To the Editor: The early medical response to the Covid-19 pandemic in the United States was limited in part by the availability of testing. Health care workers collected a swab sample from the patients’ oropharynx or nasopharynx according to testing guidelines for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus. This procedure potentially increased the risk of transmission of the virus to health care workers who lacked sufficient personal protective equipment (PPE).1
In other clinical conditions,2,3 it is faster to obtain a tongue, nasal, or mid-turbinate sample than a nasopharyngeal sample, with less potential for the patient to sneeze, cough, or gag. In addition, recent data support the validity of non-nasopharyngeal samples for detection of SARS-CoV-2.4,5 Collection by the patient reduces high exposure of the health care worker to the virus and preserves limited PPE.
We obtained swab samples from the nasopharynx and from at least one other location in 530 patients with symptoms indicative of upper respiratory infection who were seen in any one of five ambulatory clinics in the Puget Sound region of Washington. Patients were provided with instructions and asked to collect tongue, nasal, and mid-turbinate samples, in that order. A nasopharyngeal sample was then collected from the patient by a health care worker. All samples were submitted to a reference laboratory for reverse-transcriptase–polymerase-chain-reaction (RT-PCR) testing that yielded qualitative results (positive or negative) and cycle threshold (Ct) values for positive samples only (additional details are provided in the Methods section in the Supplementary Appendix, available with the full text of this letter at NEJM.org).
Our study was powered on the basis of a one-sided test to determine whether the sensitivities of the non-nasopharyngeal swabs collected by the patients themselves were significantly greater than 90%. We calculated that 48 patients with positive nasopharyngeal samples would be needed for the study, assuming a true sensitivity of 98% with 80% power. Pairwise analyses were conducted to compare each sample collected by the patient with the nasopharyngeal sample collected by a health care worker. Of the 501 patients with both tongue and nasopharyngeal samples, both swabs tested negative in 450 patients, both swabs tested positive in 44, the nasopharyngeal swab was positive and the tongue swab was negative in 5, and the tongue swab was positive and the nasopharyngeal swab was negative in 2. Of the 498 patients with both nasal and nasopharyngeal samples, both swabs were negative in 447, both swabs were positive in 47, the nasopharyngeal swab was positive and the nasal swab was negative in 3, and the nasal swab was positive and the nasopharyngeal swab was negative in 1. Of the 504 patients with both mid-turbinate and nasopharyngeal samples, both swabs were negative in 452, both swabs were positive in 50, and the nasopharyngeal swab was positive and the mid-turbinate swab was negative in 2; none of these patients had a positive mid-turbinate swab and a negative nasopharyngeal swab.
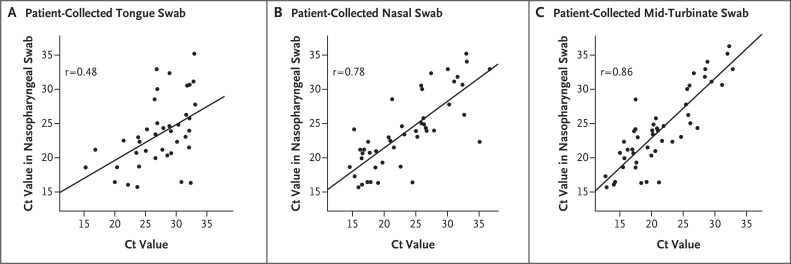
When a nasopharyngeal sample collected by a health care worker was used as the comparator, the estimated sensitivities of the tongue, nasal, and mid-turbinate samples collected by the patients were 89.8% (one-sided 97.5% confidence interval [CI], 78.2 to 100.0), 94.0% (97.5% CI, 83.8 to 100.0), and 96.2% (97.5% CI, 87.0 to 100.0), respectively. Although the estimated sensitivities of the nasal and mid-turbinate samples were greater than 90%, all the confidence intervals for the sensitivity of the samples collected by the patients contained 90%. Despite the lack of statistical significance, both the nasal and mid-turbinate samples may be clinically acceptable on the basis of estimated sensitivities above 90% and the 87% lower bound of the confidence interval for the sensitivity of the mid-turbinate sample being close to 90%. Ct values from the RT-PCR tests showed Pearson correlations between the positive results from the nasopharyngeal swab and the positive results from the tongue, nasal, and mid-turbinate swabs of 0.48, 0.78, and 0.86, respectively. Figure 1 shows the Ct values for the sites from the patient-collected swab samples relative to those for the nasopharyngeal swab samples, with a linear regression fit superimposed on the scatterplot. For patients with positive test results from both the nasopharyngeal swab and a tongue, nasal, or mid-turbinate swab, the Ct values for the swabs collected by the patient were less than the Ct values for the nasopharyngeal swab 18.6%, 50.0%, and 83.3% of the time, respectively, indicating that the viral load may be higher in the middle turbinate than in the nasopharynx and equivalent between the nose and the nasopharynx (additional details are provided in the Methods section in the Supplementary Appendix).
Figure 1. Cycle Threshold (Ct) Values from Tongue, Nasal, and Mid-Turbinate Swabs Collected by Patients Relative to Those from Nasopharyngeal Swabs Collected by Health Care Workers.
The correlation coefficient is superimposed on each panel, along with a trend line estimated with the use of simple linear regression. Plots show the available Ct values for 43 patients who had positive test results from both tongue and nasopharyngeal swabs (Panel A), 46 patients who had positive test results from both nasal and nasopharyngeal swabs (Panel B), and 48 patients who had positive test results from both mid-turbinate and nasopharyngeal swabs (Panel C). Data on 4 patients (1 patient with positive test results from both tongue and nasopharyngeal swabs, 1 patient with positive test results from both nasal and nasopharyngeal swabs, and 2 patients with positive test results from both mid-turbinate and nasopharyngeal swabs) were not included in this analysis because multiple swabs obtained from these patients were labeled with a single test site (i.e., tongue, nasopharynx, nose, or middle turbinate).
Our study shows the clinical usefulness of tongue, nasal, or mid-turbinate samples collected by patients as compared with nasopharyngeal samples collected by health care workers for the diagnosis of Covid-19. Adoption of techniques for sampling by patients can reduce PPE use and provide a more comfortable patient experience. Our analysis was cross-sectional, performed in a single geographic region, and limited to single comparisons with the results of nasopharyngeal sampling, which is not a perfect standard test. Despite these limitations, we think that patient collection of samples for SARS-CoV-2 testing from sites other than the nasopharynx is a useful approach during the Covid-19 pandemic.
Supplementary Appendix
Disclosure Forms
This letter was published on June 3, 2020, at NEJM.org.
Footnotes
Supported by a grant to Drs. Cangelosi and Wood from the Bill and Melinda Gates Foundation.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Padilla M. ‘It feels like a war zone’: doctors and nurses plead for masks on social media. New York Times. March 19, 2020. (https://www.nytimes.com/2020/03/19/us/hospitals-coronavirus-ppe-shortage.html).
- 2.Seaman CP, Tran LTT, Cowling BJ, Sullivan SG. Self-collected compared with professional-collected swabbing in the diagnosis of influenza in symptomatic individuals: a meta-analysis and assessment of validity. J Clin Virol 2019;118:28-35. [DOI] [PubMed] [Google Scholar]
- 3.Luabeya AK, Wood RC, Shenje J, et al. Noninvasive detection of tuberculosis by oral swab analysis. J Clin Microbiol 2019;57(3):e01847-e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020;323:1843-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.To KK-W, Tsang OT-Y, Chik-Yan Yip C, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis 2020. February 12 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.