Abstract
Background:
Extra-corporeal video telescope operating monitor system provides a necessary instrument to perform high-precision neurosurgical procedures that could substitute or supplement the traditional surgical microscope. The present study was designed to evaluate a compact high-definition two-dimensional exoscope system for assisting in surgical removal of large vestibular schwannoma (VS), as an alternative to a binocular surgical microscope.
Methods:
Patients with Koos grade 3 and grade 4 VS undergoing surgery were enrolled in this prospective cohort study between January 2013 and June 2018. The demographics and tumor characteristics (size, Koos grade, composition [cystic or solid mass]) were matched between the two groups of patients. The following outcome measurements were compared between the two groups: duration of surgery, volume of blood loss, extent of tumor resection, number of operating field adjustments, pre- and post-operative facial and cochlear nerve function evaluated at 3 months post-surgery, complications and surgeons’ comfortability.
Results:
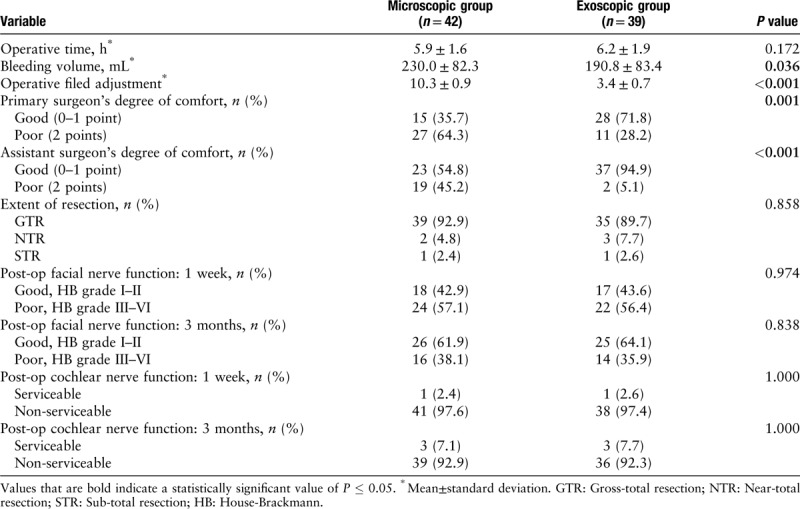
A total of 81 patients received tumor resection through the retrosigmoid approach under either an exoscope (cases, n = 39) or a surgical microscope (control, n = 42). Patients in the two groups had comparable tumor location (P = 0.439), Koos grading (P = 0.867), and composition (P = 0.891). While no significant differences in the duration of surgery (P = 0.172), extent of tumor resection (P = 0.858), facial function (P = 0.838), and hearing ability (P = 1.000), patients operated on under an exoscope had less blood loss (P = 0.036) and a fewer field adjustments (P < 0.001). Both primary and assistant surgeons reported a high level of comfort operating under the exoscope (P = 0.001 and P < 0.001, respectively).
Conclusions:
The compact high-definition two-dimensional exoscope system provides a safe and efficient means to assist in removing large VSs, as compared to a surgical microscope. After the acquaintance with a visual perception through a dynamic hint and stereoscopically viewing corresponding to the motion parallax, the exoscope system provided a comfortable, high-resolution visualization without compromising operational efficiency and patient safety.
Keywords: Vestibular schwannoma, Exoscope, Telescope video monitor, Operating microscope
Introduction
Vestibular schwannoma (VS) is a benign neoplasm that comprises 8% of all intracranial and 85% of cerebellopontine angle tumors. Large and symptomatic tumors are indicated for surgical resection, except in cases of complex comorbidities that are contraindicated for craniotomy.[1] The primary goal of surgery is to remove the tumor mass, while preserving the function of facial and cochlear nerves.[2] It can be achieved using high-precision microsurgical technique under a binocular surgical microscope, which provides optimal focal length, sufficient magnification, three-dimensional (3D) perception, and bright deep illumination. However, a binocular surgical microscope has major disadvantages: (1) it has a shallow and narrow depth of field and often require intermittently field adjustments and refocusing after each movement; (2) neurosurgeons and assistants are often in awkward positions for an extended period of time and fatigue easily; (3) the head stage and counterbalance system are bulky, occupying not only the precious space of operating field but also limiting the space for surgical procedures; and (4) it is not optimally designed for education purposes because surgical assistants and trainees may have different views and orientation from an operating surgeon.[3] Some or all of these disadvantages could be overcome by the compact high-definition (HD) exoscope system. This system has a rigid rod lens telescope above the surgical field to continuously record HD images, thus offering a longer focal length, a deeper depth of field, a wider field of view, than a traditional surgical microscope. This exoscope system has gained considerable attention in the field of neurosurgery in recent years. The two-dimensional (2D) exoscope system has been used in spine surgery, peripheral nerve schwannoma removal, intracranial hematoma evacuation, vessel anastomosis, and glioma and meningioma resection.[4–6] However, to our knowledge, the use of this 2D exoscope system has not been systematically evaluated in high-precision neurosurgical operations such as large VS resection. The primary objective of this study was to assess the clinical efficiency and safety of using a compact HD 2D exoscope to assist in surgical removal of large VS, as an alternative to the traditional binocular surgical microscope.
Methods
Patients and study design
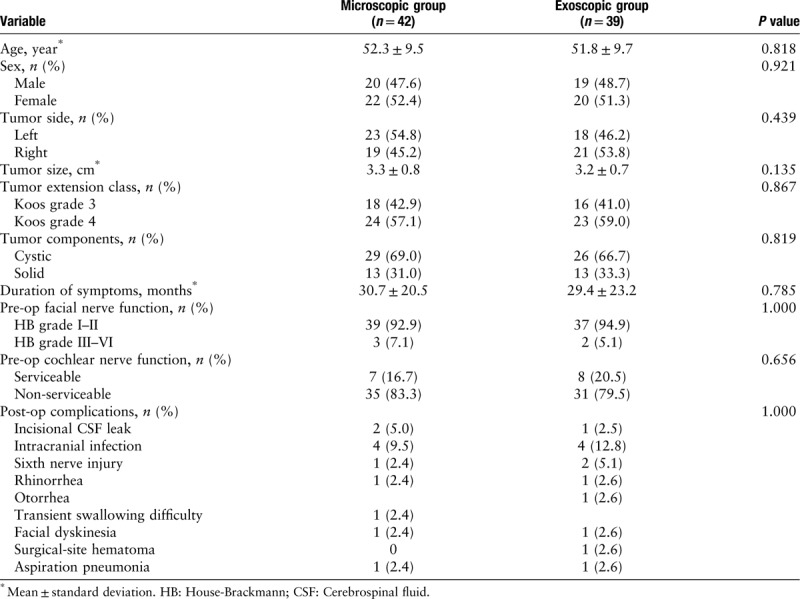
This prospective cohort study was conducted at Tianjin Medical University General Hospital (TMUGH), an academic tertiary-level medical center. The study protocol was approved by the Ethics Committee of TMUGH. The exoscope used in this study was approved by the China Food and Drug Administration. All procedures were performed by the same primary surgeon (YY). Written informed consent was obtained from patients or their next of kin. Consecutive VS patients requiring surgery were screened between January 2013 and June 2018. Patients were included in the study if they met the following inclusion criteria: Koos grade 3 and grade 4 VS, and age between 18 and 70 years. The exclusion criteria were as follows: (1) Koos grade 1 and grade 2 VS, (2) younger than 18 years or older than 70 years, (2) neurofibromatosis type 2, (3) bilateral VS, (4) prior surgery or radiation for VS, (5) pregnancy, (6) participation in other clinical trials within the past 30 days, and (7) consent refusal.
Data collection
In addition to demographic information, all patients underwent a standardized VS diagnostic workup including physical examination, audiography, and radiographic evaluations with CT and magnetic resonance imaging (MRI) scan. A tumor was defined as cystic if the following three criteria were met: (1) a hypodense area on CT or a hypointensive area on MRI were reported, (2) these hypodense or hypointense areas consisted of one to two adjacent fluid pockets, which accounted for ≥30% of tumor volume, and (3) cystic structure found during surgery.[7] Tumor extension was classified based on the Koos grading system [Table 1]. A 3-month post-operative contrast-enhanced MRI scan was used to evaluate the extent of tumor resection that included gross-total resection with no residual tumor detectable, near-total resection with a maximal tumor thickness of less than 5 mm, sub-total resection with a maximal tumor thickness exceeding 5 mm. Audiometric data were obtained before and after surgery, according to the 1995 American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) Committee reporting guidelines.[8] Facial nerve function was evaluated before and after surgery using the House-Brackmann grading scale. After surgery, surgeons were surveyed for the degree of comfort: Score 0: comfortable, no pain in the joints and muscles of neck, shoulder, and back; Score 1: acceptable, mild pain in the joints and muscles of neck, shoulder, and back; Score 2: uncomfortable, severe pain in the joints and muscles of neck, shoulder, and back.
Table 1.
Koos grading scale of vestibular schwannoma.

Microscopes used in the study
A compact HD 2D exoscope (ViTOM, Video Telescope Operating Microscopy; Karl Storz GmbH, Tuttlingen, Germany) was used in this study [Figure 1]. The 90° ViTOM telescope is held in position with a mechanical holding arm (Point Setter PSMS-2; Mitaka Kohki, Tokyo, Japan). A full-HD camera (IMAGE1, H3-Z-SPIESTM Three Chip Full-HD; Karl Storz GmbH) and a light cable (Fiber Optic Light Cable 495 TIP; Karl Storz GmbH) are attached to the ViTOM in the provided slots. Two 26 inch video monitors are placed for primary surgeon and assistant to view. The binocular surgical microscopes used in this study were Leica M525 OH4 (Leica Microsystems Schweiz AG, Heerbrugg, Switzerland) and Carl Zeiss OPMI PENTERO 900 (Carl Zeiss Meditec AG, Oberkochen, Germany).
Figure 1.
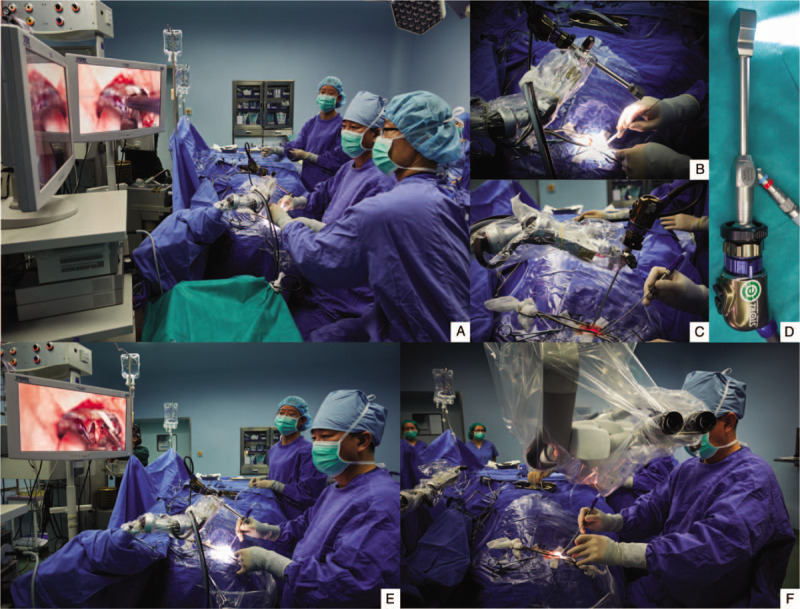
(A) The operative room setting with a compact high-definition two-dimensional (2D) exoscope for a right sub-occipital/retrosigmoid craniotomy. A 2D exoscope is attached to a mechanical holding arm and held over the surgical field. Two 26 inch video monitors are positioned 2 m in front of the primary and assistant surgeons. The assistant surgeon sits on the left side of the primary surgeon. All the surgical team has the same view with the primary surgeon. (B) A 90o 2D exoscope is held in position with a mechanical holding arm that is mounted on the operating table. (C) A 2D exoscope can be quickly switched to an endoscope. (D) A light table and high-definition camera are connected to a exoscope. (E and F) Comparison between the 2D exoscope and binocular operating microscope using in a right suboccipital/retrosigmoid craniotomy. It should be noted that the primary surgeon's head, neck, and arm are in a neutral, relaxed position when performing the surgical procedure using the 2D exoscope. The bulky head stage of the binocular operating microscope takes up the precious space of the operating field.
Tumor resection via the retrosigmoid approach
All patients underwent tumor resection via a standard retrosigmoid approach under either traditional binocular surgical microscope or the 2D exoscope by the two neurosurgeons assigned to this study. The surgical procedures and microscopic dissection technique were kept identical under both exoscope and surgical microscope. Continuous auditory brainstem response, steady-state auditory evoked potential, and facial nerve direct stimulation were monitored during the surgery. Although gross-total resection was attempted in all patients, a small piece of the capsule that extensively adhered to the facial nerve or the brainstem was intentionally left behind.
Statistical analysis
The statistical software SPSS 23.0 (SPSS statistics for Windows; IBM, Armonk, NY, USA) was used for data analysis. Significance of differences between groups was determined using the Fisher exact test and t test. The level of significance was set at a P value of 0.05 or less.
Results
A total of 81 patients were included in the study and randomly assigned to the exoscope group (n = 39) or the surgical microscope group (n = 42). The demographic and tumor characteristics of enrolled patients are listed in Table 2.
Table 2.
Demographics and general information of patients with vestibular schwannomas.
The measurements related to surgery and evaluations during the follow-up period of up to 3 months after surgery are summarized in Table 3. We found that patients have significantly less blood loss when they were operated on under the exoscope, as compared to those under the surgical microscope (230.0 ± 82.3 mL vs. 190.8 ± 83.4 mL, P = 0.036). In addition, there was a significantly fewer field adjustments with exoscope than the surgical microscope (3.4 ± 0.7 vs. 10.3 ± 0.9, P < 0.001). The operating surgeons reported a significantly higher degree of comfort when operated under the exoscope, as compared to operation under the surgical microscope (71.8% vs. 35.7%, P = 0.001). A higher degree of comfort for assistant surgeons was also reported in surgery under an exoscope than under a surgical microscope (94.9% vs. 54.8%, P < 0.001). In contrast, we found no statistical significance between the two groups in measurements related to tumor characteristics. Furthermore, no significant difference in facial nerve and cochlear nerve function were observed 1 week and 3 months after surgery.
Table 3.
Comparison between the microscopic and exoscopic group demonstrating the clinical efficacy and safety of the two-dimensional exoscope system in surgical removal of large vestibular schwannoma.
Discussion
To the best of our knowledge, this is the first prospective cohort study investigating the operational efficacy and patient safety of a compact HD 2D exoscope system (2D exoscope) in the surgical removal of large VS, as compared to a regular surgical microscope. We made the following key observations. First, the 2D exoscope has a comparable operational efficacy and safety as compared to the surgical microscope, in terms of operative time, the extent of tumor resection, post-operative facial and cochlear nerve function, and rate of post-operative complications. Second, patients experienced less blood loss during surgery under the 2D exoscope than under the surgical microscope. Third, neurosurgeons reported better ergonomical experience during operation with less adjustments of surgical fields under the exoscope.
Microsurgical resection is necessary in surgically removing as much VS mass as possible while preserving the facial and cochlear nerve function. Because of its location and relation to nearby vascular and neural structures, completely removing a large VS requires high-precision surgical techniques under high magnification, resolution, and bright deep-cavity illumination, which are traditionally offered by a binocular surgical microscope.[9–12] However, a binocular surgical microscope has a number of limitations: (1) the sizable head stage and counterbalance system dominants the surgical field and takes up the available space for surgery, (2) surgeons are often in uncomfortable positions with little freedom of motion for extended periods, increasing the level of operation fatigue, and (3) a regular surgical microscope is not optimal as an efficient training tool because trainees often have different view field from an operating surgeon.[13] An exoscope could overcome these limitations because it allows surgeons to operate through magnified video images on a high-resolution display, provides more flexible and ergonomically optional ways to operate, and offering an excellent training platform for trainees, who will have identical orientation and view as the surgeon has.[14] Recent data from a small study have indeed reported that a 2D exoscope significantly reduces operating fatigue of neurosurgeons.[15] Our study provides a prospective evolution of exoscope in a large patient cohort for removing large VSs while allowing surgeons a high level of comfort during operation because (1) a surgeon is free from the binocular eyepieces of a surgical microscope and able to operate sitting straight upright in a neutral position [Figure 1E and 1F], (2) less adjustments and refocus are needed with a deeper depth of field and a wider field of view, and (3) the compact size and long focal length give a surgeon the room for passing and using surgical instruments [Figure 1 A–D]. These might also contribute to the less blood loss during surgical operations using 2D exoscope.[16]
The results of this prospective study confirmed the benefits of using exoscope in surgically removal of large VSs. Our findings are supported by the studies on the clinical efficacy and safety of 2D exoscope in spine surgery, intracranial hematoma evacuation, surgical are in debate.[17–19] Chan et al did not find any superiority of the 3D over the 2D laparoscopic system. The only experience in laparoscopic surgery had a significant effect on an individual's performance.[17] Several skull-base neurosurgeons performing intranasal endoscopic surgery have demonstrated that lack of stereopsis does not prevent from achieving satisfactory surgical outcomes.[20] Notwithstanding, the 3D exoscope is promising innovative equipment, which has just been evaluated in the cranial and spinal procedure in the last 2 years,[21–26] clinical data comparing the 2D and 3D version in the setting of neurosurgical operation was lacking. In this study, we demonstrated that the 2D exoscope had a potentially comparable operational efficacy and safety, even with much less bleeding volume, as the operating microscope (OM) in large VS resection, which requires high hand-eye coordination.
The study is limited in its ability to determine the use of 2D exoscope in other fields of complex neurosurgical operations. These beneficial effects of the 2D exoscope observed in this study might be brought by the difference in tumor biological characteristics and heterogeneities. Nevertheless, this study suggests that the compact HD 2D exoscope system provides a safe and efficient means to assist in removing large VSs, as compared to a surgical microscope.
The compact HD 2D exoscope system provides a safe and efficient alternative or supplement to surgical microscope for large VS resection. After the acquaintance with a visual perception through a dynamic hint and stereoscopically viewing corresponding to the motion parallax, the exoscope system gives neurosurgeons with a comfortable, high-resolution visualization without compromising operational efficiency and patient safety.
Acknowledgements
The authors sincerely thank Drs. Liang Xue, Chuan Zhang, Shao-Bo Su, and Dong Wang from Department of Neurosurgery, Tianjin Medical University General Hospital, Dr. Jing-Hua Wang from Tianjin Neurological Institute for their assistance in clinical data collections, technical, and statistical support.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81671902), the Project of Tianjin Applied Basic and Cutting-edge Technological Research (No. 17JCYBJC25200), and by the Tianjin Health Care Elite Prominent Young Doctor Development Program and Young, and middle-aged innovative talent training program.
Conflicts of interest
None.
Footnotes
How to cite this article: Chen X, Gao XL, Chai Y, Shi MM, Zhang JN, Yue SY. Use of a compact high-definition two-dimensional exoscope in surgical treatment of large vestibular schwannoma. Chin Med J 2020;133:1292–1297. doi: 10.1097/CM9.0000000000000818
References
- 1.Rosahl S, Bohr C, Lell M, Hamm K, Iro H. Diagnostics and therapy of vestibular schwannomas - an interdisciplinary challenge. GMS Curr Top Otorhinolaryngol Head Neck Surg 2017; 16:Doc03.doi: 10.3205/cto000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnurman Z, Golfinos JG, Roland JT, Kondziolka D. Knowledge silos: assessing knowledge sharing between specialties through the vestibular schwannoma literature. J Neurosurg 2018; 129:1278–1285. doi: 10.3171/2017.6.JNS171182. [DOI] [PubMed] [Google Scholar]
- 3.Nishiyama K. From exoscope into the next generation. J Korean Neurosurg Soc 2017; 60:289–293. doi: 10.3340/jkns.2017.0202.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mamelak AN, Danielpour M, Black KL, Hagike M, Berci G. A high-definition exoscope system for neurosurgery and other microsurgical disciplines: preliminary report. Surg Innov 2008; 15:38–46. doi: 10.1177/1553350608315954. [DOI] [PubMed] [Google Scholar]
- 5.Mamelak AN, Nobuto T, Berci G. Initial clinical experience with a high-definition exoscope system for microneurosurgery. Neurosurgery 2010; 67:476–483. doi: 10.1227/01.NEU.0000372204.85227.BF. [DOI] [PubMed] [Google Scholar]
- 6.Shirzadi A, Mukherjee D, Drazin DG, Paff M, Perri B, Mamelak AN, et al. Use of the video telescope operating monitor (VITOM) as an alternative to the operating microscope in spine surgery. Spine (Phila Pa 1976) 2012; 37:E1517–E1523. doi: 10.1097/BRS.0b013e3182709cef. [DOI] [PubMed] [Google Scholar]
- 7.Jian BJ, Sughrue ME, Kaur R, Rutkowski MJ, Kane AJ, Kaur G, et al. Implications of cystic features in vestibular schwannomas of patients undergoing microsurgical resection. Neurosurgery 2011; 68:874–880. doi: 10.1227/NEU.0b013e318208f614. [DOI] [PubMed] [Google Scholar]
- 8.Scheller C, Wienke A, Tatagiba M, Gharabaghi A, Ramina KF, Ganslandt O, et al. Stability of hearing preservation and regeneration capacity of the cochlear nerve following vestibular schwannoma surgery via a retrosigmoid approach. J Neurosurg 2016; 125:1277–1282. doi: 10.3171/2015.10.JNS15926. [DOI] [PubMed] [Google Scholar]
- 9.Lin EP, Crane BT. The management and imaging of vestibular schwannomas. AJNR Am J Neuroradiol 2017; 38:2034–2043. doi: 10.3174/ajnr.A5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn IF, Bi WL, Mukundan S, Delman BN, Parish J, Atkins T, et al. Congress of neurological surgeons systematic review and evidence-based guidelines on the role of imaging in the diagnosis and management of patients with vestibular schwannomas. Neurosurgery 2018; 82:E32–E34. doi: 10.1093/neuros/nyx510. [DOI] [PubMed] [Google Scholar]
- 11.Sweeney AD, Carlson ML, Shepard NT, McCracken DJ, Vivas EX, Neff BA, et al. Congress of neurological surgeons systematic review and evidence-based guidelines on otologic and audiologic screening for patients with vestibular schwannomas. Neurosurgery 2018; 82:E29–E31. doi: 10.1093/neuros/nyx509. [DOI] [PubMed] [Google Scholar]
- 12.Olson JJ, Kalkanis SN, Ryken TC. Congress of neurological surgeons systematic review and evidence-based guidelines on the treatment of adults with vestibular schwannomas: executive summary. Neurosurgery 2018; 82:129–134. doi: 10.1093/neuros/nyx586. [DOI] [PubMed] [Google Scholar]
- 13.Ricciardi L, Mattogno PP, Olivi A, Sturiale CL. The exoscope era: the next technical and educational step in microneurosurgery. World Neurosurg 2019; 128:371–373. doi: 10.1016/j.wneu.2019.05.162. [DOI] [PubMed] [Google Scholar]
- 14.Ricciardi L, Chaichana KL, Cardia A, Stifano V, Rossini Z, Olivi A, et al. The exoscope in neurosurgery: an innovative “point of view". A systematic review of the technical, surgical and educational aspects. World Neurosurg 2019; 127:653.doi: 10.1016/j.wneu.2018.12.202. [DOI] [PubMed] [Google Scholar]
- 15.Garneau JC, Laitman BM, Cosetti MK, Hadjipanayis C, Wanna G. The use of the exoscope in lateral skull base surgery: advantages and limitations. Otol Neurotol 2019; 40:236–240. doi: 10.1097/MAO.0000000000002095. [DOI] [PubMed] [Google Scholar]
- 16.Mamelak AN, Drazin D, Shirzadi A, Black KL, Berci G. Infratentorial supracerebellar resection of a pineal tumor using a high definition video exoscope (VITOM(R)). J Clin Neurosci 2012; 19:306–309. doi: 10.1016/j.jocn.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Chan AC, Chung SC, Yim AP, Lau JY, Ng EK, Li AK. Comparison of two-dimensional vs three-dimensional camera systems in laparoscopic surgery. Surg Endosc 1997; 11:438–440. doi: 10.1007/s004649900385. [DOI] [PubMed] [Google Scholar]
- 18.Levy ML, Chen JC, Moffitt K, Corber Z, McComb JG. Stereoscopic head-mounted display incorporated into microsurgical procedures: technical note. Neurosurgery 1998; 43:392–395. doi: 10.1097/00006123-199808000-00141. [DOI] [PubMed] [Google Scholar]
- 19.Han KN, Kim HK, Choi YH. Application of a three-dimensional video system in the training for uniportal thoracoscopic surgery. J Thorac Dis 2018; 10:3643–3650. doi: 10.21037/jtd.2018.05.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kassam AB, Engh JA, Mintz AH, Prevedello DM. Completely endoscopic resection of intraparenchymal brain tumors. J Neurosurg 2009; 110:116–123. doi: 10.3171/2008.7.JNS08226. [DOI] [PubMed] [Google Scholar]
- 21.Nossek E, Schneider JR, Kwan K, Kulason KO, Du V, Chakraborty S, et al. Technical aspects and operative nuances using a high-definition 3-dimensional exoscope for cerebral bypass surgery. Oper Neurosurg (Hagerstown) 2019; 17:157–163. doi: 10.1093/ons/opy342. [DOI] [PubMed] [Google Scholar]
- 22.Kwan K, Schneider JR, Du V, Falting L, Boockvar JA, Oren J, et al. Lessons learned using a high-definition 3-dimensional exoscope for spinal surgery. Oper Neurosurg (Hagerstown) 2019; 16:619–625. doi: 10.1093/ons/opy196. [DOI] [PubMed] [Google Scholar]
- 23.Rossini Z, Cardia A, Milani D, Lasio GB, Fornari M, D’Angelo V. VITOM 3D: preliminary experience in cranial surgery. World Neurosurg 2017; 107:663–668. doi: 10.1016/j.wneu.2017.08.083. [DOI] [PubMed] [Google Scholar]
- 24.Oertel JM, Burkhardt BW. Vitom-3D for exoscopic neurosurgery: initial experience in cranial and spinal procedures. World Neurosurg 2017; 105:153–162. doi: 10.1016/j.wneu.2017.05.109. [DOI] [PubMed] [Google Scholar]
- 25.Smith S, Kozin ED, Kanumuri VV, Barber SR, Backous D, Flavio Nogueira J, et al. Initial experience with 3-dimensional exoscope-assisted transmastoid and lateral skull base surgery. Otolaryngol Head Neck Surg 2019; 160:364–367. doi: 10.1177/0194599818816965. [DOI] [PubMed] [Google Scholar]
- 26.Ichikawa Y, Senda D, Shingyochi Y, Mizuno H. Potential advantages of using three-dimensional exoscope for microvascular anastomosis in free flap transfer. Plast Reconstr Surg 2019; 144:726e–727e. doi: 10.1097/PRS.0000000000006088. [DOI] [PubMed] [Google Scholar]


