Abstract
Infant formula companies have been fortifying formulas with long-chain PUFA for 10 y. Long-chain PUFA are precursors of prostanoids, which stimulate recovery of intestinal barrier function. Supplementation of milk with PUFA increases the content of arachidonic acid (ARA) in enterocyte membranes; however, the effect of this enrichment on intestinal repair is not known. The objective of these experiments was to investigate the effect of supplemental ARA on intestinal barrier repair in ischemia-injured porcine ileum. One-day-old pigs (n = 24) were fed a milk-based formula for 10 d. Diets contained no PUFA (0% ARA), 0.5% ARA, 5% ARA, or 5% EPA of total fatty acids. Following dietary enrichment, ilea were subjected to in vivo ischemic injury by clamping the local mesenteric blood supply for 45 min. Following the ischemic period, control (nonischemic) and ischemic loops were mounted on Ussing chambers. Transepithelial electrical resistance (TER) was measured over a 240-min recovery period. Ischemia-injured ileum from piglets fed 5% ARA (61.0 ± 14%) exhibited enhanced recovery compared with 0% ARA (16 ± 14) and 0.5% ARA (22.1 ± 14)-fed pigs. Additionally, ischemia-injured ileum from 5% EPA (51.3 ± 14)-fed pigs had enhanced recovery compared with 0% ARA-fed pigs (P < 0.05). The enhanced TER recovery response observed with ischemia-injured 5% ARA supplementation was supported by a significant reduction in mucosal-to-serosal flux of3H-mannitol and14C-inulin compared with all other ischemia-injured dietary groups (P < 0.05). A histological evaluation of ischemic ilea from piglets fed the 5% ARA showed reduced histological lesions after ischemia compared with the other dietary groups (P < 0.05). These data demonstrate that feeding elevated levels of long-chain PUFA enhances acute recovery of ischemia-injured porcine ileum.
Introduction
Necrotizing enterocolitis (NEC)5 is a multifaceted, inflammatory, gastrointestinal disease and the most common gastrointestinal emergency facing human infants. The morbidity and mortality rates associated with NEC are high, with a 5% incidence rate for infants born at <36 wk of gestation and an overall mortality rate between 10 and 50% (1,2). Although neonatologists understand the symptoms, the characterization of disease progression and mechanisms still remains vague. The most common risk factors of the disease are premature birth and enteral feeding; however, bacterial colonization, hypoxia, and/or intestinal ischemia also have been associated with the development of NEC.
Dietary lipids are known to play a role as immunomodulators (3). Prostanoids are synthesized from arachidonic acid (ARA) and EPA, which themselves are endogenously synthesized from dietary essential linoleic acid and linolenic acid, respectively. In several studies, dietary PUFA supplementation following damage to the intestine improved recovery (4,5), possibly via PGE2 (6,7). Moreover, research has shown that PG, which are involved in inflammation and derived from bioactive lipids, play a major role in the recovery of intestinal barrier function in ischemia-injured porcine ileum (8–10). The rate-limiting step in the formation of prostanoids is conversion of ARA or EPA to PGH2 or PGH3, respectively, by cyclooxygenase (COX). The importance of ARA and ARA-derived eicosanoids in the intestinal epithelium was recently comprehensively reviewed by Ferrer and Moreno (11), including molecular mechanisms involved in the regulation of ARA release from the membrane by phospholipase A2, activation of the COX enzymes, and production of PGE2 that seems to be differentially regulated between intestinal epithelial cell proliferation and cellular differentiation pathways (11).
Because these pathways can be considerably affected via dietary manipulation, nutritional intervention could have an important impact on gastrointestinal health, possibly minimizing the use of pharmacological treatments. The nutritional requirements of essential fatty acids (linoleic and linolenic acids) for growth and development are well established. Most recently, progress has been made in the area of long-chain PUFA nutrition and supplementation. Specifically, in 2002, upon FDA approval, infant formula companies began fortifying formulas with ARA and DHA. This decision was predicated on literature describing important accumulation of these fatty acids in retina and brain of developing neonates and recommended supplementation levels were based in part on corresponding concentrations that are common in breast milk (12–16). Therefore, the goal of this study was to investigate whether supraphysiological levels of dietary ARA in suckling pigs had a protective and/or reparative effect on ischemia-injured ileum.
Materials and Methods
Piglets and dietary treatments.
All procedures were approved by the Institutional Animal Care and Use Committee of North Carolina State University. Animals and dietary treatments were the same as described previously (17,18). Briefly, colostrum-fed piglets were acquired at 12–24 h of age and individually caged. Piglets were randomly allocated to 0, 0.5, or 5% ARA or 5% EPA diets (percentage of total fatty acids) differing in fatty acid composition. Pigs were subjected to the surgical treatments described below between 10 and 12 d of age (n = 6).
Experimental surgery.
Surgeries were performed similar to those previously described (9). Briefly, piglets were anesthetized and the ileum was exposed via a midline incision and the ischemic loops were created 10 cm above the ileal cecal junction by ligating the local mesenteric blood supply. Adjacent 10-cm ileal loops not subjected to ischemia were used as nonischemic control tissues. After 45 min of ischemia, pigs were killed and the ileal loops were promptly removed and placed in oxygenated Ringer solution. Two sets of ileal mucosal samples were taken from the control and ischemic loops immediately following the 45 min of ischemia. These samples were either preserved in 10% buffered formalin for histology or were frozen in liquid nitrogen and stored at −80°C for subsequent mRNA quantification.
Ussing chamber studies.
The mucosa was stripped from the seromuscular layer and mounted in 1.14-cm2 aperture Ussing chambers. The Ussing chamber measurements were made as previously reported by Blikslager et al. (9). The spontaneous potential difference (PD) was measured using Ringer-agar bridges connected to calomel electrodes, and the PD was short-circuited through Ag-AgCl electrodes by means of a voltage clamp that corrected for fluid resistance. Resistance (Ω∙cm2) was calculated from the PD and short-circuit current. The short-circuit current and PD were recorded over 240 min.
Isotopic flux studies of epithelial permeability were performed using3H-mannitol (10 mCi/L) and14C-inulin (10 mCi/L) as previously described (8). Isotopically labeled mannitol and inulin flux data (mucosal-to-serosal) were collected in 4 successive 60-min sampling periods (from 0 to 240 min). Samples were collected in scintillation vials and assessed for β emissions (dpm). All chemicals were purchased from Sigma Chemicals.
Intestinal histology.
Tissue samples for histological analysis were collected following the 45-min ischemic injury period from both nonischemic (control) and ischemic loops of the ileum from each pig. Samples were fixed in 10% neutral buffered formalin, transferred to 70% ethanol after 24 h, embedded in paraffin, sectioned (5μm), and stained with hematoxylin and eosin. Slides were read using an Olympus Vanox-S Microscope and analyzed using SPOT Basic Imaging software (Diagnostic Instruments). For each tissue, an investigator unaware of the treatment groups evaluated 5 different villi from the 2 stained sections. Methods for measurements of villus height, width, crypt depth, and the height of the epithelial covered portion of the villus were conducted as previously described by Moeser et al. (19). In addition, the surface area formula was modified by a factor that accounted for the hemispherical shape of the upper portion of the villus (20). The percentage of the villus surface area that remained denuded was calculated from the total surface area of the villus and the surface area of the villus covered by epithelium.
mRNA analysis.
Mucosal RNA was extracted, quantified, and cDNA synthesized as previously reported with slight modifications (18). The first-strand synthesis reaction was diluted 2-fold to provide an equivalent of 500 ng of input RNA/mL of cDNA. Quantitative PCR assays were carried out in 25-μL reactions of iQ SYBR Green Supermix (Bio-Rad) with diluted first-strand cDNA equivalent to 300 ng of input RNA. The primers for pigCOX-2 andGAPDH were then used to measure mRNA abundance of these genes by qRT-PCR and the 2ΔΔCT method of quantification (21) withGAPDH as the reference standard. All samples were run in duplicate and gene of interest and reference gene were all blocked within plate by replicate of experiment. Relative expression ratios were normalized to the nonischemic 0.5% ARA treatment. The porcine-specific sense and anti-sense primer sequences (5′-3′), respectively, were as follows:COX-2 (ATAAGTGTGACTGCACCCGAAC, GGTGGGCTATCAATCAGATGTG) andGAPDH (CATCCATGACAACTTCGGCA, GCATGGACTGTGGTCATGAGTC) (18,22).
Data analysis.
All data were analyzed using the general linear model procedures of SAS according to an ANOVA for a 2 × 4 factorial design. Differences were analyzed for main effects of diet and treatment and their interaction. When the interaction was not significant, main effects were separated using Tukey's test for post hoc pairwise multiple comparisons. The α-level for significance was set atP < 0.05. Data were reported as means ± SEM for a given number (n) of animals for each experiment.
Results
The initial and final body weights of piglets did not differ among dietary treatments and were 1.73 ± 0.14 and 4.54 ± 0.76 kg, respectively.
Protective effects of dietary ARA on villus epithelium
Histopathology of time 0 tissue.
To assess the effects of pigs fed dietary ARA or EPA on ischemia-injured porcine ileum, ileal samples were resected following the 45-min ischemic injury and histologically analyzed for the percentage of denuded villus surface area. Ischemia-injured ileum had a greater percentage of denuded villus surface area compared with villi from nonischemic ileum of all dietary groups (P < 0.05) (Table 1). Diet also affected the percentage of denuded villus surface area. Ischemia-injured ileum from pigs supplemented with 5% ARA had a lower percentage of denuded villus surface area compared with all other ischemia-injured dietary groups (P < 0.05). Histologically, ischemia-injured mucosa was sloughed an average of 63% from the upper villi in the 0% ARA-, 0.5% ARA-, and 5% EPA-fed pigs, which was significantly greater than the 44% denuded villi from the ischemia-injured mucosa of 5% ARA-fed pigs (Table 1). Tissues from the control loops of pigs did not differ from each other in denuded villus surface area. Villus height and crypt depth were not affected by ischemic injury or diet (data not shown).
TABLE 1.
Denuded villi surface area, initial PGE2 concentration, andCOX-2 mRNA expression in control and ischemic ileum of piglets fed 0, 0.5, or 5% ARA or 5% EPA1
| 0% ARA | 0.5% ARA | 5% ARA | 5% EPA | Pooled SEM | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonischemic | Ischemic | Nonischemic | Ischemic | Nonischemic | Ischemic | Nonischemic | Ischemic | Diet | Treatment | Diet × treatment | ||
| Denuded villi surface area,% | 6.9c | 62.8a | 7.7c | 65.5a | 6.9c | 43.6b | 4.7c | 60.0a | 3.6 | 0.13 | <0.001 | 0.001 |
| Initial PGE2,2ng/L | 140b | 124b | 142b | 101bc | 200b | 317a | 33c | 21c | 45 | <0.001 | 0.31 | 0.27 |
| COX-2 mRNA,3relative expression ratio | 0.78b | 1.44b | 1.00b | 1.30b | 1.11b | 1.14b | 3.68a | 2.81a | 0.47 | <0.001 | 0.93 | 0.43 |
Values are mean ± SEM, = 6. Means in a row without a common letter differ,P ≤ 0.05. ARA, arachidonic acid; COX, cyclooxygenase.
Measured after 30 min on Ussing chamber.
Normalized relative to nonischemic, 0.5% ARA treatment.
Initial PGE2 concentration.
Following the 30-min equilibration, PGE2 concentrations did not differ between control and ischemia-injured groups for the 0% ARA, 0.5% ARA, and 5% EPA dietary groups, but ischemia-injured ileum from pigs fed 5% ARA produced 59% more PGE2 than the corresponding nonischemic group from the same diet (P < 0.05) (Table 1). In addition, ileum from pigs fed 5% ARA with ischemic injury produced higher concentrations of PGE2 compared with all other ischemia-injured dietary treatments (P < 0.05) (Table 1). And ilea from the 5% EPA-fed pigs had a lower PGE2 production in both the ischemic and control groups than the 0% ARA-, 0.5% ARA-, and 5% ARA-fed piglets (P < 0.05) (Table 1).
COX-2 mRNA following ischemic injury.
Contrary to predictions of ischemia-injured porcine ileum having increased mRNA ofCOX-2 compared with nonischemic groups, this was not the case (Table 1). In all diet groups, the ischemia-injured ilea did not significantly differ from the control ilea inCOX-2 mRNA expression. Interestingly, a 10-fold increase in the dietary ARA concentration did not significantly affectCOX-2 mRNA expression. In contrast, the data showed a ~2.9-fold increase inCOX-2 mRNA expression in ischemia-injured pigs fed 5% EPA diets compared with all other ischemia-injured dietary treatment groups (P < 0.05) (Table 1).
Reparative effects of diet on ischemia-injured porcine ileum
Transepithelial electrical resistance.
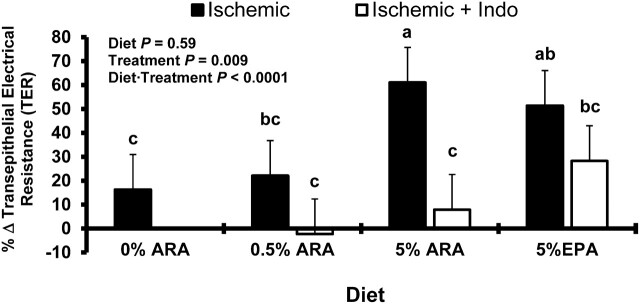
Transepithelial electrical resistance (TER) data (Fig. 1) are reported as the change from initial to 240 min and thus reflect the reparative properties of diet on ischemia-injured ileum. The TER data support that dietary long-chain PUFA can affect the recovery of TER following ischemic injury. Dietary inclusion of 5% ARA and 5% EPA resulted in 40% greater TER in ischemia-injured porcine ileum compared with the 0% ARA dietary group (P < 0.05). Moreover, tissue from piglets fed the diets supplemented with 5% ARA had greater TER than both the 0% and 0.5% ARA-fed pigs, which were 45 and 39% less, respectively (P < 0.05). In addition, in the 5% ARA ischemia-injured ileum, this reparative effect was inhibited by the addition of indomethacin, a nonselective COX inhibitor (87% reduction in TER;P < 0.01). However, in the 5% EPA-fed pigs, the effect was not altered by inhibition of COX. These differences indicate potentially different mechanisms for (n-6) and (n-3) fatty acids for increased TER following ischemic injury.
FIGURE 1.
Electrical responses of ischemia-injured porcine ileum with or without indomethacin. Data are reported as the percent change in TER from 0 to 240 min on Ussing chambers. Values are the percentage change in least square means from initial to final measurements + SEM,n = 6. Bars lacking a common letter differ,P < 0.05. ARA, arachidonic acid; TER, transepithelial electrical resistance.
Flux measurements of H3-mannitol and C14-inlulin.
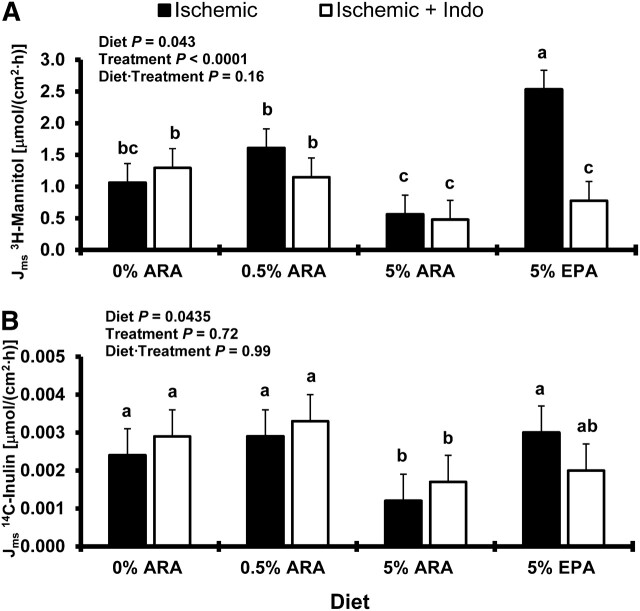
To assess the ability of dietary fatty acids to enhance barrier function of ischemia-injured porcine ileum, we measured the flux (Jms) of3H-mannitol and14C-inulin. Consistent with the changes reported in measurements of TER, ileum from the 5% ARA-fed pigs had lessJms of H3-mannitol (Fig. 2A) and C14-inulin (Fig. 2B) compared with ischemia-injured ileum from all other dietary groups (P < 0.05). Mannitol flux was greater by 50, 65, and 78% in the 0% ARA-, 0.5% ARA-, and 5% EPA-fed pigs for ischemia-injured ileum, respectively, compared with the 5% ARA-fed pigs with ischemia-injured ileum (Fig. 2A). Inulin flux was similarly higher in ischemia-injured groups by 50, 58, and 60% for the 0% ARA, 0.5% ARA, and 5% EPA dietary groups, respectively, compared with the 5% ARA dietary group (Fig. 2B). Interestingly, measurements of TER were not a predictor of H3-mannitol and C14-inulinJms in ischemia-injured tissues from 5% EPA-fed pigs. The 5% EPA-fed pigs had elevated TER measurements in the recovery but did not have reduced mannitol and inulin flux compared with ischemia-injured groups from all other dietary treatments.
FIGURE 2.
Mucosal-to-serosal flux of mannitol (A) or inulin (B) across ischemia-injured porcine ileum with or without indomethacin from 0 to 240 min. Values are least square means + SEM,n = 6. Bars lacking a common letter differ,P < 0.05. ARA, arachidonic acid.
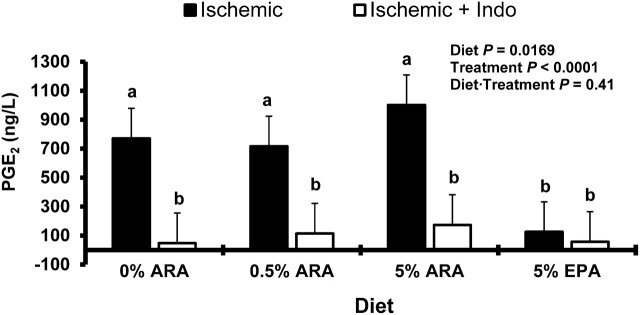
Final PGE2 concentrations.
PGE2 concentrations were measured initially at 30 min from the Ussing chambers and then again at 240 min. The concentration of the initial 30-min measurements of PGE2 were increased in the ischemic groups from pigs fed 5% ARA (P < 0.05) (Table 1). The PGE2 measurements at 240 min showed a greater PGE2 secretion in all ischemia-injured samples from all diet groups except the ischemia-injured group fed 5% EPA (Fig. 3) (P < 0.05). Additionally, in the 0, 0.5, and 5% ARA-fed pigs, ischemia-injured tissue production of PGE2 was inhibited by the addition of indomethacin to the chambers (Fig. 3) (P < 0.05).
FIGURE 3.
PGE2 production following 240-min repair on Ussing chambers by porcine ischemia-injured ileum with or without indomethacin from piglets fed diets differing in concentrations of long-chain PUFA from the (n-6) and (n-3) series. Values are least square means + SEM,n = 6. Bars lacking a common letter differ,P < 0.05. ARA, arachidonic acid.
Discussion
There are 3 phases to recovery of epithelial barrier integrity following ischemic injury:1) villus contraction to reduce the total denuded surface area;2) migration of epithelial cells to seal the basement membrane; and3) closure of leaky epithelial intercellular spaces and tight junctions (10). When the physiological repair mechanisms do not occur in a timely manner or are further exacerbated by inflammation in the injured epithelial mucosa, the increased intestinal permeability predicates the onset of acute and chronic intestinal disorders. For infants afflicted with NEC, this may have lifelong implications such as short bowel syndrome or be as severe as sepsis and multiple organ failure, leading causes of morbidity and mortality in these infants (1).
Our previous observations in weanling pigs indicated that PG, in particular PGE2, stimulate recovery of barrier function in ischemia-injured ileum (8–10). To evaluate if dietary supplementation of long-chain PUFA to the diet could affect ileum barrier function following ischemic injury, we fed formulas to 1-d-old pigs with no long-chain PUFA, 0.5% ARA (current infant formula standard), and 5% ARA or EPA for 10 d to enrich the phospholipid composition of the enterocytes (17).
Our results indicate that ilea from piglets fed supra-physiologic levels of ARA (5% of total fatty acids) in neonatal formula are less susceptible to ischemia-induced epithelial cell sloughing. Additionally, these same ARA-enriched tissues have much greater PGE2 secretion following ischemic injury. To determine if these changes were related to changes in gene transcription of enzymes involved in the oxidation of ARA to eicosanoids, we measuredCOX-2 mRNA. If the mechanism for decreased epithelial cell sloughing following ischemic injury is related to PGE2 secretion from the mucosal epithelium, our results show that it is not mediated via an acute upregulation ofCOX-2 mRNA. This was somewhat unexpected, because COX is a key enzyme involved in PG synthesis, and insults to the mucosa usually cause an upregulation of COX-2. The explanation may have multiple facets. Takeuchi et al. (23) demonstrated that indomethacin-induced lesions to rat small intestine display a 24-h delay from the onset of lesions to the actual upregulation ofCOX-2 mRNA expression. Additionally, our dietary treatments may have created tissues wherein the substrate (ARA) rather than the COX-2 enzyme dictates the rate of PGE2 synthesis. This idea is supported by the impact of 5% EPA onCOX-2 mRNA. Indeed, although unexpected, a significant and notable finding from this research was the 2.9-fold increase inCOX-2 mRNA in intestinal tissue of 5% EPA-fed pigs regardless of ischemic injury compared with all other dietary groups. Others have observed a suppressive effect of (n-3) PUFA onCOX-2 mRNA regulation in many cell types but especially in cell lines and mouse models of colon cancer (24,25). However,COX-2 mRNA and protein activity undergo complex positive and negative feedback control from the pathway-produced PG (26). In particular, the changes in regulation are closely linked to the ARA metabolites, especially increasing concentrations of PGE2, 15Δ-PGJ2(a metabolite of PGD2), and 6-keto PGF1α (a metabolite of PGI2) (26). Therefore, it seems plausible that dietary alterations causing considerable modification to eicosanoid profiles could significantly affect gene expression, especially given the importance of (n-6)-derived PG in gastrointestinal mucosa maintenance, protection, and repair processes. This mechanism, in conjunction with the protective effects of ARA, needs to be further investigated, but these observations underscore 2 very important points. First, (n-6) PUFA can have beneficial acute physiological impacts (3) and second, the imbalance of (n-6) and (n-3) dietary fatty acid profiles in either direction may have significant physiological effect.
In addition to the prophylactic, protective effects of dietary long-chain PUFA, we also were interested to know if high supplemental levels would accelerate repair of the intestinal epithelium after an ischemic insult. Previous Ussing chamber experiments established that blockade of endogenous PG synthesis with the nonselective, nonsteroidal antiinflammatory drug indomethacin impairs the ability of the intestine to recover barrier function as assessed by TER. Furthermore, application of PG analogs (PGE2 and PGI2) to ischemia-injured mucosa resulted in rapid restoration of TER values to uninjured control levels (8,9,27). The results from this study show dietary ARA and EPA included in formula at 5% of total fat acids enhanced recovery of TER following 45 min of ischemic injury in porcine ileum compared with all other ischemia-injured dietary groups. Additionally, the effect was attenuated by indomethacin in 5% ARA-fed pigs. Indomethacin did not significantly block the increase in resistance in the 5% EPA-fed pigs, but there was numerical decrease. Therefore, it is probable the increased recovery of resistance at the 5% level of (n-3) dietary PUFA is not solely due to COX-derived eicosanoids.
The potential for different mechanisms of epithelial barrier function repair for (n-6) and (n-3) fatty acids is further substantiated by the mannitol and inulin flux data, which depicted differences in full closure of tight junctions involved in the regulation of solute and macromolecule diffusion across the barrier. Although ischemia-injured ileum from pigs fed 5% ARA had significantly reducedJms of mannitol and inulin from 0 to 240 min compared with all other dietary treatments, theJms for mannitol was highest in injured ileum from the 5% EPA-fed pigs. These differences in paracellular permeability are probably related to altered expression or disorganization of tight junction proteins needed for complete repair of the barrier function. Research has shown altered tight junction protein expression and change in paracellular permeability by changes in trace elements, fatty acids, and flavonoids have a significant impact on paracellular permeability of Caco-2 monolayers (28). Moreover, Usami et al. (29,30) have shown that Caco-2 monolayers cultured with DHA and EPA have greater paracellular permeability of fluorescein sulfonic acid in a concentration-dependent manner. Interestingly, in our study, indomethacin significantly decreased the paracellular flux of3H-mannitol in the 5% EPA-fed pig ileum following ischemic injury. This would suggest that products of the COX pathway are upregulating paracellular solute diffusion in ischemia-injured tissue from suckling piglets supplemented with a high level of dietary EPA. The differential effect of dietary PUFA on resistance and flux of the intestinal mucosa implicate 2 functionally distinct pathways for molecules to traverse the tight junction: first, a high-capacity, charge-selective pore pathway that allows passage of small ions and uncharged molecules, and second, a low-capacity leak pathway that allows flux of larger ions and molecules regardless of charge (31). Our data suggest that 5% dietary ARA protects both the pore and leak pathways, whereas 5% EPA may protect only the pore pathway.
Mechanistically, the significant changes in TER and 4-h flux of mannitol and inulin are probably not directly related to PGE2 secretion. PGE2 secretion did not differ between ischemia-injured tissues for the 0% ARA, 0.5% ARA or 5% ARA diet groups. As expected, the levels of PGE2 were significantly lower in the ischemia-injured 5% EPA group compared with the ischemia-injured groups from the other dietary treatments, because the product of the pathway would be PGE3 instead of PGE2. The mechanism by which high dietary supplementation of (n-6) and (n-3) long-chain PUFA affect recovery of intestinal epithelial barrier function in neonatal pigs needs further investigation.
Altering the fatty acid composition of the diet can produce substantial changes in membrane fatty acid composition in neonatal suckling piglets (17). Differences in eicosanoid synthesis, due to differing precursor pools, could also result in alterations in cell signaling and metabolism. Although this initial study has shown a beneficial impact of supra-physiological dietary ARA in the protection and repair of porcine ischemia-injured ileum, we are not naive to the important impact that inadequacies, imbalances, or excesses of dietary PUFA could have on the nutritional, metabolic, immunologic, and endocrine functions of neonates (32). Although our study supports nutritional interventions of supra-physiological concentrations of long-chain PUFA having significant, positive impacts on reducing villi denudation, accelerating recovery of TER, and decreasing paracellular flux following injury, we also acknowledge the need to further investigate the mechanisms related to chronic physiological effects due to dietary modification.
Acknowledgments
B.A.C. and R.J.H. designed research; A.T.B. designed and conducted research; J.O. designed research and had primary responsibility for final content; S.K.J. conducted research, analyzed data, and wrote the paper; and A.J.M. conducted research. All authors read and approved the final manuscript.
Abbreviations
- ARA
arachidonic acid
- COX
cyclooxygenase
- NEC
necrotizing enterocolitis
- PD
potential difference
- TER
transepithelial electrical resistance
Footnotes
Presented in part at the 108th Annual Meetings of the American Gastroenterological Association Institute. Digestive Disease Week, Washington, DC (2007) [Jacobi SK, Moeser AJ, Corl BA, Ryan K, Blikslager AT, Harrell RJ, Odle J. Prophylactic enrichment of ileal enterocyte phospholipids with polyunsaturated fatty acids facilitates acute repair following ischemic injury in suckling piglets. Gastroenterology 2007;132:A-242] and the NCR-97 meeting for Regulation of Adipose Tissue Accretion in Meat-Producing Animals (2008) [Jacobi SK, Moeser AJ, Corl BA, Ryan K, Blikslager AT, Harrell RJ, Odle J. Impact of polyunsaturated fatty acids on transcription factors involved in regulation of COX-2 and repair of ischemia-injured porcine ileum. NCR-97 2008, San Diego, CA].
Supported by in part by Cooperative State Research, Education and Extension Service, USDA-National Research Initiative grant no. 2005-35200-16174 and by the North Carolina Agriculture Research Service.
Literature Cited
- 1. Hunter CJ,Upperman JS,Ford HR,Camerini V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC).Pediatr Res. 2008;63:117–23. [DOI] [PubMed] [Google Scholar]
- 2. Neu J,Walker WA. Medical progress: necrotizing enterocolitis.N Engl J Med. 2011;364:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fritsche K.Fatty acids as modulators of the immune response.Annu Rev Nutr. 2006;26:45–73. [DOI] [PubMed] [Google Scholar]
- 4. López-Pedrosa JM,Ramirez M,Torres MI,Gil A. Dietary phospholipids rich in long-chain polyunsaturated fatty acids improve the repair of small intestine in previously malnourished piglets.J Nutr. 1999;129:1149–55. [DOI] [PubMed] [Google Scholar]
- 5. Lopez-Pedrosa JM,Torres MI,Fernandez MI,Rios A,Gil A. Severe malnutrition alters lipid composition and fatty acid profile of small intestine in newborn piglets.J Nutr. 1998;128:224–33. [DOI] [PubMed] [Google Scholar]
- 6. Ruthig DJ,Meckling-Gill KA. Both (n-3) and (n-6) fatty acids stimulate wound healing in the rat intestinal epithelial cell line, IEC-6.J Nutr. 1999;129:1791–8. [DOI] [PubMed] [Google Scholar]
- 7. Ruthig DJ,Meckling-Gill KA. N-3 and n-6 fatty acids stimulate restitution by independent mechanisms in the IEC-6 model of intestinal wound healing.J Nutr Biochem. 2002;13:27–35. [DOI] [PubMed] [Google Scholar]
- 8. Blikslager AT,Roberts MC,Rhoads JM,Argenzio RA. Prostaglandins I-2 and E-2 have a synergistic role in rescuing epithelial barrier function in porcine ileum.J Clin Invest. 1997;100:1928–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blikslager AT,Pell SM,Young KM. PGE(2) triggers recovery of transmucosal resistance via EP receptor cross talk in porcine ischemia-injured ileum.Am J Physiol Gastrointest Liver Physiol. 2001;281:G375–81. [DOI] [PubMed] [Google Scholar]
- 10. Blikslager AT,Moeser AJ,Gookin JL,Jones SL,Odle J. Restoration of barrier function in injured intestinal mucosa.Physiol Rev. 2007;87:545–64. [DOI] [PubMed] [Google Scholar]
- 11. Ferrer R,Moreno JJ. Role of eicosanoids on intestinal epithelial homeostasis.Biochem Pharmacol. 2010;80:431–8. [DOI] [PubMed] [Google Scholar]
- 12. Innis SM.Human milk: maternal dietary lipids and infant development.Proc Nutr Soc. 2007;66:397–404. [DOI] [PubMed] [Google Scholar]
- 13. Innis SM.Essential fatty acids in infant nutrition: lessons and limitations from animal studies in relation to studies on infant fatty acid requirements.Am J Clin Nutr. 2000;71:S238–44. [DOI] [PubMed] [Google Scholar]
- 14. Gibson RA,Makrides M. The role of long chain polyunsaturated fatty acids (LCPUFA) in neonatal nutrition.Acta Paediatr. 1998;87:1017–22. [DOI] [PubMed] [Google Scholar]
- 15. Carlson SE.Long-chain polyunsaturated fatty acids and development of human infants.Acta Paediatr Suppl. 1999;88:72–7. [DOI] [PubMed] [Google Scholar]
- 16. Uauy R,Hoffman DR. Essential fat requirements of preterm infants.Am J Clin Nutr. 2000;71:S245–50. [DOI] [PubMed] [Google Scholar]
- 17. Hess HA,Corl BA,Lin X,Jacobi SK,Harrell RJ,Blikslager AT,Odle J. Enrichment of intestinal mucosal phospholipids with arachidonic and eicosapentaenoic acids fed to suckling piglets is dose and time dependent.J Nutr. 2008;138:2164–71. [DOI] [PubMed] [Google Scholar]
- 18. Jacobi SK,Lin X,Corl BA,Hess HA,Harrell RJ,Odle J. Dietary arachidonate differentially alters desaturase-elongase pathway flux and gene expression in liver and intestine of suckling pigs.J Nutr. 2011;141:548–53. [DOI] [PubMed] [Google Scholar]
- 19. Moeser AJ,Nighot PK,Ryan KA,Wooten JG,Blikslager AT. Prostaglandin-mediated inhibition of Na+/H+ exchanger isoform 2 stimulates recovery of barrier function in ischemia-injured intestine.Am J Physiol Gastrointest Liver Physiol. 2006;291:G885–94. [DOI] [PubMed] [Google Scholar]
- 20. Argenzio RA,Lecce J,Powell DW. Prostanoids inhibit intestinal Nacl absorption in experimental porcine cryptosporidiosis.Gastroenterology. 1993;104:440–7. [DOI] [PubMed] [Google Scholar]
- 21. Giulietti A,Overbergh L,Valckx D,Decallonne B,Bouillon R,Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression.Methods. 2001;25:386–401. [DOI] [PubMed] [Google Scholar]
- 22. Gabler NK,Spencer JD,Webel DM,Spurlock ME. n-3 PUFA attenuate lipopolysaccharide-induced down-regulation of toll-like receptor 4 expression in porcine adipose tissue but does not alter the expression of other immune modulators.J Nutr Biochem. 2008;19:8–15. [DOI] [PubMed] [Google Scholar]
- 23. Takeuchi K,Tanigami M,Amagase K,Ochi A,Okuda S,Hatazawa R. Endogenous prostaglandin E(2) accelerates healing of indomethacin-induced small intestinal lesions through upregulation of vascular endothelial growth factor expression by activation of EP4 receptors.J Gastroenterol Hepatol. 2010;25:S67–74. [DOI] [PubMed] [Google Scholar]
- 24. Rao CV,Hirose Y,Indranie C,Reddy BS. Modulation of experimental colon tumorigenesis by types and amounts of dietary fatty acids.Cancer Res. 2001;61:1927–33. [PubMed] [Google Scholar]
- 25. Gravaghi C,La Perle KMD,Ogrodwski P,Kang JX,Quimby F,Lipkin M,Lamprecht SA. Cox-2 expression, PGE(2) and cytokines production are inhibited by endogenously synthesized n-3 PUFAs in inflamed colon of fat-1 mice.J Nutr Biochem. 2011;22:360–5. [DOI] [PubMed] [Google Scholar]
- 26. Vichai V,Suyarnsesthakorn C,Pittayakhajonwut D,Sriklung K,Kirtikara K. Positive feedback regulation of COX-2 expression by prostaglandin metabolites.Inflamm Res. 2005;54:163–72. [DOI] [PubMed] [Google Scholar]
- 27. Blikslager AT,Roberts MC,Argenzio RA. Prostaglandin-induced recovery of barrier function in porcine ileum is triggered by chloride secretion.Am J Physiol. 1999;276:G28–36. [DOI] [PubMed] [Google Scholar]
- 28. Ulluwishewa D,Anderson RC,Mcnabb WC,Moughan PJ,Wells JM,Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components.J Nutr. 2011;141:769–76. [DOI] [PubMed] [Google Scholar]
- 29. Usami M,Muraki K,Iwamoto M,Ohata A,Matsushita E,Miki A. Effect of eicosapentaenoic acid (EPA) on tight junction permeability in intestinal monolayer cells.Clin Nutr. 2001;20:351–9. [DOI] [PubMed] [Google Scholar]
- 30. Usami M,Komurasaki T,Hanada A,Kinoshita K,Ohata A. Effect of gamma-linolenic acid or docosahexaenoic acid on tight junction permeability in intestinal monolayer cells and their mechanism by protein kinase C activation and/or eicosanoid formation.Nutrition. 2003;19:150–6. [DOI] [PubMed] [Google Scholar]
- 31. Shen L,Weber CR,Raleigh DR,Yu D,Tumer JR. Tight, junction pore and leak pathways: a dynamic duo.Annu Rev Physiol. 2011;73:283–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Innis SM.Metabolic programming of long-term outcomes due to fatty acid nutrition in early life.Matern Child Nutr. 2011;7:112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]