Summary
Background:
As HIV incidence and mortality continue to increase in Eastern Europe and Central Asia (EECA), especially among people who inject drugs (PWID), it is crucial to effectively scale-up opioid agonist therapies (OAT) to optimize HIV outcomes. With low OAT coverage among PWID, we therefore conducted an optimization assessment using current OAT procurement and allocation and then modeled the impact of increased OAT scale-up on HIV incidence and mortality for 23 administrative regions in Ukraine.
Methods:
In this modeling study, we developed a linear optimization model to estimate efficiency gains that could be achieved based on current procurement of OAT. We also developed a dynamic, compartmental population model of HIV transmission that includes both injection and sexual risk to model the impact of OAT scale-up on HIV infections and mortality over a 10-year horizon. The compartmental population model was calibrated to HIV prevalence and incidence among PWID for 23 regions. The sources for regional data include Syrex database on harm-reduction utilization, Integrated Bio-behavioral Survey and population-size estimates published by Alliance for Public Health, Ukranian Center for Socially Dangerous Disease Control of the Ministry of Health of Ukraine, Public Health Center of Ministry of Health of Ukraine and the Ukranian Census.
Findings:
Under the status quo scenario, the projected number of new HIV infections among PWID in Ukraine over the next ten years would increase by 58,565 (95% CI: 48,011-65,563), with marked regional differences. Under optimal allocation without additional increases in procurement, OAT coverage could increase from 2.7% to 3.3% by optimizing OAT dosage to ensure higher retention levels. OAT scale-up to 10% and 20% over ten years would prevent 4,368 (95% CI: 3,134-5,243) and 10,864 (95% CI: 7,787-13,038) new HIV infections and reduce deaths by 7,096 (95% CI: 5,078-9,160) and 17,863 (95% CI: 12,828-23,062) deaths, respectively, relative to the status quo. OAT expansion to 20% in five regions with the highest HIV burden would account for 56% of HIV infections and 49% of deaths prevented over 10 years.
Conclusions:
In order to optimize HIV prevention and treatment goals in Ukraine, OAT must be markedly scaled-up in all regions, which requires increased medication procurement combined with optimizing OAT dosing in Ukraine. Restricting OAT scale-up to some regions could benefit many, but the regions most impacted are not necessarily those with the highest HIV burden.
Funding:
National Institute on Drug Abuse
Keywords: HIV, mathematical modeling, Ukraine, mortality, opioid agonist therapies, scale-up, linear optimization, prevention
Introduction
Eastern Europe and Central Asia (EECA) remains the only region globally where HIV incidence and mortality continue to increase,1 in the setting of suboptimal prevention coverage.2 Ukraine is a lower-middle income country with 43 million people with low average life-expectancy (66.1 years for men). Ukraine has the highest HIV prevalence (1·2%) among adults and the second highest number of people with HIV (PWH) in the EECA region, with over 240,000 estimated HIV cases.3 The HIV epidemic in Ukraine is concentrated in people who inject drugs (PWID), who accounted for 33% of all PWH and 22% of new HIV infections in 2015, suggesting transition to other key populations. 4,5 Triangulation data from Ukraine, however, suggest that HIV risk is often misclassified with the HIV epidemic remaining concentrated in key populations, especially PWID.5 Moreover, HIV prevalence is 22·6% among the estimated 340,000 PWID (172,000-520,000) in Ukraine in 2016, with >80% injecting opioids.6–8
Opioid agonist treatment (OAT) with buprenorphine was first introduced for HIV prevention in Ukraine in 2004, followed by methadone maintenance treatment (MMT) in 2008, with access prioritized for PWH and gradually expanded broadly to include treatment for any PWID with dependence.9 Modeling from Ukraine using 2005 to 2008 data found OAT scale-up with methadone would be the most cost-effective HIV prevention strategy,10 but the model did not use real-world data from Ukraine, modeled relatively low OAT coverage that was not population-based (500 to 4,000 treatment slots, though stated range as 3.1% to 25% coverage) and included ART coverage levels reaching 80%, which has essentially not been achieved anywhere.
Despite overwhelming evidence supporting its effectiveness, OAT access in Ukraine remains limited. In 2015, only 2.7% (N=8,512) of PWID received OAT.11 Though international donors like the Global Fund to Fight AIDS, Tuberculosis and Malaria (GFFATM) ensured funding for 30,000 OAT patients by 2015, OAT scale-up failed to meet targets as early as 2010 due to multiple patient, program and structural barriers.12 Though OAT scale-up is the most cost-effective ΗIV prevention strategy for Ukraine’s transitioning epidemic, it has not been examined using real-world data, incorporated mortality as an outcome nor provided the granularity of regional differences to more effectively provide a targeted response. We therefore examined the efficiency and effectiveness of OAT programs nationally and in each region under current constraints, as well as if OAT scale-up met the minimum levels (20%) recommended by the World Health Organization (WFIO).13,14,15,16
Methods
Study Design and Setting
Our study design is simulation-based and uses a combination of mathematical modeling and real-world regional data from Ukraine to analyze efficiency and effectiveness of OAT programs. OAT is restricted to methadone, since the number of buprenorphine patients has not increased above 700 after 2008, when all OAT expansion was limited to methadone. Ukraine, is administratively divided into 26 regions (24 oblasts and 2 cities). We excluded four administrative regions (Crimea, Donetsk, Luhansk and Sevastopol) where OAT delivery is markedly influenced by on-going military conflict between Ukraine and Russia; leaving 22 remaining regions for analysis, as well as the city of Kyiv, which holds special administrative status. In 2016, OAT coverage remained low, with 2.7% of PWID nationally receiving treatment, with variable regional coverage levels (Ivano-Frankivsk, Mykolaiv, Poltava, Vinnytsia and Zhytomyr achieved coverage levels ranging between 5% to 12%, while Odesa and Zakarpattia provided <1% OAT coverage. Our modeling analysis focuses on MMT, which accounts for 91% of OAT prescribed in Ukraine in 2016. Data from the national OAT registry (SyReX) for 2016 showed mean retention on OAT after 12 months as 84.2% (range among regions: 73%-90%) (Table S3: On-line Supplement). Using epidemic profiles and regional data, including statistics on OAT enrollment and retention in each region, we developed two different mathematical models. The first model evaluated the efficiency of current OAT programs, while the second model assessed the prospective impact of expanding OAT programs to 10% and 20% coverage on HIV prevention and mortality over 10 years.
Model 1: Linear Optimization Analysis
We developed a linear optimization model to estimate the optimal size of OAT programs in each region in Ukraine from 2011-2016. Retention on and attrition from OAT was calculated using the SyReX database (2011-2016). Among 16,183 clients enrolled in OAT, retention on OAT increased with increasing average MMT dosage (<40mg, 40-60mg, 60-100mg, >100mg), with average retention and associated hazard ratio estimates reported in Tables S1/S2 (appendix p.1-2). Retention on OAT is correlated with higher primary and secondary HIV prevention levels. Nationally, the average rate of attrition from OAT programs was 16.8% in 2016, with >20% of regions having attrition rates that exceed 20%. The optimization model allowed us to assess relative efficiency gains that could be attained under current resource constraints. In our modelling formulation, each region’s central decision-maker is assumed to be in charge of a) admission policies determining the number of patients whom they project should be in OAT in each period and b) MMT dosage policies to guide patient treatment. The average annual amount of MMT procured between 2011-2016 was obtained from publicly available procurement records, and served as a resource constraint for the total dosage, as each region’s central planner was allocated an amount of MMT proportional to the number of “registered” (not estimated) PWID in each region. The optimization model was used to project the total procurement needed to attain 20% OAT coverage. In modeling the dynamics of the participation constraint, we used data from SyReX and incorporated the probability of attrition based on the observed association between dosage and the patient’s hazard of discontinuing OAT. The model was implemented using R, and the technical details of the analysis and procedure are contained in the supplementary on-line appendix (p.1-3)
Model 2: HIV Transmission and Mortality
We also developed a deterministic model of HIV transmission in order to estimate the impact of expanding OAT coverage (5%, 10%, 20% of PWID) on the number of new HIV infections and mortality in each region over 10 years. The HIV transmission model was stratified by both injection and sexual risk, assuming proportional homogenous mixing within different populations. In the model, individuals were stratified into three mutually exclusive groups: heterosexual men (HM), heterosexual women (HW) and men who have sex with men (MSM). The three population groups were disaggregated into PWID and non-PWID based on injection drug behavior. PWID were further stratified by their OAT participation status, which is reflected in differential rates of mortality, injection behaviors, propensity to share injection paraphernalia, as well as adherence to ART for PWH and the probability of attaining viral suppression. Cause-specific mortality data was not available for regions, restricting our analysis to reporting all-cause mortality. The summary of parameters used in the models, as well as data sources, is available in Table 1. To calibrate the model, we developed a step-wise minimization algorithm which is described in the supplementary appendix (p. 11). The calibration parameters, which we allowed to vary when fitting the model, included rates of injection in PWID, probability of HIV transmission per sexual act and per injection, conditional on population group, injection frequency among PWID not on OAT, and average number of injections per day among them.
Table 1:
Summary HIV transmission compartment model parameters*
| Parameters | Definition | Median Value (Regional Range) | Distribution | Source |
|---|---|---|---|---|
| p | HIV Prevalence | 20.4 (4.3-34.5) | - | Estimated from IBBS data (2009-2015) from Alliance for Public Health6 (Description in the Appendix p.6-7) |
| i | HIV incidence, PWID not on OAT | 0.022 (0.004-0.07) | - | Ukrainian CDC 28 |
| d1 | Mortality rate per year among non-PWID, HIV− | 0.011 (0.009,0.012) | LogNormal | Ukrainian Census 29 |
| d2 | Mortality rate per year among non-PWID, HIV+ | 0.021 (0.008 — 0.037) | LogNormal | Ukrainian CDC28 |
| d3 | Mortality rate per year among non-OAT PWID, HIV− | 0.061 (0.012-0.18) | Calculated | Calculation Based on Sordo et al20 |
| d4 | Mortality rate per year among non-OAT PWID, HIV+ | 0.14 (0.04-0.26) | Calculated | Calculation Based on Sordo et al20 |
| d5 | Mortality rate per year among OAT PWID, HIV− | 0.019 (0.0036-0.057) | LogNormal | Syrex database (Description in the Appendix p.4) |
| d6 | Mortality rate per year among OAT PWID, HIV+ | 0.05 (0.014-0.083) | LogNormal | Syrex database (Description in the Appendix p.4) |
| mrroat | Mortality Risk Ratio: PWID on OAT vs Non-OAT | 3.2 (2.6-3.86)* | Uniform | Global Meta-analysis 20 |
| α | Average number of sexual act frequency per month | 6.27 (1-11.2)* | Poisson | Kiev International Institute of Sociology30 |
| β1 | Average number of injections per day among PWID not on OAT | 0.90 (0.3 — 1.31) | Varied to fit the model | Calibrated to fit the model; validated against ExMAT data |
| β2 | Average number of injections per day among PWID on OAT | 0.12 (0.095 — 0.14) | Beta | ExMAT data (Description in the Appendix p.4) |
| f1 | Probability of sharing needles among PWID not on OAT | 0.16 (0.04 — 0.28) | - | Varied to fit the model |
| f2 | Probability of sharing needles among PWID on OAT | 0.009 (0.0006 —0.02) | Beta | ExMAT data (Description in the Appendix p.4) |
| Voat | Probability of Viral Suppression among HIV+ PWID on OAT | 0.446 (0.11—0.99) | LogNormal | SyReX (Description in the Appendix p.4) |
| vNon—oat | Proportion of HIV+ PWID who attain viral suppression among non-OAT PWID on ART | 0.668 (0.49- 0.77) | LogNormal | Ukraine’s Ministry of Health 31 |
| RART | Proportion of PWID receiving ART among Non-OAT, HIV+ PWID | 0.5455 (0.48- 0.72) | LogNormal | Ukraine’s Ministry of Health 31 |
| THIV | Proportion of HIV status awareness in Ukraine | 0.56 | LogNormal | UNAIDS8 |
Legend: The complete list of parameters are in the supplementary online appendix p.6-7. The following abbreviations are used: MSM – men who have sex with men, PWID – people who inject drugs, OAT – opioid agonist therapy, ART – antiretroviral therapy
The model was validated against HIV prevalence in each of the compartments, as well as the number of new HIV Infections in each region in 2015 and 2016. The estimates of HIV prevalence among PWID were obtained from published reports from the 2011-2015 IBBS survey data. HIV incidence was calculated based on the statistics reported by Ukraine’s Health Ministry in 2016 on new HIV cases diagnosed, which was comparable to empiric incidence estimates obtained from the 2017 IBBS data. Additional technical details on the calibration and validation procedures, as well as the results are contained in the appendix (p.11-17). The model was implemented in the mathematical programming language Matlab 2018b.
Sensitivity and Uncertainty Analysis
In order to account for parameter uncertainty we sampled all the parameters from their respective distributions except for specific parameters which were calibrated in the process of fitting the model (Table 1). For the sensitivity analysis, we varied the size of PWID population by using the previously published estimates, which are displayed in the supplementary appendix (, p.19-41). In addition we varied the coverage of ART among PWID by changing the probability of viral suppression among OAT, as well as PWID not on OAT by +/−30% from the levels observed in 2016. We similarly varied ART prescription and viral suppression among PWID not on OAT by +/−30%. Finally, we examined the sensitivity of the outcomes to the population heterogeneity, by aggregating MSM and non-MSM populations.
Role of the funding source
The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The first and corresponding authors had full access to all study data, computer code and take final responsibility for the decision to submit the manuscript for publication.
Results
In the linear optimization model, OAT participation could achieve gains from 7,262 to 8,682 patients, (2.7% to 3.3% of PWID population) due to strategies that favored higher rates of admission of patients as well as improved retention through higher administered doses of MMT, if MMT supply was kept constant, with notable regional differences (Figure 1). Using this strategy, new infections (N=1,132) and deaths (N=1,932) averted over 10 years would be negligible. The largest gains in OAT enrolment could be made in Odesa (N=585; 197% increase), Mykolaiv (N=126; 19% increase) and Cherkasy (N=81; 49% increase) while the smallest gains were in Dnipro (N=27; 2%). By procuring enough MMT (1,967kg) to achieve 20% coverage levels, the linear optimization model estimates that every region can improve OAT retention by increasing average OAT dosage to at least 80 mg or higher, as only 8 out of 23 regions (Khmelnytskyi, Kyiv, Odesa, Sumy, Ternopil, Vinnytsia, and Zaporizhia) have average doses of at least 80 mg.
Figure 1:
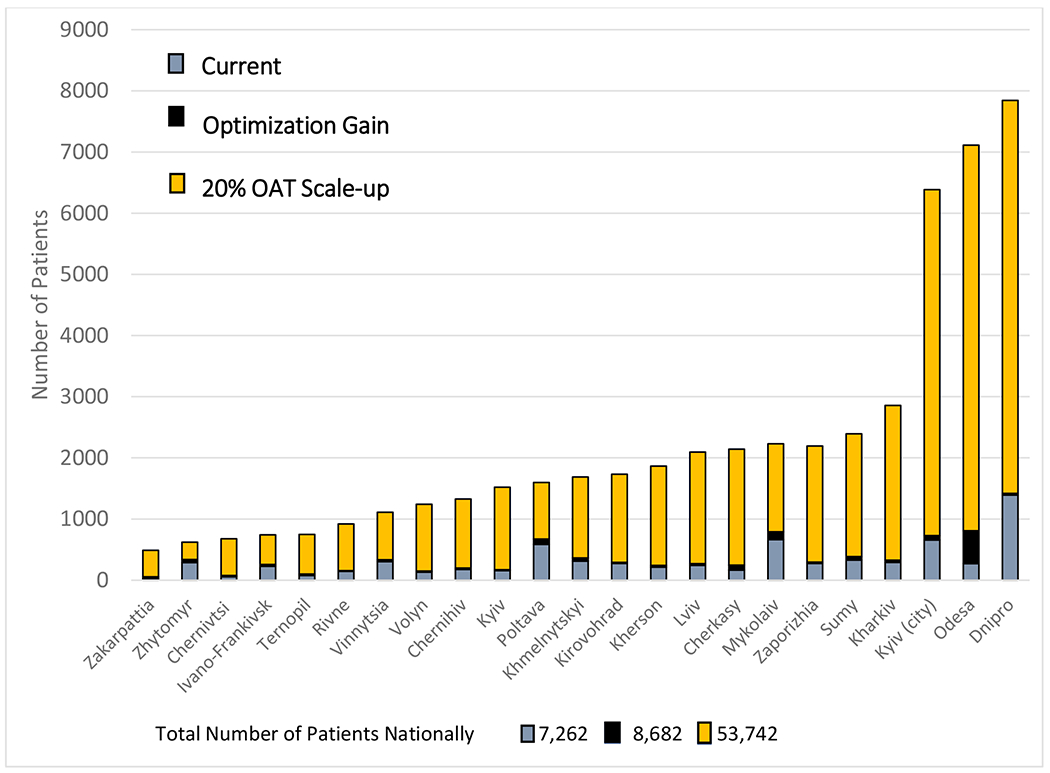
Modeling Opioid Agonist Treatment Optimization and Scale up Regionally in Ukraine
Legend: Modeling Scenarios A. Current OAT coverage based on observed levels of OAT enrollment (grey); B. Optimization gain using no additional procurement for the country, but with optimal dosing allocations, treatment entry and retention strategies (black); C. Scaling up OAT coverage to 20% in each region (orange).
In the HIV transmission model, the HIV prevalence and incidence rates for 2016 match the data well for each region (,Appendix p.16-17). Under the status quo (OAT coverage=2.7%), new HIV infections and number of deaths among PWID will increase by 58,565 (95% CI: 48,011- 65,563) and 191,531 (95% CI: 131,553-268,845), respectively, over 10 years. Five regions, Dnipro (N=11,032), Odesa (N=6,912), Kyiv oblast (N=6,305), Kyiv City (N=5,434), and Poltava (N=3,043) would experience the highest increases in the number of HIV infections among PWID, accounting for 56% of new HIV infections over 10 years (Table 2).
Table 2:
Aggregate Number of Estimated New HIV Infections and Deaths among PWID over 10 years Without Scale-up of Opioid Agonist Therapies
| Region | New Infections | New Deaths |
|---|---|---|
| Dnipro | 11,032 (9,745, 13,180) | 34,943 (30,538, 39,600) |
| Odesa | 6,912 (4,485, 12,127) | 26,052 (15,257, 37,954) |
| Kyiv (city) | 5,434 (4,289, 6,225) | 20,289 (13,661, 28,199) |
| Kharkiv | 2,490 (1,353, 3,110) | 8,809 (3,577, 15,890) |
| Sumy | 1,113 (651, 1,320) | 4,579 (1,956, 8,183) |
| Zaporizhia | 2,710 (1,485, 3,815) | 9,794 (5,317, 14,816) |
| Mykolaiv | 2,589 (1,578, 3,179) | 8,761 (6,619, 11,278) |
| Cherkasy | 2,262 (1,137, 2,850) | 6,297 (2,987, 12,284) |
| Lviv | 2,127 (1,161, 2,657) | 12,510 (7,121, 18,228) |
| Kherson | 1,711 (1,058, 2,345) | 7,731 (4,590, 11,035) |
| Kirovohrad | 2,231 (1,660, 2,652) | 3,492 (1,187, 6,904) |
| Khmelnytskyi | 460 (324, 569) | 6,248 (3,698, 9,380) |
| Poltava | 3,043 (2,118, 3,594) | 5,458 (3,390, 7,953) |
| Kyiv (Region) | 6,305 (3,167, 7,688) | 6,236 (3,325, 10,661) |
| Chernihiv | 2,923 (2,013, 3,363) | 2,976 (656, 6,746) |
| Volyn | 582 (347, 1,190) | 4,829 (3,495, 6,330) |
| Vinnytsia | 751 (526, 924) | 1,253 (250, 3,414) |
| Rivne | 645 (318, 856) | 2,431 (781, 5,780) |
| Ternopil | 154 (112, 198) | 6,349 (3,449, 8,884) |
| Ivano-Frankivsk | 458 (207, 592) | 4,095 (2,000, 6,628) |
| Chernivtsi | 257 (145, 358) | 2,391 (341, 6,123) |
| Zhytomyr | 2,502 (1,208, 3,026) | 3,133 (1,714, 4,748) |
| Zakarpattia | 129 (89, 195) | 2,667 (954, 4,782) |
| Total | 58,820 (47,968, 65,535) | 191,330 (131,321, 270,605) |
Note: Results are based on Means of 1,000 simulated runs, with 95% Confidence Interval reported in parentheses
Relative to the status quo, scaling up OAT to 10% and 20% over ten years would prevent 4,368 (95% CI: 3,134- 5,243) and 10,864 (95% CI: 7,787-13,038) new HIV infections and reduce deaths by 7,096 (95% CI: 5,078-9,160) and 17,863 (95% CI: 12,828-23,062), respectively. Expanding OAT coverage to 10% and 20% in regions with the highest HIV burden (Dnipro, Mykolaiv, Odesa, Kyiv City and Kherson), which contain 50% of the PWID population and are designated as high priority regions by PEPFAR, would reduce the total number of new HIV infections [2,207 (3.8%) and 5,224 (8.9%)] and deaths [3,778 (2.0%) and 9,335 (4.9%)], respectively, over 10 years. Expanding OAT coverage to 10% and 20% in remaining 18 regions, the remaining 50% of estimated PWID, would reduce the total new HIV infections by 2,151 (3.7%) and 5,619 (9.6%,), respectively, while the number of deaths averted among PWID would be 3,317 (1.7%) and 8,527 (4.5%), respectively (Figures 3 & 4).
Figure 3:
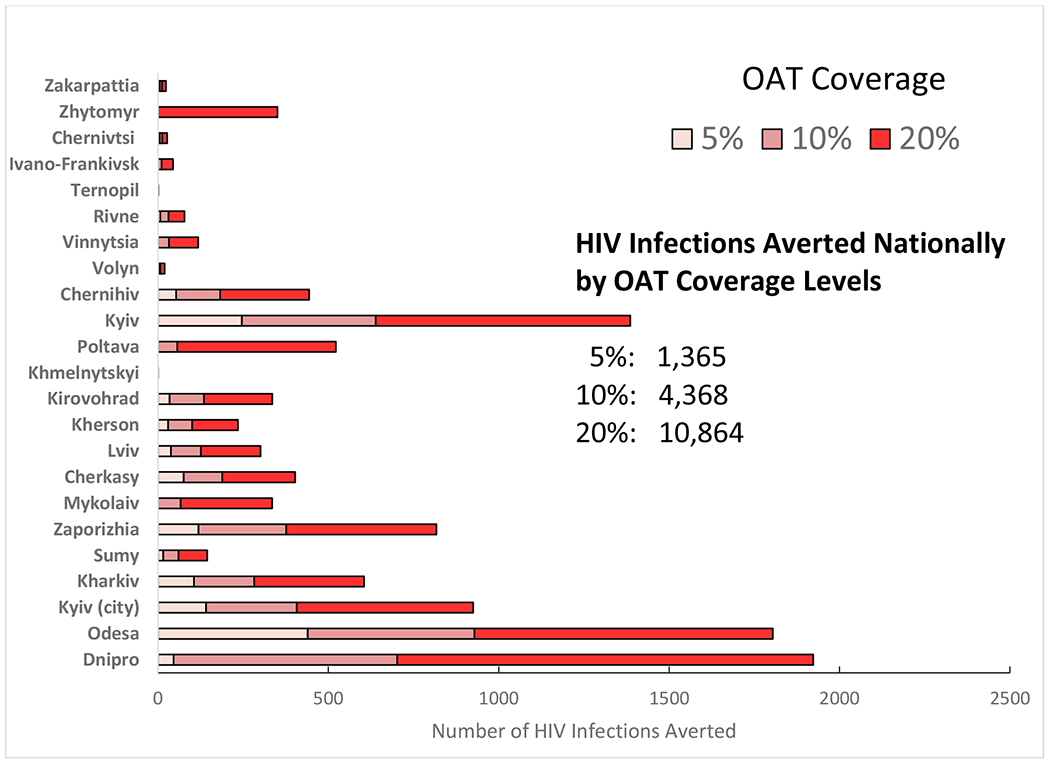
Number of HIV Infections Averted Nationally and in Each Region of Ukraine Over 10 years
Figure 4.
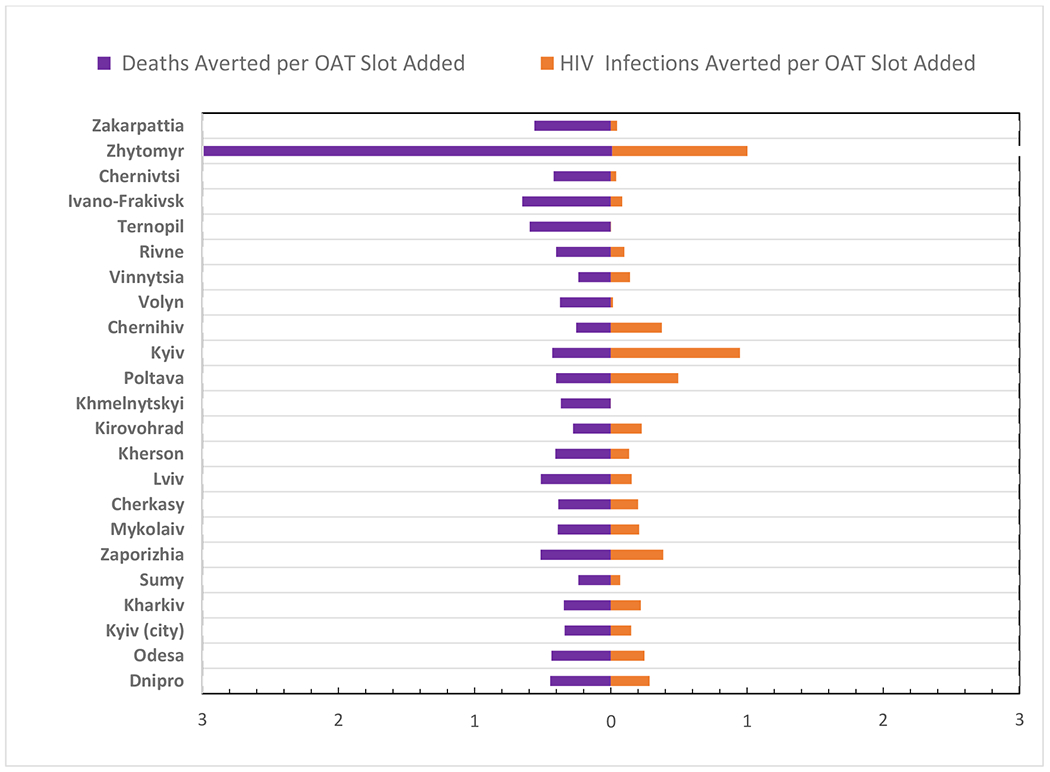
Number of Deaths and HIV Infections Averted per Additional OAT Slot in a 20% Scale-up Scenario
OAT coverage levels would have a differential impact regionally when examining the number of new HIV infections and deaths averted as a ratio (R) of the total OAT slots added and filled to attain the 20% coverage benchmark. Zhytomyr (R=0.99), Poltava (R=0.49) and Kyiv regions (R=0.82) would have the highest marginal impact from scale-up in terms of HIV infections, while less marginal impact would be observed in the small regions of Chernivtsi (R=0.04), Ternopil (R=0.001) and Khmelnytskyi (R=0.0003). Under the same scenario, Ivano-Frankovsk (R=0.65), Ternopil (R=0.59), Zakarpattia (R=0.56), Cherkasy (R=0.51), and Lviv (R=0.51) are likely to have highest ratio of the number of deaths averted per OAT slot added, with the lowest marginal effects observed in Zhytomyr (R=0.28), Chernihiv (R=0.25), Sumy (R=0.23) and Vinnytsia (R=0.23).
We also performed sensitivity analyses assuming different rates of ART coverage and viral suppression among PWID on and off OAT. For most regions, predictions about mortality and new infections were not sensitive to changes in the rates of viral suppression among PWID on OAT, with higher number of HIV infections and deaths averted based on higher viral suppression levels. The predictions for mortality and new infections were more sensitive to the variations in the probability of viral suppression among PWID not on OAT. On average, increases in viral suppression and ART coverage among PWID not on OAT by 30%, would reduce the estimate of new HIV infections by 5%-6%, while a reduction in viral suppression and ART coverage among PWID not on OAT would increase new HIV infections and mortality by 10-12%, on average, per region. In addition, the estimates for the number of HIV infections averted were not sensitive to the variation in the population size (<10% relative difference) for most of the regions. However the predicted number of new deaths was sensitive to large increases in the population size. Finally, the model for the impact of OAT was not sensitive to the aggregation of MSM and non-MSM groups (less than10% relative difference). The results of the sensitivity analysis are depicted in Figures S2–S28 of the supplementary appendix (p.19-41).
Discussion
Without OAT expansion in Ukraine, HIV incidence and mortality are projected to worsen, with markedly diverse regional effects over a 10-year horizon. Reallocating OAT throughout the country would minimally increase OAT coverage and have limited influence on averted new infections and mortality, suggesting that shifting current resources is reasonably effective, but do little to avert new infections or death. Instead, policy-informed strategies that facilitate increases in OAT coverage, including improved medication procurement practices and ones that optimize quality OAT-prescribing practices to optimize methadone dosing have the highest likelihood to substantially reduce HIV incidence and mortality. Even doubling OAT coverage from 2.7% to 5% nationally would not markedly reduce HIV transmission and mortality in PWID. Increasing OAT scale-up to 20%, the minimum recommended coverage level by the WHO and already achieved throughout most of Western Europe may prevent 20% of new HIV infections and 9.5% of deaths in PWID over the 10-year horizon. This translates to prevention of 10,843 new infections and saving 17,862 lives. In addition to saving lives, OAT is one of the most effective treatments that has been shown to bring a wide range of health and social benefits, including reduction or elimination of injection drug use, reduced crime, and higher social stability linked with better employment outcomes and higher quality of life.17 Findings in this study suggest a reappraisal of current HIV prevention and treatment strategies.
Currently, PEPFAR limits support for HIV treatment and prevention efforts in 12 regions (six high-burden and six medium-burden), which account for nearly 80% of all PWH and newly reported infections in 2016.18 Findings from our data that examines both new infections and death, however, suggest that a more nuanced approach be considered if resources must be constrained. For example, unsupported regions like Kharkiv , Poltava, Zaporizhia and Zhytomyr merit inclusion for targeted funding as these regions appear to be vulnerable to volatile HIV transmission where in the absence of OAT scale-up, new infections might increase most. In the case of mortality, unsupported regions of Kharkiv, Lviv, Ternopil and Zaporizhia remain vulnerable in the absence of OAT scale-up.
The benefits to scaling up OAT in regions with large PWID populations (e.g., Dnipro, Kyiv city, Odesa) would prevent the largest number of deaths and HIV infections in absolute terms, while the scale-up in regions with smaller PWID populations remains important, especially for prevention of mortality. On average, the number of deaths averted per OAT slot created was higher than the number of infections averted per OAT slot created, which in many cases, favored some smaller regions.
In systematic reviews, OAT not only decreases mortality among PWID,18 but also increases the likelihood of improved HIV treatment outcomes (i.e., ART receipt and adherence and viral suppression19,20), including data from Ukraine21. Our findings generally support previous modeling studies that confirm benefit that OAT reduces HIV transmission and mortality, but here we have incorporated real-world estimates of OAT coverage and achievable ART strategies that incorporate HIV treatment as prevention principles. With concerns about the sustainability of continued international support from GFTFATM and PEPFAR,22 findings suggest that more effective implementation and sustained commitment of domestic and international resources are needed to reverse the trend of increased HIV incidence and mortality – a problem plaguing the EECA region. Despite low OAT coverage in Ukraine, 2.7% is higher than other countries in the region where it exists mostly as demonstration programs and where there is no OAT at all in Russian and Turkmenistan.2 Key findings here may serve as a guide to other EECA countries that have similar HIV epidemics and support several-fold increases in OAT coverage along with quality treatment that optimizes adequate methadone dosages to promote OAT retention;23 both strategies are that aligned with WHO recommendations.
Real-world OAT scale-up requires navigating numerous structural, clinical and patient-level barriers that have slowed down the implementation of the OAT expansion in Ukraine. Barriers to OAT expansion include rigid government regulation, fiscal constraints, and lack of coordination between regions for developing treatment guidelines.24 As of March 2016, the Ukrainian Ministry of Health adopted an amendment under Order 200, which expanded OAT access by allowing PWID to obtain buprenorphine in pharmacies through prescriptions and to forego the official registration “as a drug users” the office of National Narcology requires, which is likely to improve access to OAT and improve entry into substance abuse treatment.25 In 2017, the Ukrainian Parliament approved the Health Finance Reform law in an effort to reform the healthcare system.26 There is a need for Ukraine to continue increasing local and national funding for HIV services and improve access to HIV care and treatment. Moreover, as Ukraine is a leader in HIV prevention and treatment in the EECA region, a region that shares similar epidemiology of HIV among PWID, findings here should be used to guide HIV prevention and treatment strategies elsewhere in EECA.2
Our study has several limitations. First, there is parameter uncertainty stemming from a lack of consistent estimates, potentially due to sampling as well as measurement error in the population data, which we have previously reported,27 which may cause us to underestimate the impact of OAT expansion, as well underestimate mortality and incidence. We attempted to mitigate uncertainty through parameter sampling and additional sensitivity analysis, which showed the overall findings were robust. Even though our model incorporates the latest regional data, the transmission of HIV and injection drug use behavior may be subject to stochastic fluctuations not fully captured by the models, or assessed in the data. Despite our rigorous effort to validate the model against HIV prevalence in different population groups, the absence of reliable time series data constrains our ability to validate the model over longer time periods and account for stochastic trends. In addition, the absence of intervention effect of OAT on mortality in Ukraine has constrained us to rely on published estimates based on a global meta-analysis, which may be different in Ukraine, especially if in the presence of non-random selection into OAT programs that may induce selection bias. In addition, due to the observational nature of the study that may contain measurement errors, our estimate of the impact of dosing on average retention may be not be accurate, especially if lower dosage is recorded prior to patients’ departure. The constant hazard assumption used in the optimization model, while serving as an approximation, may ignore time-dependent variation in retention.
Conclusions
The results highlight the critical need to markedly scale-up OAT for prevention of new HIV infections and mortality in all regions, which would be accomplished by the combination of increased procurement of medication, as well as a provision of adequate dosing to promote treatment in Ukraine. As more affordable and effective forms of treatment for opioid use disorder become available, like buprenorphine or extended-release naltrexone, this should extend the benefits of treatment that we modeled.28–31
Supplementary Material
Figure 2:
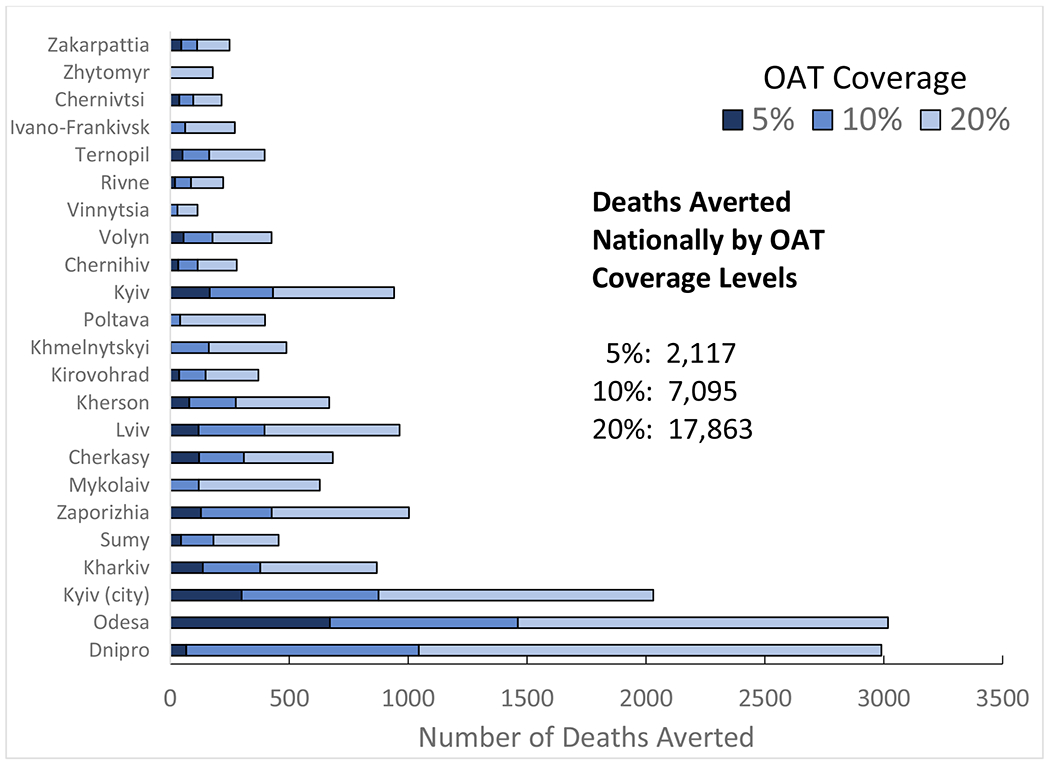
Number of Deaths Averted Nationally and in Each Region of Ukraine Over 10 Years
Figure 5:
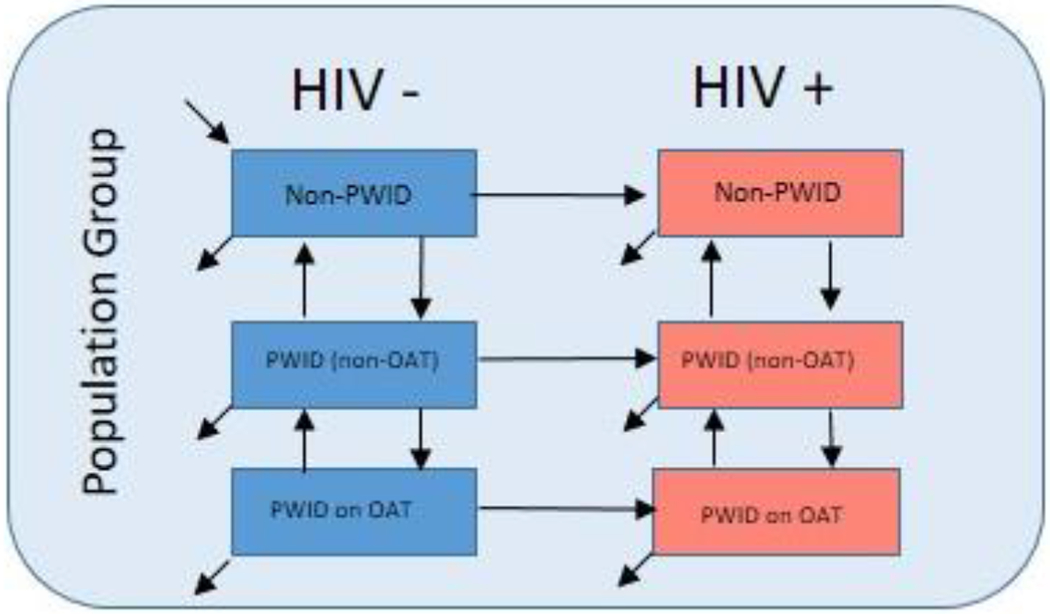
Model Schematic HIV Transmission model.
Note: The model is stratified by 3 population groups: Heterosexual Men, Heterosexual Women, MSM (See model in appendix for complete details)
Research in Context
The Eastern Europe and Central Asia (EECA) region remains one of the few UNAIDS regions where the HIV epidemic continues to expand, with Ukraine being an emblematic country with considerable support from international donors. With an HIV epidemic concentrated among people who inject drugs (PWID), and with evidence of transition to other key populations, opioid agonist therapies (OAT) remain the most cost-effective strategy for HIV prevention. Currently, OAT coverage remains woefully inadequate, with <3% of the current PWID receiving treatment. OAT coverage is diverse throughout the regions of the country. We searched PubMed on October 1,2018 using the following MeSH terms (“modeling” or “modelling”) AND “HIV” AND (“harm reduction” OR “opioid substitution therapy” OR “medically assisted treatment” OR “opioid agonist therapies”). We identified three studies that included dynamic epidemic models of HIV among PWID in Ukraine. Only one of the studies assessed the impact of scaling up OAT nationally14, while other studies focused on selected cities and did not include OAT as an intervention or was focused on OAT scale-up in prisons15. Previous research based on mathematical modeling has found OAT to be among the most cost-effective strategies for reducing new HIV infections in Ukraine, yet granular data on how to best optimize prevention has not been applied16.
Valued Added
To our knowledge, this is one of the first studies to use real-world OAT data from Ukraine to optimize current treatment efficiency and to model treatment dynamics, reflecting the diversity between regional settings. In addition, this is one of the first studies to empirically evaluate the operational efficiency of current OAT programs. Using real-world regional data and targets achieved elsewhere, we show that without additional interventions, the number of new HIV infections is expected to increase by 58,565 (95% CI: 48,011- 65,563) over ten years. Increasing total procurement of OAT and optimizing OAT dosage (which is associated with higher retention levels) constitute crucial strategies for expanding OAT coverage in each region. Without an increase in the procurement constraint, OAT coverage can only be raised from 2.7% to 3.3%. An increase in procurement to provide coverage to 10% and 20% of PWID in each region can prevent 4,368 (95% Cl: 3,134- 5,243) and 10,864 (95% Cl: 7,787-13,038) new HIV infections, reduce deaths by 7,096 (95% Cl: 5,078-9,160) and 17,863 (95% Cl: 12,828-23,062) and deaths relative to the status quo. The reductions in death and mortality, however, differ markedly by region
Implications
Findings from this study provide important insights into regional and national OAT scale-up strategies to further strengthen HIV prevention in Ukraine. The results highlight the importance of markedly and strategically scaling up OAT in Ukraine, in combination with providing adequate dosing to promote retention on OAT (longer retention is associated with higher primary and secondary prevention impact). While emphasizing scale-up in the five highest HIV burden regions would avert 56% of preventable new HIV infections and 49% of preventable deaths in 10 years, ignoring the other regions, especially those not targeted by international donors, has the unintended consequence of increasing HIV incidence and mortality.
Acknowledgements:
The National Institute on Drug Abuse provided support for research (R01 DA033679, R01 DA043125, R01 DA029910) and NIH career development (K01 DA037826 for AZ). Simulations were run at the Yale University High Performance Computing Center, which is supported by National Institutes of Health grants RR19895 and RR029676-0.
Disclosures: FLA reports grants from Gilead, Merck, NIH, SAMHSA, HRSA, personal fees from Gilead, Merck, Practice Point Communications, outside the submitted work. JT, LM, AZ declare no competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2016. Geneva, Switzerland, 2016. https://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016. [Google Scholar]
- 2.LaMonaca K, Dumchev K, Dvoriak S, Azbel L, Morozova O, Altice FL., HIV, Drug Injection, and Harm Reduction Trends in Eastern Europe and Central Asia: Implications for International and Domestic Policy. Curr Psychiatry Rep 2019; 21(7): 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. Ending AIDS: progress towards the 90-90-90 targets., 2017. www.unaids.org/en/resources/documents/2017/20170720_Global_AIDS_update_2017.
- 4.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. Geneva, Switzerland, 2010. https://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016. [Google Scholar]
- 5.Cakalo JI, Bozicevic I, Vitek C, Mandel JS, Salyuk T, Rutherford GW. Misclassification of Men with Reported HIV Infection in Ukraine. AIDS Behav 2015; 19(10): 1938–40. [DOI] [PubMed] [Google Scholar]
- 6.Barska J, Sazanova Y. Monitoring of Behavior and HIV Prevalence in People Who Inject Drugs and Their Sexual Partners in Ukraine, 2015. Kiev, Ukraine: Alliance for Public Health, http://aph.org.ua/wp-content/uploads/2015/09/Monitoryng-povedinky-SIN_PROEKT.pdf [Google Scholar]
- 7.Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Global Health 2017; 5: 1192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UNAIDS. Country factsheets- UKRAINE, http://www.unaids.org/en/regionscountries/countries/ukraine.
- 9.Schaub M, Chtenguelov V, Subata E, Weiler G, Uchtenhagen A. Feasibility of buprenorphine and methadone maintenance programmes among users of home made opioids in Ukraine. International Journal of Drug Policy 2010; 21(3): 229–33. [DOI] [PubMed] [Google Scholar]
- 10.Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. PLoS Med 2011; 8(3): e1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alliance for Public Health. Annual Report 2015. Kiev, Ukraine, 2016. [Google Scholar]
- 12.Bojko MJ, Mazhnaya A, Marcus R, et al. The Future of Opioid Agonist Therapies in Ukraine: A Qualitative Assessment of Multilevel Barriers and Ways Forward to Promote Retention in Treatment. J Subst Abuse Treat 2016; 66: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Good practices in Europe: HIV prevention for People Who Inject Drugs implemented by the International HIV/AIDS Alliance in Ukraine. Copenhagen, Denmark: WHO regional Office for Europe, 2014. http://www.euro.who.int/en/countries/ukraine/publications/good-practices-in-europe-hiv-prevention-for-people-who-inject-drugs-implemented-by-the-international-hivaids-alliance-in-ukraine-2014. [Google Scholar]
- 14.Vickerman P, Kumaranayake L, Balakireva 0, Guinness L, Artyukh O, Semikop T, Yaremenko O, Watts C. The cost-effectiveness of expanding harm reduction activities for injecting drug users in Odessa, Ukraine, Sexual Transmitted Disease 2006; 33(10 Suppl): S89–102. [DOI] [PubMed] [Google Scholar]
- 15.Altice FL, Azbel L, Stone J, et al. The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and Central Asia. Lancet 2016; 388(10050): 1228–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. Plos Medicine 2011; 8(3): e1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet 2010; 376(9738): 59–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.PEPFAR. Ukraine county Operational Plan (COP) 2018 Strategic Direction Summary, https://www.pepfar.gov/documents/organization/285850.pdf.
- 19.Low A, Mburu G, Welton N, May M, Davies CF, French C, Turner KM, Looker KJ, Christensen H, McLean S, Rhodes T, Platt L, Hickman M, Guise A, Vickerman P1. Impact of Opioid Substitution Therapy on Antiretroviral Therapy Outcomes: A Systematic Review and Meta-Analysis. Clinical Infectious Diseases 2017; 15(63): 1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, Pastor-Barriuso R Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 2017; 357(j1550): 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazhnaya A, Marcus R, Bojko MJ, et al. Opioid Agonist Treatment and Improved Outcomes at Each Stage of the HIV Treatment Cascade in People Who Inject Drugs in Ukraine. Journal of acquired immune deficiency syndromes 2018; 79(3): 288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Lancet HIV. HIV funding key for sustainable development. Lancet HIV 2016; 3(11): e499. [DOI] [PubMed] [Google Scholar]
- 23.Makarenko IM A, Marcus R, Pykalo I, Madden L, Filippovich S, Dvoriak S, Altice F Concurrent drug injection during opioid agonist treatment among people who inject drugs in Ukraine. Journal of Substance Abuse Treatment 2018; 87: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madden L, Bojko M, Farnum S, Mazhnaya A, Fomenko T, Marcus RM, Barry B, Ivanchuk I, Kolomiets V, Filippovych S, Dvoryak S, Altice FL. Using nominal group technique among clinical providers to identify barriers and prioritize solutions to scaling up opioid agonist therapies in Ukraine. International Journal of Drug Policy 2017; 49: 48–53. [DOI] [PubMed] [Google Scholar]
- 25.Zelenev A, Shea P, Mazhnaya A, et al. Assessment of barrier severity and willingness to enter opioid agonist treatment among people who inject drugs in Ukraine. Drug and Alcohol Dependence 2018; 190: 82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Twigg J Ukraine’s Health Sector: Sustaining momentum for Reform. Washington, DC: Center for Strategic and International Studies, 2017https://csis-prod.s3.amazonaws.com/s3fs-public/publication/170817_Twigg_UkrainesHealthSector_Web.pdf [Google Scholar]
- 27.Zelenev A, Shea P, Mazhnaya A, et al. Estimating HIV and HCV prevalence among people who inject drugs in 5 Ukrainian cities using stratification-based respondent driven and random sampling. Int J Drug Policy 2019; 67: 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ukrainian Center for Socially Dangerous Disease Control of the Ministry of Health of Ukraine & Gromashevsky Institute of Epidemiology and Infectious Diseases of the Academy of Medical Sciences of Ukraine. HIV Infection in Ukraine, Information Bulletin 45. Kiev, Ukraine, 2016. [Google Scholar]
- 29.State Statistical Service of Ukraine. Population of Ukraine 2017. Demographic Yearbook; Kiev, Ukraine: 2017. [Google Scholar]
- 30.Kiev International Institute of Sociology. 44% of Ukrainians consider sex to be an important part of their lives . Kiev, Ukraine, 2013https://www.kiis.com.ua/?lang=eng&cat=reports&id=144&page=42 [Google Scholar]
- 31.Public Health Center of Ukraine’s Ministry of Health. Statistics on the Number of Patients on ART in Ukraine, Informational Bulletin. Kyiv, Ukraine, 2016https://phc.org.ua/pages/diseases/hiv_aids/statistics/art. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


