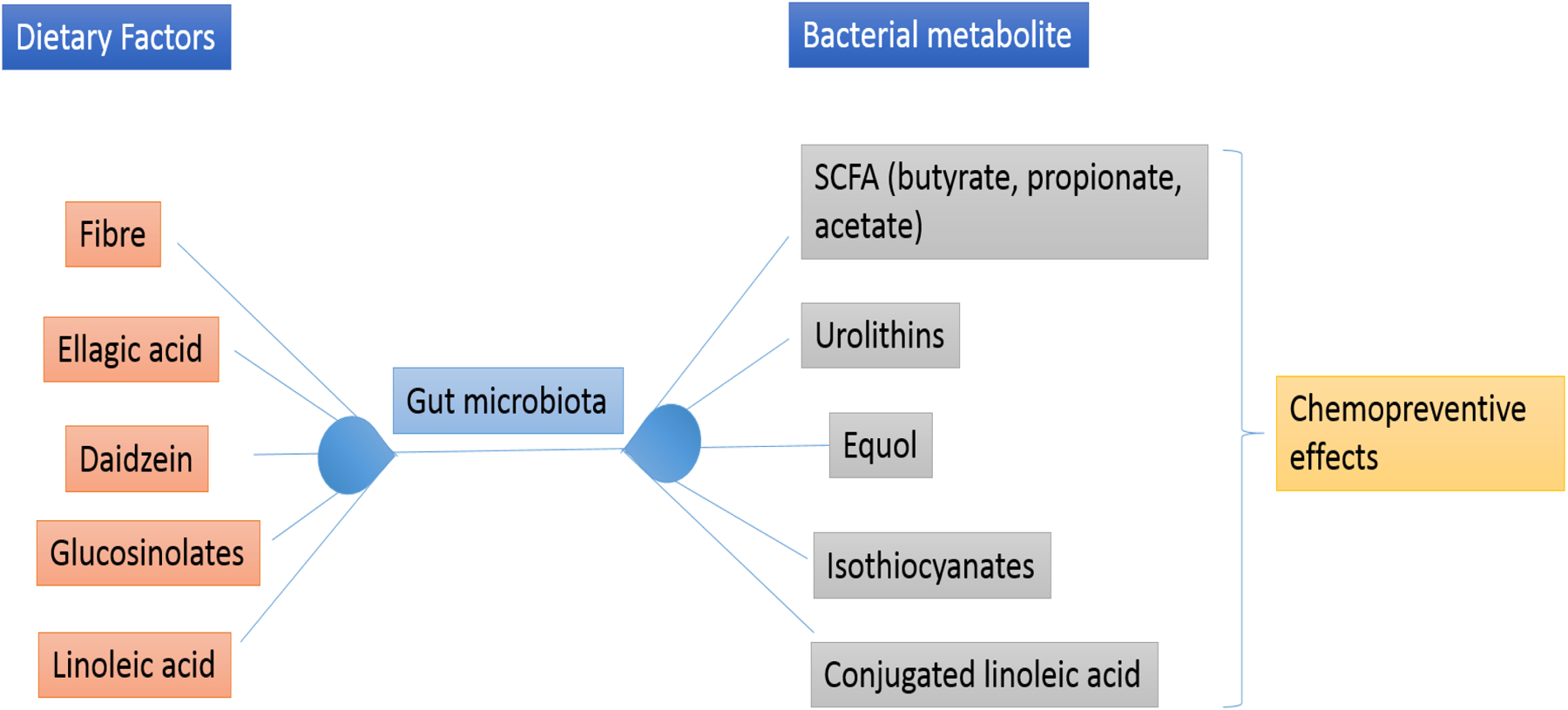
Various dietary components are converted to bioactive food components by gut microbiota that are useful in chemoprevention.
The above section reviews the role of gut microbiome in cancer chemoprevention. It can be speculated that skin microbiome can also perform similar functions. History has witnessed the use of the same dietary factors when applied topically have led to reduced blemishes, tanning and gave rise to healthy and glowing skin. Moreover, dietary factors like polyphenolic antioxidants, isoflavones, green tea and grape extracts etc. have been found to protect against skin cancer when applied topically (79). Interestingly, butyrate, the SCFA produced upon fermentation by gut microbiota was found to induce apoptosis of keratinocytes (80). This gives an indication that, in a manner similar to gut microbiome, microbiota constituting skin microbiome may convert the plant fibers into metabolites having anti-cancer/anti-inflammatory potential that gives them chemopreventive properties. Lack of literature in this regard speaks about the little work done in this area, therefore, it can be an active area of research as the research outcome may lead to development of novel chemopreventive strategies and targets against skin cancer.
Vitamin D and Skin Microbiome
UVB radiation exposure is required for the synthesis of Vitamin D3 in the skin epidermis from 7-dehydrocholesterol (81, 82). 7-dehydrocholesterol absorbs UVB and gets converted to pre-vitamin D3 that is thermally isomerized to form vitamin D3. Vitamin D3 enters liver from circulation and undergoes hydroxylation reaction to form 25-hydroxyvitamin D3 (25(OH)D). It is transported to kidneys where a second hydroxylation reaction occurs to convert 25(OH)D to 1,25 dihydroxyvitamin D (1,25(OH)2D) which is the active metabolite (81, 82) (Figure 2).
Figure 2.
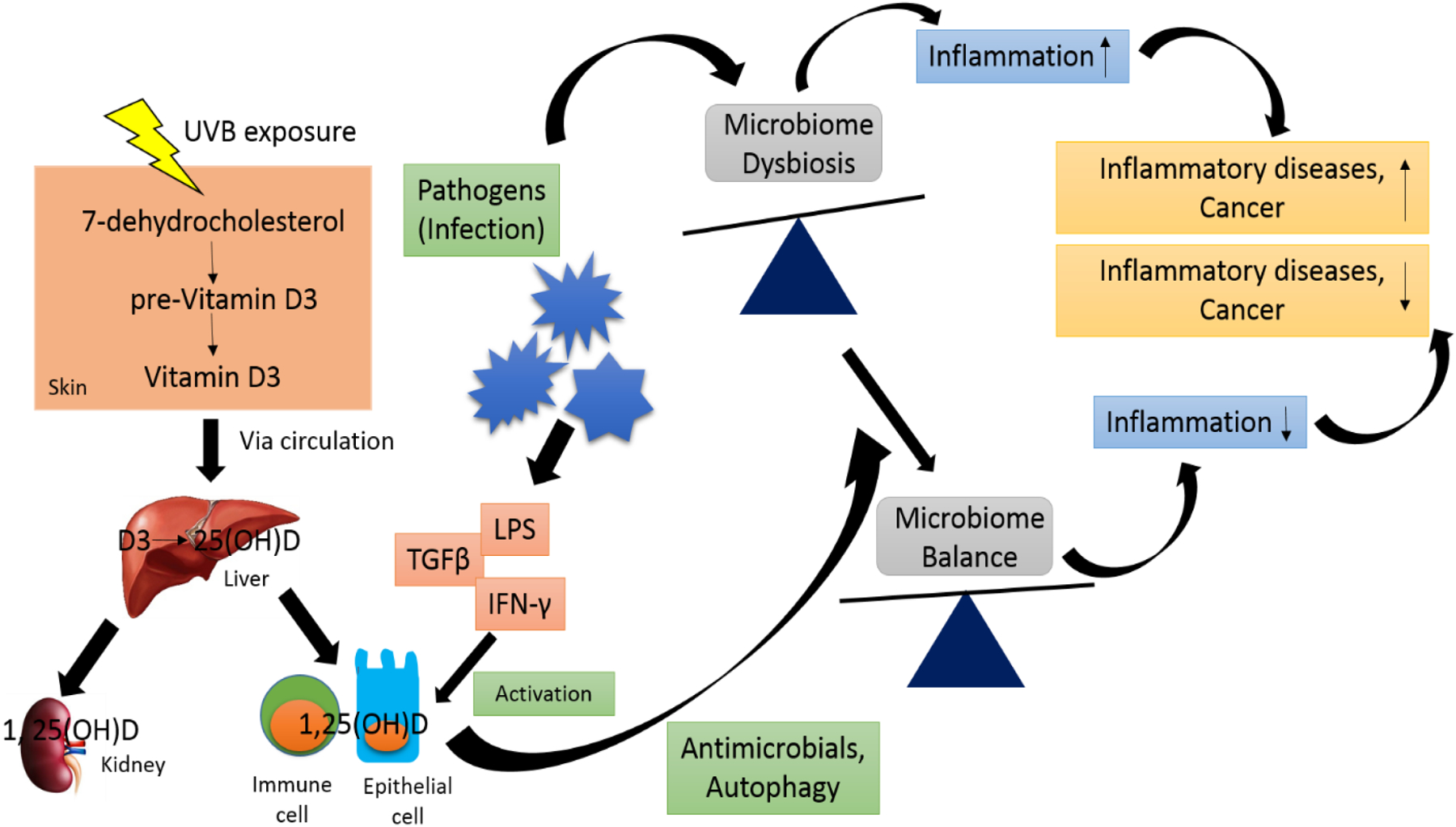
UVB exposure leads to conversion of 7-dehydrocholesterol to pre-vitamin D3 in the epidermis that gets isomerized to vitamin D3. Vitamin D3 enters circulation and gets transported to liver where it hydrolyses to 25(OH)D. Second hydroxylation reaction occurs predominantly in kidneys where 25(OH)D is converted to 1,25(OH)D. Immune and epithelial cells also synthesize 1,25(OH)D from 25(OH)D upon activation by bacterial products like lipopolysaccharide (LPS) and cytokines such as transforming growth factor (TGF)-β and interferon (IFN)-γ. 1,25(OH)D leads to production of antimicrobials and induce autophagy in bacterial cells that may help restore normal microbiome. Normal microflora may lead to decreased inflammation and reduced inflammatory diseases and cancer. On the other hand, microbiome dysbiosis in response to infection may induce inflammatory diseases.
Vitamin D3 status may regulate the bacterial flora constituting the microbiome that may further influence the outcome of immune function. Upon interaction with pathogens, immune cells also synthesize 1,25(OH)2D seemingly through activation of TLRs or stimulation by cytokines (82). Bacteria derived products like lipopolysaccharide (LPS) and cytokines such as transforming growth factor (TGF)-β and interferon (IFN)-γ induce activation of immune cells such as monocytes and epithelial cells that lead to local synthesis of 1,25(OH)2D, and induce the production of regulatory T cells (T-regs) for immune tolerance (82, 83). 1,25(OH)2D may indirectly modulate the local microbiome by influencing innate pathways such as antimicrobial synthesis and activation of autophagy (Figure 1). The crosstalk between vitamin D, microbiome and immune tolerance may reduce inflammation as is observed in gut and lungs. Microbiome dysbiosis is observed in psoriasis and atopic dermatitis (84). Interestingly, topical application of 1,25(OH)2D has been found to be effective in treating psoriasis, atopic dermatitis and other inflammatory skin diseases (85). Therefore, it can be speculated that vitamin D3 may help restore normal microbiome which may inhibit inflammatory responses as seen in psoriasis and other inflammatory diseases. Studies exploring a connection between vitamin D3 and microbiome of the skin may give us an insight about vitamin D3 being a possible agent against skin cancer as microbiome controls the responsiveness of immune cells to vitamin D3 which reduces tissue inflammation that in turn may lead to reduction in cancer incidence.
Prebiotics, Probiotics and Skin Microbiome
Proprionibacteria (P. acnes, P. avidum and P. granulosum), Staphylococci (Staphylococcus epidermidis), Micrococci, Corynebacteria and Acinetobacter are the normal microflora that colonize the human skin as resident species while Staphylococcus aureus, Escherichia coli, Pseudomonas aeroguinosa and Bacillus species are some of the transient species (86). The resident microflora are considered to occupy the space that may be taken by pathogenic microorganisms that can cause infection but at the same time resident microflora themselves become pathogenic under conditions of trauma, injury and when the host becomes immuno-compromised. The type of species to colonize the skin is regulated by several factors including flow of secretions, pH, osmotic potential, integrity of barrier function and biochemical products like lipids, amino acids, vitamins etc (86). As reported, neutral pH favors most resident bacteria while acidic pH favors the growth of Propionibacterium spp (86). It is important to keep into consideration the crosstalk between skin microflora and skin immunity, which is of immense importance in overall health of skin and prevention of various cutaneous diseases including skin cancer. Interestingly, skin microflora have been observed to be decorated with immunoglobulins found in eccrine gland secretions (87, 88). This gives a slight indication towards activation of adaptive immune response by skin microflora. Innate immune responses have also been found to control microbial colonization. Upon activation of TLRs on epidermal keratinocytes, β-defensins as well as cathelicidin are released to inhibit microbial population. These data indicate that skin microflora, skin integrity and skin immune system work coherently to control cutaneous functions and prevent skin diseases (86).
Prebiotics, the soluble and fibrous food ingredients that selectively stimulate/limit the growth of a particular microbial species for the benefit of the host, once considered in the context of altering/modulating gut microflora are now being developed to modulate the skin microorganisms (86). As stated above, various factors lead to dysbiosis i.e. imbalance of normal microflora on the skin and administration of prebiotics helps restore normal balance that decreases disease (Figure 2). In case of overgrowth of P. acnes, the pre-biotic approach is to provide a cosmetic strategy that inhibits the growth of P. acnes but preserves the growth of beneficial bacteria (89). For instance, application of Ginseng or Black currant or pine extracts to human skin was found to be effective against growth of P. acnes keeping the population of coagulase negative Staphylococci unaffected (90). This is indeed superior to various anti-biotic approaches that focus only at the killing of the pathogenic organisms that leads to elimination of the beneficial microbiota as well.
Prebiotic studies are in infancy but a good amount of literature is available for probiotics for skin. It is now a general belief that probiotics are beneficial for overall health of the digestive system by improving the features of gut microflora, in other words, restoring normal microbiome balance (Figure 3). Oral probiotics have been found to be beneficial in the management of gastroenteritis, diarrhoea, inflammatory bowel disease, irritable bowel syndrome, etc (18). They have also been reported to be effective against colon cancer (18). Similarly, oral probiotic supplements given to infants were found to be useful in treatment of atopic dermatitis (91–94). Administration of oral probiotics is found to be effective against atopic eczema in children (95). Intake of Lactobacilli have been linked to reduced UV damage and risk of skin cancer (18). Therefore, it seems that probiotics may exert their effect beyond the gut to the skin level. Alteration in the systemic immune responses seems to be involved in the effects induced by probiotic gut flora. For instance, oral administration of Lactobacillus casei reduced contact hypersensitivity only when CD4 cells were present (96). Administration of Lactobacillus johnsonii to healthy hairless mice induced protection against immunosuppressive effects of UVB-radiation (97). It indicates that modulation of microbiome may be directly involved in modulating tumor microenvironment.
Figure 3.
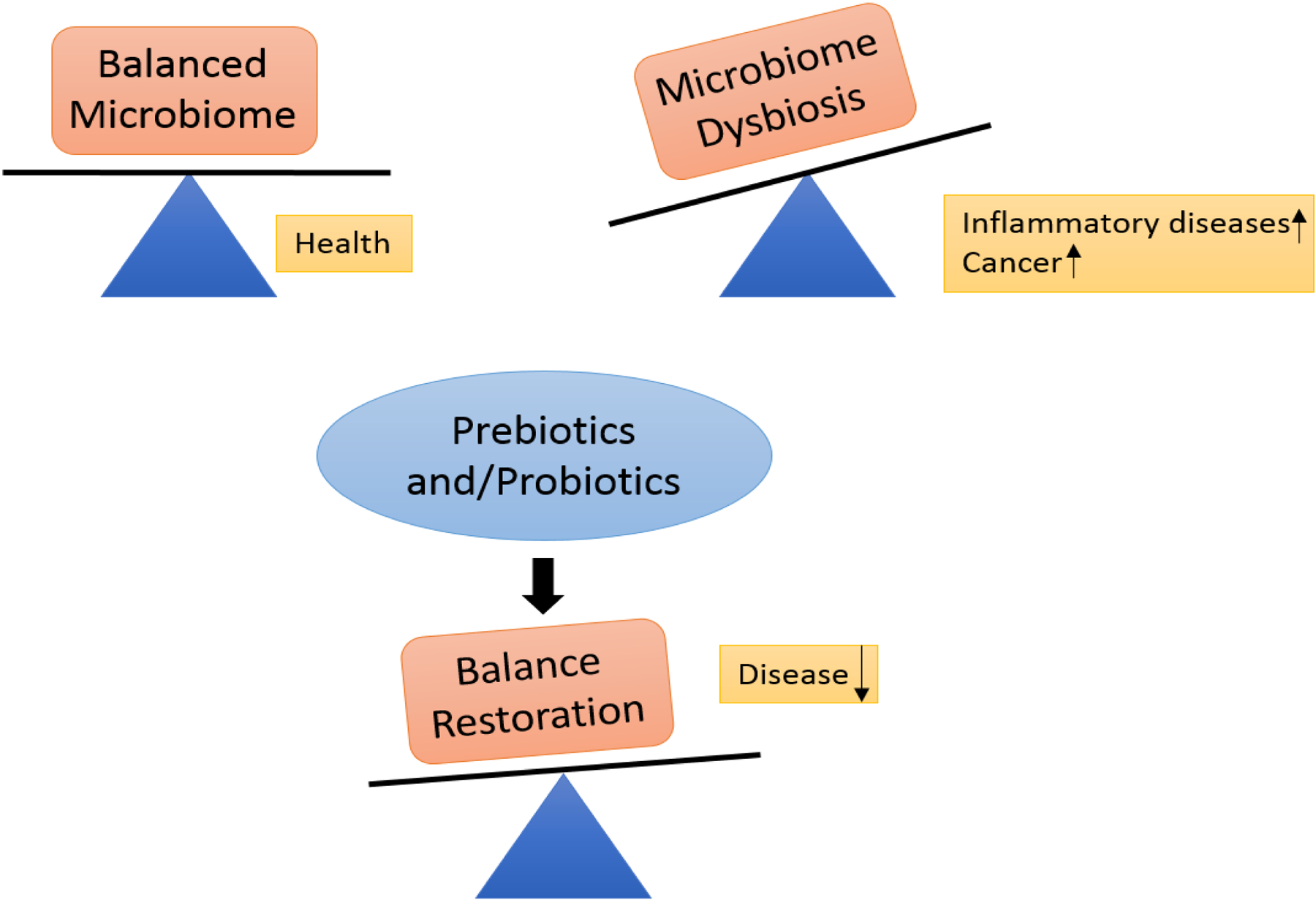
Imbalance in normal microbiome or dysbiosis leads to diseased state. Administration of prebiotics and/probiotics helps restore normal balance that reduces disease.
In addition to oral administration of probiotics, topical probiotics may influence skin microbiome and modulate immune response of the skin to other diseases in a better manner. As far as penetrating stratum corneum is concerned, topical probiotic may be of reduced efficacy but the self-replicating nature of bacteria makes small doses enough for beneficial effects (18). Topical application of Lactobacillus plantarum was effective in reducing P. aeruginosa infections of the skin (98). Moreover, Bifidobacterium longum reduced inflammatory responses caused by substance P when applied topically (28). Topical application of Vitreoscilla filiformis and Lactococcus is found to be beneficial in the treatment of seborrheic dermatitis and atopic eczema by reducing mast cells and increasing T-reg cytokines (18). Application of topical probiotic has also been linked to reduced risk of atopic dermatitis in pregnant women and L. plantarum when applied topically has been found to reduce burn infections (98). It can therefore be suggested that topical probiotics may also reduce the risk of skin cancer by increasing immune-surveillance and reducing chronic inflammation as is done by gut microbiome. Moreover, if a topical probiotic is applied to a cutaneous tumor or injected into it, it may modulate tumor microenvironment by altering immune responses which may lead to therapeutic effects.
Future Studies
Cancer chemoprevention in respect to gut microbiome is an active area of research but we are very far when it comes to cancer chemoprevention in the context of skin microbiome. It is said that diet can change the gut microbiome that influences chemoprevention, so, the question arises can the same diet also lead to alteration in skin microbiome, does skin microbiome convert the plants fibers to same metabolites as obtained in gut and can those metabolites produce chemopreventive effects as those observed for colon. To address these queries, further studies are needed which will open new avenues in the skin microbiome-chemoprevention area and may help developing novel strategies to combat cancer.
Conclusion
In summary, the relationship between microbiome and skin cancer is the subject of ongoing study, and there are considerable gaps in our knowledge about the subject. Continued investigations with advanced sequencing technology will be useful in further understanding the complex human microbiome and its relationship with host. Much of the current data on microbiota and cancer focus on the gut microbiome, and similar research within the skin microbiome could be highly relevant for skin cancer studies. Utilizing the microbiome to study environmental risks of skin cancer may eventually provide excellent ways to diagnose skin cancers early and create novel skin cancer prevention and treatment plans. Investigating the effects of environmental UV, the main risk factor of skin cancers, on commensal bacteria, such as P. acnes, seems to be a promising step to efficient, pre-symptomatic diagnosis of radiation risk. Studying the link between vitamin D and microbiome of skin may seemingly help in identifying vitamin D as skin cancer therapeutic. It is interesting to find association of several bacterial species with cutaneous T cell lymphoma and Marjolin’s ulcer. On the other hand, reduction in UV damage and skin cancer risk upon oral intake of probiotic containing Lactobacilli indicates that modulation of microbiome may directly influence tumor microenvironment. Advances in viral metagenomics has helped identify new virus-cancer interactions, such as MCPyV in MCC and HPV in BCC, and a better understanding of the specific roles these viruses play in the development of specific skin cancers is needed to develop applicable therapies. Association between gut microbiome and chemoprevention to colon cancer indicates that a similar link may be present between skin microbiome and skin cancer prevention. Research focused on the complex relationships between microbes, immune system, and skin cancer may eventually provide novel insight into microbial therapies. There are many unanswered questions regarding the complex relationship between the human microbiome and skin cancer, and attempting to answer these questions shows great promise in potential skin cancer therapies.
Acknowledgement:
Saba Tufail acknowledges University Grants Commission (UGC), Govt. of India for postdoctoral fellowship.
Grant support: This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases 1R01AR071157-01A1 (to N.Y.).
Footnotes
Conflicts of Interest: None of the authors have a potential conflict of interest with this submission.
References:
- 1.Cancer Facts and Figures 2017. American Cancer Society http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-048738.pdf. Accessed May 13, 2017.
- 2.Guy GP, Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am J Prev Med 2014; 104:e69–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV Radiation and the Skin. Int J Mol Sci 2013:doi: 10.3390/ijms140612222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong HH. Skin microbiome: genomics-based insights into the diversity and role of skin microbes. Trends Mol Med 2011; 17:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett C, Knight R, Gordon JI. The human microbiome project: exploring the microbial part of ourselves in a changing world. Nature 2007; 449:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwase T, Uehara Y, Shinji H et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 2010; 465:346–349. [DOI] [PubMed] [Google Scholar]
- 7.Tlaskalová-Hogenová H, Štepánková R, Hudcovic T et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett 2004; 93:97–108. [DOI] [PubMed] [Google Scholar]
- 8.Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- 9.Handelsman J, Rondon MR, Brady SF, Clardy J. Goodman RM Molecular biological access to the chemistry of unknown soil microbes: a new frontier for natural products. Chem Biol 1998;5:R245–249 [DOI] [PubMed] [Google Scholar]
- 10.Eisen JA. Environmental Shotgun Sequencing: It’s potential and challenges for studying the hidden world of microbes. PLoS Biology 2007; 5(3):e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett C, Knight R, Gordon JI. The human microbiome project: exploring the microbial part of ourselves in a changing world. Nature 2007; 449:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Hertzen LC, Joensuu H, Haahtela T. Microbial deprivation, inflammation and cancer. Cancer Metastasis Rev 2011; 30:211–223. [DOI] [PubMed] [Google Scholar]
- 13.Gallimore AM, Simon AK. Positive and negative influences of regulatory T cells on tumour immunity. Oncogene 2008; 27:5886–5893. [DOI] [PubMed] [Google Scholar]
- 14.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (T reg) in human organ transplantation and autoimmune diseases. Clin Exp Immunol 2007; 148:32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns EM, Yusuf N. Toll-like receptors and skin cancer. Front Immunol 2014; 5:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science 2014; 346:954–959. [DOI] [PubMed] [Google Scholar]
- 17.Belkaid Y, Tamoutounour S. The influence of skin microorganisms on cutaneous immunity. Nat Rev Immunol 2016; 16:353–366. [DOI] [PubMed] [Google Scholar]
- 18.Yu Y, Champer J, Beynet D, Kim J, Friedman AJ. The role of the cutaneous microbiome in skin cancer: lessons learned from the gut. J Drugs Dermatol 2015; 14: 461–5. [PubMed] [Google Scholar]
- 19.Bedoya SK, Lam B, Lau K, Larkin J 3rd. Th17 cells in immunity and autoimmunity. Clin Dev Immunol 2013; 2013:986789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agak GW, Qin M, Nobe J et al. Propionibacterium acnes Induces an IL-17 Response in Acne Vulgaris that Is Regulated by Vitamin A and Vitamin D. J Invest Dermatol 2014;134:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol 2001; 79:547–68. [DOI] [PubMed] [Google Scholar]
- 22.Sacksteder MR. Occurrence of spontaneous tumors in the germfree F344 rat. J Natl Cancer Inst 1976; 57:1371–1373. [DOI] [PubMed] [Google Scholar]
- 23.Swann JB, Vesely MD, Silva A et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci USA 2008; 105:652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mittal D, Saccheri F, Vénéreau E, Pusterla T, Bianchi ME, Rescigno M. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J 2010; 29:2242–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cataisson C, Salcedo R, Hakim S et al. IL-1R–MyD88 signaling in keratinocyte transformation and carcinogenesis. J Exp Med 2012; 209:1689–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akaza H, Iwasaki A, Ohtani M et al. Expression of antitumor response. Role of attachment and viability of bacillus Calmette-Guerin to bladder cancer cells. Cancer 1993; 72:558–563. [DOI] [PubMed] [Google Scholar]
- 27.Juckett DA, Aylsworth CF, Quensen JM. Intestinal protozoa are hypothesized to stimulate immunosurveillance against colon cancer. Med Hypotheses 2008; 71:104–110. [DOI] [PubMed] [Google Scholar]
- 28.Khazaie K, Zadeh M, Khan MW et al. Abating colon cancer polyposis by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci USA 2012; 109:10462–10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schellhammer PF, Ladaga LE, Fillion MB. Bacillus Calmette-Guérin for superficial transitional cell carcinoma of the bladder. J Urol 1986; 135:261–264. [DOI] [PubMed] [Google Scholar]
- 30.Chen YE, Tsao H. The skin microbiome: current perspectives and future challenges. J Am Acad Dermatol 2013; 69:143–155.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enterline PE, Sykora JL, Keleti G, Lange JH. Endotoxins, cotton dust, and cancer. Lancet 1985;2:934–935 [DOI] [PubMed] [Google Scholar]
- 32.Vijh AK. Inverse trend between estimated worldwide frequency of major cancers and inferred infectious burdens of populations: possible role of adaptive immune system. Med Hypothesis 2004; 62:880–888. [DOI] [PubMed] [Google Scholar]
- 33.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature 2004; 432:324–331. [DOI] [PubMed] [Google Scholar]
- 34.Lai Y, Di Nardo A, Nakatsuji T et al. Commensal bacteria regulate TLR3-dependent inflammation following skin injury. Nat Med 2009; 15:1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lecuit M, Abachin E, Martin A et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med 2004; 350:239–248. [DOI] [PubMed] [Google Scholar]
- 36.Gold JS, Bayar S, Salem RR. Association of Streptococcus bovis bacteremia with colonic neoplasia and extracolonic malignancy. Arch Surg 2004; 139:760–765. [DOI] [PubMed] [Google Scholar]
- 37.Xie FJ, Zhang YP, Zheng QQ et al. Helicobacter pylori infection and esophageal cancer risk: an updated meta-analysis. World J Gastroenterol 2013; 19:6098–6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Zhang FC, Wang YJ. Helicobacter pylori and pancreatic cancer risk: a meta- analysis based on 2,049 cases and 2,861 controls. Asian Pac J Cancer Prev 2014; 15:4449–4454. [DOI] [PubMed] [Google Scholar]
- 39.Zhong L, Zhang X, Covasa M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J Gastroenterol 2014; 20:7878–7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali IA, Foolad N, Sivamani RK. Considering the Gut-Skin Axis for Dermatological Diseases. Austin J Dermatolog 2014; 1:1024. [Google Scholar]
- 41.Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes 2014; 5:185–99. [DOI] [PubMed] [Google Scholar]
- 42.Benyacoub J, Bosco N, Blanchard C et al. Immune modulation property of Lactobacillus paracasei NCC2461 (ST11) strain and impact on skin defences. Benef Microbes 2014; 5:129–136. [DOI] [PubMed] [Google Scholar]
- 43.Mirvish JJ, Pomerantz RG, Falo LD Jr, Geskin LJ. Role of infectious agents in cutaneous T-cell lymphoma: facts and controversies. Clin Dermatol 2013; 31(4):423–31. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen V, Huggins RH, Lertsburapa T et al. Cutaneous T-cell lymphoma and Staphylococcus aureus colonization. J Am Acad Dermatol 2008; 59:949–952. [DOI] [PubMed] [Google Scholar]
- 45.Coursaget P Viruses associated with skin and mucosal cancers. Presse Med 2014; 43:401–403. [DOI] [PubMed] [Google Scholar]
- 46.Mokili JL, Rohwer F Dutilh BE. Metagenomics and future perspectives in virus discovery. Curr Opin Virol 2012; 2:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samimi M, Touzé A. Viruses and skin cancers. Presse Med 2014; 43:e405–e411. [DOI] [PubMed] [Google Scholar]
- 48.Houben R, Shuda M, Weinkam R et al. Merkel cell polyomavirus infected Merkel cell carcinoma cells require expression of viral T antigens. J Virol 2010; 84:7064–7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bichakjian CK, Lowe L, Lao CD et al. Merkel cell carcinoma: Critical review with guidelines for multidisciplinary management. Cancer 2007; 110:1–12. [DOI] [PubMed] [Google Scholar]
- 50.Munde PB, Khandekar SP, Dive AM, Sharma A. Pathophysiology of merkel cell. J Oral Maxillofac Pathol 2013; 17:408–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jenson AB, Geyer S, Sundberg JP, Ghim S. Human papillomavirus and skin cancer. J Investig Dermatol Symp Proc 2001; 6:203–206. [DOI] [PubMed] [Google Scholar]
- 52.Ferenczy A, Jenson AB. Tissue effects and host response. The key to the rational triage of cervical neoplasia. Obstet Gynecol Clin North Am 1996; 23:759–782. [DOI] [PubMed] [Google Scholar]
- 53.Karagas MR, Waterboer T, Li Z et al. Genus beta human papillomaviruses and incidence of basal cell and squamous cell carcinomas of skin: population based case-control study. BMJ 2010; 341:c2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma Y, Madupu R, Karaoz U et al. Human Papillomavirus Community in Healthy Persons, Defined by Metagenomics Analysis of Human Microbiome Project Shotgun Sequencing Data Sets. J Virol 2014; 88:4786–4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang MH, You SL, Chen CJ et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst 2009;101:1348–1355 [DOI] [PubMed] [Google Scholar]
- 56.Tang MS. Ultraviolet A light: potential underlying causes of melanoma. Future Oncol, 2010; 6:1523–1526. [DOI] [PubMed] [Google Scholar]
- 57.González Maglio DH, Paz ML, Leoni J. Sunlight Effects on Immune System: Is There Something Else in addition to UV-Induced Immunosuppression? Biomed Res Int 2016; 2016:1934518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moan J, Dahlback A, Setlow RB. Epidemiological support for an hypothesis for melanoma induction indicating a role for UVA radiation. Photochem Photobiol 1999; 70:243–247. [PubMed] [Google Scholar]
- 59.Talbott EO, Craun GF. Introduction to Environmental Epidemiology. New York: CRC Lewis Publishers, 1995. [Google Scholar]
- 60.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B 2001; 63:8–18. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Zhu W, Shu M et al. The Response of Human Skin Commensal Bacteria as a Reflection of UV Radiation: UV-B Decreases Porphyrin Production. PLoS One 2012; 7:e47798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Gruijl FR. p53 mutations as a marker of skin cancer risk: comparison of UVA and UVB effects. Exp Dermatol 2002; 11 Suppl: 137–39. [DOI] [PubMed] [Google Scholar]
- 63.Muthusamy V, Piva TJ. The UV response of the skin: a review of the MAPK, NFκB and TNFα signal transduction pathways. Arch Dermatol Res 2010; 302:5–17. [DOI] [PubMed] [Google Scholar]
- 64.McGinley KJ, Webser GF, Leyden JJ. Regional variations of cutaneous propionic bacteria. Appl Environ Microbiol 1987; 35:62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramstad S, Futsaether CM, Johnsson A. Porphyrin sensitization and intracellular calcium changes in the prokaryote Propionibacterium acnes. J Photochem Photobiol B 1997; 40:141–148. [DOI] [PubMed] [Google Scholar]
- 66.Bultman SJ. The microbiome and its potential as a cancer preventive intervention. Semin Oncol 2016; 43:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greiner AK, Papineni RV, Umar S. Chemoprevention in gastrointestinal physiology and disease. Natural products and microbiome. Am J Physiol Gastrointest Liver Physiol 2014; 307:G1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krumholz LR, Crawford RL, Hemling ME, Bryant MP. A rumen bacterium degrading quercetin and trihydroxybenzenoids with concurrent use of formate or H2. Progr Clin Biol Res 1986; 213:211–214. [PubMed] [Google Scholar]
- 69.Schneider H, Schwiertz A, Collins MD, Blaut M. Anaerobic transformation of quercetin-3-glucoside by bacteria from the human intestinal tract. Arch Microbiol 1999; 171:81–91. [DOI] [PubMed] [Google Scholar]
- 70.Winter J, Moore LH, Dowell VR Jr, Bokkenheuser VD. C-ring cleavage of flavonoids by human intestinal bacteria. Appl Environ Microbiol 1989; 55:1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donohoe DR, Holley D, Collins LB et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectaltumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov 2014; 4:1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bode LM, Bunzel D, Huch M et al. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am J Clin Nutr 2013; 97:295–309. [DOI] [PubMed] [Google Scholar]
- 73.Larrosa M, Gonzalez-Sarrias A, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem 2006; 54:1611–1620. [DOI] [PubMed] [Google Scholar]
- 74.Gonzalez-Sarrias A, Larrosa M, Tomas-Barberan FA, Dolara P, Espin JC. NF-κB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br J Nutr 2010; 104:503–512. [DOI] [PubMed] [Google Scholar]
- 75.Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood) 2005; 230:155–170. [DOI] [PubMed] [Google Scholar]
- 76.Davis CD, Milner JA. Gastrointestinal microflora, food components and colon cancer prevention. J Nutr Biochem 2009; 20:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hullar MA, Burnett-Hartman AN, Lampe JW. Cancer Treat Res 2014; 159:377–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bolca S, Possemiers S, Herregat A et al. Microbial and dietary factors are associated with the equol producer phenotype in healthy postmenopausal women. J Nutr 2007; 137:2242–2246. [DOI] [PubMed] [Google Scholar]
- 79.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 2003; 3:768–80. [DOI] [PubMed] [Google Scholar]
- 80.Daehn IS, Varelias A, Rayner TE. Sodium butyrate induced keratinocyte apoptosis. Apoptosis 2006; 11:1379–1390. [DOI] [PubMed] [Google Scholar]
- 81.Burns EM, Elmets CA, Yusuf N. Invited Review Vitamin D and skin cancer. Photochem Photobiol 2015; 91:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lucas RM, Gorman S, Geldenhuys S, Hart PH. Vitamin D and immunity. F1000Prime Rep. 2014; 6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nature Reviews Immunology 2008; 8:685–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zeeuwen PL, Kleerebezem M, Timmerman HM, Schalkwijk J. Microbiome and skin diseases. Curr Opin Allergy Clin Immunol 2013; 13:514–520. [DOI] [PubMed] [Google Scholar]
- 85.Tremezaygues L, Reichrath J. Vitamin D analogs in the treatment of psoriasis: Where are we standing and where will we be going? Dermatoendocrinol 2011; 3:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krutmann J Pre- and probiotics for human skin. J Dermatol Sci 2009; 54:1–5. [DOI] [PubMed] [Google Scholar]
- 87.Okada T, Konishi H, Ito M, Nagura H, Asai J. Identification of secretory immunoglobulin A in human sweat and sweat glands. J Invest Dermatol 1988; 90:648–51. [DOI] [PubMed] [Google Scholar]
- 88.Metze D, Kerssten A, Jurecka W, Gebhart W. Immunoglobulins coat microorganisms of skin surface: a comparative immunohistochemical and ultrastructural study of cutaneous and oral microbial symbionts. J Invest Dermatol 1991; 96:439–45. [DOI] [PubMed] [Google Scholar]
- 89.Leyden JJ, McGinley KJ, Bowels B. Propionibacterium acnes colonization in acne and nonacne. Dermatology 1998; 196:55–8. [DOI] [PubMed] [Google Scholar]
- 90.Bockmuhl D, Jasoy C, Nieveler S, Scholtyssek R, Wadle A, Waldmann-Laue M. Prebiotic cosmetics: an alternative to antibacterial products. IFSSC Mag 2006; 9:1–5. [Google Scholar]
- 91.Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin Exp Allergy 2000; 30:1605–1610. [DOI] [PubMed] [Google Scholar]
- 92.Rosenfeldt V, Benfeldt E, Nielsen DS et al. Effect of probiotic Lactobacillus strains in children with atopic eczema. J Allergy Clin Immunol 2003; 111:389–95. [DOI] [PubMed] [Google Scholar]
- 93.Viljnen M, Savilahti E, Haahtela et al. Probiotics in the treatment of atopic eczema/dermatitis syndromein infants: a double-blind placebo-controlled trial. Allergy 2005; 60:494–500. [DOI] [PubMed] [Google Scholar]
- 94.Weston S, Halbert A, Rihmond P, Prescott SL. Effects of prebiotics on atopic dermatitis: a randomized controlled trial. Arch Dis Child 2005; 90:892–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kalliomaki M, Salminen S, Arvilommi H, Kero P, Kaskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomized placebo controlled trial. Lancet 2001; 357:1076–1079. [DOI] [PubMed] [Google Scholar]
- 96.Chapat L, Chemin K, Dubois B, Bourdet-Sicard R, Kaiserlian D. Lactobacillus casei reduces CD8+ T-cell-mediated skin inflammation. Eur J Immunol 2004; 34:2520–8. [DOI] [PubMed] [Google Scholar]
- 97.Gueniche A, Benyacoub J, Buetler TM, Smola H, Blum S. Supplementation with oral probiotic bacteria maintains cutaneous immune homeostasis after UV exposure. Eur J Dermatol 2006; 16:511–7. [PubMed] [Google Scholar]
- 98.Iannitti T, Palmieri B. Therapeutical use of probiotic formulations in clinical practice. Clin Nutr 2010; 29:701–725. [DOI] [PMC free article] [PubMed] [Google Scholar]


