Abstract
BACKGROUND
Reproductive disorders and infertility are associated with the risk of obstetric complications and have a negative impact on pregnancy outcome. Affected patients often require assisted reproductive technologies (ART) to conceive, and advanced maternal age is a further confounding factor. The challenge is to dissect causation, correlation and confounders in determining how infertility and reproductive disorders individually or together predispose women to poor pregnancy outcomes.
METHODS
The published literature, to June 2015, was searched using PubMed, summarizing all evidences concerning the perinatal outcome of women with infertility and reproductive disorders and the potential mechanisms that may influence poor pregnancy outcome.
RESULTS
Reproductive disorders (endometriosis, adenomyosis, polycystic ovary syndrome and uterine fibroids) and unexplained infertility share inflammatory pathways, hormonal aberrations, decidual senescence and vascular abnormalities that may impair pregnancy success through common mechanisms. Either in combination or alone, these disorders results in an increased risk of preterm birth, fetal growth restriction, placental pathologies and hypertensive disorders. Systemic hormonal aberrations, and inflammatory and metabolic factors acting on endometrium, myometrium, cervix and placenta are all associated with an aberrant milieu during implantation and pregnancy, thus contributing to the genesis of obstetric complications. Some of these features have been also described in placentas from ART.
CONCLUSIONS
Reproductive disorders are common in women of childbearing age and rarely occur in isolation. Inflammatory, endocrine and metabolic mechanisms associated with these disorders are responsible for an increased incidence of obstetric complications. These patients should be recognized as ‘high risk’ for poor pregnancy outcomes and monitored with specialized follow-up. There is a real need for development of evidence-based recommendations about clinical management and specific obstetric care pathways for the introduction of prompt preventative care measures.
Keywords: polycystic ovary syndrome, endometriosis, uterine fibroids, unexplained infertility, assisted reproductive technologies, preterm birth, pre-eclampsia, placenta, inflammation, sex steroids
Introduction
A major challenge of modern women's health is to define maternal or fetal factors associated with the risk of adverse obstetric outcomes. A growing number of studies are revealing that infertility and reproductive disorders, such as endometriosis, adenomyosis, polycystic ovary syndrome (PCOS) and uterine fibroids, may have a negative impact on pregnancy, from implantation until term. In addition, many patients with reproductive disorders and/or infertility require assisted reproductive technologies (ART), which independently may affect pregnancy outcomes. Thus, it is a difficult task to distinguish the contribution of specific reproductive disorders or infertility to poor pregnancy outcomes relative to the interventions required for pregnancy success (Talaulikar and Arulkumaran, 2012). In addition, women are delaying commencement of a family until later in life, resulting in an increased rate of infertility due to advanced maternal age, which constitutes an additional obstetric risk factor (Balasch and Gratacós, 2012). Therefore, women with reproductive disorders often have multiple risk factors (advanced maternal age, use of ART) contributing to negative obstetric outcomes. It is important to understand the causes of this effect and develop new care pathways to ensure adequate management of their reproductive health.
Hormones and inflammatory mechanisms are implicated in the major events of female reproductive function, including ovulation, menstruation, embryo implantation and pregnancy. Increasing evidence shows that hormonal aberrations and a hyperinflammatory state may lead to derangements of the immune-endocrine cross talk among endometrium, myometrium and cervix, and between the decidua and trophoblast, predisposing to pregnancy complications. Therefore, the aim of the current review was to assess whether inflammatory mechanisms and hormonal and metabolic dysfunctions occurring in uterine (endometrium, myometrium, cervix) and placental tissues in women with uterine fibroids, endometriosis, adenomyosis, PCOS and unexplained infertility may contribute to pregnancy disorders. Since other uterine conditions associated with obstetric complications, such as uterine malformations (Chanet al., 2011), synechiae (Tuuliet al., 2012) and Asherman syndrome (March, 2011), work mainly through mechanisms other than inflammatory, endocrine and metabolic pathways, they are not part of the present review.
Methods
The published literature was searched using PubMed summarizing all evidence concerning the obstetric and neonatal outcome of women with infertility and reproductive disorders and the potential mechanisms that may influence pregnancy complications. In particular, the literature research, up to June 2015, was focused on endometriosis, adenomyosis, PCOS, uterine fibroids and ART, while the pathogenic mechanisms contributing to adverse pregnancy outcome were related to hormonal and neurohormonal aberration, inflammatory pathways and metabolic dysfunction.
Adverse maternal and neonatal outcomes in pregnant women with reproductive disorders
Polycystic ovary syndrome
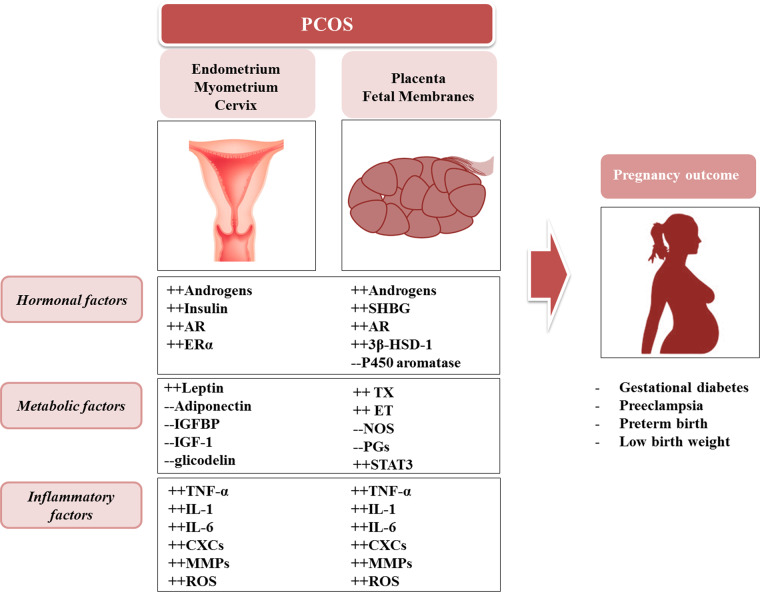
PCOS, one of the most common disorders in women of reproductive age (affecting 4–7% of women), is characterized by hyperandrogenism and ovarian dysfunction (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004). Approximately 50% of women with PCOS are overweight or obese and have reduced insulin sensitivity (Normanet al., 2004). Growing evidence demonstrates that PCOS has a negative impact on fertility and pregnancy outcome (Boomsmaet al., 2006;Kjerulffet al., 2011;Rooset al., 2011;Fauseret al., 2012;Qinet al., 2013). An increased risk of pregnancy and neonatal complications, including early pregnancy loss, gestational diabetes (GDM), gestational hypertensive disorders, preterm birth (PTB), low birthweight (LBW) and need for Cesarean section, independent of obesity, has been demonstrated (Fig.1) . The severity of adverse pregnancy outcome is related to the different phenotypes and features of PCOS (Touliset al., 2009;Palombaet al., 2010;De Frèneet al., 2014).
Figure 1.
Hormonal, inflammatory and metabolic factors occurring in the uterus (endometrium, myometrium, cervix) and in placental tissues (trophoblast and membranes) mediate the mechanisms of pregnancy complications in women with PCOS. AR, androgen receptor; ERα, estrogen receptor alpha; SHBG, sex hormone-binding globulin; 3β-HSD-1, 3beta-hydroxysteroid dehydrogenase type 1; IGFBP, insulin-like growth factor-binding protein; IGF-1, insulin-like growth factor type 1; TX, thromboxane; ET, endothelin; NOS, nitric oxide synthase; PGs, prostaglandins; STAT3, signal transducer and activator of transcription 3: TNF-α, tumor necrosis factor-alpha; IL-1, interleukin 1; IL-6, interleukin 6; CXCs, chemokines; MMPs, matrix metalloproteinases; ROS, reactive oxygen species.
Women with PCOS and glucose intolerance may develop GDM (5–40% risk;Touliset al., 2009). PTB affects 6–15% of pregnancies of women with PCOS (Yamamotoet al., 2012) and in hyperandrogenic women with PCOS a 2-fold increased risk of PTB and pre-eclampsia (PE) occurs, suggesting the role of androgens in the pathogenesis in these complications, although metabolic abnormalities must also be considered (Naveret al., 2014). Furthermore, in a large cohort of pregnant women with PCOS, a surprisingly high frequency of cervical insufficiency, particularly in South Asian and African women, is described (Feigenbaumet al., 2012).
Neonates of women with PCOS are at greater risk of neonatal complications, including perinatal mortality, prematurity and higher neonatal intensive care unit admission (Rooset al., 2011).
Endometriosis and adenomyosis
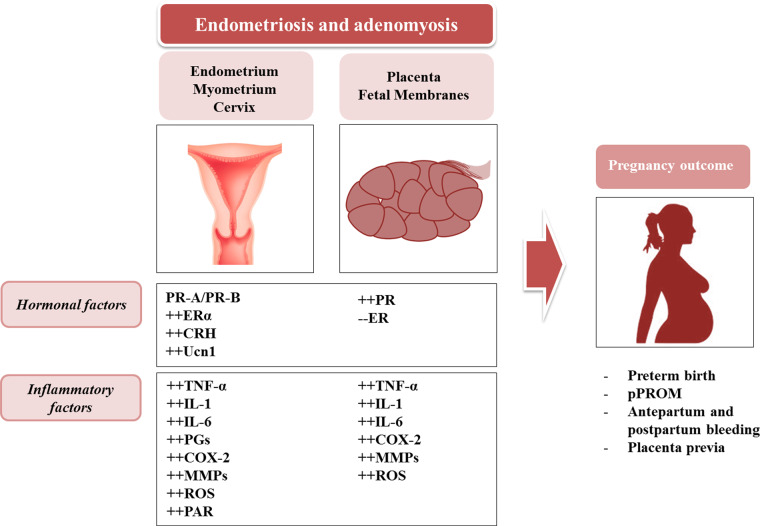
Endometriosis is a benign, chronic, inflammatory disease that affects 10% of reproductive age women and up to 50% of women with infertility (Burney and Giudice, 2012). The incidence of obstetric complications in patients with endometriosis, achieving pregnancy spontaneously or through ART, is controversial. Patients with an ovarian endometrioma achieving pregnancy by ART are twice as likely to have PTB or a small for gestational age (SGA) neonate (Fernandoet al., 2009), when compared with other forms of endometriosis. Patients with endometriosis requiring IVF treatment have a higher risk of placenta previa and post-partum hemorrhage (Healyet al., 2010;Takemuraet al., 2013), while in those women who conceived spontaneously, there was a higher incidence of miscarriage, PTB and placental complications (Vercelliniet al., 2012). In particular, women with endometriosis in their first pregnancy have greater risk of SGA babies, GDM, premature preterm rupture of membranes (pPROMs) and PTB, with longer hospitalization for mother and neonate (Contiet al., 2014). Also women with adenomyosis have increased risk of PTB and pPROM (Juanget al., 2007). In large population studies, including both spontaneous and ART pregnancies, an increased rate of PTB in women with endometriosis has been described (Stephanssonet al., 2009; Fig.2). Regarding neonatal outcome, endometriosis increases the incidence of stillbirth, irrespective of the use of ART (Aris, 2014). Conversely, a lack of negative pregnancy outcome in women with endometriosis has been shown in other studies (Kortelahtiet al., 2003;Benagliaet al., 2012;Mekaruet al., 2014).
Figure 2.
Hormonal and inflammatory factors occurring in uterus (endometrium, myometrium, cervix) and in placental tissues (trophoblast and membranes) mediate the mechanisms of pregnancy complications in women with endometriosis and adenomyosis. PR-A, progesterone receptor isoform A; PR-B, progesterone receptor isoform B; CRH, corticotropin-releasing hormone; Ucn1, urocortin 1; COX-2, cyclooxygenase 2; PAR, protease-activated receptor.
Uterine fibroids
Uterine fibroids (leiomyomas) are the most common benign tumors of the female reproductive tract, affecting 30–70% of reproductive-age women and therefore are common in pregnancy (from 0.1 to 12.5% of all pregnancies) (Cooper and Okolo, 2005).
Current evidence suggests that fibroids are associated with adverse obstetric outcomes, with antepartum, intrapartum and post-partum complications. Uterine fibroids are related to PTB (Coronadoet al., 2000;Qidwaiet al., 2006;Contiet al., 2013), and large fibroids (>5 cm) have been shown to be significantly associated with earlier delivery (Shavellet al., 2012). Uterine fibroids are also associated with fetal malpresentations, fetal growth restriction (FGR), placenta previa or placental abruption (Koikeet al., 1999;Ouyanget al., 2006;Somiglianaet al., 2007;Klatskyet al., 2008;Deveeret al., 2012;Lamet al., 2014) and a higher incidence of post-partum hemorrhage (Coronadoet al., 2000;Andreaniet al., 2009).
The most common cause of neonatal morbidity in pregnant women with fibroids is preterm delivery (Laiet al., 2012), with a longer neonatal hospitalization (Contiet al., 2013), but this should be adjusted for confounding factors, such as maternal age and the use of ART (Khalafet al., 2006;Luyckxet al., 2014).
Unexplained infertility and ART
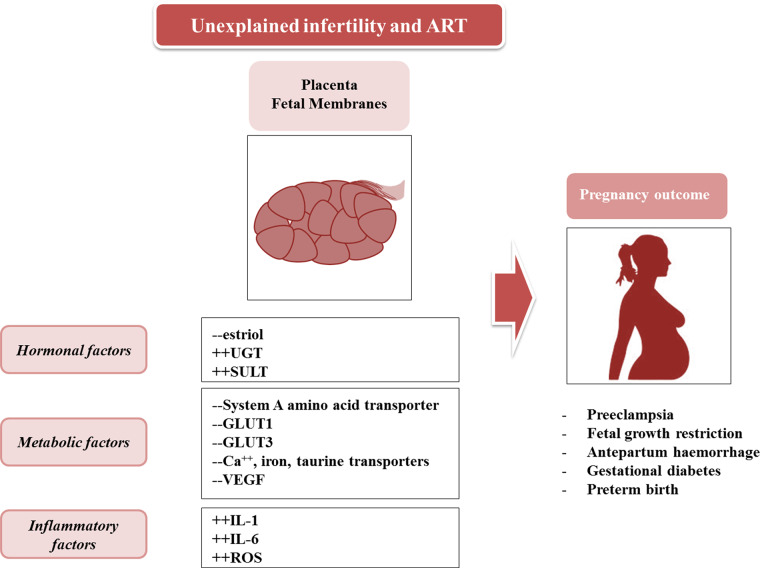
Unexplained infertility affects 10–20% of women (Sunderamet al., 2012), and regardless of treatment, is associated with an increased risk of pregnancy-induced hypertension (PIH) and PE, antepartum hemorrhage, PTB and Caesarean delivery (Pandianet al., 2003;Thomsonet al., 2005;Jaqueset al., 2010;Raatikainenet al., 2012;Messerlianet al., 2013). Risks for these disorders are also increased in pregnancies conceived by ART (Schieveet al., 2002;Kovalevskyet al., 2003;Helmerhorstet al., 2004;Romundstadet al., 2008;Klemettiet al., 2010). Note that twin pregnancies, irrespective of mode of conception, are associated with an increased risk of morbidity and mortality for mother and babies (Geisleret al., 2014), and thus only singleton ART should to be compared with spontaneously conceived singleton pregnancies in risk assessment. In fact, singleton pregnancies achieved by IVF or ICSI show an increased risk of antepartum hemorrhage, hypertensive disorders, GDM, FGR, induction of labor, pPROM, PTB and Caesarean section (Helmerhorstet al., 2004;Jacksonet al., 2004;Pinborget al., 2013; Fig.3). The increased risk persists even when the effect of stimulation is removed (in frozen embryo transfers) and single embryo transfer is performed (Pandeyet al., 2012). An increased risk for other maternal complications, including placenta praevia, placental abruption and vaginal bleeding, is also observed (Jacksonet al., 2004;Romundstadet al., 2006;Healyet al., 2010).
Figure 3.
Hormonal, inflammatory and metabolic factors occurring in placental tissues (trophoblast and membranes) mediate the mechanisms of pregnancy complications in women with unexplained infertility and requiring ART. UGT, uridine 5′-diphospho (UDP)-glucuronosyltransferase; SULT, sulfotransferase; GLUT1, glucose transporter type 1; GLUT3, glucose transporter type 3; VEGF, vascular endothelial growth factor.
In the last decade, oocyte donation has enabled couples to overcome infertility due to advanced maternal age, diminished ovarian reserve, primary ovarian insufficiency and surgical menopause (Luket al., 2010). Controversial results are reported in infertile women undergoing IVF with donor oocytes. Similar rates of prematurity, hypertensive disorders of pregnancy, GDM and placental abnormalities were found when donor oocyte cycles were compared with IVF cycles with autologous oocytes in women with advanced maternal age (Krieget al., 2008;Malchauet al. 2013). Conversely, donor oocyte recipients are at higher risk for untoward obstetric outcomes than their IVF counterparts, with the highest rate of PIH (Keeganet al., 2007;Le Rayet al., 2012). Moreover, in women who conceive twin pregnancies using IVF, oocyte donation increases the risk of PIH and PE, independent of age, BMI and parity (Sekhonet al., 2014). However, oocyte donation has no impact on the overall perinatal outcome (Stoopet al., 2012).
Neonates from women with unexplained infertility have a higher risk of perinatal morbidity even without the use of ART. An increased rate of prematurity, SGA and ‘poor neonatal health’ was observed as the time to conception (without medical assistance) increased beyond 6 months (Jaqueset al., 2010;Raatikainenet al., 2010). The same adverse neonatal outcomes are observed in singleton IVF neonates (Moiniet al., 2012;Kawwasset al., 2013;Kondapalli and Perales-Puchalt, 2013). Singletons born after intrauterine insemination (IUI) had a higher risk of adverse perinatal outcomes compared with spontaneously conceived children, similar to ICSI, but more favorable outcomes compared with IVF. Stimulation with clomiphene citrate is associated with higher risk of SGA compared with natural-cycle IUI, but FSH treatment is not associated with adverse outcomes (Malchauet al., 2014).
Pathogenic mechanisms contributing to adverse pregnancy outcome in women with reproductive disorders
Pathogenic mechanisms that contribute to uterine fibroids, endometriosis, adenomyosis, PCOS and unexplained infertility may impair pregnancy outcome through common pathways associated with hormonal aberrations, inflammation, metabolic disorders, decidual dysfunction and vascular disorders. As mentioned, either in combination or alone, these disorders result in an increased risk of PTB, FGR, GDM, placental pathologies and/or hypertensive disorders, which are also collected as a syndrome (Romeroet al., 2014). Endometrium, myometrium, cervix or placenta may be the anatomical sites where an aberrant milieu predisposes to the onset of specific obstetric complications.
Endometrium
Endometrial alterations are in part responsible for infertility and suboptimal uterine receptivity linked to uterine fibroids, endometriosis/adenomyosis and PCOS (Cakmak and Taylor, 2011;Fauseret al., 2012;Lesseyet al., 2013) and the abnormal endometrial milieu may contribute to adverse pregnancy outcome through hormonal, metabolic and inflammatory mechanisms. The establishment and development of pregnancy requires the coordinated implantation of the embryo and trophoblast invasion into the receptive maternal decidua, followed by remodeling of the spiral arteries. Proliferation, migration and invasion of trophoblastic cells into the maternal endometrium are essential steps, and failure of one of these due to endometrial dysfunction may be the basis for developing obstetric complications.
Hormonal abnormalities, including altered endometrial receptor expression, are present in PCOS and endometriosis and drive endometrial dysfunction leading to altered trophoblast decidual invasion. Endometrial growth and differentiation in women with PCOS are influenced by hyperandrogenism, in view of overexpression of androgen and estrogen receptors (ER) (Makievaet al., 2014). Endometrium of PCOS also shows altered progesterone receptor (PR) gene expression (Piltonenet al., 2015). Indeed, endometrial stromal fibroblasts from women with PCOS have impaired progesterone-mediated decidualization. A proinflammatory cytokine profile, chemokine and matrix metalloproteinase (MMP) release and immune cell chemoattraction are also observed (Piltonenet al., 2015). These gene abnormalities may induce a significant impairment of decidual endovascular trophoblast invasion, responsible for increased incidence of PTB, FGR and hypertensive disorders in pregnancy (Fig.1).
Similarly, endometriosis is characterized by abnormal ER- and PR-mediated signaling pathways associated with progesterone resistance (Al-Sabbaghet al., 2012;Lessey and Young, 2014). In case of pregnancy in Fkbp52–/– mice, characterized by progesterone resistance, decidualization is inhibited and inefficiency in maintaining pregnancy to full term are observed (Tranguchet al., 2007;Yanget al., 2012; Fig.2).
Apart from sex steroids, an abnormal decidual/trophoblast interaction may also be driven by metabolic dysfunction. Indeed, adiponectin, leptin and other fat tissue hormones, mainly related to obesity and PCOS, may have an impact on decidua/trophoblast interaction in PCOS and obese patients (Crujeiras and Casanueva, 2015). Furthermore, PCOS patients in the first trimester of pregnancy show a reduction of serum insulin-like growth factor-binding protein 1 and glycodelin that significantly correlate with reduced trophoblast invasion (Palombaet al., 2012). Moreover, lower glycodelin may contribute to a more proinflammatory environment in women with PCOS or endometriosis during early gestation, further impairing trophoblast invasiveness (Irwinet al., 2001;Alok and Karande, 2009). These alterations may lead to epigenetic changes in endometrium that persist long after the insult is removed or corrected. Widespread alterations in endometrial gene methylation, and therefore gene expression, affect endometrial function (Leeet al., 2009,Naqviet al., 2014; Fig.1).
The activation of inflammatory pathways could be subsequently associated with immune and vascular dysfunction in placenta/decidua interactions, leading to FGR, PE and PTB. PCOS is associated with a state of chronic low-grade inflammation shown by increased serum concentrations of tumor necrosis factor (TNF)-alpha, interleukin (IL)-6 and IL-1, adhesion molecules, follistatin and C-reactive protein (Piltonenet al., 2013;Palombaet al., 2014). Moreover, in PCOS, an increased oxidative stress index activated by inflammatory transcription factor NF-kappa B has been observed (Agarwalet al., 2012; Fig.1).
Increased local and systemic inflammatory pathways and reactive oxygen species are considered a major causal factor for explaining the poor pregnancy outcomes in women with endometriosis (Reiset al., 2013). Altered cell proliferation and apoptosis, increased oxidative stress, increased endometrial prostaglandin (PG) production (PGE2 and PGF2α) and expression of cyclooxygenase 2 (COX-2) (Burneyet al., 2007;Tamaresiset al., 2014) ultimately impact decidualization, reducing endometrial receptivity and thereby influencing pregnancy outcomes (Brosenset al., 2012;Vilellaet al., 2013;Tamaresiset al., 2014). Inflammation may be enhanced by the altered ratio of PR isoform A (PR-A) to PR isoform B (PR-B) in eutopic endometrium. The hyperinflammatory state in endometriosis influences the decidua/trophoblast interactions early in gestation as well as chorion–decidua interactions that could activate mechanisms of PTB later in pregnancy (Petragliaet al., 2012;Tamaresiset al., 2014;Marcellinet al., 2015) (Fig.2).
The same mechanisms may affect decidual/trophoblast invasion in women with adenomyosis, who showed increased expression of MMP-2 and MMP-9, E-cadherin, HOXA-10 and leukemia inhibitory factor (Fischeret al., 2011;Benagianoet al., 2012;Gallianoet al., 2015; Fig.2).
Corticotropin-releasing hormone (CRH) and urocortin (Ucn) are examples of neurohormones/neuropeptides related to stress and inflammation in reproductive organs and women with endometriosis share deranged CRH and Ucn mRNA expression associated with an impaired CRH receptor (CRH-R1) activity in modulating the process of decidualization (Novembriet al., 2011).
Uterine cavities containing leiomyomas also show excess inflammation, with up-regulation of MMPs and inflammatory cytokines such as IL-1, transforming growth factor-β and TNF-α, possibly contributing to the increased incidence of PTB (Horne and Critchley, 2007;Sinclairet al., 2011;Tamaresiset al., 2014;Doherty and Taylor, 2015). Indeed, endometrium of women with submucosal and intramural fibroids displays significantly higher macrophage infiltration and increased expression of the chemokine CCL2 and PGF2 compared with women without fibroids (Miuraet al., 2006). These molecular alterations lead to impaired decidualization and may contribute to adverse pregnancy outcomes.
Myometrium
Myometrium is a major reproductive tissue involved in pregnancy maintenance as well as in labor onset and progression: a very complex biomolecular communication system exists within myometrium which is actively coordinated through endocrine, paracrine and immunoregulatory factors (Challiset al., 2009;Hirotaet al., 2010). Progesterone is essential for the maintenance of pregnancy by regulating myometrial quiescence and the withdrawal of this hormone from the maternal circulation, or at the receptor level, may lead to the onset of labor (Patelet al., 2015). An increased myometrial cell PR-A to PR-B ratio, through an epigenetic mechanism, eliminates PR-B-mediated inhibition (Chaiet al., 2014;Patelet al., 2015) and up-regulates pro-inflammatory genes in myometrial cells. The same PR myometrial expression changes are evident in women with preterm labor and endometriosis, supporting that this aberrant hormonal milieu may predispose women to increased risk of PTB.
Several metabolic factors may influence the myometrium in reproductive disorders. For example, obesity, often associated with PCOS, is characterized by systemic vascular endothelial dysfunction, common to PE as well. In particular, vasoconstriction and vasodilatation are impaired in myometrial arteries from obese women, probably involving prothrombotic, proinflammatory and vasoactive (leptin, TNF, IL-6 and IL-8) and vasoprotective (adipokines) factors (Denisonet al., 2010). These changes may stimulate utero- and fetoplacental vascular endothelial dysfunction also via an imbalance in nitric oxide bioavailability and increased oxidative stress (Crujeiras and Casanueva, 2015; Fig.1).
Pro-inflammatory cytokines and PGs are crucial in laboring myometrium (Smith, 2007;Challiset al., 2009). Of note, the uterus with fibroids is characterized by a chronic inflammatory milieu and decreased oxytocinase activity (Ciavattiniet al., 2013), potentially predisposing women with uterine fibroids to PTB and pPROM, especially if multiple fibroids are present or if placentation occurs adjacent to or overlying a fibroid. In addition, dysfunctional uterine contractility, anatomical distortion of the uterine cavity and subsequent poor placentation may contribute to premature activation of parturition (Cakmak and Taylor, 2011).
Also in endometriosis inflammation may play a major role in activating premature uterine contractility. TNF-α and IL-1β increase the expression of COX-2 and the production of PGE2 by myometrial cells, while IL-6 up-regulates the expression of oxytocin receptors in the myometrial cellsin vitro (Benagianoet al., 2014). CRH and CRH-R play a role for priming and preparing myometrium for the onset of labor by regulating cell adaptation to increasing activity of inflammatory cytokines and switch to a procontractile phenotype with increased responsiveness to hormonal signals and mechanical forces (Markovicet al., 2013;Youet al., 2014).
Another inflammatory pathway involved in causing threatening preterm contractions leading to PTB in endometriosis may be mediated by protease-activated receptor 2 (PAR-2) that during pregnancy is associated with production/release of COX pathway products activating thromboxane (TX)/PGH2 receptors and TXA2/PGH2 receptors (Freerksenet al., 2005). The observation that uterine deletion of transformation-related protein (TRp53) increases the incidence of PTB, a condition corrected by oral administration of the selective COX2 inhibitor celecoxib, further supports the findings that PGs are essential for myometrial contraction (Hirotaet al., 2010; Fig.2).
Cervix
Uterine cervix is the other uterine compartment that undergoes extensive changes through gestation and parturition acting as a gatekeeper, and collagens, elastin, proteoglycans and hyaluronate are responsible for the full tensile strength (Gonzalezet al., 2011).
Hormonal dysfunction and inflammatory mechanisms associated with gynecological disorders may play an important role in degrading the cervical extracellular matrix and promoting cervical insufficiency. Indeed, the altered hormonal milieu of women with PCOS may influence the mechanical properties of the cervix by destabilizing cervical collagen, resulting in cervical insufficiency (Feigenbaumet al., 2012). In this context, increased androgens and dehydroepiandrosterone sulfate may have a role in promoting cervical modifications by enhancing collagenase activity and thus decreasing fibril collagen organization (Makievaet al., 2014). Although the mechanism of this action is not well established, there is some evidence that it is likely mediated via metabolism of 5α-reductase type 1 (which converts testosterone to dihydrotestosterone (DHT)), the predominant enzyme expressed by cervix at term (Mahendroo, 2012). Notably DHT, which cannot be metabolized to estrogens, promotes cervical ripening, implying an androgen-specific effect in remodeling throughout pregnancy (Makievaet al., 2014).
Cervical ripening is induced by PGE2, IL-1, platelet-activating factor, by mechanical stretch and migration of macrophages and neutrophils (Mahendroo, 2012) and may explain the high incidence of PTB by cervical incompetence in PCOS (Feigenbaumet al., 2012; Fig.1).
Placenta and membranes
Abnormal placentation is considered crucial for poor obstetric outcomes and the potential placental mechanisms underlying the association between adverse pregnancy outcomes and reproductive disorders is a new area of investigation.
The endometrial–myometrial junctional zone (JZ) plays a critical role in human placentation and women with endometriosis or adenomyosis have defective deep placentation because of defective remodeling of the spiral arteries (Brosenset al., 2010). In the absence of adequate decidual transformation, endovascular trophoblast cells arrest at the level of the endometrial–myometrial JZ and fail to progress into the myometrial spiral arteries, explaining the vascular resistance in pPROM and PTB (Brosenset al., 2013). Defective endovascular trophoblast invasion may also be secondary to absence of natural killer cells in the thickened myometrial JZ that usually regulate the depth of trophoblast invasion (Robsonet al., 2012;Wallaceet al., 2012;Moffett and Colucci, 2014).
The rate and the extent of endovascular trophoblast invasion (proportion between areas immunoreactive to cytokeratin 7 and to CD34) are significantly reduced also in pregnant women with PCOS (Palombaet al., 2012). Placenta in PCOS patients had a reduced weight, thickness, density and volume and a more irregular shape. The macroscopic findings of placentae could be interpreted as an epiphenomenon of the microscopic placental changes detected such as utero-placental vascular lesions, chronic villitis and intervillositis, abnormal villus maturity and absence of physiological change of the spiral vessels (Palombaet al., 2013). Transferrin, fibrinogen variants, kininogen-1, annexin 2 and peroxiredoxin 2 are hyperexpressed in women with PE and in women with PCOS and are suggested to be the link between these diseases (Khanet al., 2015).
Impaired utero-placental growth and vascular development in pregnancies following ART have been suggested from research involving animal models. ART and the transfer of embryos decreases vascular cell proliferation, the density of blood vessels and angiogenic factors, resulting in reduced placental vascular development, poor placental function and compromised fetal growth and development (Grazul-Bilskaet al., 2014). Gene expression is significantly altered in the placenta of mice models (>6% of the transcripts), with excessive gene repression of the complete transcriptome (Fauqueet al., 2010a;Zhanget al., 2010). ART triggers the induction of placental genes involved in metabolism, immune response, transmembrane signaling and cellular proliferation and an alteration of genes involved in apoptosis pathways (Fauqueet al., 2010b;Zhanget al., 2010;Nelissenet al., 2014). An epigenetic disruption of DNA methylation may also contribute to inhibiting human trophoblastic invasionin vitro by disturbing expression of epigenetically regulated genes such as E-Cadherin (Rahnamaet al., 2006;Chelbi and Vaiman, 2008; Fig.3).
The increased rate of PIH and PE suggests that there may be immunological maladaptation with oocyte donation. In fact, in pregnancies achieved after egg donation, the fetus may be viewed as a total allogeneic graft for the gravid woman and no longer a semi-allogeneic graft (Martínez-Vareaet al., 2014). These findings support the ‘immunologic theory’ suggesting that immunological intolerance between mother and fetus may affect placental function (Levronet al., 2014).
Sex steroids and receptors
The steroidogenic function of placenta in PCOS women is altered, with a higher 3β-hydroxysteroid dehydrogenase type 1 and lower P450 aromatase activity, contributing to the high androgen concentrations observed in maternal blood of PCOS patients (Maliqueoet al., 2013;Patelet al., 2015). Interestingly, in the rat model the excess of maternal, fetal and placental androgens is associated with decreased placental size, affecting the ability of placenta to deliver nutrients to the fetus (Sunet al., 2012). Despite the protective mechanisms of increased circulating maternal sex hormone-binding globulin and progesterone levels, maternal hyperandrogenemia may contribute to the development of PE or PTB by affecting endovascular trophoblast invasion and induce placental alterations (Makievaet al., 2014; Fig.1).
Hormonal changes associated with ovulation induction in ART persist during the peri-implantation and early placentation periods, by affecting trophoblast differentiation and changing the distribution of cell types in the placenta (Mainigiet al., 2014). Placentas from ART pregnancies were overrepresented in the highest quartile of weight, and the placental weight/birthweight ratio was commonly higher, even after adjusting for confounding factors (Haavaldsenet al., 2012). Even though normal phenotypes and no microscopic alterations were observed, ultra-structural modifications, such as degenerative alterations of terminal villi, mainly in syncytiotrophoblasts, including a thicker placental barrier, decreased apical microvilli and increased multiple vacuoles in human term ART-derived placentas than in control placentae were observed (Zhanget al., 2011). It was proposed that increased placental weight after IVF could be a compensatory process to ensure normal fetal growth. Similarly, in a mouse model, down-regulation of nutrient transport pathways, such as system A amino acid transporter and GLUT3 protein, have been demonstrated in blastocysts developedin vitro (Rinaudo and Schultz, 2004). Mouse placental weight correlated inversely with amino acid transport during late pregnancy, and the least efficient placenta (per unit of weight), had the greatest degree of enlargement (Bloiseet al., 2012). In most cases, successful compensation leads to the normal progress of pregnancy and the development of healthy offspring. If compensatory mechanisms are overwhelmed, improper maternal–fetal exchanges occur, potentially leading to abortion or adverse pregnancy outcomes such as growth restriction (Bloiseet al., 2014). Recent evidence in a mouse model confirmed that ART placentae exhibit down-regulation of a majority of placental nutrient transporters, including not only amino acid and glucose transporter but also the genes for the calcium, iron, thiamine and taurine transporters (Chenet al., 2015). Moreover, ART placentae have histomorphological alterations with defects in placental layer segregation and glycogen cell migration, with a significantly greater glycogen-positive area rate. Thus, the disrupted expression of a majority of imprinted genes important for placental development and function results in structural abnormalities of the placenta (Chenet al., 2015). Indeed, a murine model showed that placentas from ART had lower estriol levels and significantly higher activities of the steroid metabolizing enzymes UDP-glucuronosyltransferase and sulfotransferase. Thus, the ART placenta has a higher metabolism and clearance of steroids, affecting the passage of essential hormones for fetal growth (Collieret al., 2009; Fig.3).
Metabolic pathways
Metabolic mechanisms also mediate PCOS-related placental vascular dysfunction via increased sensitivity to vasoconstrictor substances (TX and endothelin (ET)) and blunting vasodilatory influences (PGs and NO). Gestational hyperinsulinemia induces vasoconstriction, resulting in shallower implantation and altered placental expression of the three isoforms of nitric oxide synthase (NOS) (neuronal-nNOS, inducible-iNOS and endothelial-eNOS) (Skarzinskiet al., 2009). Furthermore, placentae exposed to hyperinsulinemia have increased expression of endothelin converting enzyme 1 and the ET-A receptors, thus potentially contributing to the pathogenesis of FGR (Khamaisiet al., 2012). Metabolic inflammation represents a newer concept, combining chronic metabolic disturbances with low-grade inflammatory responses, which engage in the release of pro-inflammatory cytokines by several organs (Hotamisligil, 2006). An activator of transcription 3 (STAT3) modulates placental nutrient transport and its signaling is increased in placentae of women with PCOS, independent of pregnancy-related complications and is activated by inflammatory and metabolic factors related to obesity (Maliqueoet al., 2015; Fig.1).
Inflammatory mechanisms
As shown in endometrium, myometrium and cervix, altered inflammatory mechanisms are found in placentas of women with reproductive disorders. Syncytiotrophoblast apoptosis and shedding of products that extensively damage endothelial integrity can decrease utero-placental flow and activate a cascade of molecular effects leading to hypoxia, thrombosis, and endothelial cell dysfunction and adverse pregnancy outcomes. Excessive oxidative stress and complement activation can lead to placental damage, abnormal placental development, generalized endothelial activation and release of antiangiogenic factors (Menonet al., 2014). Thrombin significantly up-regulates proinflammatory chemokines, resulting in endothelial dysfunction or inappropriate endothelial cell activation, enhanced endothelial cell permeability and platelet aggregation which are common clinical manifestations in PE (Lockwoodet al., 2011).
Placentae of obese women have increased infiltration of proinflammatory macrophages, which express high levels of IL-1, TNF-α and IL-6, associated with increased expression of chemotactic cytokines and neutrophils in the maternal interstitial space and in muscularity of placental vessels (Challieret al., 2008). This pro-inflammatory maternal and fetal environment may play a role in mediating adverse pregnancy outcomes for both mother and fetus, such as GDM and PE (Robertset al., 2011).
Inflammation appears to be the most obvious link between endometriosis and adenomyosis and PTB, as suggested by chorioamniotic or systemic inflammations (Blanket al., 2008). Recently, endometriotic-like lesions (glandular components in the choriodecidual layer surrounded by enlarged decidualized cells) have been described along the entire membrane surface within the decidual side of the chorion–decidua of the fetal membranes from women affected with severe endometriosis (Marcellinet al., 2015). Significant alterations were observed for 2773 genes involved in glandular function, the endocrine and nervous systems, neoangiogenesis and autoimmune disease in membranes. CpG methylation analysis revealed 5999 differentially methylated regions. These data support the hypothesis that maternal endometriosis persists during pregnancy and affects the decidual side of the chorion–decidua, possibly involved in PTB (Marcellinet al., 2015; Fig.2).
In extravillous trophoblasts PAR-1 and PAR-2 are likely to play a role in the maintenance of the placental circulation (O'Brienet al., 2003). Interestingly, amnion mesenchymal cells from pregnancies with PTB show activation of PAR-1 (Mogamiet al., 2014), an enzyme also involved in endometriosis pathogenesis.
Placental inflammation and oxidative stress are significantly raised in the mouse model of ART. Specific testing revealed significantly lower placental lipid loading, increased placental cell death (apoptosis) and compromised intracellular nucleotides (lower RNA levels and integrity, higher DNA damage) (Rauniget al., 2011). Furthermore, ART manipulation resulted in increased inflammation through the IL-6 pathway and greater oxidative stress in placentas, both of which were particularly apparent in placentae from pregnancies achieved through ICSI. Excessive placental inflammation and oxidative stress may be a critical factor in the higher incidence of PTB, LBW and pediatric imprinting disorders reported following ART (Rauniget al., 2011; Fig.3).
Conclusions
From the present data, it is evident that patients with PCOS, endometriosis, adenomyosis, uterine fibroids and/or unexplained infertility show a series of endometrial, myometrial, cervical and placental alterations that underlie the poor obstetric outcomes observed in these patients. Pre-pregnancy hormonal dysfunction that includes hyperandrogenism, progesterone resistance and hyperinsulinism appears to impair uterine placentation mechanisms. Metabolic dysfunction prevails in PCOS, while inflammatory mechanisms are more relevant in endometriosis, adenomyosis and uterine fibroids, although endometrial inflammation also is present women with PCOS. When ART is utilized, an effect on placental adaptive function is shown. We therefore hypothesize that abnormal endometrial and myometrial hormonal/inflammatory mechanisms lead to a greater risk of PTB; whereas, hormonal/metabolic derangement lead to PE or GDM.
In clinical practice, many women of child-bearing age have more than one reproductive disorder, as these rarely occur in isolation (Holochet al., 2014). Therefore, concomitant reproductive disorders should be considered to increase the risk of poor pregnancy and neonatal outcomes once a pregnancy is achieved. Understanding the inflammatory, endocrine and metabolic mechanisms responsible for the increased incidence of obstetric complications associated with reproductive disorders may help in developing new therapies. Studies to date suggest that patients with reproductive disorders and/or undergoing ART belong to a ‘high risk’ category for pregnancy. It is thus important that evidence-based preconception and prenatal guidelines be developed to minimize pre-pregnancy abnormalities and risk factors, and for advising women, alerting the health care teams to the high risk status of the pregnancy for the mother and the neonate, and to optimize pregnancy outcomes overall.
Authors' roles
All authors contributed equally to manuscript drafting and critical discussion and approved the final version.
Funding
The authors declare no relationship with funding sources or sponsorships.
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- Agarwal A,Aponte-Mellado A,Premkumar BJ,Shaman A,Gupta S. The effects of oxidative stress on female reproduction: a review.Reprod Biol Endocrinol 2012;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alok A,Karande AA. The role of glycodelin as an immune-modulating agent at the feto-maternal interface.J Reprod Immunol 2009;83:124–127. [DOI] [PubMed] [Google Scholar]
- Al-Sabbagh M,Lam EW,Brosens JJ. Mechanisms of endometrial progesterone resistance.Mol Cell Endocrinol 2012;358:208–215. [DOI] [PubMed] [Google Scholar]
- Andreani M,Vergani P,Ghidini A,Locatelli A,Ornaghi S,Pezzullo JC. Are ultrasonographic myoma characteristics associated with blood loss at delivery? Ultrasound Obstet Gynecol 2009;34:322–325. [DOI] [PubMed] [Google Scholar]
- Aris A.A 12-year cohort study on adverse pregnancy outcomes in Eastern Townships of Canada: impact of endometriosis.Gynecol Endocrinol 2014;30:34–37. [DOI] [PubMed] [Google Scholar]
- Balasch J,Gratacós E. Delayed childbearing: effects on fertility and the outcome of pregnancy.Curr Opin Obstet Gynecol 2012;24:187–193. [DOI] [PubMed] [Google Scholar]
- Benagiano G,Brosens I,Habiba L. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis.Human Reprod Update 2014;20:386–402. [DOI] [PubMed] [Google Scholar]
- Benagiano G,Habiba M,Brosens I. The pathophysiology of uterine adenomyosis: an update.Fertil Steril 2012;98:572–579. [DOI] [PubMed] [Google Scholar]
- Benaglia L,Bermejo A,Somigliana E,Scarduelli C,Ragni G,Fedele L,Garcia-Velasco JA. Pregnancy outcome in women with endometriomas achieving pregnancy through IVF.Hum Reprod 2012;27:1663–1667. [DOI] [PubMed] [Google Scholar]
- Blank V,Hirsch E,Challis JR,Romero R,Lye SJ. Cytokine signaling, inflammation, innate immunity and preterm labour—a workshop report.Placenta 2008;29:S102–S104. [DOI] [PubMed] [Google Scholar]
- Bloise E,Lin W,Liu X,Simbulan R,Kolahi KS,Petraglia F,Maltepe E,Donjacour A,Rinaudo P. Impaired placental nutrient transport in mice generated by in vitro fertilization.Endocrinology 2012;153:3457–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloise E,Feuer SK,Rinaudo PF. Comparative intrauterine development and placental function of ART concepti: implications for human reproductive medicine and animal breeding.Hum Reprod Update 2014;20:822–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma CM,Eijkemans MJ,Hughes EG,Visser GH,Fauser BC,Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome.Hum Reprod Update 2006;12:673–683. [DOI] [PubMed] [Google Scholar]
- Brosens I,Derwig I,Brosens J,Fusi L,Benagiano G,Pijnenborg R. The enigmatic uterine junctional zone: the missing link between reproductive disorders and major obstetrical disorders? Hum Reprod 2010;25:569–574. [DOI] [PubMed] [Google Scholar]
- Brosens I,Brosens JJ,Fusi L,Al-Sabbagh M,Kuroda K,Benagiano G. Risks of adverse pregnancy outcome in endometriosis.Fertil Steril 2012;98:30–35. [DOI] [PubMed] [Google Scholar]
- Brosens I,Pijnenborg R,Benagiano G. Defective myometrial spiral artery remodelling as a cause of major obstetrical syndromes in endometriosis and adenomyosis.Placenta 2013;34:100–105. [DOI] [PubMed] [Google Scholar]
- Burney RO,Giudice LC. Pathogenesis and pathophysiology of endometriosis.Fertil Steril 2012;98:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney RO,Talbi S,Hamilton AE,Vo KC,Nyegaard M,Nezhat CR,Lessey BA,Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis.Endocrinology 2007;148:3814–3826. [DOI] [PubMed] [Google Scholar]
- Cakmak H,Taylor HS. Implantation failure: molecular mechanisms and clinical treatment.Hum Reprod Update 2011;17:242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai SY,Smith R,Fitter JT,Mitchell C,Pan X,Ilicic M,Maiti K,Zakar T,Madsen G. Increased progesterone receptor A expression in labouring human myometrium is associated with decreased promoter occupancy by the histone demethylase JARID1A.Mol Hum Reprod 2014;20:442–453. [DOI] [PubMed] [Google Scholar]
- Challier JC,Basu S,Bintein T,Minium J,Hotmire K,Catalano PM,Hauguel-de Mouzon S. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta.Placenta 2008;29:274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis JR,Lockwood CJ,Myatt L,Norman JE,Strauss JF III,Petraglia F. Inflammation and pregnancy.Reprod Sci 2009;16:206–215. [DOI] [PubMed] [Google Scholar]
- Chan YY,Jayaprakasan K,Tan A,Thornton JG,Coomarasamy A,Raine-Fenning NJ. Reproductive outcomes in women with congenital uterine anomalies: a systematic review.Ultrasound Obstet Gynecol 2011;38:371–382. [DOI] [PubMed] [Google Scholar]
- Chelbi ST,Vaiman D. Genetic and epigenetic factors contribute to the onset of preeclampsia.Mol Cell Endocrinol 2008;282:120–129. [DOI] [PubMed] [Google Scholar]
- Chen S,Sun FZ,Huang X,Wang X,Tang N,Zhu B,Li B. Assisted reproduction causes placental maldevelopment and dysfunction linked to reduced fetal weight in mice.Sci Rep 2015;5:10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciavattini A,Di Giuseppe J,Stortoni P,Montik N,Giannubilo SR,Litta P,Islam MS,Tranquilli AL,Reis FM,Ciarmela P. Uterine fibroids: pathogenesis and interactions with endometrium and endomyometrial junction.Obstet Gynecol Int 2013;2013:173184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AC,Miyagi SJ,Yamauchi Y,Ward MA. Assisted reproduction technologies impair placental steroid metabolism.J Steroid Biochem Mol Biol 2009;116:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti N,Tosti C,Pinzauti S,Tomaiuolo T,Cevenini G,Severi FM,Di Tommaso M,Petraglia F. Uterine fibroids affect pregnancy outcome in women over 30 years old: role of other risk factors.J Matern Fetal Neonatal Med 2013;26:584–587. [DOI] [PubMed] [Google Scholar]
- Conti N,Cevenini G,Vannuccini S,Orlandini C,Valensise H,Gervasi MT,Ghezzi F,Di Tommaso M,Severi FM,Petraglia F. Women with endometriosis at first pregnancy have an increased risk of adverse obstetric outcome.J Matern Fetal Neonatal Med 2014;9:1–4. [DOI] [PubMed] [Google Scholar]
- Cooper NP,Okolo S. Fibroids in pregnancy—common but poorly understood.Obstet Gynecol Surv 2005;60:132–138. [DOI] [PubMed] [Google Scholar]
- Coronado GD,Marshall LM,Schwartz SM. Complications in pregnancy, labor, and delivery with uterine leiomyomas: a population-based study.Obstet Gynecol 2000;95:764–769. [DOI] [PubMed] [Google Scholar]
- Crujeiras AB,Casanueva FF. Obesity and the reproductive system disorders: epigenetics as a potential bridge.Hum Reprod Update 2015;21:249–261. [DOI] [PubMed] [Google Scholar]
- De Frène V,Vansteelandt S,T'Sjoen G,Gerris J,Somers S,Vercruysse L,De Sutter P. A retrospective study of the pregnancy, delivery and neonatal outcome in overweight versus normal weight women with polycystic ovary syndrome.Hum Reprod 2014;29:2333–2338. [DOI] [PubMed] [Google Scholar]
- Denison FC,Roberts KA,Barr SM,Norman JE. Obesity, pregnancy, inflammation, and vascular function.Reproduction 2010;140:373–385. [DOI] [PubMed] [Google Scholar]
- Deveer M,Deveer R,Engin-Ustun Y,Sarikaya E,Akbaba E,Senturk B,Danisman N. Comparison of pregnancy outcomes in different localizations of uterine fibroids.Clin Exp Obstet Gynecol 2012;39:516–518. [PubMed] [Google Scholar]
- Doherty LF,Taylor HS. Leiomyoma-derived transforming growth factor-β impairs bone morphogenetic protein-2-mediated endometrial receptivity.Fertil Steril 2015;103:845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauque P,Mondon F,Letourneur F,Ripoche MA,Journot L,Barbaux S,Dandolo L,Patrat C,Wolf JP,Jouannet P et al. . In vitro fertilization and embryo culture strongly impact the placental transcriptome in the mouse model.PLoS One 2010a;5:e9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauque P,Ripoche MA,Tost J,Journot L,Gabory A,Busato F,Le Digarcher A,Mondon F,Gut I,Jouannet P et al. . Modulation of imprinted gene network in placenta results in normal development of in vitro manipulated mouse embryos.Hum Mol Genet 2010b;19:1779–1790. [DOI] [PubMed] [Google Scholar]
- Fauser BC,Tarlatzis BC,Rebar RW,Legro RS,Balen AH,Lobo R,Carmina E,Chang J,Yildiz BO,Laven JS et al. . Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group.Fertil Steril 2012;97:28–38. [DOI] [PubMed] [Google Scholar]
- Feigenbaum SL,Crites Y,Hararah MK,Yamamoto MP,Yang J,Lo JC. Prevalence of cervical insufficiency in polycystic ovarian syndrome.Hum Reprod 2012;27:2837–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando S,Breheny S,Jaques AM,Halliday JL,Baker G,Healy D. Preterm birth, ovarian endometrioma, and assisted reproduction technologies.Fertil Steril 2009;91:325–330. [DOI] [PubMed] [Google Scholar]
- Fischer CP,Kayisili U,Taylor HS. HOXA10 expression is decreased in endometrium of women with adenomyosis.Fertil Steril 2011;95:1133–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freerksen N,Betancourt A,Maul H,Wentz M,Orise P,Günter HH,Sohn C,Vedernikov Y,Saade G,Garfield R. PAR-2 activating peptide-induced stimulation of pregnant rat myometrium contractile activity partly involves the other membrane receptors.Eur J Obstet Gynecol Reprod Biol 2007;130:51–59. [DOI] [PubMed] [Google Scholar]
- Galliano D,Bellver J,Díaz-García C,Simón C,Pellicer A. ART and uterine pathology: how relevant is the maternal side for implantation? Hum Reprod Update 2015;21:13–38. [DOI] [PubMed] [Google Scholar]
- Geisler ME,O'Mahony A,Meaney S,Waterstone JJ,O'Donoghue K. Obstetric and perinatal outcomes of twin pregnancies conceived following IVF/ICSI treatment compared with spontaneously conceived twin pregnancies.Eur J Obstet Gynecol Reprod Biol 2014;181:78–83. [DOI] [PubMed] [Google Scholar]
- Gonzalez JM,Dong Z,Romero R,Girardi G. Cervical remodeling/ripening at term and preterm delivery: the same mechanism initiated by different mediators and different effector cells.PLoS One 2011;6:e26877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazul-Bilska AT,Johnson ML,Borowicz PP,Bilski JJ,Cymbaluk T,Norberg S,Redmer DA,Reynolds LP. Placental development during early pregnancy in sheep: effects of embryo origin on vascularization.Reproduction 2014;147:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haavaldsen C,Tanbo T,Eskild A. Placental weight in singleton pregnancies with and without assisted reproductive technology: a population study of 536,567 pregnancies.Hum Reprod 2012;27:576–582. [DOI] [PubMed] [Google Scholar]
- Healy DL,Breheny S,Halliday J,Jaques A,Rushford D,Garrett C,Talbot JM,Baker HW. Prevalence and risk factors for obstetric haemorrhage in 6730 singleton births after assisted reproductive technology in Victoria Australia.Hum Reprod 2010;25:265–274. [DOI] [PubMed] [Google Scholar]
- Helmerhorst FM,Perquin DA,Donker D,Keirse MJ. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies.Br Med J 2004;328:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y,Daikoku T,Tranguch S,Xie H,Bradshaw HB,Dey SK. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice.J Clin Invest 2010;120:803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoch KJ,Savaris RF,Forstein A,Miller PB,Higdon HL,Likes CE,Lessey BA. Coexistence of PCOS and endometriosis in women with infertility.J Endometriosis 2014;6:79–83. [Google Scholar]
- Horne AW,Critchley HO. The effect of uterine fibroids on embryo implantation.Semin Reprod Med 2007;25:483–489. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS.Inflammation and metabolic disorders.Nature 2006;444:860–867. [DOI] [PubMed] [Google Scholar]
- Irwin JC,Suen LF,Faessen GH,Popovici RM,Giudice LC. Insulin-like growth factor (IGF)-II inhibition of endometrial stromal cell tissue inhibitor of metalloproteinase-3 and IGF-binding protein-1 suggests paracrine interactions at the decidua: trophoblast interface during human implantation.Clin Endocrinol Metab 2001;86:2060–2064. [DOI] [PubMed] [Google Scholar]
- Jackson RA,Gibson KA,Wu YW,Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis.Obstet Gynecol 2004;103:551–563. [DOI] [PubMed] [Google Scholar]
- Jaques AM,Amor DJ,Baker HW,Healy DL,Ukoumunne OC,Breheny S,Garrett C,Halliday JL. Adverse obstetric and perinatal outcomes in subfertile women conceiving without assisted reproductive technologies.Fertil Steril 2010;94:2674–2679. [DOI] [PubMed] [Google Scholar]
- Juang CM,Chou P,Yen MS,Twu NF,Horng HC,Hsu WL. Adenomyosis and risk of preterm delivery.BJOG 2007;114:165–169. [DOI] [PubMed] [Google Scholar]
- Kawwass JF,Monsour M,Crawford S,Kissin DM,Session DR,Kulkarni AD,Jamieson DJ;National ART Surveillance System (NASS) Group.Trends and outcomes for donor oocyte cycles in the United States, 2000–2010.J Am Med Assoc 2013;310:2426–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan DA,Krey LC,Chang HC,Noyes N. Increased risk of pregnancy-induced hypertension in young recipients of donated oocytes.Fertil Steril 2007;87:776–781. [DOI] [PubMed] [Google Scholar]
- Khalaf Y,Ross C,El-Toukhy T,Hart R,Seed P,Braude P. The effect of small intramural uterine fibroids on the cumulative outcome of assisted conception.Hum Reprod 2006;21:2640–2644. [DOI] [PubMed] [Google Scholar]
- Khamaisi M,Skarzinski G,Mekler J,Zreik F,Damouni R,Ariel I,Bursztyn M. Hyperinsulinemia increases placenta endothelin-converting enzyme-1 expression in trophoblasts.Am J Hypertens 2012;25:109–114. [DOI] [PubMed] [Google Scholar]
- Khan GH,Galazis N,Docheva N,Layfield R,Atiomo W. Overlap of proteomics biomarkers between women with pre-eclampsia and PCOS: a systematic review and biomarker database integration.Hum Reprod 2015;30:133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjerulff LE,Sanchez-Ramos L,Duffy D. Pregnancy outcomes in women with polycystic ovary syndrome: a meta-analysis.Am J Obstet Gynecol 2011;204:558. [DOI] [PubMed] [Google Scholar]
- Klatsky PC,Tran ND,Caughey AB,Fujimoto VY. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery.Am J Obstet Gynecol 2008;198:357–366. [DOI] [PubMed] [Google Scholar]
- Klemetti R,Sevón T,Gissler M,Hemminki E. Health of children born after ovulation induction.Fertil Steril 2010;93:1157–1168. [DOI] [PubMed] [Google Scholar]
- Koike T,Minakami H,Kosuge S,Usui R,Matsubara S,Izumi A,Sato I. Uterine leiomyoma in pregnancy: its influence on obstetric performance.J Obstet Gynaecol Res 1999;25:309–313. [DOI] [PubMed] [Google Scholar]
- Kondapalli LA,Perales-Puchalt A. Low birth weight: is it related to assisted reproductive technology or underlying infertility? Fertil Steril 2013;99:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortelahti M,Anttila MA,Hippeläinen MI,Heinonen ST. Obstetric outcome in women with endometriosis—a matched case–control study.Gynecol Obstet Invest 2003;56:207–212. [DOI] [PubMed] [Google Scholar]
- Kovalevsky G,Rinaudo P,Coutifaris C. Do assisted reproductive technologies cause adverse fetal outcomes? Fertil Steril 2003;79:1270–1272. [DOI] [PubMed] [Google Scholar]
- Krieg SA,Henne MB,Westphal LM. Obstetric outcomes in donor oocyte pregnancies compared with advanced maternal age in in vitro fertilization pregnancies.Fertil Steril 2008;90:65–70. [DOI] [PubMed] [Google Scholar]
- Lai J,Caughey AB,Qidwai GI,Jacoby AF. Neonatal outcomes in women with sonographically identified uterine leiomyomata .J Matern Fetal Neonatal Med 2012;25:710–713. [DOI] [PubMed] [Google Scholar]
- Lam SJ,Best S,Kumar S. The impact of fibroid characteristics on pregnancy outcome.Am J Obstet Gynecol 2014;211:395. [DOI] [PubMed] [Google Scholar]
- Le Ray C,Scherier S,Anselem O,Marszalek A,Tsatsaris V,Cabrol D,Goffinet F. Association between oocyte donation and maternal and perinatal outcomes in women aged 43 years or older.Hum Reprod 2012;27:896–901. [DOI] [PubMed] [Google Scholar]
- Lee B,Du H,Taylor HS. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium.Biol Reprod 2009;80:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey BA,Young SL. Homeostasis imbalance in the endometrium of women with implantation defects: the role of estrogen and progesterone.Semin Reprod Med 2014;32:365–375. [DOI] [PubMed] [Google Scholar]
- Lessey BA,Lebovic DI,Taylor RN. Eutopic endometrium in women with endometriosis: ground zero for the study of implantation defects.Semin Reprod Med 2013;31:109–124. [DOI] [PubMed] [Google Scholar]
- Levron Y,Dviri M,Segol I,Yerushalmi GM,Hourvitz A,Orvieto R,Mazaki-Tovi S,Yinon Y. The immunologic theory of preeclampsia revisited: a lesson from donor oocyte gestations.Am J Obstet Gynecol 2014;211:383.e1– 383.e5. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ,Huang SJ,Krikun G,Caze R,Rahman M,Buchwalder LF,Schatz F. Decidual hemostasis, inflammation, and angiogenesis in pre-eclampsia.Semin Thromb Hemost 2011;37:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk J,Greenfeld DA,Seli E. Third party reproduction and the aging couple.Maturitas 2010;66:389–396. [DOI] [PubMed] [Google Scholar]
- Luyckx M,Squifflet JL,Jadoul P,Votino R,Dolmans MM,Donnez J. First series of 18 pregnancies after ulipristal acetate treatment for uterine fibroids.Fertil Steril 2014;102:1404–1409. [DOI] [PubMed] [Google Scholar]
- Mahendroo M.Cervical remodeling in term and preterm birth: insights from an animal model.Reproduction 2012;143:429–438. [DOI] [PubMed] [Google Scholar]
- Mainigi MA,Olalere D,Burd I,Sapienza C,Bartolomei M,Coutifaris C. Peri-implantation hormonal milieu: elucidating mechanisms of abnormal placentation and fetal growth.Biol Reprod 2014;90:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makieva S,Saunders PT,Norman JE. Androgens in pregnancy: roles in parturition.Hum Reprod Update 2014;20:542–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchau SS,Loft A,Larsen EC,Aaris Henningsen AK,Rasmussen S,Andersen AN,Pinborg A. Perinatal outcomes in 375 children born after oocyte donation: a Danish national cohort study.Fertil Steril 2013;99:1637–1643. [DOI] [PubMed] [Google Scholar]
- Malchau SS,Loft A,Henningsen AK,Nyboe Andersen A,Pinborg A. Perinatal outcomes in 6,338 singletons born after intrauterine insemination in Denmark, 2007 to 2012: the influence of ovarian stimulation.Fertil Steril 2014;102:1110–1116. [DOI] [PubMed] [Google Scholar]
- Maliqueo M,Lara HE,Sánchez F,Echiburú B,Crisosto N,Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome.Eur J Obstet Gynecol Reprod Biol 2013;166:151–155. [DOI] [PubMed] [Google Scholar]
- Maliqueo M,Sundström Poromaa I,Vanky E,Fornes R,Benrick A,Åkerud H,Stridsklev S,Labrie F,Jansson T,Stener-Victorin E. Placental STAT3 signaling is activated in women with polycystic ovary syndrome.Hum Reprod 2015;30:692–700. [DOI] [PubMed] [Google Scholar]
- Marcellin L,Santulli P,Gogusev J,Lesafre C,Jacques S,Chapron C,Goffinet F,Vaiman D,Mehats C. Endometriosis also affects the decidua in contact with the fetal membranes during pregnancy.Hum Reprod 2015;30:392–405. [DOI] [PubMed] [Google Scholar]
- March CM.Management of Asherman's syndrome.Reprod Biomed Online 2011;23:63–76. [DOI] [PubMed] [Google Scholar]
- Markovic D,Bari MF,Lu B,Vatish M,Grammatopoulos DK. Corticotropin-releasing hormone interacts with interleukin-1β to regulate prostaglandin H synthase-2 expression in human myometrium during pregnancy and labor.J Clin Endocrinol Metab 2013;98:2864–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Varea A,Pellicer B,Perales-Marín A,Pellicer A. Relationship between maternal immunological response during pregnancy and onset of preeclampsia.J Immunol Res 2014;2014:210241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekaru K,Masamoto H,Sugiyama H,Asato K,Heshiki C,Kinjyo T,Aoki Y. Endometriosis and pregnancy outcome: are pregnancies complicated by endometriosis a high-risk group? Eur J Obstet Gynecol 2014;172:36–39. [DOI] [PubMed] [Google Scholar]
- Menon R,Boldogh I,Hawkins HK,Woodson M,Polettini J,Syed TA,Fortunato SJ,Saade GR,Papaconstantinou J,Taylor RN. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro.Am J Pathol 2014;184:1740–1751. [DOI] [PubMed] [Google Scholar]
- Messerlian C,Maclagan L,Basso O. Infertility and the risk of adverse pregnancy outcomes: a systematic review and meta-analysis.Hum Reprod 2013;28:125–137. [DOI] [PubMed] [Google Scholar]
- Miura S,Khan KN,Kitajima M,Hiraki K,Moriyama S,Masuzaki H,Samejima T,Fujishita A,Ishimaru T. Differential infiltration of macrophages and prostaglandin production by different uterine leiomyomas.Hum Reprod 2006;21:2545–2554. [DOI] [PubMed] [Google Scholar]
- Moffett A,Colucci F. Uterine NK cells: active regulators at the maternal–fetal interface.J Clin Invest 2014;124:1872–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogami H,Keller PW,Shi H,Word RA. Effect of thrombin on human amnion mesenchymal cells, mouse fetal membranes, and preterm birth.J Biol Chem 2014;289:13295–13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moini A,Shiva M,Arabipoor A,Hosseini R,Chehrazi M,Sadeghi M. Obstetric and neonatal outcomes of twin pregnancies conceived by assisted reproductive technology compared with twin pregnancies conceived spontaneously: a prospective follow-up study.Eur J Obstet Gynecol Reprod Biol 2012;165:29–32. [DOI] [PubMed] [Google Scholar]
- Naqvi H,Ilagan Y,Krikun G,Taylor HS. Altered genome-wide methylation in endometriosis.Reprod Sci 2014;21:1237–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naver KV,Grinsted J,Larsen SO,Hedley PL,Jørgensen FS,Christiansen M,Nilas L. Increased risk of preterm delivery and pre-eclampsia in women with polycystic ovary syndrome and hyperandrogenaemia.BJOG 2014;121:575–581. [DOI] [PubMed] [Google Scholar]
- Nelissen EC,Dumoulin JC,Busato F,Ponger L,Eijssen LM,Evers JL,Tost J,van Montfoort AP. Altered gene expression in human placentas after IVF/ICSI.Hum Reprod 2014;29:2821–2831. [DOI] [PubMed] [Google Scholar]
- Norman RJ,Noakes M,Wu R,Davies MJ,Moran L,Wang JX. Improving reproductive performance in overweight/obese women with effective weight management.Hum Reprod Update 2004;10:267–280. [DOI] [PubMed] [Google Scholar]
- Novembri R,Borges LE,Carrarelli P,Rocha AL,De Pascalis F,Florio P,Petraglia F. Impaired CRH and urocortin expression and function in eutopic endometrium of women with endometriosis.J Clin Endocrinol Metab 2011;96:1145–1150. [DOI] [PubMed] [Google Scholar]
- O'Brien PJ,Koi H,Parry S,Brass LF,Strauss JF III,Wang LP,Tomaszewski JE,Christenson LK. Thrombin receptors and protease-activated receptor 2 in human placentation: receptor activation mediates extravillous trophoblast invasion in vitro.Am J Pathol 2003;163:1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang DW,Economy KE,Norwitz ER. Obstetric complications of fibroids.Obstet Gynecol Clin North Am 2006;33:153–169. [DOI] [PubMed] [Google Scholar]
- Palomba S,Falbo A,Russo T,Tolino A,Orio F,Zullo F. Pregnancy in women with polycystic ovary syndrome: the effect of different phenotypes and features on obstetric and neonatal outcomes.Fertil Steril 2010;94:1805–1811. [DOI] [PubMed] [Google Scholar]
- Palomba S,Russo T,Falbo A,Di Cello A,Amendola G,Mazza R,Tolino A,Zullo F,Tucci L,La Sala GB. Decidual endovascular trophoblast invasion in women with polycystic ovary syndrome: an experimental case–control study.J Clin Endocrinol Metab 2012;97:2441–2449. [DOI] [PubMed] [Google Scholar]
- Palomba S,Russo T,Falbo A,Di Cello A,Tolino A,Tucci L,La Sala GB,Zullo F. Macroscopic and microscopic findings of the placenta in women with polycystic ovary syndrome.Hum Reprod 2013;28:2838–2847. [DOI] [PubMed] [Google Scholar]
- Palomba S,Falbo A,Chiossi G,Orio F,Tolino A,Colao A,La Sala GB,Zullo F. Low-grade chronic inflammation in pregnant women with polycystic ovary syndrome: a prospective controlled clinical study.J Clin Endocrinol Metab 2014;99:2942–2951. [DOI] [PubMed] [Google Scholar]
- Pandey S,Shetty A,Hamilton M,Bhattacharya S,Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis.Hum Reprod Update 2012;18:485–503. [DOI] [PubMed] [Google Scholar]
- Pandian Z,Bhattacharya S,Nikolaou D,Vale L,Templeton A. The effectiveness of IVF in unexplained infertility: a systematic Cochrane review 2002.Hum Reprod 2003;18:2001–2007. [DOI] [PubMed] [Google Scholar]
- Patel B,Elguero S,Thakore S,Dahoud W,Bedaiwy M,Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology.Hum Reprod Update 2015;21:155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia F,Arcuri F,de Ziegler D,Chapron C. Inflammation: a link between endometriosis and preterm birth.Fertil Steril 2012;98:36–40. [DOI] [PubMed] [Google Scholar]
- Piltonen TT,Chen J,Erikson DW,Spitzer TL,Barragan F,Rabban JT,Huddleston H,Irwin JC,Giudice LC. Mesenchymal stem/progenitors and other endometrial cell types from women with polycystic ovary syndrome display inflammatory and oncogenic potential.J Clin Endocrinol Metab 2013;98:3765–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piltonen TT,Chen JC,Khatun M,Kangasniemi M,Liakka A,Spitzer TB,Tran N,Huddleston H,Irwin JC,Giudice LC. Endometrial stromal fibroblasts from women with polycystic ovary syndrome have impaired progesterone-mediated decidualization, aberrant cytokine profiles and promote enhanced immune cell migration in vitro.Hum Reprod 2015;30:1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinborg A,Wennerholm UB,Romundstad LB,Loft A,Aittomaki K,Söderström-Anttila V,Nygren KG,Hazekamp J,Bergh C. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis.Hum Reprod Update 2013;19:87–104. [DOI] [PubMed] [Google Scholar]
- Qidwai GI,Caughey AB,Jacoby AF. Obstetric outcomes in women with sonographically identified uterine leiomyomata.Obstet Gynecol 2006;107:376–382. [DOI] [PubMed] [Google Scholar]
- Qin JZ,Pang LH,Li MJ,Fan XJ,Huang RD,Chen HY. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta-analysis.Reprod Biol Endocrinol 2013;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raatikainen K,Harju M,Hippeläinen M,Heinonen S. Prolonged time to pregnancy is associated with a greater risk of adverse outcomes.Fertil Steril 2010;94:1148–1151. [DOI] [PubMed] [Google Scholar]
- Raatikainen K,Kuivasaari-Pirinen P,Hippeläinen M,Heinonen S. Comparison of the pregnancy outcomes of subfertile women after infertility treatment and in naturally conceived pregnancies.Hum Reprod 2012;27:1162–1169. [DOI] [PubMed] [Google Scholar]
- Rahnama F,Shafiei F,Gluckman PD,Mitchell MD,Lobie PE. Epigenetic regulation of human trophoblastic cell migration and invasion.Endocrinology 2006;147:5275–5283. [DOI] [PubMed] [Google Scholar]
- Raunig JM,Yamauchi Y,Ward MA,Collier AC. Placental inflammation and oxidative stress in the mouse model of assisted reproduction.Placenta 2011;32:852–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis FM,Petraglia F,Taylor RN. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis.Hum Reprod Update 2013;19:406–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaudo P,Schultz RM. Effects of embryo culture on global pattern of gene expression in preimplantation mouse embryos.Reproduction 2004;128:301–311. [DOI] [PubMed] [Google Scholar]
- Roberts KA,Riley SC,Reynolds RM,Barr S,Evans M,Statham A,Hor K,Jabbour HN,Norman JE,Denison FC. Placental structure and inflammation in pregnancies associated with obesity.Placenta 2011;32:247–254. [DOI] [PubMed] [Google Scholar]
- Robson A,Harris LK,Innes BA,Lash GE,Aljunaidy MM,Aplin JD,Baker PN,Robson SC,Bulmer JN. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy.FASEB J 2012;26:4876–4885. [DOI] [PubMed] [Google Scholar]
- Romero R,Dey SK,Fisher SJ. Preterm labor: one syndrome, many causes.Science 2014;345:760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romundstad LB,Romundstad PR,Sunde A,von Düring V,Skjaerven R,Vatten LJ. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother.Hum Reprod 2006;21:2353–2358. [DOI] [PubMed] [Google Scholar]
- Romundstad LB,Romundstad PR,Sunde A,von Düring V,Skjaerven R,Gunnell D,Vatten LJ. Effects of technology or maternal factors on perinatal outcome after assisted fertilisation: a population-based cohort study.Lancet 2008;372:737–743. [DOI] [PubMed] [Google Scholar]
- Roos N,Kieler H,Sahlin L,Ekman-Ordeberg G,Falconer H,Stephansson O. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study.Br Med J 2011;343:d6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS).Hum Reprod 2004;191:41–47. [DOI] [PubMed] [Google Scholar]
- Schieve LA,Meikle SF,Ferre C,Peterson HB,Jeng G,Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology.N Engl J Med 2002;346:731–737. [DOI] [PubMed] [Google Scholar]
- Sekhon LH,Gerber RS,Rebarber A,Saltzman DH,Klauser CK,Gupta S,Fox NS. Effect of oocyte donation on pregnancy outcomes in in vitro fertilization twin gestations.Fertil Steril 2014;101:1326–1330. [DOI] [PubMed] [Google Scholar]
- Shavell VI,Thakur M,Sawant A,Kruger ML,Jones TB,Singh M,Puscheck EE,Diamond MP. Adverse obstetric outcomes associated with sonographically identified large uterine fibroids.Fertil Steril 2012;97:107–110. [DOI] [PubMed] [Google Scholar]
- Sinclair DC,Mastroyannis A,Taylor HS. Leiomyoma simultaneously impair endometrial BMP-2-mediated decidualization and anticoagulant expression through secretion of TGF-β3.J Clin Endocrinol Metab 2011;96:412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarzinski G,Khamaisi M,Bursztyn M,Mekler J,Lan D,Evdokimov P,Ariel I. Intrauterine growth restriction and shallower implantation site in rats with maternal hyperinsulinemia are associated with altered NOS expression.Placenta 2009;30:898–906. [DOI] [PubMed] [Google Scholar]
- Smith R.Parturition.N Engl J Med 2007;356:271–283. [DOI] [PubMed] [Google Scholar]
- Somigliana E,Vercellini P,Daguati R,Pasin R,De Giorgi O,Crosignani PG. Fibroids and female reproduction: a critical analysis of the evidence.Hum Reprod Update 2007;13:465–476. [DOI] [PubMed] [Google Scholar]
- Stephansson O,Kieler H,Granath F,Falconer H. Endometriosis, assisted reproduction technology, and risk of adverse pregnancy outcome.Hum Reprod 2009;24:2341–2347. [DOI] [PubMed] [Google Scholar]
- Stoop D,Baumgarten M,Haentjens P,Polyzos NP,De Vos M,Verheyen G,Camus M,Devroey P. Obstetric outcome in donor oocyte pregnancies: a matched-pair analysis.Reprod Biol Endocrinol 2012;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M,Maliqueo M,Benrick A,Johansson J,Shao R,Hou L,Jansson T,Wu X,Stener-Victorin E. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring.Am J Physiol Endocrinol Metab 2012;303:E1373–E1385. [DOI] [PubMed] [Google Scholar]
- Sunderam S,Kissin DM,Flowers L,Anderson JE,Folger SG,Jamieson DJ,Barfield WD;Centers for Disease Control and Prevention (CDC).Assisted reproductive technology surveillance—United States, 2009.MMWR Surveill Summ 2012;61:1–23. [PubMed] [Google Scholar]
- Takemura Y,Osuga Y,Fujimoto A,Oi N,Tsutsumi R,Koizumi M,Yano T,Taketani Y. Increased risk of placenta previa is associated with endometriosis and tubal factor infertility in assisted reproductive technology pregnancy.Gynecol Endocrinol 2013;29:113–115. [DOI] [PubMed] [Google Scholar]
- Talaulikar VS,Arulkumaran S. Reproductive outcomes after assisted conception.Obstet Gynecol Surv 2012;67:566–583. [DOI] [PubMed] [Google Scholar]
- Tamaresis JS,Irwin JC,Goldfien GA,Rabban JT,Burney RO,Nezhat C,DePaolo LV,Giudice LC. Molecular classification of endometriosis and disease stage using high-dimensional genomic data.Endocrinology 2014;155:4986–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson F,Shanbhag S,Templeton A,Bhattacharya S. Obstetric outcome in women with subfertility.BJOG 2005;112:632–637. [DOI] [PubMed] [Google Scholar]
- Toulis KA,Goulis DG,Kolibianakis EM,Venetis CA,Tarlatzis BC,Papadimas I. Risk of gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and a meta-analysis.Fertil Steril 2009;92:667–677. [DOI] [PubMed] [Google Scholar]
- Tranguch S,Wang H,Daikoku T,Xie H,Smith DF,Dey SK. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific.J Clin Invest 2007;117:1824–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuuli MG,Shanks A,Bernhard L,Odibo AO,Macones GA,Cahill A. Uterine synechiae and pregnancy complications.Obstet Gynecol 2012;119:810–814. [DOI] [PubMed] [Google Scholar]
- Vercellini P,Parazzini F,Pietropaolo G,Cipriani S,Frattaruolo MP,Fedele L. Pregnancy outcome in women with peritoneal, ovarian and rectovaginal endometriosis: a retrospective cohort study.BJOG 2012;119:1538–1543. [DOI] [PubMed] [Google Scholar]
- Vilella F,Ramirez L,Berlanga O,Martínez S,Alamá P,Meseguer M,Pellicer A,Simón C. PGE2 and PGF2α concentrations in human endometrial fluid as biomarkers for embryonic implantation.J Clin Endocrinol Metab 2013;98:4123–4132. [DOI] [PubMed] [Google Scholar]
- Wallace AE,Fraser R,Cartwright JE. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership.Hum Reprod Update 2012;18:458–e71.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M,Feigenbaum SL,Crites Y,Escobar GJ,Yang J,Ferrara A,Lo JC. Risk of preterm delivery in non-diabetic women with polycystic ovarian syndrome.J Perinatol 2012;32:770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H,Zhou Y,Edelshain B,Schatz F,Lockwood CJ,Taylor HS. FKBP4 is regulated by HOXA10 during decidualization and in endometriosis.Reproduction 2012;143:531–538. [DOI] [PubMed] [Google Scholar]
- You X,Liu J,Xu C,Liu W,Zhu X,Li Y,Sun Q,Gu H,Ni X. Corticotropin-releasing hormone (CRH) promotes inflammation in human pregnant myometrium: the evidence of CRH initiating parturition.J Clin Endocrinol Metab 2014;99:E199–E208. [DOI] [PubMed] [Google Scholar]
- Zhang Y,Cui Y,Zhou Z,Sha J,Li Y,Liu J. Altered global gene expressions of human placentae subjected to assisted reproductive technology treatments.Placenta 2010;31:251–258. [DOI] [PubMed] [Google Scholar]
- Zhang Y,Zhao W,Jiang Y,Zhang R,Wang J,Li C,Zhao H,Gao L,Cui Y,Zhou Z et al. . Ultrastructural study on human placentae from women subjected to assisted reproductive technology treatments.Biol Reprod 2011;85:635–642. [DOI] [PubMed] [Google Scholar]