To the Editor: Identifying the pathogen from clinical samples is crucial for the diagnosis of a newly emergent infectious disease, such as 2019 novel coronavirus (2019-nCoV), which has posed great threats to global public health.[1] In some cases, despite the positive epidemiological, clinical, and radiographic evidence, coronavirus disease 2019 (COVID-19) diagnosis can still be restricted by inconclusive polymerase chain reaction (PCR) results.[2] For samples of suspicious patients, where the fluorescence quantitative PCR (FQ-PCR) results in a few respiratory tract specimens were inconclusive, high-throughput sequencing (HTS) can be an effective confirmation method. Here, we report our experience of applying HTS to confirm a suspected 2019-nCoV infection.
Three upper respiratory tract specimens including throat or nasal swabs were collected from a 65-year-old pneumonia patient with suspected history of contact with COVID-19 patients in Guangzhou on January 22 and 23, 2019. Patient consent was not applicable for this study as the samples were obtained as part of a routine diagnostic procedure. All the clinical specimens were handled at the State Key Laboratory of Respiratory Disease, Guangzhou, China. The initial 2019-nCoV screening results of the sample was negative by FQ-PCR with a commercial FQ-PCR kit (cycle threshold [CT] values of open reading frame 1ab [ORF1ab] and nucleoprotein [N] genes were >40 [#1 throat swab sample], where CT > 40 is considered negative). In view of the epidemiological link and clinical symptoms of the patient, a second screening using another FQ-PCR kit was performed and the range of CT value was weakly positive for the 2019-nCoV viral RNA (CT values of ORF1ab/ envelope protein [E]/N genes of three samples were 34.44/31.58/35.50 [#1 throat swab sample], >40/36.37/38.45 [#2 nasal swab sample], and 33.40/34.36/33.10 [#3 throat swab sample]). The test results of the two kits were inconsistent; therefore, to make a definite diagnosis, we performed meta-transcriptomic sequencing using the same throat swab sample (#1 throat swab sample) from the patient. A meta-transcriptomic library was constructed for single-end (75 base pairs) sequencing on the Illumina NextSeq 550Dx (Illumina, San Diego, CA, USA). The sequencing data were analyzed with the rapid pathogen detection (RPD-seq) system of the Sagene Company in Guangzhou, China, as previously reported.[3] In total, we obtained ten unique sequence reads that completely mapped onto the 2019-nCoV genome (GenBank accession No. MN908947.3). We considered this sample to be weakly positive rather than contaminated, based on the results that the ten reads are evenly distributed throughout the entire gene sequence of the virus. It is worth noting that these regions of ORF1ab, E and N genes were used as the standard PCR targets [Figure 1].
Figure 1.
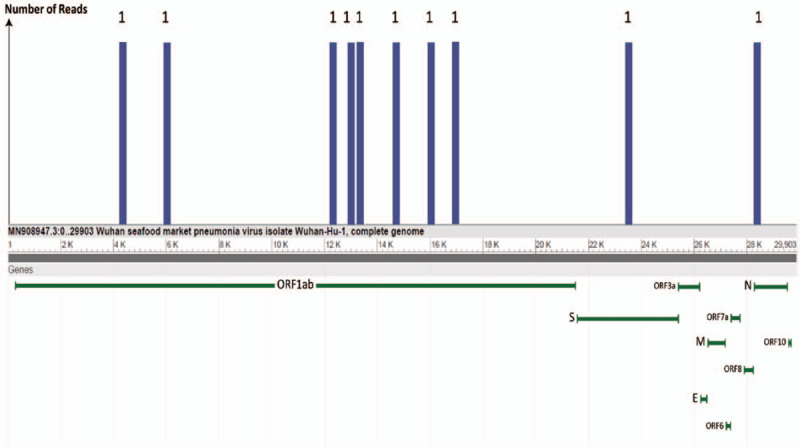
High-throughput sequencing for 2019 novel coronavirus (2019-nCoV) of the throat swab sample (#1) from the patient. Ten unique reads were found, and eight of them on the largest ORF1ab gene, while one of them was located on the second largest spike protein gene-S and another one on the envelope protein gene-E of the 2019-nCoV genome.
According to the national diagnosis and treatment plan released by the National Health Commission of the People's Republic of China, positive viral RNA FQ-PCR or virus gene sequencing results were the diagnostic criteria of COVID-19.[4] However, the different performance of FQ-PCR kits may lead to inconsistent results. Here we reported a case with inconsistent FQ-PCR results, for which the HTS was used to make a further diagnosis of the 2019-nCoV infection. Although HTS may be too costly and labor intensive for routine diagnosis, we believe that it can be used for further diagnosis of patients with COVID-19 with unclear FQ-PCR results under the condition of strict operation and quality control.
Acknowledgements
The authors are greatly indebted to Prof. Sooksan Wong, for her valuable suggestions on writing of the manuscript.
Funding
This work was supported by grants from the National Key Research and Development Program of China (Nos. 2018YFC1200100 and 2018YFC1311900), science research project of the Guangdong Province (No. 2019B030316028), and Guangzhou Medical University High-level University Clinical Research and Cultivation Program (Nos. [2017]159 and 160).
Conflicts of interest
None.
Footnotes
How to cite this article: Guan WD, Chen LP, Ye F, Ye D, Wu SG, Zhou HX, He JY, Yang CG, Zeng ZQ, Wang YT, Li RF, Du QL, Liang XL, Ma QH, Yang ZF. High-throughput sequencing for confirmation of suspected 2019-nCoV infection identified by fluorescence quantitative polymerase chain reaction. Chin Med J 2020;133:1385–1386. doi: 10.1097/CM9.0000000000000792
Wen-Da Guan and Li-Ping Chen contributed equally to the work.
References
- 1.Ren LL, Wang YM, Wu ZQ, Xiang ZC, Guo L, Xu T, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J 2020; 133.[Epub ahead of print]. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Ye G, Chen L, Wang J, Li Y. Analysis of false-negative results for 2019 novel coronavirus nucleic acid test and related countermeasures [in Chinese]. Chin J Lab Med 2020; 43.[Epub ahead of print]. doi: 10.3760/cma.j.issn.1009-9158.2010.0006. [Google Scholar]
- 3.Zhang N, Wang L, Deng X, Liang R, Su M, He C, et al. Recent advances in the detection of respiratory virus infection in humans. J Med Virol 2020; 92:408–417. doi: 10.1002/jmv.25674.3.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. [[Accessed February 18, 2020]]. COVID-19 Diagnosis and Treatment Plan (Trial Version 6) [in Chinese]. Beijing: National Health Commission of the People's Republic of China, 2020. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf. [Google Scholar]


