Abstract
Background
Helicobacter pylori (HP) has been considered to be one of the primary causes of gastric mucosa-associated lymphoid tissue (MALT) lymphoma since 1993. Low-grade gastric MALT lymphoma with HP is widely treated with HP eradication therapy, according to each specific clinical situation. However, several studies and guidelines indicate that the modified HP eradication therapy is also valid for HP-negative gastric MALT lymphoma. The aim of this study was to perform a meta-analysis of the clinical efficacy of the modified HP eradication therapy for gastric MALT lymphoma without HP.
Methods
We searched studies that reported the response rate of the modified HP eradication therapy regimen for gastric MALT lymphoma without HP by using PubMed, Medline, and Ebsco from January 1971 until February 2019. All statistical analyses were carried out using R 3.5.3 (Mathsoft Company, Cambridge, MA, USA). The pooled response rate was expressed as a decimal. The heterogeneity test was performed using the I-squared (I2) statistic.
Results
A total of 14 studies were selected with a total of 148 patients with HP-negative gastric MALT lymphoma. The overall pooled response rate was 0.38 (95% confidence interval [CI]: 0.29–0.47). The combined estimate is I2 = 57% (P < 0.01). The study subjects were categorized by factors (area of patients). The pooled response rate of the sub-groups (Korea, Japan, China, and Western countries) was 0.63 (95% CI: 0.50–0.76), 0.16 (95% CI: 0.05–0.30), 0.38 (95% CI: 0.20–0.55), and 0.57 (95% CI: 0.08–1.00). The response rate showed that the modified HP eradication therapy was effective for patients with HP-negative gastric MALT lymphoma, especially in Korea and Western countries.
Conclusion
Therefore, the modified HP eradication therapy can be considered an optional therapy for patients with low-grade HP-negative gastric MALT lymphoma. However, several limitations were revealed in the meta-analysis. Further systematic reviews and research are required.
Keywords: Therapy, Helicobacter pylori, Gastric MALT lymphoma, Meta-analysis
Introduction
Extra-nodal lymphoma refers to a malignant tumor of the lymphoid tissue or lymphocytes other than lymph nodes. The stomach is the most preferred site, in extra-nodal marginal zone lymphoma of both mucosa-associated lymphoid tissue (MALT) lymphoma and diffuse large B cell lymphoma.[1]Helicobacter pylori (HP) is one of the most common infectious agents in the world,[2] and has been considered by Hussell et al[3] and Wotherspoon et al[4] to be one of the primary causes of gastric MALT lymphoma since 1993. Several studies have also shown that HP eradication therapy for gastric MALT lymphoma is indispensable.[5] Moreover, HP eradication therapy is preferred for low-grade (I-II) HP-positive gastric MALT lymphoma.[6–8] Although HP eradication therapy is recommended to be administered to all gastric MALT lymphoma, it is not widely used clinically.[9]
In comparison, HP-negative gastric MALT lymphoma is more likely to invade the submucosal layer,[10] there are increased challenges associated with treatment and the higher non-response rate against HP eradication therapy since the patients are HP-negative. Radiotherapy or rituximab is preferred according to the guidelines for HP-negative gastric MALT lymphoma, regardless of the stage.[6,11] In addition, if the preferred therapy fails, treatment with either monoclonal antibodies alone or combined with chemotherapy is also an option[11]; however, it has been well-established that radiation therapy can lead to radiation gastroenteritis and gastrointestinal perforation, hemorrhage, and other complications. Chemotherapy can also lead to bone marrow depression, gastrointestinal symptoms, and allergic reactions. In addition, monotherapy can cause allergic reactions, angioedema, and pain at the tumor site, a temporary drop in blood pressure, bronchospasm, and progressive multifocal leukoencephalopathy. It has been well-established that HP eradication therapy is associated with minimal side effects.
HP eradication therapy can be divided into triple and quadruple therapy. The current Guidelines indicate that quadruple therapy with bismuth can have a higher HP eradication rate,[12] namely proton pump inhibitor (PPI) + bismuth + two antibiotics (eg, metronidazole, clarithromycin, amoxicillin, and tetracycline). Of course, some experts believe that quadruple therapy can consist of quadruple therapy without bismuth, namely PPI + amoxicillin + clarithromycin + metronidazole[13]; however, it has not yet reached widespread recognition.
Some articles have shown that the HP eradication therapy exhibits significantly superior efficacy against HP-positive gastric MALT lymphoma compared with HP-negative MALT lymphoma; however, there was no statistical difference between the two groups.[14] Moreover, the clinical efficacy of the modified HP eradication therapy for HP-negative gastric MALT lymphoma remains inconclusive. Thus, novel data demonstrating the efficacy of the modified HP eradication therapy for HP-negative gastric MALT lymphoma is required. The purpose of this study was to evaluate the concrete response rate of the modified HP eradication therapy for HP-negative gastric MALT lymphoma.
Methods
Search strategy
The meta-analysis was managed by searching databases, including PubMed, Medline, and Ebsco from January 1971 to February 2019. The search terms consisted of “gastric mucosa-associated lymphoid tissue lymphoma,” “gastric MALT lymphoma and HP,” “Gastric MALT lymphoma,” and “Helicobacter pylori.” We modified the search terms to satisfy each database.
Study selection
Studies were included if they satisfied the following criteria: (1) studies investigating the clinical efficacy of the modified HP eradication therapy for patients with HP-negative gastric MALT lymphoma; (2) studies only published in English; (3) studies in full-text format; and (4) studies providing concrete data. The excluded criteria were as follows: (1) case reports, review articles, and letters; (2) patients who used the modified HP eradication therapy and other treatments simultaneously; (3) ineligible research objects (eg, animals, children, and HP-positive patients); (4) insufficient reported data; and (5) duplicated data.
Data extraction and quality assessment
The data were independently extracted by two authors (Xie YL and Guan WJ) using a standard data extraction form. The extracted data from included study characteristics (first author, year of publication, time interval of patients, treatment regimen, follow-up duration and sample size and patient information (eg, median age, stage, and status of t[11;18] [q21;q21]).
The study quality was individually assessed by two authors (Xie YL and Guan WJ) using the Newcastle-Ottawa quality assessment scale (NOS). The NOS is recommended by the Cochrane Collaboration for a quality evaluation of the non-randomized studies.[15,16] A score of ≥6 points on the NOS scale was considered to be high quality.
Statistical analysis
R 3.5.3 was used to perform all statistical analyses (Mathsoft Company, Cambridge, MA, USA). A Freeman-Tukey double anti-sinusoidal transformation or un-transformation method was used to combine the response rate. The pooled response rate was expressed as a decimal. The heterogeneity test was first performed using the I-squared (I2) statistic. Heterogeneity was divided into four categories (very low, low, moderate, and high), with the corresponding I2 of I2 ≤ 25%, 25% < I2 ≤ 50%, 50% < I2 ≤ 75%, and I2 > 75%, respectively. A sub-group analysis was performed to analyze the sources of heterogeneity if there was moderate or high heterogeneity. The association between the clinical efficacy and patients’ information was assessed using a sub-group analysis. Moreover, using a fixed effect model, a P > 0.05 indicated that there was a homogeneous inclusion of the studies. Conversely, using a random effect model, a P < 0.05 indicated that the studies were heterogeneous. An Egger test was used to assess any potential publication bias. P > 0.05 in the Egger test was considered to indicate no publication bias.
Results
Selection and summary of studies
Finally, 14 out of 2419 studies reporting the clinical efficacy of the modified HP eradication therapy in HP-negative patients with gastric MALT lymphoma were considered to be qualified for inclusion in the meta-analysis.[10,14,17–28] The majority of the responding patients had low-grade gastric MALT lymphoma. A total of 62 studies were identified after the initial screening. We excluded 48 out of 62 studies because they satisfied the exclusion criteria: two presented duplicated data; 14 did not provide concrete data; 14 were case reports; nine were reviews; two were editorials; one was a letter; one researched ineligible subjects; one merged with other therapies; and four were not written in English. The general characteristics of the included studies are summarized in Table 1 . The included studies were cohort studies reported from 2000 to 2017, which totaled 148 patients. The follow-up duration ranged from 0 to 20.5 years. The majority of the included patients had low-grade gastric MALT lymphoma. The majority of the included studies considered that an HP infection was considered negative only when all HP tests were negative.[10,14,19,21,24,28] Moreover, some articles defined response as complete remission, partial remission, or histologic residual disease,[22,24,28] while only complete remission is defined as a response.[10] We extracted data for t(11;18)(q21;q21) in the included studies. This shows that patients displaying t(11;18)(q21;q21) seldom respond to the modified HP eradication therapy.[19–21,23,28] The details regarding the study quality are presented in Table 2. All of the included studies were of high quality.
Table 1.
Characteristics of the included studies investigating the clinical efficacy of the therapy for patients with HP-negative gastric MALT lymphoma.
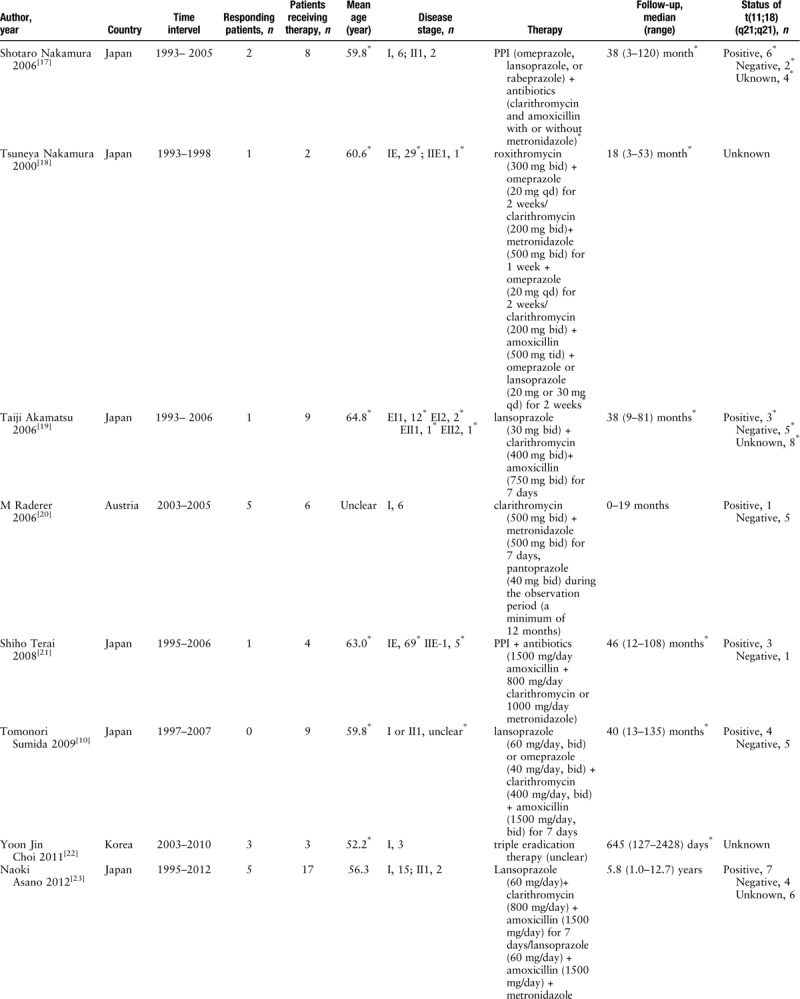
Table 1 (Continued).
Characteristics of the included studies investigating the clinical efficacy of the therapy for patients with HP-negative gastric MALT lymphoma.
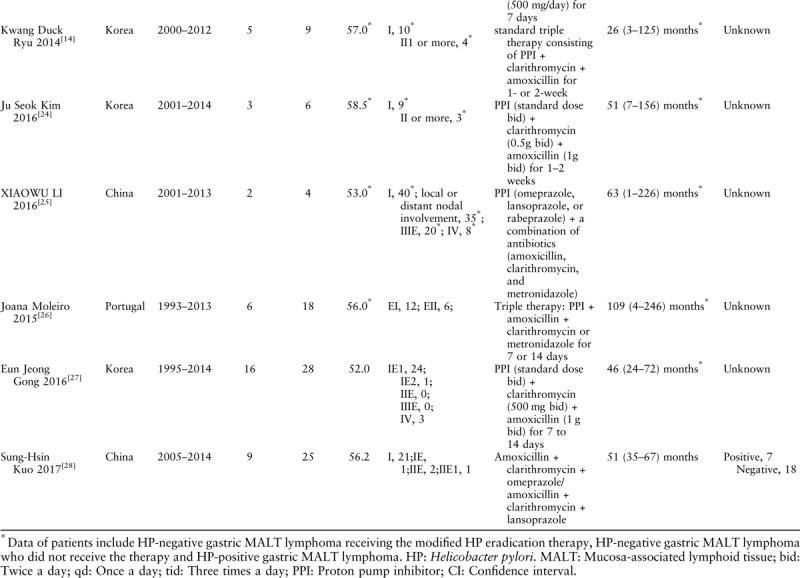
Table 2.
Newcastle-Ottawa quality assessment scale of the quality of the included studies.
Pooled analysis of the efficacy of the modified HP eradication therapy in HP-negative patients with gastric MALT lymphoma
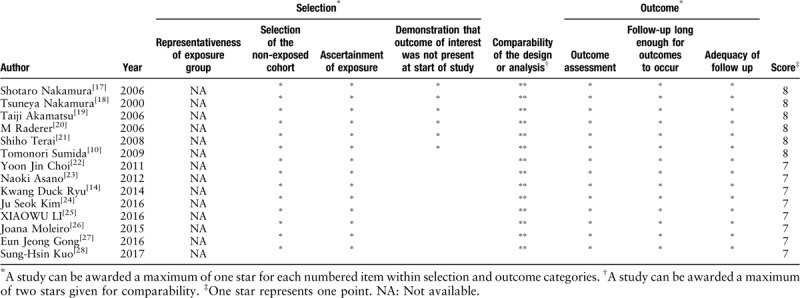
A Freeman-Tukey double anti-sinusoidal transformation was used to perform a pooled analysis of the 14 studies. The response rate of the modified HP eradication therapy for HP-negative gastric MALT lymphoma was 0.38 (95% confidence interval [CI]: 0.29–0.47) [Figure 1]. There was a significant heterogeneity in the 14 included studies (I2 = 57%; P < 0.01). The results of the Egger test indicated that there was no publication bias (P = 0.69) [Figure 2].
Figure 1.
Forest plot showing the overall pooled response rate of the modified HP eradication therapy for HP-negative gastric MALT lymphoma. The individual response rate of the studies is indicated by a square, with the size of the square is proportional to the weight given to the study. The lines indicate the 95% confidence interval of the studies. The diamonds denote the pooled response rate. HP: Helicobacter pylori; MALT: Mucosa-associated lymphoid tissue.
Figure 2.
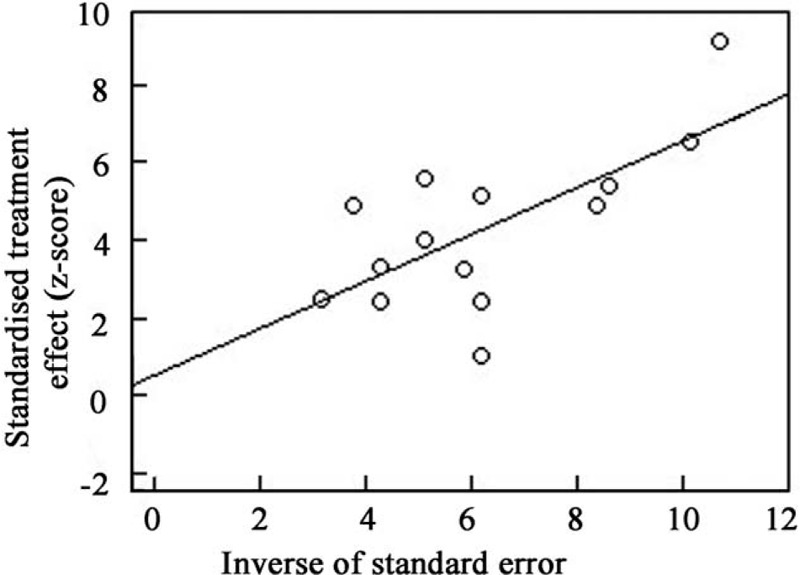
Egger test showing the publication bias of the meta-analysis. (P = 0.69).
Sub-group analysis of the efficacy of the modified HP eradication therapy in patients with HP-negative gastric MALT lymphoma
We recognized that the pooled analysis for the efficacy of the modified HP eradication therapy in patients with HP-negative gastric MALT lymphoma displayed moderate heterogeneity. Therefore, a sub-group analysis was performed based on the area of the included patients. The sources of heterogeneity were analyzed using a sub-group analysis. We divided groups into both Asian (Korea,[14,22,24,27] Japan,[10,17–19,21,23] and China[25,28]) and Western countries.[20,26]
Except for the Freeman-Tukey double anti-sinusoidal transformation method used in Japan, all of the other groups used an un-transformation method to perform the efficacy in all sub-group analyses [Figures 3 and 4]. The pooled response rate of the modified HP eradication therapy in HP-negative gastric MALT lymphoma in the sub-group was higher than that in the pooled analysis, except for in Japan (0.16; 95% CI: 0.05–0.30). The pooled response rate of other sub-groups (Korea, China, and Western countries) was 0.63 (95% CI: 0.50–0.76), 0.38 (95% CI: 0.20–0.55), and 0.57 (95% CI: 0.08–1.00), respectively. There was extremely low heterogeneity in the sub-groups, except for that in the Western countries (I2 = 85%; P < 0.01) and Korea (I2 = 51%; P = 0.11). The results of the Egger test in the sub-group indicated that there was no publication bias (P > 0.05). Moreover, China and Western countries could not perform an Egger test because relatively few studies were included.
Figure 3.
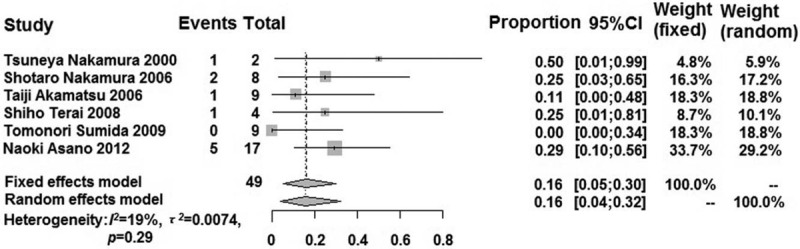
Forest plot showing the response rate of the modified HP eradication therapy for HP-negative gastric MALT lymphoma in Japan using the Freeman-Tukey double anti-sinusoidal transformation method. HP: Helicobacter pylori; MALT: Mucosa-associated lymphoid tissue.
Figure 4.
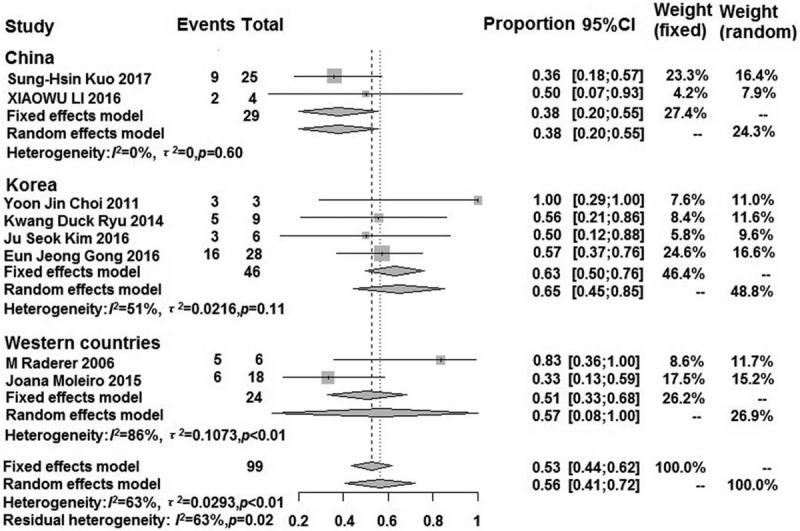
Forest plot showing the response rate of the modified HP eradication therapy for HP-negative gastric MALT lymphoma in sub-groups using the un-transformation method. HP: Helicobacter pylori; MALT: Mucosa-associated lymphoid tissue.
Influential analysis of the efficacy of the modified HP eradication therapy in patients with HP-negative gastric MALT lymphoma
In consideration of the moderate heterogeneity of the pooled analysis regarding the efficacy of the modified HP eradication therapy in patients with HP-negative gastric MALT lymphoma, an influential analysis was performed. The influential analysis showed that there was only one study that had a substantial impact on heterogeneity.[10]
Discussion
Main findings
This meta-analysis investigated the therapeutic efficacy of the modified HP eradication therapy in patients with HP-negative gastric MALT lymphoma. The combined response rate of the overall studies was 0.38, while the sub-groups of Korea, Japan, China, and Western countries were 0.63, 0.16, 0.38, and 0.57, respectively. Since the response rate of the HP eradication therapy regimen in gastric MALT lymphoma with HP was 0.50 to 0.80,[11,29] we hypothesized that the modified HP eradication therapy could be considered an optional therapy for patients with low-grade HP-negative gastric MALT lymphoma, at least in Korea and Western countries. However, we noted that there was high heterogeneity associated with the studies overall and for two sub-groups (Korea and Western countries). Using an influential analysis, we found that there was one study that had a substantial impact on heterogeneity.[10] This study may be the source of heterogeneity in the analysis. In comparison to the remaining studies, the response rate in the study by Tomonori Sumida was 0.[10] Moreover, Tomonori Sumida et al[10] defined the therapy a failure if two consecutive histological examinations of the gastric mucosa indicate no improvement. This suggests that the study by Tomonori Sumida et al[10] may have had insufficient data and a different follow-up duration. Heterogeneity may be derived from a small sample size, which does not exclude different follow-up durations, countries, criteria for HP infection and remission, or the medicine and duration of the modified HP eradication therapy.
Interpretation of the results
The results of the meta-analysis revealed that the modified HP eradication therapy is useful for low-grade HP-negative gastric MALT lymphoma. The possible reasons for the efficacy of the modified HP eradication therapy for HP-negative gastric MALT lymphoma requires further exploration.
False negatives in the HP detection methods
Currently, HP detection methods are classified as either invasive or non-invasive. Invasive detection has the advantages of a high level of sensitivity and accuracy.[30] However, it is difficult to perform invasive testing, and there is a certain possibility that HP infection sites cannot be obtained through detection, resulting in HP false negatives. Non-invasive detection is convenient and rapid[31]; however, the sensitivity and accuracy are lower compared to invasive detection, and susceptible to various environmental factors (eg, urea breath test and the history of taking anti-secretion drugs, such as PPI and H2 receptor inhibitors). The first symptoms of most gastric MALT lymphoma are gastrointestinal symptoms, and the patients are generally advised to take anti-secretion drugs before the biopsy. However, anti-secretion drugs will lead to false negatives in many test methods, including histological examinations.[32] Therefore, in the case of the detection of HP false negatives, the HP eradication therapy regimen can act on existing HP to treat gastric MALT lymphoma.
Special mechanism of action of antibiotics
Antibiotics are indispensable in the HP eradication therapy regimen, with clarithromycin being used more frequently.[33] Both clarithromycin and azithromycin are macrolides, which can induce the apoptosis of activated lymphocytes by inhibiting BCL-XL expression.[34] In CD4+ T cells, azithromycin can effectively inhibit cellular proliferation and cytokine secretion by down-regulating the activity of the target protein of rapamycin in mammals. The above immunosuppressive effect was also observed when the concentration of clarithromycin was high (40 mg/L).[35] With a high dose of clarithromycin (2 g per day for 14 days), the CR rates in patients with relapsed or refractory extra-nodal MALT lymphoma can reach 26.9%.[36] In summary, clarithromycin exhibits a direct anti-tumor or immunomodulatory effect.
Presence of other pathogens
The pathogenesis of extra-nodal MALT lymphoma is unclear and may be related to infectious factors through chronic inflammation of the stomach. Therefore, the active eradication of pathogenic pathogens is considered to play an important role in disease mitigation.[37] Studies have reported HP-negative but Helicobacter heilmannii-positive gastric MALT lymphoma.[38,39] Moreover, Takuma Okamura et al[39] treated one case of H. heilmannii-positive gastric MALT lymphoma with treatment similar to HP eradication therapy (10 mg rabeprazole, 750 mg amoxicillin, 400 mg clarithromycin twice daily for 7 days) and reached complete remission. In addition, H. heilmannii was also confirmed to have no histological evidence. Moreover, studies have confirmed that H. heilmannii can indeed cause gastric MALT lymphoma in rats.[40]
Helicobacter suis is the most common Helicobacter species in the human stomach other than HP, which causes disease by altering mucin composition and glycosylation.[41,42]H. suis may also cause gastric MALT lymphoma.[41] Since H. suis has lower urease activity, partial detections are less sensitive to H. suis detection (eg, breath test). Therefore, there is no definitive research showing that H. suis-positive gastric MALT lymphoma can achieve remission by treatment similar to HP eradication therapy. However, studies suggest that metronidazole is effective for H. suis, especially when clarithromycin-containing therapy is unsuccessful.[42]
Gene trans-location
Many recent studies have reported that if the gene for MALT lymphoma is trans-located, the resistance to antibiotic therapy is higher, which may lead to poor efficacy of the modified HP eradication therapy and a higher recurrence rate.[19–21,23,28] Tomonori Sumida et al[10] showed that the positive rate of API2-MALT1 gene expression in HP-negative gastric MALT lymphoma (44.4%) was substantially higher than that of HP-positive gastric MALT lymphoma (5.26%). The result of t(11;18)(q21;q21) is the API2-MALT1 gene. Moreover, the trans-located gene includes t(11;18)(q21;q21), t(1;14)(p22;q32), t(14;18)(q32;q21), t(3;14)(q27;q32), and t(3;14)(p14.1;q32).[43] Most of these gene trans-locations lead to fusion of the related gene, resulting in BCL10 over-expression, which leads to cell transformation and facilitates tumor B cell survival.[44] However, studies have shown that HP-negative gastric MALT lymphoma with t(11;18)(q21;q21) positive responds to the modified HP eradication therapy.[19,23] If the gene trans-location is negative, the patients with HP-negative gastric MALT lymphoma will have a higher response rate to the modified HP eradication therapy.
State of chemokine receptors
Chemokine receptors mediate the immigration, activation, and enhancement of lymphocytes through binding to their ligand, and their expression is differentially regulated in each lymphocyte subset.[45] CXCR3 is a chemokine receptor that consists of activated T cells and is expressed on B lymphocytes in MALT lymphoma. Studies have shown that CXCR3 expression is a predictive factor for non-responsiveness to the HP eradication therapy to gastric MALT lymphoma. This has implied that CXCR3-positive tumors are less sensitive to the HP eradication therapy regimen compared to that of CXCR3-negative tumors.[46] In HP-negative patients with CXCR3-negative tumors are likely to respond to the HP eradication therapy regimen, similar to genetic translocation.
Implications of the meta-analysis
Gastric MALT lymphoma is known to be an indolent lymphoma that develops slowly and even cases of spontaneous remission exist.[47] Conservative medical therapy for HP-negative gastric MALT lymphoma will not delay treatment, can make patients obtain cheaper, more effective therapy, and can ensure a better quality of life; however, the specific allowable follow-up time still needs to be studied. Studies have shown that the follow-up time to achieve histological remission be delayed by more than 30 months after administering the HP eradication therapy.[48] Studies have also shown that with the extension of the follow-up period, there will be a significant increase in the remission rate. Moreover, Wundish et al[49] reported that the histological remission rate at a follow-up period of three months and 1 year was 61% and 88%, respectively. Currently, the maximum allowable follow-up period is inconclusive. Therefore, if no disease progression is identified during the follow-up period, the follow-up time can be extended to 1 year before deciding to undergo additional therapy.[48,50–52]
Due to the above-mentioned causes, clarithromycin can even be changed to azithromycin, which has a longer half-life.[53] To avoid the occurrence of false negatives, based on the history of taking anti-secretion drugs, HP-negative diagnostic indicators should be determined to be negative for multiple assays, including both invasive and non-invasive tests. Since other pathogenic bacteria are not easily detected using existing HP detection methods, if HP is definitely diagnosed as negative, infection with other pathogenic bacteria should be considered to provide a basis for conservative treatment and a tendency for antibiotic selection. The relationship between the response rate of the HP eradication therapy regimen for HP-negative gastric MALT lymphoma and gene translocation must be further explored and studied. Before the protocol, genetic testing for determining the presence or absence of a gene translocation may provide new clues for the cure of MALT lymphoma. Chemokine receptors also affect the efficacy of the HP eradication therapy for HP-negative gastric MALT lymphoma. In addition, studies have shown that chemokine receptors can be used for targeted therapy and diagnosis.[54] Similar to gene translocation, altering the status of chemokine receptors can enhance the efficacy of the HP eradication therapy regimen for HP-negative gastric MALT lymphoma. Unlike gene translocation, there are fewer studies that have investigated chemokine receptors. In addition to CXCR3, many chemokine receptors are associated with the migration of lymphoma. Therefore, more comprehensive studies are required to research the follow-up time, gene translocation, chemokine receptors, and antibiotic selection.
Limitations
The limitations of the meta-analysis include: (1) the small sample size due to the limited number of patients in each of the included studies; (2) the possibility of selection bias; (3) different diagnostic criteria, evaluation criteria of the therapy efficacy, and follow-up duration; (4) high heterogeneity in the sub-group analysis of the Western countries; and (5) ignoring many influencing factors, including the age of patients, diseased part, lesion depth, and type of antibiotics. Thus, these limitations limit the reliability of the results.
Conclusion
The meta-analysis showed that the modified HP eradication therapy regimen is effective for patients with low grade HP-negative gastric MALT lymphoma, especially in Western countries and Korea. Based on the meta-analysis results, the HP eradication therapy or other therapy, including PPI and antibiotics (eg, one of clarithromycin, metronidazole, tetracycline, and amoxicillin) can be used as an optional therapy for low grade HP-negative gastric MALT lymphoma. However, the sample size of the included studies was small, and the characteristics of the patients with HP-negative gastric MALT lymphoma were incomplete. Further clinical and laboratory research is required to explore the efficacy of the modified HP eradication therapy in patients with low grade HP-negative gastric MALT lymphoma.
Conflicts of interest
None.
Footnotes
How to cite this article: Xie YL, He CY, Wei Sq, Guan WJ, Jiang Z. Clinical efficacy of the modified Helicobacter pylori eradication therapy for Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma: a meta analysis. Chin Med J 2020;133:1337–1346. doi: 10.1097/CM9.0000000000000813
References
- 1.Juárez-Salcedo LM, Sokol L, Chavez JC, Dalia S. Primary gastric lymphoma, epidemiology, clinical diagnosis, and treatment. Cancer Control 2018; 25:107327481877825.doi: 10.1177/1073274818778256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruno G, Rocco G, Zaccari P, Porowska B, Mascellino MT, Severi C. Helicobacter pylori infection and gastric dysbiosis: can probiotics administration be useful to treat this condition? Can J Infect Dis Med Microbiol 2018; 2018:6237239.doi: 10.1155/2018/6237239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussell T, Isaacson PG, Spencer J, Crabtree JE. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet 1993; 342:571–574. doi: 10.1016/0140-6736(93)91408-E. [DOI] [PubMed] [Google Scholar]
- 4.Wotherspoon AC, Diss TC, Pan L, Isaacson PG, Doglioni C, Moschini A, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 1993; 342:575–577. doi: 10.1016/0140-6736(93)91409-F. [DOI] [PubMed] [Google Scholar]
- 5.Diaconu S, Predescu A, Moldoveanu A, Pop CS, Fierbinţeanu-Braticevici C. Helicobacter pylori infection: old and new. J Med Life 2017; 10:112–211. [PMC free article] [PubMed] [Google Scholar]
- 6.Zelenetz AD, Gordon LI, Abramson JS, Advani RH, Bartlett NL, Caimi PF, et al. NCCN guidelines insights: B-cell lymphomas, version 3.2019. J Natl Compr Canc Netw 2019; 17:650–661. doi: 10.6004/jnccn.2019.0029. [DOI] [PubMed] [Google Scholar]
- 7.Song Y, Jiang K, Su S, Wang B, Chen G. Clinical manifestations and epigenetic mechanisms of gastric mucosa associated lymphoid tissue lymphoma and long-term follow-up following Helicobacter-pylori eradication. Exp Ther Med 2018; 15:553–561. doi: 10.3892/etm.2017.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Q, Zhang Y, Zhang X, Fu K. Gastric mucosa-associated lymphoid tissue lymphoma and Helicobacter pylori infection: a review of current diagnosis and management. Biomark Res 2016; 4:15.doi: 10.1186/s40364-016-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zucca E, Copie-Bergman C, Ricardi U, Thieblemont C, Raderer M, Ladetto M. Gastric marginal zone lymphoma of MALT type: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24:vi144–vi148. doi: 10.1093/annonc/mdt343. [DOI] [PubMed] [Google Scholar]
- 10.Sumida T, Kitadai Y, Hiyama T, Shinagawa K, Tanaka M, Kodama M, et al. Antibodies to Helicobacter pylori and CagA protein are associated with the response to antibacterial therapy in patients with H. pylori-positive API2–MALT1-negative gastric MALT lymphoma. Cancer Sci 2009; 100:1075–1081. doi: 10.1111/j.1349-7006.2009.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi Y. JSH practical guidelines for hematological malignancies, 2018: II. Lymphoma-2. Marginal zone lymphoma (MALT lymphoma/extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue and splenic marginal zone lymphoma). Int J Hematol 2019; 110:393–405. doi: 10.1007/s12185-019-02719-6. [DOI] [PubMed] [Google Scholar]
- 12.Sheu BS, Wu MS, Chiu CT, Lo JC, Wu DC, Liou JM, et al. Consensus on the clinical management, screening-to-treat, and surveillance of Helicobacter pylori infection to improve gastric cancer control on a nationwide scale. Helicobacter 2017; 22: doi: 10.1111/hel.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosques-Padilla FJ, Remes-Troche JM, González-Huezo MS, Pérez-Pérezd G, Torres-Lópeze J, Abdo-Francisf JM, et al. The fourth Mexican consensus on Helicobacter pylori. Rev Gastroenterol Mex 2018; 83:325–341. doi: 10.1016/j.rgmxen.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Ryu KD, Kim GH, Park SO, Lee KJ, Moon JY, Jeon HK, et al. Treatment outcome for gastric mucosa-associated lymphoid tissue lymphoma according to Helicobacter pylori infection status: a single-center experience. Gut Liver 2014; 8:408–414. doi: 10.5009/gnl.2014.8.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Wiley Online Library: The Cochrane Collaboration; 2008. [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta analyses. Eur J Epidemiol 2010; 25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura S, Matsumoto T, Ye H, Nakamura S, Suekane H, Matsumoto H, et al. Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma: a clinicopathologic and molecular study with reference to antibiotic treatment. Cancer 2006; 107:2770–2778. doi: 10.1002/cncr.22326. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T, Nakamura S, Yonezumi M, Suzuki T, Matsuura A, Yatabe Y, et al. Helicobacter pylori and the t(11;18)(q21;q21) translocation in gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type. Jpn J Cancer Res 2000; 91:301–309. doi: 10.1111/j.1349-7006.2000.tb00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akamatsu T, Mochizuki T, Okiyama Y, Matsumoto A, Miyabayashi H, Ota H. Comparison of localized gastric mucosa-associated lymphoid tissue (MALT) lymphoma with and without Helicobacter pylori infection. Helicobacter 2006; 11:86–95. doi: 10.1111/j.1523-5378.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 20.Raderer M, Streubel B, Wöhrer S, Häfner M, Chott A. Successful antibiotic treatment of Helicobacter pylori negative gastric mucosa associated lymphoid tissue lymphomas. Gut 2006; 55:616–618. doi: 10.1136/gut.2005.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terai S, Iijima K, Kato K, Dairaku N, Suzuki T, Yoshida M, et al. Long-term outcomes of gastric mucosa-associated lymphoid tissue lymphomas after Helicobacter pylori eradication therapy. Tohoku J Exp Med 2008; 214:79–87. doi: 10.1620/tjem.214.79. [DOI] [PubMed] [Google Scholar]
- 22.Yoon Jin C, Dong Ho L, Ji Yeon K, Ji Eun K, Jae Yeon K, Hyun Jin J, et al. Low grade gastric mucosa-associated lymphoid tissue lymphoma: clinicopathological factors associated with Helicobacter pylori eradication and tumor regression. Clin Endosc 2011; 44:101–108. doi: 10.5946/ce.2011.44.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asano N, Iijima K, Terai S, Jin X, Ara N, Chiba T, et al. Eradication therapy is effective for Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma. Tohoku J Exp Med 2012; 228:223–227. doi: 10.1620/tjem.228.223. [DOI] [PubMed] [Google Scholar]
- 24.Seok KJ, Hyung KS, Seok MH, Kyu SJ, Yong JH. Clinical outcome of eradication therapy for gastric mucosa-associated lymphoid tissue lymphoma according to H. pylori infection status. Gastroenterol Res Pract 2016; 2016:1–7. doi: 10.1155/2016/6794848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Wang X, Zhan Z, Zhang L, Sun B, Zhang Y. Evaluation of the clinical characteristics, management, and prognosis of 103 patients with gastric mucosa-associated lymphoid tissue lymphoma. Oncol Lett 2016; 11:1713–1718. doi: 10.3892/ol.2016.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moleiro J, Ferreira S, Lage P, Dias Pereira A. Gastric malt lymphoma: analysis of a series of consecutive patients over 20 years. United European Gastroenterol J 2016; 4:395–402. doi: 10.1177/2050640615612934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong EJ, Ahn JY, Jung HY, Park H, Ko YB, Na HK, et al. Helicobacter pylori eradication therapy is effective as the initial treatment for patients with H. pylori-negative and disseminated gastric mucosa-associated lymphoid tissue lymphoma. Gut Liver 2016; 10:706–713. doi: 10.5009/gnl15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo SH, Yeh KH, Wu MS, Lin CW, Wei MF, Liou JM, et al. First-line antibiotic therapy in Helicobacter pylori-negative low-grade gastric mucosa-associated lymphoid tissue lymphoma. Sci Rep 2017; 7:14333.doi: 10.1038/s41598-017-14102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen SM, Petryk M, Varma M, Kozuch PS, Grossbard ML. Non-Hodgkin's lymphoma of mucosa-associated lymphoid tissue. Oncologist 2006; 11:1100–1117. doi: 10.1634/theoncologist.11-10-1100. [DOI] [PubMed] [Google Scholar]
- 30.Chen XZ, Huang CZ, Hu WX, Liu Y, Yao XQ. Gastric cancer screening by combined determination of serum Helicobacter pylori antibody and pepsinogen concentrations: ABC method for gastric cancer screening. Chin Med J 2018; 131:1232–1239. doi: 10.4103/0366-6999.231512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koichiro K, Shujiro Y, Reiko K, Katsuhisa N. Detection capability of the stool Helicobacter pylori antigen kit using gastric juice collected during esophagogastroduodenoscopy. Chin Med J 2018; 131:2252–2253. doi: 10.4103/0366-6999.240818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaberg KB, Evans MF, Wilcox R, Lewis MR. Antisecretory medication is associated with decreased Helicobacter pylori detection in gastric marginal zone lymphoma. Ann Diagn Pathol 2015; 19:397–402. doi: 10.1016/j.anndiagpath.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Bilgilier C, Simonitsch-Klupp I, Kiesewetter B, Raderer M, Dolak W, Makristathis A, et al. Prevalence of clarithromycin-resistant Helicobacter pylori strains in gastric mucosa-associated lymphoid tissue lymphoma patients. Ann Hematol 2016; 95:1115–1120. doi: 10.1007/s00277-016-2672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizunoe S, Kadota JI, Tokimatsu I, Kishi K, Nagai H, Nasu M. Clarithromycin and azithromycin induce apoptosis of activated lymphocytes via down-regulation of bcl-xl. Int Immunopharmacol 2004; 4:0–1207. doi: 10.1016/j.intimp.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Ratzinger F, Haslacher H, Poeppl W, Hoermann G, Kovarik JJ, Jutz S, et al. Azithromycin suppresses cd4+ t-cell activation by direct modulation of mtor activity. Sci Rep 2014; 4:7438.doi: 10.1038/srep07438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreri AJM, Sassone M, Kiesewetter B, Govi S, Scarfò L, Donadoni G, et al. High-dose clarithromycin is an active monotherapy for patients with relapsed/refractory extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT): the HD-K phase II trial. Ann Oncol 2015; 26:1760–1765. doi: 10.1093/annonc/mdv214. [DOI] [PubMed] [Google Scholar]
- 37.Cheah CY, Opat S, Trotman J, Marlton P. Front-line management of indolent non-Hodgkin lymphoma in Australia. Part 2: mantle cell lymphoma and marginal zone lymphoma. Intern Med J 2019; 49:1070–1080. doi: 10.1111/imj.14268. [DOI] [PubMed] [Google Scholar]
- 38.Morgner A, Lehn N, Andersen LP, Thiede C, Bennedsen M, Trebesius K, et al. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterol 2000; 118:821–828. doi: 10.1016/S0016-5085(00)70167-3. [DOI] [PubMed] [Google Scholar]
- 39.Okamura T, Iwaya Y, Yokosawa S, Suga T, Arakura N, Matsumoto T, et al. A case of Helicobacter heilmannii-associated primary gastric mucosa-associated lymphoid tissue lymphoma achieving complete remission after eradication. Clin J Gastroenterol 2013; 6:38–45. doi: 10.1007/s12328-012-0355-9. [DOI] [PubMed] [Google Scholar]
- 40.O’Rourke JL, Dixon MF, Jack A, Enno A, Lee A. Gastric B-cell mucosa-associated lymphoid tissue (MALT) lymphoma in an animal model of ’Helicobacter heilmannii’ infection. J Pathol 2004; 203:896–903. doi: 10.1002/path.1593. [DOI] [PubMed] [Google Scholar]
- 41.Padra M, Adamczyk B, Flahou B, Erhardsson M, Chahal G, Smet A, et al. Helicobacter suis infection alters glycosylation and decreases the pathogen growth inhibiting effect and binding avidity of gastric mucins. Mucosal Immunol 2019; 12:784–794. doi: 10.1038/s41385-019-0154-4. [DOI] [PubMed] [Google Scholar]
- 42.Satoru N, Tadashi S, Masahiko N, Daisuke C, Hidezumi K, Manabu S, et al. The resolution of Helicobacter suis-associated gastric lesions after eradication therapy. Intern Med 2018; 57:203–207. doi: 10.2169/internalmedicine.8971-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raderer M, Kiesewetter B, Ferreri AJM. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J Clin 2016; 66:152–171. doi: 10.3322/caac.21330. [DOI] [PubMed] [Google Scholar]
- 44.Willis TG, Jadayel DM, Du MQ, Peng H, Perry AR, Abdulrauf M, et al. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell 1999; 96:35–45. doi: 10.1016/S0092-8674(00)80957-5. [DOI] [PubMed] [Google Scholar]
- 45.Ohshima K, Suefuji H, Karube K, Hamasaki M, Hatano B, Tutiya T, et al. Expression of chemokine receptor CXCR3 and its ligand, mig, in gastric and thyroid marginal zone lymphomas. Possible migration and autocrine mechanism. Leuk Lymphoma 2003; 44:329–336. doi: 10.1080/1042819031000060546. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto H, Nakamura T, Matsuo K, Tajika M, Kawai H, Ohmiya N, et al. Significance of CXCR3 expression in gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type for predicting responsiveness to Helicobacter pylori eradication. Cancer Sci 2010; 99:1769–1773. doi: 10.1111/j.1349-7006.2008.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugiyama T, Arita K, Shinno E, Nakajima T. Spontaneous remission of diffuse large B cell lymphoma in the stomach and the continuation of remission for 10 years. Case Rep Gastroenterol 2018; 12:699–703. doi: 10.1159/000494750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee SK, Lee YC, Chung JB, Chon CY, Yang WI. Low grade gastric mucosa associated lymphoid tissue lymphoma: treatment strategies based on 10 year follow-up. World J Gastroenterol 2004; 10:223–226. doi: 10.3748/wjg.v10.i2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morgner A, Thiede C, Bayerdörffer E, Alpen B, Wündisch T, Neubauer A, et al. Long-term follow-up of gastric MALT lymphoma after H. pylori eradication. Curr Gastroenterol Rep 2002; 3:516–522. doi: 10.1007/s11894-001-0073-9. [DOI] [PubMed] [Google Scholar]
- 50.Hong SS, Jung HY, Choi KD, Song HJ, Lee GH, Oh TH, et al. A prospective analysis of low-grade gastric malt lymphoma after Helicobacter pylori eradication. Helicobacter 2010; 11:569–573. doi: 10.1111/j.1523-5378.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 51.Stathis A, Chini C, Bertoni F, Proserpio I, Capella C, Mazzucchelli L, et al. Long-term outcome following Helicobacter pylori eradication in a retrospective study of 105 patients with localized gastric marginal zone B-cell lymphoma of MALT type. Ann Oncol 2009; 20:1086–1093. doi: 10.1093/annonc/mdn760. [DOI] [PubMed] [Google Scholar]
- 52.Zucca E, Dreyling M. Gastric marginal zone lymphoma of MALT type: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann of Oncol 2008; 19: Suppl 2: ii70–ii71. doi: 10.1093/annonc/mdn094. [DOI] [PubMed] [Google Scholar]
- 53.Lagler H, Kiesewetter B, Dolak W, Obermüller M, Simonitsch-Klupp I, Lukas J, et al. Treatment of mucosa associated lymphoid tissue lymphoma with a long-term once-weekly regimen of oral azithromycin: results from the phase II MALT—a trial. Hematol Oncol 2019; 37:22–26. doi: 10.1002/hon.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stollberg S, Kämmerer D, Neubauer E, Schulz S, Simonitsch-Klupp I, Kiesewetter B, et al. Differential somatostatin and CXCR4 chemokine receptor expression in MALT-type lymphoma of gastric and extragastric origin. J Cancer Res Clin Oncol 2016; 142:2239–2247. doi: 10.1007/s00432-016-2220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


