Abstract
Aggregation of an amyloid protein, α-synuclein (αS), is a critical step in the neurodegenerative pathway of Parkinson’s diseases (PD). Specific detection of amyloid conformers (i.e., monomers, oligomers and fibrils) produced during αS aggregation is critical in better understanding a molecular basis of PD and developing a diagnostic tool. While various molecular probes are available for detection of αS fibrils, which may serve as a reservoir of toxic αS aggregate forms, these probes suffer from limited conformer-specificity and operational flexibility. In the present study, we explored the potential of non-self-aggregating peptides derived from the highly aggregation-prone KLVFFAE region of an amyloid protein, β-amyloid, as molecular probes for αS aggregates. We show that of the four peptides tested (KLVFWAK, ELVFWAE and their C-terminal capping variants, all of which were attached with fluorescein isothiocyanate at their respective N-termini), KLVFWAK with C-terminal capping was selectively bound to αS fibrils over monomers and oligomers, and readily used for monitoring αS fibrilization. Our analyses suggest that binding of the peptide to αS fibrils is mediated by both electrostatic and hydrophobic interactions. We anticipate that our peptide can readily be optimized for conformer-specificity and operational flexibility. Overall, this study presents the creation of a KLVFFAE-based molecular probe for αS fibrils and demonstrates fine-tuning of its conformer-specificity by terminal mutations and capping.
Keywords: α-synuclein, Amyloid aggregation, Fibril, Peptide probe, Protein engineering
Introduction
The aggregation of β-amyloid (Aβ) and α-synuclein (αS) is implicated in the neurodegenerative pathways leading to the onset of Alzheimer’s and Parkinson’s diseases (AD and PD), respectively [1, 2]. Aggregation of these proteins in monomeric forms produces soluble oligomers, which are known as primary toxic agents in AD and PD [1, 2]. Oligomers can further aggregate into fibrillar species, which can serve as the catalytic surface promoting the formation of soluble oligomeric aggregates [3, 4]. Moreover, fibrils can dissociate into oligomers, acting as reservoirs for the toxic amyloid aggregates [4–7]. The existence of the distinct conformers and interchange among them present heterogeneity and complexity, hindering the development of effective therapeutic and diagnostic approaches [8–11]. Therefore, specific detection of different amyloid conformers is critical in better understanding of a molecular basis of amyloid diseases and the development of a diagnostic tool [8–11].
Among various molecular probes recognizing amyloid oligomers and fibrils [12], Thioflavin T (ThT) is a fluorescent dye widely used to detect the presence of amyloid aggregates in vitro [13]. While self-quenched on its own, ThT displays a significant increase in fluorescence upon binding to the grooves of the cross-β-sheet structures, which are rich in hydrophobic residues and commonly found in amyloid fibrils [13, 14]. Despite the common usage of ThT for detecting amyloid fibrils, ThT has several limitations. For example, ThT is not truly fibrillar conformer-specific, as it has been shown to bind to amyloid oligomers, though with lower fluorescence signals than for binding to fibrils [13, 15, 16]. In addition, ThT has fixed wavelengths for excitation (λex: 440 nm) and emission (λem: 487 nm), which are often overlapped with other commonly used optical dyes [17–21]. The limited operational flexibility may make it difficult to adopt ThT for detection of multiple targets using fluorescence with different wavelengths of light. Unfortunately, a design principle of derivatizing ThT for enhanced conformer-specificity and different wavelengths of fluorescence is yet to be fully established [22]. Other molecular probes for amyloid aggregates suffer from similar limitations [12]. For example, Congo red (CR) and Thioflavin S (ThS) are also limited by fixed excitation and emission wavelengths. The excitation and emission wavelengths of CR are λex: 380–490 nm and λem: 540 nm, respectively, and λex: 430 nm and λem: 550 nm for ThS [23]. Moreover, CR has been shown to bind to both oligomeric [16] and fibrillar Aβ aggregates [24], suggesting that CR shares similar limitations as ThT. Antibody or antibody fragment-based molecular probes require precise protein folding in order for target binding but their application and optimization are often limited by insufficient conformational stability [25]. As an alternative, peptide-based molecular probes can serve as a versatile molecular framework, where conformer-specificity and operational flexibility can be systematically optimized by engineering their primary sequences and attaching a dye with desired optical properties, respectively. Moreover, a peptide probe requires no precise protein folding for binding to targets, highlighting its structural simplicity.
The peptide, KLVFWAK, derived from a highly amyloidogenic Aβ fragment, 16KLVFFAE22, has been shown to bind to Aβ oligomers and fibrils rather than monomers [26]. Despite significant sequence similarity with the highly aggregation-prone KLVFFAE, KLVFWAK does not self-aggregate and remains monomeric during prolonged incubation due to intermolecular electrostatic repulsion mediated by the terminal lysines [27]. Similarly, ELVFWAE, a variant of KLVFWAK created by terminal mutations, is non-self-aggregating [27]. Interestingly, both KLVFWAK and ELVFWAE have been shown to influence αS aggregation though in a different manner [28]: when compared to samples of αS alone, αS aggregation was delayed in the presence of KLVFWAK while the extent of aggregation, as judged by the final ThT value, was greater with ELVFWAE. The observed interactions of KLVFWAK and ELVFWAE with αS suggest that these peptides may serve as a molecular framework upon which αS conformer-specific probes can be developed. The different impacts of each peptide on αS aggregation upon co-incubation also suggest that electrostatic interactions may determine a mechanism by which KLVFWAK or ELVFWAE interacts with αS. Unfortunately, binding characteristics of KLVFWAK and ELVFWAE for different αS conformers have yet to be examined. As shown with KLVFFAE [29], electrostatic interactions among amyloid polypeptides can be further modified by terminal capping, such as amidation at the C-terminus and acetylation at the N-terminus, which removes the terminal backbone charges of the peptide and thus restricts the electrostatic interactions to side chains only.
In this study, we examine binding profiles of KLVFWAK and ELVFWAE and their terminal capping variants using different αS conformers. Our results demonstrate that KLVFWAK with the N-terminal attachment of a fluorescent dye, fluorescein isothiocyanate (FITC), and C-terminal capping (the resulting peptide referred to as capped FITC-KLVFWAK) was selectively bound to αS fibrils among other αS conformers. No other peptides we tested showed similar αS fibrillar conformer-specificity, demonstrating the critical role of electrostatic interactions in binding of capped FITC-KLVFWAK to αS fibrils. We also show that capped FITC-KLVFWAK successfully detected αS fibrils during aggregation of freshly prepared αS samples. Overall, this study demonstrates the ability to fine-tune binding characteristics of KLVFFAE-derived peptides for amyloid aggregates by point mutations of charged residues and capping at the termini.
Materials and Methods
Materials
ELVFWAE and KLVFWAK tagged with fluorescein isothiocyanate (FITC) at the N-terminal α-amine (referred to as FITC-ELVFWAE and FITC-KLVFWAK, respectively) were synthesized using solid-phase chemistry and purified using reverse-phase HPLC by GenScript (Piscataway, NJ, USA). FITC-ELVFWAE and FITC-KLVFWAK variants with C-terminal amidation (referred to as capped FITC-ELVFWAE and capped FITC-KLVFWAK, respectively) were similarly synthesized and produced by GenScript. FPLC columns used for αS purification were purchased from GE Healthcare (Piscataway, NJ, USA). Anti-αS antibodies, F11, 5C2, 211 and D10 were purchased from Santa Cruz Biotechnology, Inc (Dallas, TX, USA) or Novus International (Saint Charles, MO, USA). An anti-amyloid oligomer antibody, A11, was purchased from Life Technologies (Grand Island, NY, USA). Unless otherwise stated, all other materials were purchased from Fisher Scientific (Pittsburgh, PA, USA).
αS Expression and Purification
BL21(DE3) cells harboring the plasmid pRK172 [30–32] which contains the human αS sequence was used for expression of αS, as described previously [33, 34]. The cells were grown at 37°C with orbital shaking at 250 rpm in 1 L of Luria Broth with 100 μg/mL of ampicillin. Upon reaching an OD600 of ~0.6, αS expression was induced by addition of 1 mM isopropyl β-D-1-thiogalactopyranoside and then the cell culture moved to 25°C and shaken at 250 rpm overnight. The cells were centrifuged for 10 minutes at 4,000 rpm in a Beckman Coulter Avanti JE centrifuge (Fullerton, CA, USA) and resuspended in 8 mL of 50 mM Tris-HCl (pH ~8.0) per gram of cells. A Hielscher Ultrasonic Processor UP200S (Teltow, Germany) was used to lyse the cells by sonication, followed by centrifugation at 18,000 rpm and 4°C for 1 hour. The supernatant was heat-treated at 80°C for 20 minutes to denature the endogenous cellular proteins other than αS, which is heat-resistant [33, 34]. The heat-treated supernatant was then centrifuged for 1 hour at 4°C and 18,000 rpm. After centrifugation, the supernatant was passed through an anion-exchange column (HiTrap Q XL; GE Healthcare) from which proteins including αS were eluted with NaCl in Tris buffer at 0.35 mM. The fraction containing αS was then concentrated approximately ten-fold and run through a size exclusion column (SEC; HiPrep 16/60 Sephacryl S-100, GE Healthcare). The purified αS was then desalted using a HiPrep 26/10 desalting column (GE Healthcare) and eluted in deionized water. Identity of αS was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie Blue staining and dot blot assays using anti-αS antibodies, F11, 5C2, 211 and D10. αS purity was determined to be ≥ 95% by SDS-PAGE. The purified αS was frozen and lyophilized using a Labconco Freezone 6 Freeze Dryer System (Kansas City, MO, USA) and stored in −80°C until needed.
αS Monomer Preparation
Lyophilized αS was dissolved in 1X PBSA (20 mM Na2HPO4/NaH2PO4, 150 mM NaCl, and 0.02% (w/v) NaN3, with a final pH of ~7.4) at ~10 mg/mL and room temperature. The αS solution was then filtered with a 0.45 μm filter (MilliporeSigma, Burlington, MA, USA) and its concentration was determined using absorbance at 280 nm, as described previously [33]. The αS samples prepared in this manner were previously found to contain predominantly (~95%) monomeric αS as determined by native-PAGE and SEC [33], which was also confirmed in this study (data not shown). Filtrates were used for subsequent experiments without any additional separation.
αS Oligomer Preparation
For preparation of αS oligomers, αS monomer samples prepared as described above were incubated at 350 μM for 6 hours at 37°C in a glass vial with constant shaking at 250 rpm. Following the 6-hour incubation, the αS solution was filtered using Amicon Ultra 100 kDa ultracentrifugation filters (MilliporeSigma) and the filtrate discarded. The retentate, containing αS oligomers, was washed three times with 1X PBSA and then collected. αS concentration was determined using absorbance at 280 nm. As reported previously [33, 35], the αS samples prepared in this manner contained oligomeric αS, which migrated more slowly than αS monomers in native-PAGE and was recognized by an anti-amyloid oligomer antibody, A11 (data not shown).
αS Fibril Preparation
αS fibrils were prepared by incubating αS monomer samples at 350 μM and 37°C in a glass vial with constant stirring using a magnetic stir bar at 250 rpm for 4–6 weeks. The αS samples were then pelleted by centrifugation at 14,000 rpm for 5 minutes following the incubation period. The supernatant was removed, and the pellet resuspended and washed with 1X PBSA, followed by additional centrifugation. The resulting pellet containing αS fibrils was washed similarly two more times, replacing the 1X PBSA each time. The concentration of αS in the supernatant after each centrifugation was determined using absorbance at 280 nm. The final concentration of αS fibrils was then back-calculated by subtracting concentration of αS lost during washing from the initial αS concentration of samples prior to incubation. As reported previously [33, 35], αS fibril samples prepared in this manner were A11-negative (data not shown).
Peptide Sample Preparation
One mg each of lyophilized FITC-ELVFWAE and FITC-KLVFWAK was dissolved in 100 μL of dimethyl sulfoxide (DMSO) at room temperature and subsequently diluted with 1X PBSA to a final peptide concentration of 150 μM and final DMSO concentration of 2% v/v. Each of capped FITC-ELVFWAE and capped FITC-KLVFWAK was similarly dissolved in DMSO and then diluted into deionized water, followed by addition of 10X PBSA to prepare peptide solution at 150 μM with residual DMSO at 2% v/v. All four peptide solutions have the same DMSO, phosphate, sodium chloride and sodium azide concentrations.
Fluorescent Dot Blot Assays
αS samples were dotted onto a nitrocellulose membrane and allowed to dry at room temperature for 5 minutes. The membrane was kept at 4°C until needed. Blocking solution and antibody wash solutions from the Invitrogen WesternBreeze Chemiluminescent Western Blot Immunodetection Kit (Fisher Scientific) were used to block and wash the membrane, respectively. Briefly, the membrane was blocked in 10 mL of blocking solution for 1 hour with constant shaking at room temperature. After blocking, the membrane was washed with 10 mL of antibody wash solution for 5 minutes and decanted. The membrane was washed three more times and the antibody solution replaced each time. The membrane was then incubated for 1 hour in 10 mL of 10 μM FITC-tagged peptide in blocking solution. While greater sensitivity might be achieved when a higher concentration of FITC-tagged peptide is used, we chose the concentration (10 μM) to minimize any undesired self-aggregation of peptides, possibly facilitated by membrane surface [36]. After incubation, the membrane was washed with 10 mL antibody wash solution for 5 minutes. The membrane was decanted and washed four more times, replacing the antibody wash solution each time. Fluorescent images were taken of the membrane using a GE Typhoon Trio Phosphoimaging System of the Small Instrument Fleet located at the NYU Langone Medical Center. The fluorescence signals were quantified by a MATLAB-based home-made image analysis algorithm.
The blocking and antibody wash solutions used for the dot blots were prepared using the manufacturer’s protocol and pre-made solutions, Blocker/Diluent (Part A), Blocker/Diluent (Part B), and Antibody Wash Solution (16X), purchased with the Invitrogen WesternBreeze Chemiluminescent Western Blot Immunodetection Kit. The Blocker/Diluent (Part A), Blocker/Diluent (Part B), and Antibody Wash Solution (16X) are described by the manufacturer as concentrated buffered saline with detergent, concentrated Hammarsten casein solution, and concentrated buffered saline with detergent, respectfully. The blocking solution was prepared by mixing a ratio of 7:2:1 deionized water: Blocker/Diluent (Part A): Blocker/Diluent (Part B). Antibody wash solution was prepared by diluting the Antibody Wash Solution (16X) with deionized water to a 1X concentration.
Circular Dichroism (CD) Spectroscopy
Immediately prior to CD measurements, αS samples were diluted to 17.5 μM with 1X PBSA and put into a 1 mm pathlength quartz cuvette. The CD spectra of αS samples in the far-UV range were obtained using a Jasco (Easton, MD, USA) J-815 spectropolarimeter. The CD spectra of buffer controls were determined similarly and subtracted from those obtained from αS samples.
Thioflavin T Fluorescence
An αS sample and stock ThT solution (0.1 mM) were added to 1X PBSA, for final αS and ThT concentrations at 5 μM each. ThT fluorescence intensity of the sample was measured immediately using a Photon Technology QuantaMaster QM-4 spectrofluorometer (HORIBA Scientific, Piscataway, NJ), with excitation and emission wavelengths at 440 nm and 487 nm, respectively.
Time-Course αS Aggregation
Monomeric αS was prepared as described above and diluted to 100 μM in 1X PBSA. The sample was then incubated in a glass vial at 37°C while being continuously stirred using a magnetic stir bar at 250 rpm for 7 days. The sample was aliquoted each day and subjected to ThT fluorescence measurements and fluorescent dot blot assays.
Transmission Electron Microscopy (TEM)
Morphology of αS samples was examined by transmission electron microscopy (TEM). Five μl of the samples were pipetted onto grids, followed by negative staining with 1% uranyl acetate solution and multiple washing. Images of the samples were taken on a Phillips CM12 Transmission Electron Microscope (FEI Corp., Hillsboro, OR, USA) at 120 kV with a 4k × 2.67k GATAN digital camera, located at the Skirball Institute for Biomolecular Medicine at the NYU Langone Medical Center.
Results and Discussion
Characterization of αS samples
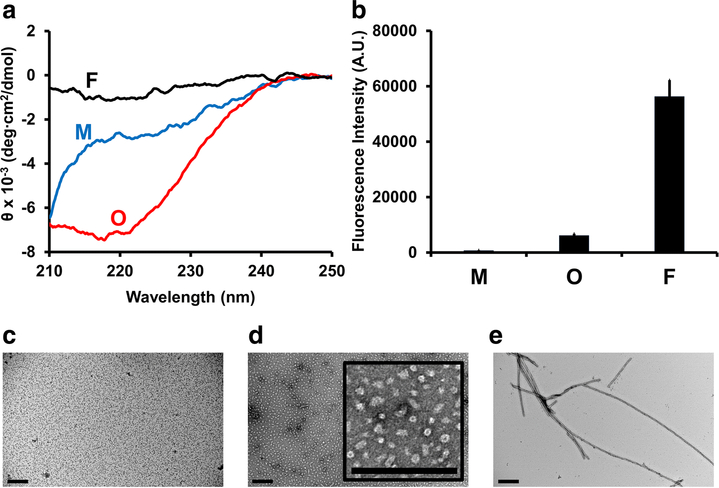
Monomeric, oligomeric, and fibrillar αS samples were characterized by circular dichroism (CD) spectroscopy, Thioflavin T (ThT) fluorescence and transmission electron microscopy (TEM) (Fig. 1). As expected, monomeric αS samples lacked an ordered secondary structure, while αS oligomers were rich in β-sheets, as judged by the CD spectra (Fig. 1A). The CD signals of insoluble αS fibrils was weak (Fig. 1A), due to the low concentration of αS remaining in solution. A slight minimum in the spectrum of αS fibrils at 215–220 nm was observed (Fig. 1A), which is indicative of the presence of β-sheet structures in this sample [37]. ThT is a fluorescent dye that binds to amyloid β-sheets found in fibrils and some oligomers with stronger signals upon binding to fibrillar rather than oligomeric conformers [12, 13, 15, 16]. As expected, no significant ThT fluorescence signal was detected for monomeric αS, demonstrating the absence of amyloid aggregates, in contrast to αS oligomers and fibrils with greater fluorescence signals detected for fibrils (Fig. 1B). The lack of significant amyloid aggregates in αS monomer samples was further confirmed by TEM (Fig. 1C) while globular and annular assemblies were detected in αS oligomer samples (Fig. 1D), as described elsewhere [33, 35]. Aggregates in αS fibril samples exhibited fibrillar morphology (Fig. 1E), as expected.
Figure 1.
Characterization of αS samples using (A) circular dichroism (CD) spectroscopy and (B) Thioflavin T (ThT) fluorescence. (C-E) Representative transmission electron microscopy (TEM) images of (C) αS monomer, (D) αS oligomer and (E) αS fibril samples. In (A) and (B), M, O and F represent monomers, oligomers and fibrils, respectively. In (B), Error bars: 1 standard deviation of triplicates. In (C), (D) and (E), scale bars: 200 nm. In (D), the inset image illustrates globular and annular morphology of αS oligomers.
No Strong Binding of ELVFWAE and KLVFWAK to αS
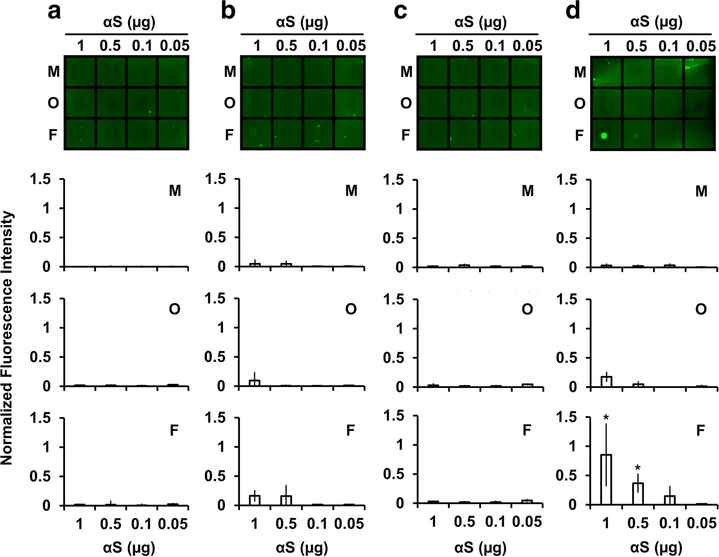
ELVFWAE was N-terminally tagged with fluorescein isothiocyanate (FITC; the resulting peptide referred to as FITC-ELVFWAE) in order to optically detect the peptide bound to monomeric, oligomeric, and fibrillar αS in fluorescent dot blot assays. For this examination, αS samples at 0.05 – 1 μg each in the three conformers were dotted onto a membrane, which was subsequently incubated with solution containing FITC-ELVFWAE. After washing off the unbound fraction, the FITC-ELVFWAE peptides bound to αS was subsequently visualized using fluorescence. Fluorescence imaging of the membrane shows that FITC-ELVFWAE was not bound to any of the three αS conformers (Fig. 2A). The effects of terminal charged residues on the peptide’s binding to αS were then examined by point mutations of the terminal glutamates to lysines. The resulting peptide, KLVFWAK, was also tagged with FITC at the N-terminus (referred to as FITC-KLVFWAK). No strong binding of FITC-KLVFWAK to the three αS conformers was detected (Fig. 2B), indicating that the terminal point mutations caused no notable change in binding to αS. It should be noted that, despite the lack of strong binding to αS in the fluorescent dot blot assays, both KLVFWAK and ELVFWAE were previously found to affect αS aggregation kinetics when each of the peptides was co-incubated with αS in solution [28]. The results are consistent with the previous finding that strong binding is not required for a short KLVFFAE-derived peptide to affect Aβ aggregation [38]. In other words, KLVFWAK or ELVFWAE could still alter αS aggregation kinetics even when they are readily released from, rather than remain bound to αS. Moreover, binding of KLVFWAK or ELVFWAE to αS may be weakened on the membrane surface relative to in solution due to mass transfer limitations.
Figure 2.
Fluorescent dot blot assays of αS using (A) FITC-ELVFWAE, (B) FITC-KLVFWAK, (C) capped FITC-ELVFWAE, and (D) capped FITC-KLVFWAK. Top: representative membrane images where αS in monomeric (M), oligomeric (O) and fibrillar (F) conformers were dotted at 0.05 – 1 μg each, and then incubated with FITC-labeled peptide solutions, followed by washing off unbound peptides and subsequent visualization of the bound peptides using fluorescence. Bottom three plots: fluorescence intensities of the membranes for monomeric (M), oligomeric (O), and fibrillar (F) αS at 0.05 – 1 μg were quantified by a MATLAB-based image analysis. Fluorescence intensities were normalized to the fluorescence signals of each FITC-labeled peptide at 1 μg directly dotted onto the membrane. Error bars: 1 SD from at least two independent experiments. *: p < 0.05 relative to normalized background intensity (0 ± 0.08, n = 4)
αS Fibrillar Conformer-Specific Binding of KLVFWAK upon C-terminal Capping
To further study the impact of terminal charges of the peptides on αS binding, the two peptides were chemically modified at their C-termini. Specifically, FITC-ELVFWAE was terminally capped at the C-terminus in order to examine the role of electrostatic interactions due to side chains only. The terminal capping removes negative charges from the backbone carboxyl terminus, leaving in only those originating from glutamate sidechains. When fluorescent dot blot assays were performed similarly for capped FITC-ELVFWAE, no difference in binding profiles for αS conformers was observed when compared to the corresponding uncapped peptide (Fig. 2C vs. 2A). In contrast, when FITC-KLVFWAK was similarly capped at the C-terminus, the resulting peptide exhibited significant binding to αS with selectivity for fibrils over other αS conformers (Fig. 2D). Under our experimental setup, the capped FITC-KLVFWAK successfully detected αS fibrils at ≥ 0.5 μg (Fig. 2D). We confirmed no significant binding of unconjugated FITC (i.e., FITC control) to the three αS conformers in a similar fluorescent dot blot assay (data not shown), indicating that binding of capped FITC-KLVFWAK to αS fibrils was mediated by the non-FITC portion of the peptide. It must be noted that the observed selectivity of capped FITC-KLVFWAK for αS fibrils might be compromised when the peptide concentration is too high: under such a condition, significant fluorescence signals are also likely to result from non-specific and weak bindings (for example, to other αS conformers). Thus, a concentration of capped FITC-KLVFWAK needs to be properly optimized for the αS fibrillar conformer-specificity.
Capped FITC-KLVFWAK contains a highly amyloidogenic segment (LVFWA) [28], which may readily interact with other hydrophobic amyloid sequences. When in the fibrillar form, αS molecules, except for the C-termini, assemble in parallel cross β-sheet structures [39–41]. These parallel structures allow for hydrophobic residues of adjacent αS molecules to be aligned, specifically between αS residues 34–101 [39]. The parallel alignment of these hydrophobic residues creates solvent-exposed hydrophobic surfaces on the αS fibrils [42], which could serve as binding sites for capped FITC-KLVFWAK, particularly, via its hydrophobic residues (LVFWA). The results from our comparative studies with the four peptides sharing the hydrophobic LVFWA but varying in charged states at the termini suggest that electrostatic interactions should also play a significant role in binding to αS fibrils. Only capped FITC-KLVFWAK displayed significant binding to αS fibrils, and its non-FITC portion has the highest net positive charge (+2) at neutral pH among the four peptides tested in this study. The inability for both capped and uncapped FITC-ELVFWAE to bind to αS fibrils suggests that the positively charged lysine side chains at the termini are essential in binding to αS fibrils. Additionally, the comparison between capped and uncapped FITC-KLVFWAK indicates that elimination of the negative charge from the C-terminal backbone is critical for strong binding to αS fibrils. Interestingly, the C-terminus of αS is rich in negatively charged glutamic acids, and has been shown to rotate freely and remain unfolded, rather than become embedded in the parallel β-sheet structure of fibrils [39, 41], implying that the αS C-terminus may be available for electrostatic interaction with the non-FITC portion of capped FITC-KLVFWAK. Electrostatic interactions between the αS C-terminus and the fibrillar core residues (αS 34–101) have also been proposed [43]. Thus, the αS C-terminus may have similar electrostatic interactions with the capped FITC-KLVFWAK peptides upon their binding to the surface of the αS fibril core. Collectively, the results suggest that binding of capped FITC-KLVFWAK to αS fibrils may be assisted by a combined effect of electrostatic and hydrophobic interactions. In contrast to αS fibrils, αS oligomers exhibit an antiparallel β-sheet structure [35, 44]. The antiparallel nature of αS oligomers may imply that hydrophobic residues on the surface are relatively spread-out in oligomeric conformers rather than locally aligned as is on the surface of the fibril. For a similar reason, local areas with strong net charges are less likely to exist in oligomeric relative to fibrillar αS conformers. Taken together, the structural feature of αS oligomers might weaken their hydrophobic and electrostatic interactions with our peptides. Similarly, the absence of local areas with strong hydrophobicity and net charges created across multiple proximal αS chains could limit binding of αS monomers to the peptide. No significant binding of all four peptides to αS monomers may also be due to high energetic costs needed for the binding between the structurally disordered αS monomeric conformers and the peptides, which were previously found to lack typical secondary structures (i.e., α-helix and β-sheet) [35, 39]. It should however be noted that detailed structural studies on capped FITC-KLVFWAK bound to αS fibrils will be required to better understand molecular factors critical in the peptide’s observed αS fibrillar conformer-specificity.
The selective binding of capped FITC-KLVFWAK to αS fibrils motivated us to study the ability of this probe to recognize the αS conformers in complex samples, such as those obtained during the αS aggregation process. For this examination, monomeric αS in solution was incubated at 37 oC with constant stirring for 7 days and allowed to form fibrillar aggregates, while samples were withdrawn daily for ThT fluorescence measurements (Fig. 3A). Aliquots of the withdrawn samples were also dotted onto a membrane, which was then incubated with capped FITC-KLVFWAK, followed by fluorescence imaging (Fig. 3B). As reported elsewhere [33], αS aggregation kinetics followed a sigmoidal change over time (Fig. 3A). The αS samples remained mostly unaggregated until Day 1, as judged by the low ThT fluorescence signal. Subsequently, the ThT fluorescence signals increased rapidly and leveled off after Day 3, indicating significant formation of αS aggregates, presumably in fibrillar forms, during this incubation period, which was also confirmed by TEM (Fig. 3A inset). The time-course ThT fluorescence change was largely mirrored by a signal change over time in the fluorescent dot blot assay (Fig. 3A), as expected based on the natures of the αS fibril-dependent signals (Fig. 1B and 2D). The fluorescent dot blot assay shows no or weak signals on Days 0 and 1, followed by an increase in signals on Day 2 with the signals remaining mostly unchanged thereafter. A slight difference between normalized fluorescence signals from ThT and capped FITC-KLVFWAK was noticed for αS samples collected on Day 2 (Fig. 3A). The discrepancy may be associated with the different natures of the two assays. For example, the ThT fluorescence assay is solution-based while the fluorescence dot blot assay requires immobilization of αS on membrane surface. Also, the mechanisms by which fluorescence signals are generated differ in the two assays. ThT is self-quenched and displays negligible fluorescence on its own [13]. However, upon binding to amyloid aggregates, ThT molecules become sterically hindered, relieving fluorescence quenching and thus generating fluorescence signals [13, 45]. By contrast, FITC attached to capped KLVFWAK remains fluorescent whether the peptide binds to αS fibrils or not: thus, the unbound peptide fractions should be removed to detect αS fibril-specific signals in fluorescent dot blot assays as described in Materials and Methods. Additionally, the number of ThT molecules or capped FITC-KLVFWAK peptides that bind to each αS molecule is unknown, implying that there may exist differences in their avidity for αS fibrils. Overall, the time course aggregation results demonstrate that capped FITC-KLVFWAK can selectively recognize αS fibrils in a dynamic sample undergoing aggregation from monomeric to fibrillar conformers.
Figure 3.
Time course αS aggregation monitored by fluorescence from ThT and capped FITC-KLVFWAK. Aliquots of αS samples were withdrawn during incubation and subjected to ThT fluorescence measurements in (A) (red squares). The remaining aliquots were blotted onto a membrane, and then incubated in solution containing capped FITC-KLVFWAK, followed by washing off unbound peptides and subsequent visualization using fluorescence, as shown in a representative membrane image in (B). Fluorescence signals in (B) were normalized and shown with green circles in (A). In (A), error bars are 1 SD from triplicate measurements. In (A), the red square symbols are larger than the size of the error bars. αS was incubated at 100 μM and 37°C for 7 days with continuous stirring at 250 rpm. In (A), the inset: a representative TEM image of αS samples after the 7-day incubation (scale bar: 200 nm).
CONCLUSION
We show that a KLVFFAE-derived peptide can be fine-tuned for αS fibril-specific binding by capping the peptide’s C-terminal backbone and mutating terminal charge residues. Our study also suggests that binding of capped FITC-KLVFWAK to αS fibrils is assisted by both hydrophobic and electrostatic interactions. We anticipate that conformer-specificity of our peptide probe can further be optimized by systematic sequence variations, for example, by phage display [46]. Sensitivity of αS detection using our peptide probe can also be improved by engineering multivalency of the peptide, which has proven effective for increasing affinity of peptide ligands [47]. The results of the present study provide significant insight into the behavior of KLVFFAE-derived peptides and the potential of terminal mutations and capping in engineering their binding characteristics for distinct amyloid conformers.
ACKNOWLEDGEMENTS
The research reported in this article was supported by the NIH/NIA Grant R21AG049137. We thank the Small Instrument Fleet of New York University (NYULMC) for the use of the Typhoon Trio Phosphoimaging System.
Footnotes
DISCLOSURES
The authors indicate no potential conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Reiss AB, Arain HA, Stecker MM, Siegart NM & Kasselman LJ (2018). Amyloid toxicity in Alzheimer’s disease. Rev. Neurosci, 29(6), 613–627. [DOI] [PubMed] [Google Scholar]
- 2.Lashuel HA, Overk CR, Oueslati A & Masliah E (2013). The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat. Rev. Neurosci, 14(1), 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buell AK, Galvagnion C, Gaspar R, Sparr E, Vendruscolo M, Knowles TP, Linse S & Dobson CM (2014). Solution conditions determine the relative importance of nucleation and growth processes in alpha-synuclein aggregation. Proc. Natl. Acad. Sci. USA, 111(21), 7671–7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tipping KW, van Oosten-Hawle P, Hewitt EW & Radford SE (2015). Amyloid Fibres: Inert End-Stage Aggregates or Key Players in Disease? Trends Biochem. Sci, 40(12), 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tipping KW, Karamanos TK, Jakhria T, Iadanza MG, Goodchild SC, Tuma R, Ranson NA, Hewitt EW & Radford SE (2015). pH-induced molecular shedding drives the formation of amyloid fibril-derived oligomers. Proc. Natl. Acad. Sci. USA, 112(18), 5691–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT & Spires-Jones TL (2009). Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl. Acad. Sci. USA, 106(10), 4012–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cremades N, Cohen SI, Deas E, Abramov AY, Chen AY, Orte A, Sandal M, Clarke RW, Dunne P, Aprile FA, Bertoncini CW, Wood NW, Knowles TP, Dobson CM & Klenerman D (2012). Direct observation of the interconversion of normal and toxic forms of alpha-synuclein. Cell, 149(5), 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho JE & Kim JR (2011). Recent approaches targeting beta-amyloid for therapeutic intervention of Alzheimer’s disease. Recent Pat. CNS Drug Discov, 6(3), 222–233. [DOI] [PubMed] [Google Scholar]
- 9.Madav Y, Wairkar S & Prabhakar B (2019). Recent therapeutic strategies targeting beta amyloid and tauopathies in Alzheimer’s disease. Brain Res. Bull, 146, 171–184. [DOI] [PubMed] [Google Scholar]
- 10.Irvine GB, El-Agnaf OM, Shankar GM & Walsh DM (2008). Protein aggregation in the brain: the molecular basis for Alzheimer’s and Parkinson’s diseases. Mol. Med, 14(7–8), 451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Y, Su B, Zheng H & Kim JR (2012). A peptide probe for detection of various beta-amyloid oligomers. Mol. Biosyst, 8(10), 2741–2752. [DOI] [PubMed] [Google Scholar]
- 12.Reinke AA & Gestwicki JE (2011). Insight into amyloid structure using chemical probes. Chem. Biol. Drug Des, 77(6), 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biancalana M & Koide S (2010). Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta, 1804(7), 1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C, Wang Z, Lei H, Duan Y, Bowers MT & Shea JE (2008). The binding of thioflavin T and its neutral analog BTA-1 to protofibrils of the Alzheimer’s disease Abeta(16–22) peptide probed by molecular dynamics simulations. J. Mol. Biol, 384(3), 718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan DA, Narrow WC, Federoff HJ & Bowers WJ (2010). An improved method for generating consistent soluble amyloid-beta oligomer preparations for in vitro neurotoxicity studies. J. Neurosci. Methods, 190(2), 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maezawa I, Hong HS, Liu R, Wu CY, Cheng RH, Kung MP, Kung HF, Lam KS, Oddo S, Laferla FM & Jin LW (2008). Congo red and thioflavin-T analogs detect Abeta oligomers. J. Neurochem, 104(2), 457–468. [DOI] [PubMed] [Google Scholar]
- 17.Naiki H, Higuchi K, Hosokawa M & Takeda T (1989). Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal. Biochem, 177(2), 244–249. [DOI] [PubMed] [Google Scholar]
- 18.Crystal AS, Giasson BI, Crowe A, Kung MP, Zhuang ZP, Trojanowski JQ & Lee VM (2003). A comparison of amyloid fibrillogenesis using the novel fluorescent compound K114. J. Neurochem, 86(6), 1359–1368. [DOI] [PubMed] [Google Scholar]
- 19.Lindgren M, Sorgjerd K & Hammarstrom P (2005). Detection and characterization of aggregates, prefibrillar amyloidogenic oligomers, and protofibrils using fluorescence spectroscopy. Biophys. J, 88(6), 4200–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt ML, Schuck T, Sheridan S, Kung MP, Kung H, Zhuang ZP, Bergeron C, Lamarche JS, Skovronsky D, Giasson BI, Lee VM & Trojanowski JQ (2001). The fluorescent Congo red derivative, (trans, trans)-1-bromo-2,5-bis-(3-hydroxycarbonyl-4-hydroxy)styrylbenzene (BSB), labels diverse beta-pleated sheet structures in postmortem human neurodegenerative disease brains. Am. J. Pathol, 159(3), 937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volkova KD, Kovalska VB, Balanda AO, Vermeij RJ, Subramaniam V, Slominskii YL & Yarmoluk SM (2007). Cyanine dye-protein interactions: looking for fluorescent probes for amyloid structures. J. Biochem. Biophys. Methods, 70(5), 727–733. [DOI] [PubMed] [Google Scholar]
- 22.Reinke AA, Abulwerdi GA & Gestwicki JE (2010). Quantifying prefibrillar amyloids in vitro by using a “thioflavin-like” spectroscopic method. Chembiochem, 11(13), 1889–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee D, Kim SM, Kim HY & Kim Y (2019). Fluorescence Chemicals To Detect Insoluble and Soluble Amyloid-beta Aggregates. Acs Chem. Neurosci, 10(6), 2647–2657. [DOI] [PubMed] [Google Scholar]
- 24.Klunk WE, Jacob RF & Mason RP (1999). Quantifying amyloid beta-peptide (A beta) aggregation using the Congo red A beta (CR-A beta) spectrophotometric assay. Anal. Biochem, 266(1), 66–76. [DOI] [PubMed] [Google Scholar]
- 25.Rouet R, Lowe D & Christ D (2014). Stability engineering of the human antibody repertoire. FEBS Lett, 588(2), 269–277. [DOI] [PubMed] [Google Scholar]
- 26.Aoraha E, Candreva J & Kim JR (2015). Engineering of a peptide probe for beta-amyloid aggregates. Mol. Biosyst, 11(8), 2281–2289. [DOI] [PubMed] [Google Scholar]
- 27.Candreva J, Chau E, Aoraha E, Nanda V & Kim JR (2018). Hetero-assembly of a dual beta-amyloid variant peptide system. Chem. Commun. (Camb), 54(49), 6380–6383. [DOI] [PubMed] [Google Scholar]
- 28.Charlton T, Shah V, Lynch T, Candreva J, Chau E, Yang Y, Kim H, Wood A & Kim JR (2018). Amyloid aggregation of Bacillus circulans xylanase under native conditions and its modulation by beta-amyloid-derived peptide fragments. Chembiochem, 19(24), 2566–2574. [DOI] [PubMed] [Google Scholar]
- 29.Tao K, Wang J, Zhou P, Wang C, Xu H, Zhao X & Lu JR (2011). Self-assembly of short abeta(16–22) peptides: effect of terminal capping and the role of electrostatic interaction. Langmuir, 27(6), 2723–2730. [DOI] [PubMed] [Google Scholar]
- 30.Conway KA, Harper JD & Lansbury PT (1998). Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat. Med, 4(11), 1318–1320. [DOI] [PubMed] [Google Scholar]
- 31.Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW, Axelsen PH & Giasson BI (2005). The E46K mutation in alpha-synuclein increases amyloid fibril formation. J. Biol. Chem, 280(9), 7800–7807. [DOI] [PubMed] [Google Scholar]
- 32.Tashiro M, Kojima M, Kihara H, Kasai K, Kamiyoshihara T, Ueda K & Shimotakahara S (2008). Characterization of fibrillation process of alpha-synuclein at the initial stage. Biochem. Biophys. Res. Commun, 369(3), 910–914. [DOI] [PubMed] [Google Scholar]
- 33.Hernandez M, Hu Y & Kim JR (2013). A conformation-switching fluorescent protein probe for detection of alpha synuclein oligomers. Chem. Commun. (Camb), 49(91), 10712–10714. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez M, Golbert S, Zhang LG & Kim JR (2011). Creation of aggregation-defective alpha-synuclein variants by engineering the sequence connecting beta-strand-forming domains. Chembiochem, 12(17), 2630–2639. [DOI] [PubMed] [Google Scholar]
- 35.Celej MS, Sarroukh R, Goormaghtigh E, Fidelio GD, Ruysschaert JM & Raussens V (2012). Toxic prefibrillar alpha-synuclein amyloid oligomers adopt a distinctive antiparallel beta-sheet structure. Biochem. J, 443(3), 719–726. [DOI] [PubMed] [Google Scholar]
- 36.McMasters MJ, Hammer RP & McCarley RL (2005). Surface-induced aggregation of beta amyloid peptide by co-substituted alkanethiol monolayers supported on gold. Langmuir, 21(10), 4464–4470. [DOI] [PubMed] [Google Scholar]
- 37.Greenfield NJ (2006). Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc, 1(6), 2876–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JR & Murphy RM (2004). Mechanism of accelerated assembly of beta-amyloid filaments into fibrils by KLVFFK(6). Biophys. J, 86(5), 3194–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Der-Sarkissian A, Jao CC, Chen J & Langen R (2003). Structural organization of alpha-synuclein fibrils studied by site-directed spin labeling. J. Biol. Chem, 278(39), 37530–37535. [DOI] [PubMed] [Google Scholar]
- 40.Chen M, Margittai M, Chen J & Langen R (2007). Investigation of alpha-synuclein fibril structure by site-directed spin labeling. J. Biol. Chem, 282(34), 24970–24979. [DOI] [PubMed] [Google Scholar]
- 41.Heise H, Hoyer W, Becker S, Andronesi OC, Riedel D & Baldus M (2005). Molecular-level secondary structure, polymorphism, and dynamics of full-length alpha-synuclein fibrils studied by solid-state NMR. Proc. Natl. Acad. Sci. USA, 102(44), 15871–15876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuttle MD, Comellas G, Nieuwkoop AJ, Covell DJ, Berthold DA, Kloepper KD, Courtney JM, Kim JK, Barclay AM, Kendall A, Wan W, Stubbs G, Schwieters CD, Lee VM, George JM & Rienstra CM (2016). Solid-state NMR structure of a pathogenic fibril of full-length human alpha-synuclein. Nat. Struct. Mol. Biol, 23(5), 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin Z, Hu D, Han S, Hong DP & Fink AL (2007). Role of different regions of alpha-synuclein in the assembly of fibrils. Biochemistry, 46(46), 13322–13330. [DOI] [PubMed] [Google Scholar]
- 44.Apetri MM, Maiti NC, Zagorski MG, Carey PR & Anderson VE (2006). Secondary structure of alpha-synuclein oligomers: characterization by raman and atomic force microscopy. J. Mol. Biol, 355(1), 63–71. [DOI] [PubMed] [Google Scholar]
- 45.Stsiapura VI, Maskevich AA, Kuzmitsky VA, Turoverov KK & Kuznetsova IM (2007). Computational study of thioflavin T torsional relaxation in the excited state. J. Phys. Chem. A, 111(22), 4829–4835. [DOI] [PubMed] [Google Scholar]
- 46.Rahbarnia L, Farajnia S, Babaei H, Majidi J, Veisi K, Ahmadzadeh V & Akbari B (2017). Evolution of phage display technology: from discovery to application. J. Drug Target, 25(3), 216–224. [DOI] [PubMed] [Google Scholar]
- 47.Wan J & Alewood PF (2016). Peptide-decorated dendrimers and their bioapplications. Angew. Chem. Int. Ed. Engl, 55(17), 5124–5134. [DOI] [PubMed] [Google Scholar]