Abstract
All mammalian uteri have luminal (LE) and glandular epithelia (GE) in their endometrium. The LE mediates uterine receptivity and blastocyst attachment for implantation, and the GE synthesize and secrete or transport bioactive substances involved in blastocyst implantation, uterine receptivity, and stromal cell decidualization. However, the mechanisms governing uterine epithelial development after birth and their function in the adult are not fully understood. Here, comprehensive microarray analysis was conducted on LE and GE isolated by laser capture microdissection from uteri on Postnatal Day 10 (PD 10) and day of pseudopregnancy (DOPP) 2.5 and 3.5. This data was integrated with analysis of uteri from gland-containing control and aglandular progesterone-induced uterine gland knockout mice from PD 10 and DOPP 3.5. Many genes were expressed in both epithelia, but there was greater expression of genes in the LE than in the GE. In the neonate, GE-expressed genes were enriched for morphogenesis, development, migration, and retinoic acid signaling. In the adult, LE-expressed genes were enriched for metabolic processes and steroid biosynthesis, whereas retinoid signaling, tight junction, extracellular matrix, and regulation of kinase activity were enriched in the GE. The transcriptome differences in the epithelia support the idea that each cell type has a distinct and complementary function in the uterus. The candidate genes and regulatory networks identified here provide a framework to discover new mechanisms regulating development of epithelia in the postnatal uterus and their functions in early pregnancy.
Keywords: endometrium; gene expression; implantation; rodents (rats, mice, guinea pigs, voles); uterus
Introduction
The endometrium of the adult rodent uterus consists of a simple columnar luminal epithelium (LE) supported by stromal cells that contain coiled endometrial glands lined with glandular epithelia (GE) [1]. Endometrial gland development or adenogenesis is uniquely or primarily a postnatal event in laboratory animals, domestic animals, and humans [2,3]. At birth, the mouse uterus lacks endometrial glands and consists of a simple LE supported by undifferentiated mesenchyme [1]. Between birth (i.e., Postnatal Day 0 or PD 0) and PD 9, GE cells differentiate and bud from the LE [4]. By PD 15, the histoarchitecture of the uterus resembles that of the adult [3,5]. Gland morphogenesis also occurs during endometrial regeneration following menstruation in humans and/or parturition in both mice and humans [6,7]. The mechanisms regulating endometrial gland development or adenogenesis are not well understood in any species but involve cell-cell interactions, morphogens, and transcription factors in the mouse (for a review, see [5,8]).
In mice, the uterus is receptive on Day 4 of pregnancy or pseudopregnancy (Day 1 = observation of a postcoital vaginal plug), whereas it is prereceptive on Days 1–3 and, by the afternoon of Day 5, becomes nonreceptive (refractory) to blastocyst implantation [9,10]. Dynamic changes in ovarian estrogen and progesterone secretion regulate endometrial function and blastocyst implantation. The implantation process, which is initiated by blastocyst trophectoderm attachment to the receptive LE, occurs prior to or right after midnight in the evening of Day 4 and becomes more prominent on the morning of Day 5. Recent evidence suggests that there are two separate uterine signals regulating the trophectoderm during blastocyst implantation, one that primes the trophectoderm for attachment to the LE and another that results in uptake of amino acids by the embryo and initiates its motility for invasion; however, the nature of those signals are not well-defined [11]. Signaling within the endometrium that regulates receptivity of the LE to blastocyst attachment involves actions of ovarian steroids as well as a myriad of genes expressed within the different endometrial cell types (for a review, see [9,10].
Research in glandless sheep and mouse models established the importance of endometrial glands and their secretions for blastocyst implantation because they are infertile and exhibit recurrent early pregnancy loss [12,13]. Endometrial glands and their secretions are presumed to be important mediators of endometrial receptivity, blastocyst implantation (trophoblast attachment, growth, and invasion), and stromal cell decidualization in humans and mice [10,12,14]. In mice, endometrial glands and their secretions, such as leukemia inhibitory factor (LIF), are required for blastocyst implantation and uterine receptivity and also influence stromal cell decidualization [12,15,16].
Based on results from available studies, the LE and GE of the mouse uterus have common and differentially expressed genes [10,17–21]. The LE has more examples of genes that are solely expressed at peri-implantation stages (Areg, Calb1, Hdc, Hegf1, Irg1, Ptgs2) than are known in the underlying GE (Lif, Calca, I16st). Both epithelial cell types can uniquely express certain genes, while other genes appear to be coordinately expressed (Cdh1, Ihh, Klf5, Msx1/Msx2, Ptgs1) in both LE and GE. Under receptive conditions, both LE and GE can express the same genes important for implantation, includingCdh1, Tro, Ihh, andPtgs1. Furthermore, genes are also downregulated in either or both epithelial cell types during the receptive window such asMuc1 andPgr [9,10]. In adult rodents, blastocyst implantation defects arise from the loss of expression of genes expressed only in the GE (LIF and calcitonin/calcitonin-related polypeptide, alpha [CALCA] [22,23]) as well as those specifically expressed in the LE and GE (e.g.,Ihh, Klf5, Msx1/Msx2) [24–26] and LE and stroma (e.g.,Ptgs2) [27]. Given the cellular complexity of the uterus, analysis of the entire uterine transcriptome is not entirely advantageous given the preponderance of stroma and myometrium relative to the endometrial epithelia. Only one study has used laser-capture microdissection (LCM) and microarray technology to explore differences in the LE and GE of the mouse uterus [20]. In that study, transcriptome of the epithelia of the receptive mouse uterus was determined 2 h before blastocyst attachment using a noncomprehensive microarray. The hypothesis is that distinct sets of genes are expressed in the epithelia of the uterus that govern their development in the neonate and function in the adult. In the present study, we sought to interrogate the endometrial epithelial transcriptome of the developing neonatal and adult uteri using LCM and a comprehensive microarray analysis coupled with use of the progesterone-induced uterine gland knockout (PUGKO) mouse model. The results provide novel insights into mechanisms regulating uterine epithelial development in the neonate and their function in the adult uterus and support the hypothesis that LE and GE have unique genetic signatures that dictate their differential and synergistic function in the uterus [20].
Materials and Methods
Animals and Hormonal Treatments
All the animal procedures were approved by the Institutional Animal Care and Use Committee of Washington State University and conducted according to the Guide for the Care and Use of Laboratory Animals and institutional guidelines. For LCM, uteri were collected from CD-1 female mice on PD 10 and at 1600 h on day of pseudopregnancy (DOPP) 2.5 and 3.5, quickly frozen in Tissue-Tek optimal cutting temperature (O.C.T.) compound (Sakura Finetek, Torrance, CA), and stored at −80°C. Generation of control and PUGKO mice was performed using previously described methods by our laboratory [28]. Briefly, litters of C57BL/6J pups received daily subcutaneous injections from PD 2 to PD 10 of sesame oil vehicle alone (Sigma-Aldrich, St. Louis, MO) as a control or progesterone (P4; 50 μg/g body weight) in sesame oil. At 8 wk of age, control and PUGKO female mice were mated to a vasectomized male. The day of the postcoital vaginal plug was designated as DOPP 0.5. Mice were killed at 1600 h on DOPP 3.5, and whole uteri were snap frozen in liquid nitrogen and stored at −80°C.
LCM and RNA Extraction
Uteri frozen in O.C.T compound were cryosectioned (12 μm) using a Leica CM1950 cryostat (Leica Microsystems, Wetzlar, Germany). Sections were mounted onto room temperature RNase-free polyethylene naphthalate-coated slides (Carl Zeiss, Munich, Germany) and immediately placed on dry ice and fixed/stained on the same day using previously described method [29]. Briefly, slides were transferred from dry ice into ice-cold 95% ethanol for 30 sec and incubated in 75% ethanol for 30 sec. Specimens were briefly stained in 1% cresyl violet solution in 75% ethanol. Tissue sections were dehydrated through 75% ethanol (30 sec), 95% ethanol (30 sec), followed by two 30 sec and one 5 min incubation in anhydrous 100% ethanol. Slides were dried for 5 min at room temperature and stored in vacuum-sealed containers at −80°C until use. The LE and GE were separately captured from 8 to 10 slides of uterine sections for no longer than 60 min using PALM MicroBeam LCM microscope (Carl Zeiss). Total RNA was extracted from collected cells using RNeasy MinElute kit (Qiagen, Valencia, CA) and eluted with 14 μl of RNase-free water. The integrity and concentration of RNA was determined using RNA 6000 Pico Kit and Bioanalyzer (Agilent Technologies, Santa Clara, CA). The RNA yield ranged from 100 to 700 ng for each cell type with RNA integrity number (RIN) above 7.
Transcriptional Profiling by Microarray
For LCM-derived samples (n = 2 per cell type and day), total RNA was amplified using Ovation Pico WTA System V2 (Nugen, San Carlos, CA). For control and PUGKO DOPP 3.5 mouse uteri (n = 4 mice per type), total RNA was extracted from uteri using Trizol reagent (Invitrogen, Carlsbad, CA) and on-column DNase treatment and cleanup was performed (Qiagen). Total RNA quality and quantity was determined using a Bioanalyzer (Agilent Technologies) and a NanoDrop 1000 (Thermo Fisher Scientific Inc., Wilmington, DE). Samples with a RIN of greater than 8.0 were analyzed using microarrays. Total RNA was labeled using a Gene Chip One-Cycle Target Labeling Kit (Affymetrix, Santa Clara, CA) and then hybridized to a Mouse Gene 1.0 ST microarray (Affymetrix). For the hybridization, wash, and staining process, the GeneChip Hybridization, Wash, and Stain Kit (Affymetrix) and a Fluidic Station 450 (Affymetrix) were used. All the steps were done according to the manufacturer's protocol. The processed arrays were scanned with a GeneChip Scanner 3000 (Affymetrix). Microarray data can be accessed on the Gene Expression Omnibus Web site (GSE48239 and GSE48340).
GeneSpring 7.0 software (Agilent Technologies) was used for analysis of microarray data. Array output was normalized via the robust multiarray method [30], and probe sets were filtered based on expression calls. Data analysis was conducted using ANOVA (P = 0.05) with a Benjamini and Hochberg false discovery rate multiple test correction to determine differentially expressed genes. Integrated analysis of different functional databases was done using functional annotation tools of the database for annotation, visualization, and integrated discovery (DAVID) [31,32].
Semiquantitative Real-Time RT-PCR
Microarray results were validated by real-time quantitative PCR (qPCR) using methods described previously [28]. Primers used for PCR analysis are provided inSupplemental Table S1 (all the Supplemental Tables are available online atwww.biolreprod.org). The qPCR was carried out in triplicate using SsoAdvanced SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) and a CFX Connect Real-Time PCR Detection System (Bio-Rad laboratories). Data (Ct value) was subjected to least-squares analyses of variance (ANOVA) using the general linear models procedures of the Statistical Analysis System (SAS Institute Inc., Cary, NC). In all the analyses, theGapdh values were used as a covariate, and error terms used in the test of significance were identified according to the expectation of the mean squares for error. Significance (P < 0.05) was determined by probability differences of least-squares means.
Results
Identification and Functional Categorization of LE-Enriched Genes in the Neonatal and Adult Mouse Uterus
To identify genes expressed in the LE of the neonatal and adult uteri, the LE and GE of PD 10, DOPP 2.5, and DOPP 3.5 uteri were isolated by LCM, and total RNA was subjected to microarray analysis. Pseudopregnant mice were used for this study because they exhibit the same gene expression changes as pregnant mice in terms of uterine receptivity, but their uteri can be isolated without the presence of a blastocyst [33]. Of the more than 28 000 genes present in the microarray, 7827, 6975, and 8067 genes were expressed (probe intensity more than 100) in the LE of the PD 10, DOPP 2.5, and DOPP 3.5 uteri, respectively. Genes enriched in the LE were determined by analyzing (P < 0.05, >2-fold) lists of expressed genes in the isolated samples of LE and GE (Supplemental Tables S2,S3, andS4). Real-time qPCR validated the results of this approach coupling LCM with a comprehensive microarray for PD 10, DOPP 2.5, and DOPP 3.5 GE and LE (Fig. 1). The top 30 genes enriched in the LE of the neonatal and adult mouse uteri are illustrated inFigure 2A. A comparative evaluation of LE-enriched genes across days is shown inFigure 2B.
Fig. 1.
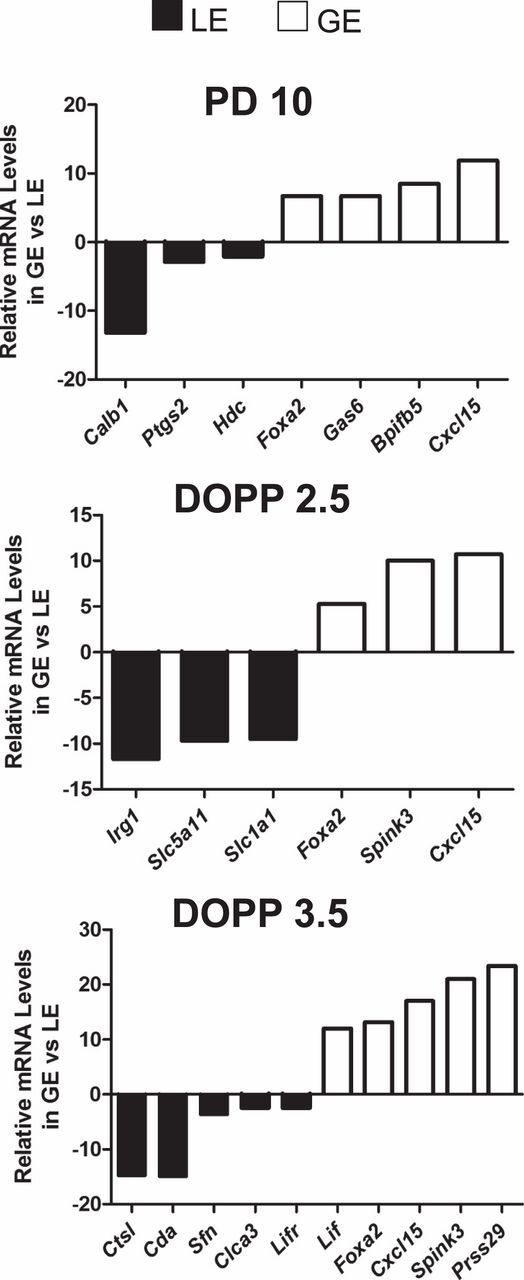
Quantitative PCR validation of selected genes identified by LCM and microarray analysis. The mRNA levels of the indicated genes were measured in microdissected luminal epithelial (LE) and glandular epithelial (GE) cells of the uteri from PD 10, DOPP 2.5, and DOPP 3.5 mice by semiquantitative RT-PCR analysis (n = 4 mice/cell type/day). Data are presented as fold change of target mRNA levels in GE as compared to LE.
Fig. 2.
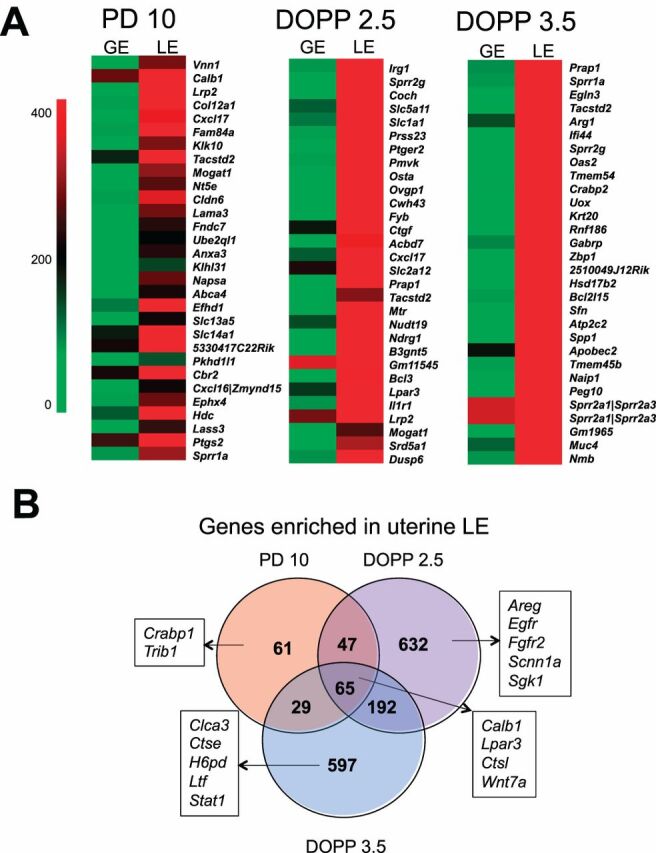
Luminal epithelial (LE) genes expressed in the neonatal and adult mouse uteri.A) Heat map of the most LE-enriched genes in PD 10, DOPP 2.5, and DOPP 3.5. Normalized probe intensity values are presented for the top 30 genes significantly enriched in LE.B) Venn diagram comparing LE-enriched genes between neonatal (PD 10) and adult uteri (DOPP 2.5 and 3.5).
In the neonatal PD 10 uterus, 203 genes were enriched in the LE, including known LE-specific genes such aswingless-related MMTV integration site 7A (Wnt7a) [34]. A number of novel LE-enriched genes were identified, includingvanin 1 (Vnn1) andcarbonyl reductase 2 (Cbr2). As summarized inTable 1, DAVID functional annotation analysis revealed that keratinocyte differentiation, immune response, cell morphogenesis involved in differentiation, and eicosanoid biosynthetic process were the most significantly enriched among LE-enriched genes in the neonatal uterus (Supplemental Table S5). Interestingly, gene ontology (GO) analysis distinguished genes encoding secreted proteins (e.g.,Muc20,Prap1,Wnt7a), transporters (e.g.,Cfh,Slc2a1,Slc5a11), and enzymes (e.g.,Cbr2,Hdc,Ptgs2) (seeSupplemental Table 6 for the complete lists).
Table. 1.
Selected results of DAVID functional annotation clustering for differentially expressed genes in the LE of the PD 10 and DOPP 2.5 and 3.5 mouse uteri.
| Representative functional terms of overrepresented annotation clustersa | Enrichment scoreb |
|---|---|
| PD 10 | |
| Keratinocyte differentiation (5, 10.8) | 2.8 |
| Fibronectin, type III (9, 4.7) | 2.8 |
| Cell morphogenesis involved in differentiation (8, 3.8) | 2.0 |
| Membrane (81, 1.4); transmembrane region (65, 1.3) | 1.9 |
| Basement membrane (4, 11.2); extracellular matrix part (5, 4.8); EGF-like, laminin (3, 7.0) | 1.6 |
| Organic acid transport (3, 2.9) | 1.4 |
| Eicosanoid biosynthetic process (4, 15.3); arachidonic acid metabolism (4, 4.1) | 1.4 |
| Keratin (4, 3.7) | 1.2 |
| Acute inflammatory response (4, 5.1); complement activation (3, 8.8); protein processing (4,4.5) | 1.1 |
| DOPP 2.5 | |
| Membrane organization (32, 2.4); endocytosis (25, 2.7) | 5.0 |
| Vasculature development (30, 2.4) | 4.5 |
| Phosphorus metabolic process (68, 1.6) | 4.0 |
| Protein amino acid phosphorylation (49, 1.5) | 3.0 |
| Cytoplasmic vesicle (40, 1.6) | 2.9 |
| Dioxygenase (11, 3.2) | 2.8 |
| Ion homeostasis (28, 1.9); chemical homeostasis (31, 1.7) | 2.8 |
| Cell death (40, 1.6) | 2.7 |
| Lysosome (18, 2.1) | 2.4 |
| Metal ion binding (210, 1.1) | 2.0 |
| DOPP 3.5 | |
| Cholesterol biosynthetic process (11, 10.8); steroid biosynthesis (11, 6.4) | 5.9 |
| Cell death (38, 1.7) | 3.4 |
| Positive regulation of cell death (25, 2.2) | 3.4 |
| 2-5-oligoadenylate synthetase, conserved site (5, 14.6) | 3.0 |
| Guanylate-binding protein, C-terminal (5, 13.0) | 2.8 |
| Blood vessel development (22, 2.0) | 2.7 |
| Phosphate metabolic process (59, 1.5) | 2.7 |
| Ion binding (205, 1.2) | 2.7 |
| Regulation of cell death (40, 1.6) | 2.6 |
| Regulation of phosphorylation (25, 1.9) | 2.5 |
Values within the parentheses indicate the number of genes and fold enrichment of the functional term.
Geometric mean of member'sP values of the corresponding annotation cluster (in −log10 scale).
In the adult DOPP 2.5 and 3.5 uteri, 936 and 883 genes were enriched in the LE, respectively, including several genes shown to be expressed in the LE such asimmunoresponsive gene 1 (Irg1) [35],lysophosphatidic acid (LPA) receptor (Lpar3) [36],proline-rich acidic protein 1 (Prap1) [37], andsodium channel, nonvoltage-gated 1 alpha (Scnn1a [38]). Several novel LE-enriched genes were found, includingsolute carrier family 5 member 11 (Slc5a11), asodium/glucose cotransporter, andEGL nine homolog 3 (Egln3). Genes enriched in LE on DOPP 2.5 were associated with endocytosis, metabolic processes, and cell death using DAVID functional annotation analysis (Table 1 andSupplemental Table S7). In addition, a number of genes encoding transporters (e.g.,Cfb, Kcnk1, Slc2a12) and enzymes (e.g.,Egfr, Fgfr2, Hdc, Lipa) were LE-enriched on DOPP 2.5 (Supplemental Table S6). Surprisingly, the set of LE-specific genes on DOPP 2.5 was not particularly enriched for genes encoding secretory proteins. On the contrary, GO analysis identified numerous genes in LE on DOPP 3.5 that encoded secretory proteins (e.g.,Clca3, Coch, Ltf, Muc4, Prap1, Spp1) in addition to transporters (e.g.,Aqp4, Cftr, Kcnn4) and enzymes (e.g.,Cda, Lipf, Ptgs2) (Supplemental Table S6). DAVID functional annotation analysis revealed that LE-enriched genes on DOPP 3.5 were mainly associated with steroid biosynthesis, cell death, and metabolic processes (Table 1 andSupplemental Table S8).
Identification and Functional Categorization of GE-Enriched Genes in the Neonatal and Adult Mouse Uteri
Of the 28 000 genes present in the microarray, 8352, 7615, and 8931 genes were expressed (probe intensity more than 100) in the GE of the PD 10, DOPP 2.5, and DOPP 3.5 uteri, respectively. Genes enriched in the GE were determined by comparing (P < 0.05, >2-fold) lists of genes expressed in the isolated samples of LE and GE (Supplemental Tables S2,S3, andS4). The top 30 genes enriched in the GE of the neonatal and adult mouse uteri are illustrated inFigure 3A. A comparative evaluation of GE-enriched genes across days is shown inFigure 3B for the different uteri.
Fig. 3.
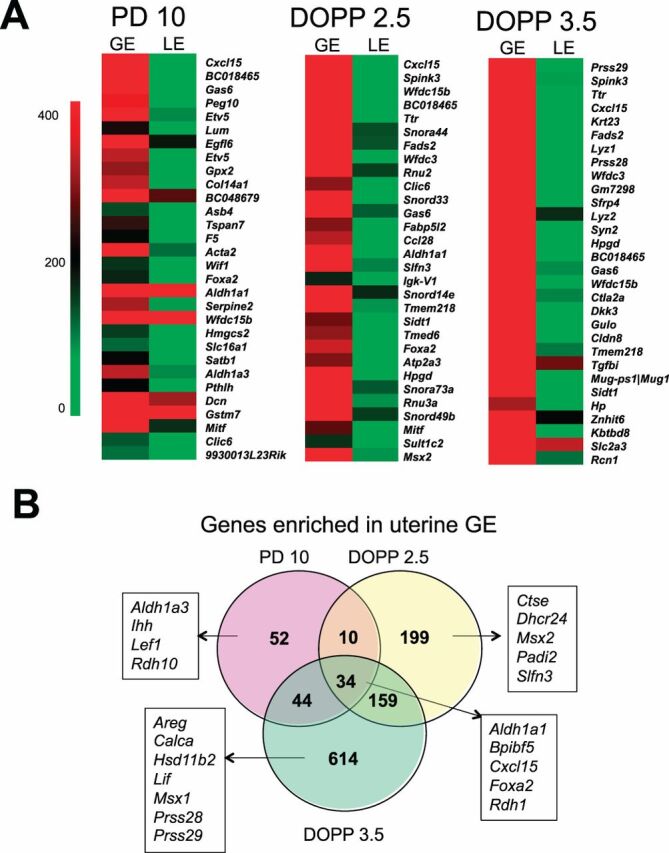
Glandular epithelial (GE) genes expressed in neonatal and adult mouse uteri.A) Heat map of the most GE-enriched genes in PD 10, DOPP 2.5, and DOPP 3.5. Normalized probe intensity values are presented for the top 30 genes significantly enriched in GE.B) Venn diagram comparing GE-enriched genes between neonatal (PD 10) and adult uteri (DOPP 2.5 and 3.5).
In the neonatal PD 10 uterus, 120 genes were enriched in the GE, including known GE-specific genes such asFoxa2 [12,15],Cxcl15 [39], andLef1 [40]. Interestingly, 52 of those genes (e.g.,Aldh1a3, Ihh, Lef1) were expressed in the glands of the neonatal but not adult uteri. DAVID functional annotation analysis revealed that the GE-enriched genes in the PD 10 uterus were associated with branching morphogenesis, growth and retinoic acid (RA) biosynthesis (Table 2 andSupplemental Table S9). Several of those genes were found to encode secretory proteins (e.g.,Cxcl15, Ihh, Sfrp2, Wif1, Wfdc15b), transporters (e.g.,Abcc4, F5, Slc6a2), or enzymes (e.g.,Aldh1a1, Rdh1, Maob, Soat1) (seeSupplemental Table S10 for the complete lists).
Table. 2.
Selected results of DAVID functional annotation clustering for differentially expressed genes in the GE of the PD 10 and DOPP 2.5 and 3.5 mouse uteri.
| Representative functional terms of overrepresented annotation clustersa | Enrichment scoreb |
|---|---|
| PD 10 | |
| Morphogenesis of a branching structure (10, 9.8) | 6.0 |
| Lung development (6, 6.6); respiratory tube development (6, 6.5) | 3.2 |
| EGF-like calcium-binding (6, 8.9) | 3.1 |
| Isoprenoid metabolic process (6, 15.3); retinoid metabolic process (5, 21.8) | 2.7 |
| Concanavalin A-like lectin/glucanase, subgroup (5, 8.9) | 2.4 |
| Glycosaminoglycan binding (6, 6.3); carbohydrate binding (7, 2.6) | 2.2 |
| Mesenchymal cell differentiation (4, 9.9) | 1.9 |
| Branching involved in ureteric bud morphogenesis (3, 13.0) | 1.6 |
| Cell migration | 1.2 |
| DOPP 2.5 | |
| Cell cycle (26, 2.5) | 5.1 |
| Condensed chromosome (7, 3.5) | 2.5 |
| Retinoic acid metabolic process (4, 14.1); cellular hormone metabolic process (4, 4.7) | 2.0 |
| Collagen (4, 11.4) | 1.9 |
| Alcohol dehydrogenase (3, 18.8) | 1.8 |
| Sulfatase (3, 13.9) | 1.6 |
| NAD(P)-binding domain (6, 2.3) | 1.5 |
| Tight junction (5, 4.9) | 1.4 |
| EGF-like 4; calcium-binding (5, 8.9) | 1.1 |
| DOPP 3.5 | |
| Proteinaceous extracellular matrix (49, 3.4) | 13.9 |
| EGF-like calcium-binding, conserved site (18, 4.6) | 6.0 |
| Polysaccharide binding (20, 3.5) | 5.2 |
| Egf-like domain (26, 2.7) | 4.7 |
| Proteinase inhibitor I1, Kazal (11, 5.6) | 4.5 |
| Regulation of locomotion (14, 3.0) | 2.9 |
| Transmembrane (243, 1.11) | 2.6 |
| Regulation of kinase activity (18, 2.2); regulation of phosphorylation (23, 1.9) | 2.4 |
Values within parenthess indicate the number of genes and fold enrichment of the functional term.
Geometric mean of member'sP values of the corresponding annotation cluster (in −log10 scale).
As illustrated inFigure 3B, 199 genes (e.g.,Ctse, Msx2) were expressed in GE predominantly on DOPP 2.5 whereas 614 genes (e.g.,Lif, Prss28) were unique for GE on DOPP 3.5. A total of 34 genes (e.g.,Aldh1a1, Foxa2) were GE-enriched in both developing neonatal and adult endometrial glands (Fig. 3). Importantly, a number of known GE-specific genes were identified as GE-enriched on either DOPP 2.5 or 3.5 includingFoxa2,Lif,Spink3, andTtr [12,15,22,41,42]. Genes enriched in GE on DOPP 2.5 encoded secretory proteins (e.g.,Arsj, Calca, Spink3), transporters (e.g.,Cldn2, Slc1a5, Ttr), and enzymes (e.g.,Aldh1a1, Ctse, Hp, Lyz1, Tst) (Supplemental Table S10). DAVID functional annotation analysis revealed that cell cycle, RA metabolism, and tight junctions were significantly enriched in the GE of DOPP 2.5 uterus (Table 2 andSupplemental Table S11). On the contrary, DAVID functional annotation analysis found that regulation of proteinaceous extracellular matrix, polysaccharide binding cascade, and regulation of kinase activity as overrepresented among GE-enriched genes on DOPP 3.5 (Table 2 andSupplemental Table S12). Based on GO analysis, several of those genes encode secreted proteins (e.g.,Calca2, Cxcl15, Lif, Lipf, Serpina3n, Spink3), transporters (e.g.,Acsl1, Abcc4, Heph, Slc2a3, Ttr), and enzymes (e.g.,Arsj, Agr2, F5, Hp, Mmp2, Prss28, Prss29) (Supplemental Table S10).
Transcriptome Analysis of the Adult Control and PUGKO Uteri
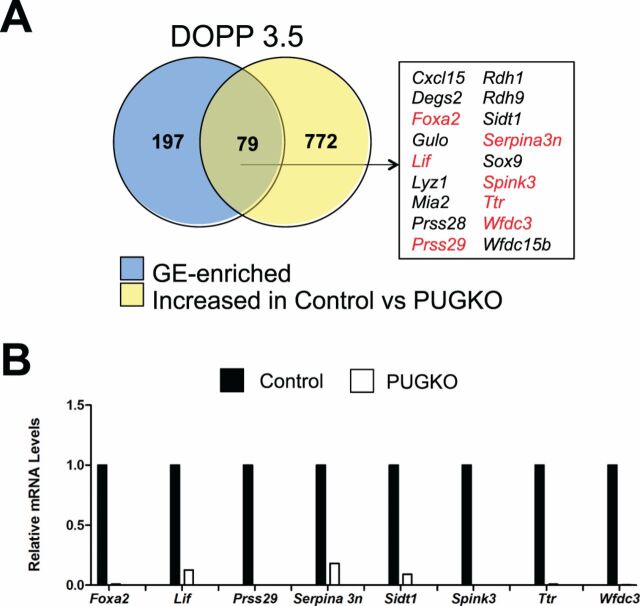
Transient exposure of neonatal C57/BL6J mice to progesterone abrogates postnatal endometrial adenogenesis and generates adult mice with glandless uteri, termed the PUGKO mouse [12,28,43]. The PUGKO uterus is an excellent model to discover gland-specific genes, as the uteri are completely devoid of glands. The list of GE-enriched genes identified in the PD 10 uterus, using LCM and microarray analysis, was integrated with 478 genes previously found to be significantly (P < 0.05) and numerically (fold change > 1.5) increased in control as compared to PUGKO mouse uteri from PD 10 provided by our previous study [28]. As presented inFigure 4A, this comparison produced a set of 14 genes, including the previously known GE-specific transcription factorFoxa2 [12,15]. Real-time qPCR analysis validated the differential expression of several genes in the control and PUGKO uteri (Fig. 4B).
Fig. 4.
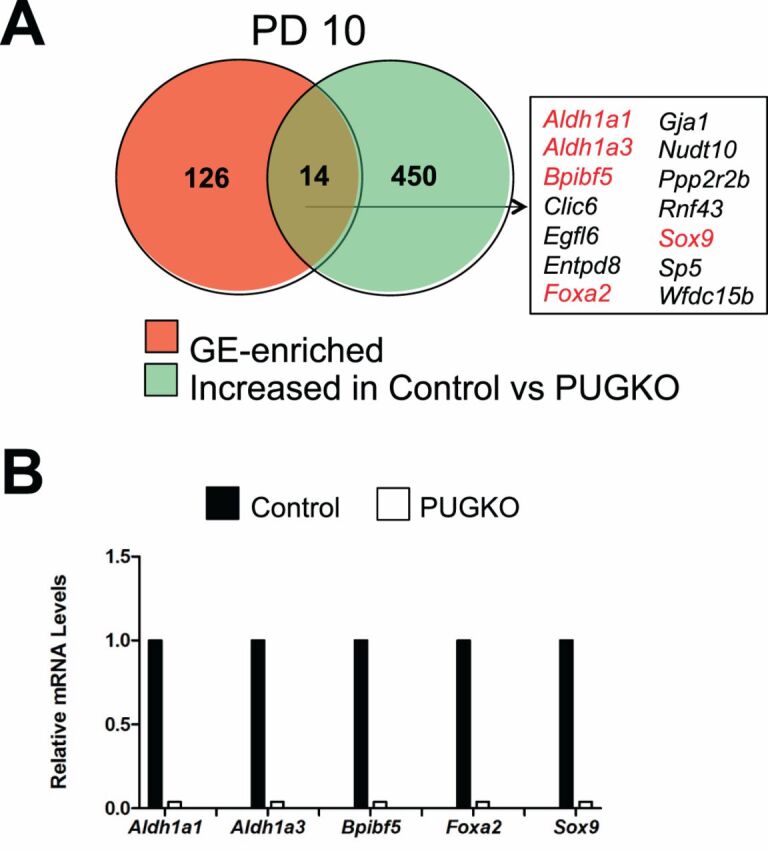
Genes enriched in the uterine glands of neonatal mice.A) Venn diagram is presented showing intersection between GE-enriched genes and genes increased in the uteri of control as compared to PUGKO mice on PD 10 as determined by microarray analysis. Genes marked in red were validated by RT-PCR analysis.B) Validation of selected GE-enriched genes by semiquantitative RT-PCR analysis. Relative mRNA levels of the indicated genes were measured in the uteri of control and PUGKO mice on PD 10 (n = 4 mice/day/treatment). Real-time PCR data are presented as fold change relative to the mRNA level in uteri from control mice.
Next, microarray analysis was performed on uteri of control and PUGKO mice on DOPP 3.5. The expression of 287 genes was greater (P < 0.05, fold change > 1.5) in control as compared to PUGKO mice (Fig. 5A andSupplemental Table S13). Selected genes were verified by qPCR analysis for DOPP 3.5 control and PUGKO uteri (Fig. 5B). DAVID functional annotation analysis revealed that cytokine-cytokine receptor signaling (e.g.,Ccl7, Cxcl15, Il13ra2, Lif), steroid hormone biosynthesis (Akr1c18, Cyp3a16, Cyp3a25, Srd5a1), JAK-STAT signaling (e.g.,Il6, Lif, Socs3), cytosolic DNA-sensing (Zbp1, Il33, Il6, Tmem173), and chemokine signaling (e.g.,Gng12, Hck, Vav1) were enriched in that set of genes. In contrast, the expression of only 29 genes (e.g.,Krt19, Lass3, Stc1, Tbx18) was greater (P < 0.05, fold change > 1.5) in the uterus of PUGKO as compared to control DOPP 3.5 mice. As shown inFigure 5A, integration of cell type-specific microarray results with the list of genes increased in control mice identified 79 candidate GE-specific genes in the adult DOPP 3.5 mouse uterus. Indeed,Lif andSpink3 are expressed solely in glands of the uterus in mice [16,42]. Many of those genes encode secreted proteins (e.g.,Lif, Prss28, orSpink3), enzymes (e.g.,Arg2, Lipf, Lyz1, Rdh10), or transporters (e.g.,Clcn5, Slco2a1, Ttr) (Table 3).
Fig. 5.
Genes enriched in the uterine glands of adult pseudopregnant mice.A) Venn diagram is presented showing intersection between GE-enriched genes and genes increased in the uteri of control as compared to PUGKO mice on DOPP 3.5 as determined by microarray gene expression analysis. Genes marked in red were validated by RT-PCR analysis.B) Validation of selected GE-enriched genes by semiquantitative RT-PCR analysis. Relative mRNA levels of the indicated genes were measured in the uteri of control and PUGKO mice on DOPP 3.5 (n = 4 mice/day/treatment). Real-time PCR data are presented as fold change relative to the mRNA level in uteri from control mice.
Table. 3.
Partial list of uterine gland-enriched genes that encode enzymes, secretory proteins, or transport proteins.
| Gene symbol | Gene description |
|---|---|
| Encodes a secreted protein | |
| Acpp | Acid phosphatase, prostate |
| Cxcl15 | Chemokine (C-X-C motif) ligand 15 |
| Lif | Leukemia inhibitory factor |
| Lipf | Lipase, gastric |
| Mia2 | Melanoma inhibitory activity 2 |
| Gm7298 | Murinoglobulin 1; predicted gene 7298 |
| Pla2g10 | Phospholipase A2, group X |
| Pigr | Polymeric immunoglobulin receptor |
| Gm106 | Predicted gene 106 |
| Prss28 | Protease, serine, 28 |
| Serpina3n | Serine (or cysteine) peptidase inhibitor, clade A, member 3N |
| Spink3 | Serine peptidase inhibitor, Kazal type 3 |
| Prss29 | Similar to implantation serine proteinase 2; protease, serine, 29 |
| Ttr | Transthyretin |
| Wfdc15b | WAP four-disulfide core domain 15B |
| Encodes a transport protein | |
| Clic6 | Chloride intracellular channel 6 |
| Slc12a3 | Solute carrier family 12, member 3 |
| Slc39a4 | Solute carrier family 39 (zinc transporter), member 4 |
| Slco2a1 | Solute carrier organic anion transporter family, member 2a1 |
| Sv2b | Synaptic vesicle glycoprotein 2 b |
| Stx18 | Syntaxin 18 |
| Ttr | Transthyretin |
| Encodes an enzyme | |
| Arg2 | Arginase type II |
| Degs2 | Degenerative spermatocyte homolog 2 (Drosophila), lipid desaturase |
| Gclm | Glutamate-cysteine ligase, modifier subunit |
| Gulo | Gulonolactone (L-) oxidase |
| Lipf | Lipase, gastric |
| Lyz1 | Lysozyme 1 |
| Pla2g10 | Phospholipase A2, group X |
| Prss28 | Protease, serine, 28 |
| Prss29 | Similar to implantation serine proteinase 2; protease, serine, 29 |
| Rdh1 | Retinol dehydrogenase 1 |
| Rdh9 | Retinol dehydrogenase 9 |
| Tdo2 | Tryptophan 2,3-dioxygenase |
| Tmprss11a | Transmembrane protease, serine 11a |
Discussion
This work represents a comprehensive discovery of genetic networks that are active in the uterine epithelia in the developing neonatal and peri-implantation adult uteri. This study provides strong support for the idea that each epithelial compartment has a unique genetic signature that dictates their differential and synergistic function in the uterus [20]. Thus, these results significantly extend and fill a gap in our knowledge of the endometrial epithelial transcriptome [17–21]. The studies provide new insights into the mechanisms governing uterine adenogenesis in the neonate and the biological roles of uterine epithelia in regulation of uterine receptivity, blastocyst implantation, and stromal cell decidualization. Further, this data can be used to develop mouse models useful for the epithelial cell-specific modulation of genes to determine their biological roles in uterine function using the mouse as a model organism.
In the present study, 65 genes were enriched in LE of both the neonatal and adult mouse uteri. Those genes are associated with epithelial cell differentiation (e.g.,Wnt7a, Sprr1a) and innate immune response (e.g.,Il18r1, C3). As anticipated,Wnt7a was found among LE-enriched genes in mouse uterus [34,44,45]. Indeed,Wnt7a is expressed specifically in the LE of the mouse uterus, and null and conditionalWnt7a deleted mice lack endometrial glands and are infertile [46]. TheWnt7a gene encodes a secreted protein, and WNT receptors are present in all endometrial cell types in the neonatal and adult mouse uteri [45,47]. Thus, WNT7A likely acts in an autocrine and paracrine manner to govern endometrial development and function. Indeed, epithelial-mesenchymal interactions are critical for uterine development and function [5,9,10]. In the present study, at least 15 additional secretory protein-encoding genes (e.g.,Cxcl17, Enpp3) were identified as enriched in the LE of neonatal and adult uteri and thus represent potential mediators of epithelial-stromal interactions in the developing and adult mouse uteri. The effect of deleting most of those novel LE-enriched genes on mouse uterine development and function has not been reported. Therefore, future studies are needed to elucidate functional role of those LE-enriched genes and their products in postnatal uterine development.
In the neonatal PD 10 mouse uterus, more than 80 genes were more abundantly or exclusively expressed in the GE. Those genes are associated with processes important for uterine gland development including epithelial development (e.g.,Gja1), growth (e.g.,Ihh), and morphogenesis of an epithelial bud (e.g.,Pthlh) [5,8].Lymphoid-enhancing factor one (Lef1) was found among genes specifically enriched in the GE of the neonatal uterus. LEF1 is a CTNNB1-regulated transcription factor that is expressed in the uterine stroma as early as PD 3 and then in the uterine GE as they develop after PD 7 [40]. Of note, LEF1 is essential for normal endometrial gland development becauseLef1-null mice lack endometrial glands whereas all other uterine cell types appear normal [40]. As expected,Foxa2 was one of GE-enriched genes that were also found to be more abundant in the uteri from control rather than PUGKO mice on PD 10. Immunoreactive FOXA2 is present specifically in the GE cells of the mouse uterus [12,15,28]. Conditional deletion ofFoxa2 after birth inhibits endometrial adenogenesis. Thus, FOXA2 is an important regulator of endometrial adenogenesis in mice. Other pathways involved in endometrial gland development in the neonatal mouse uterus include WNT signaling (canonical CTNNB1 and noncanonical pathways) as well as cell-cell adhesion (CDH1) [48,49]. However, deletion of other GE-enriched or -specific genes, such asCxcl15 orTtr, has no effect on endometrial development [41,50]. Results of the present studies support the idea that other genes and pathways are involved in endometrial adenogenesis and postnatal development of the mouse uterus. Components of RA signaling, includingaldehyde dehydrogenase 1 (Aldh1a1),Aldh1a3,retinol dehydrogenase 1 (Rdh1), andRdh10, were enriched in the GE of the neonatal uterus. Further, expression ofAldh1a1 andAldh1a3 was much lower in PUGKO than in control mice on PD 10. Although not investigated in the neonatal mouse, RA is involved in the patterning and development of a number of different organs [51]. Mice with null mutation ofAldh1a1 orRdh1 are viable and fertile [52,53]. In contrast, complete deletion ofRdh10 orAldh1a3 result in prenatal lethality, precluding investigation of their role in postnatal organogenesis and homeostasis [52,54,55]. It is well established that the biological effects of RA during development and postnatal life are transduced by two families of nuclear receptors, the RA receptors (RARs) and the retinoid X receptor (RXRs). Interestingly, compound null mutations ofRar genes in mice lead to lethality in utero or shortly after birth and to numerous developmental abnormalities, including the genitourinary system [56]. The involvement of RA signaling in postnatal mouse uterine development and endometrial adenogenesis will require conditional postnatal deletion models such as the progesterone receptor Cre (PGR-Cre) [57].
In the peri-implantation mouse uterus, the LE of the uterus has a number of different biological roles in uterine receptivity and blastocyst implantation. The LE is the site of trophectoderm attachment for implantation after hatching of the blastocyst from the zona pellucida. Reciprocal interactions between the LE and the trophectoderm, as well as the adjacent stroma, are important for early pregnancy success. In the present study,Irg1 andScnn1a were LE-enriched in the adult uterus (DOPP 2.5 and/or 3.5).Immune-responsive gene 1 (Irg1), a progesterone-induced gene, is expressed in the LE of pregnant uterus between Days 3 and 5. Knockdown ofIrg1 impaired embryo implantation, implying that IRG1 is involved in early events of pregnancy establishment in mice [35].Sodium channel, nonvoltage-gated 1 alpha (Scnn1a) encodes amiloride-sensitive epithelial Na+ channel (ENaC), and ENaC protein was predominantly localized to the apical side of both LE and GE in both mated and unmated uteri, particularly on Day 3 postmating. Recently, ENaC was found to regulate prostaglandin E2 production and release for embryo implantation [27,38]. In agreement, blocking or knocking down uterine ENaC in mice resulted in implantation failure [38].
Interestingly, genes involved in sterol biosynthetic pathway (Cyp51, Hmgcr, Insig1, Fdps, Hmgcs1, Idi1, Sc4mol, Nsdhl, Fdft1) were overrepresented in LE on DOPP 3.5. Cytochrome P450, family 51 (CYP51) is an essential enzyme in the biosynthesis of cholesterol [58]. Interestingly, CYP51 was previously reported in peri-implantation mouse uterus [59]. Estrogen treatment of ovariectomized mice was shown to induce CYP51 in LE and GE. Interestingly, immunoreactive CYP51 was observed in subluminal stroma immediately surrounding the implanting blastocyst and then in decidual cells on Days 6 and 8 of pregnancy. Thus, de novo biosynthesis of cholesterol by CYP51 may increase the production of progesterone and/or estrogen to promote embryo implantation and the establishment of pregnancy [59]. Indeed, a recent study indicated that estrogen is synthesized de novo in the decidualizing mouse uterus [60]. Expression offarnesyl diphosphate synthetase (Fdps) was previously reported in the uterine epithelium of nonpregnant mouse [61]; however, its functional role is unknown. In addition, null mutation ofHmgcr, Nsdhl, orFdft1 are embryonic lethal. Thus, future studies using conditional gene targeting approaches are necessary to determine the precise nature of LE-enriched genes associated with steroid biosynthesis.
The present study indicated numerous genes (e.g.,Ihh andKlf5) coordinately expressed by LE and GE. Indian hedgehog (IHH), a major effector of PGR action in the uterus, acts as a paracrine signal for epithelial-stromal interaction for achieving uterine receptivity and implantation [14].Ihh is expressed in the uterine LE and GE with an expression peak right before the time of implantation.Ihh-deficient female mice are infertile [24]. The uteri of these mice are unable to support embryo implantation and fail to undergo the artificially induced decidualization [62]. Kruppel-like factor 5 (KLF5) is a zinc finger-containing transcription factor. In mice, KLF5 is present in LE and GE until decidualization is initiated on Day 5. In the present study, KLF5 was identified as LE-enriched gene on both DOPP 2.5 and 3.5. KLF5 is a steroid hormone-independent factor that is indispensable for normal implantation in mice [63]. Mice with uterine deletion ofKlf5 are severely subfertile as a result of defective implantation.
Uterine epithelia are responsible for secretion of bioactive substances (e.g., amino acids and glucose) into the uterine lumen and their transport from the lumen to stromal compartment, and thus they establish an adequate uterine environment that is a prerequisite for embryo implantation. Amino acid (leucine and arginine) activation of mTORC1 is an important aspect of blastocyst activation, in particular for induction of motile, invasive behavior in the trophectoderm [11]. Of particular note, numerous amino acid transporters (Slc1a5, Slc7a4, Slc1a4, Slc7a5), facilitated glucose transporters (Slc2a1, Slc2a3, Slc2a12), and sodium/glucose cotransporters (Slc5a1, Slc5a11) were expressed in adult uterine LE and/or GE in the present study. In addition, glucose metabolism is important for the preparation of the epithelium and stroma for embryo implantation during early pregnancy (for a review, see [64]). For example, SLC2A1 is expressed in the LE, GE, and stroma of rodent uterus, and its basolateral localization in epithelial cells likely facilitates transport of glucose into the decidualizing stroma [65,66].
In contrast to the biological roles of genes expressed in the LE and stroma of the peri-implantation mouse uterus, relatively little is known about genes enriched in the uterine glands [9,10,20]. Notably, endometrial gland secretions (e.g., LIF) play vital biological roles in regulating uterine receptivity blastocyst implantation and stromal cell decidualization [12,15].Lif expression is first induced in the GE in response to the preovulatory E2. LIF binds to its receptor LIFR present in LE, which partners with coreceptor gp130 (I16st) to activate downstream signaling via signal transducer and activator of transcription 3 (STAT3) [67]. As a result, LIF induces expression of LE-enriched genes, includingHegf1, Coch, Igfbp3, andIrg1, that are associated with uterine receptivity (for a review, see [68]). Furthermore, a recent study found that conditional ablation ofStat3 causes implantation failure because of defective uterine receptivity and decidual response [69]. Strikingly, knowledge about the function of adult GE-specific genes other thanLif is limited [9,10]. Therefore, the GE transcriptome analysis conducted here for the prereceptive (DOPP 2.5) and receptive (DOPP 3.5) uteri is novel and provides a foundation for future studies on the role of GE genes in uterine function. In the adult pseudopregnant uterus, a large number of genes (6975 on DOPP 2.5 and 8067 on DOPP3.5) were expressed in the GE as determined by LCM and microarray analysis; many of them (6% on DOPP 2.5 and 11% on DOPP 3.5) are abundantly or exclusively expressed in the GE. In addition, GE-expressed genes were identified in microarray analysis of the uteri of control and gland-containing and aglandular PUGKO uteri in the present study. The lack of uterine glands appears to be the key defect that underlies the infertility and lack of blastocyst implantation and stromal cell decidualization defect in the uterus of PUGKO mice [12]. Numerous genes encoding enzymes (e.g.,Lyz1, Gulo, Prss28) and secretory (e.g.,Pigr, Spink3) and transport proteins (e.g.,Slco2a1, Ttr) were found to be GE-specific with reduced or absent expression in the adult PUGKO uteri. Interestingly, secreted tryptasesPrss28 andPrss29 are secreted together into the uterine lumen at peri-implantation period of pregnancy where they likely promote blastocyst activation and invasion [70,71].Spink3 mRNA is expressed only in the endometrial GE of the mouse uterus; however, SPINK3 protein is present in the LE and decidual cells as well as uterine glands [42]. Thus, SPINK3 is secreted in both an apical and basal manner, as found for many other proteins secreted by polarized epithelia. Novel GE-markers identified in the present study includedpolymeric immunoglobulin receptor (Pigr) andsolute carrier organic anion transporter family, member 2a1 (Slco2a1). The PIGR is a transmembrane secretory component that was shown to regulate extracellular transport of secretory immunoglobulins A and M in the gut epithelium [72]. Deletion ofPigr is associated with the leakage of serum-derived proteins into the intestinal lumen [72]. Further studies will be necessary to determine whether PIGR controls transport of serum proteins across the GE in the adult mouse uterus. SLCO2A1 was identified as a major carrier for prostaglandin transport in mouse and human [73,74]. Here,Slco2a1 was identified as a GE-enriched gene on DOPP 3.5 with decreased expression in the absence of uterine glands. Thus, uterine glands might contribute to synthesis and transport of prostaglandins that are essential for blastocyst implantation and decidualization [27]. The novel set of GE-expressed, -enriched, and -specific genes discovered in the present study provide a framework for future investigations into the biological roles of endometrial glands and their secretions in uterine function, homeostasis, and pregnancy.
Our previous study using pseudopregnant PUGKO mice found no differences in patterns of steroid receptor expression and steroid hormone-regulated genes (Igf1, Muc1, Hand2, Hoxa10, Il13ra2) [12]. In the present study, the expression of 29 genes, includingTbx18 andStc1, was increased in the uteri of aglandular PUGKO as compared with control mice on DOPP 3.5. T-box transcription factor 18 (TBX18) was shown to be expressed in prospective ureteral mesenchyme and regulate the development of the ureteral mesenchyme [75]. Mice carrying a null allele of (Tbx18) die shortly after birth, thus, its role in postnatal uterine morphogenesis or adult function is unknown [75]. Stanniocalcin (STC1) expression was found to shift from the uterine LE to the mesometrial decidua during implantation in mice [76]. Collectively, these results support the idea that endometrial glands and their products play a biological role in the function of other cell types in the uterus, particularly the endometrial LE and stroma, and may be necessary for overall homeostasis of the uterus. Thus, the lack of proper endometrial gland generation and regeneration may have multiple impacts on uterine function and early pregnancy.
The development of the Cre/loxP system provided the means to conditionally ablate genes and determine their biological role in many organs, including the uterus [57]. Although several Cre mouse models (Amhr2-mesenychme;Wnt7a, Krt8, andSprr2f-epithelium;Myh11-myometrium;Pgr-postnatal epithelium, stroma, and myometrium) can be used to conditionally delete genes in the uterus, none are specific for the endometrial glands. Thus, there is a distinct need for new Cre models exhibiting uterine compartment specificity that would facilitate investigation of important questions concerning morphogenesis and adult function of the uterus [14]. Of particular note, the present study identified numerous genes with spatially and temporally restricted expression patterns in the uterine epithelia. For example,Bpibf5, Cxcl15, andGas6 are enriched in GE as early as PD 10. In contrast,Spink3 andPrss29 expression is restricted to adult GE withPrss29 expressed uniquely on DOPP 3.5. Therefore, knowledge gained from this work can be utilized to generate novel Cre mouse lines for cell-specific and time-dependent gene targeting in the mouse uterus.
Collectively, this work strongly supports the idea that LE and GE exhibit distinct genetic signatures not only in the adult uterus but also in the neonatal mouse uterus. These studies along with others provide a foundation for mechanistic investigations into the biology of epithelia development and function in the uterus using the mouse as a model system. Endometrial gland secretions play vital biological roles in regulating uterine receptivity and stromal cell decidualization [8,12,15]. Indeed, deficient glandular activity described as a secretory-phase defect is linked to early pregnancy failure in humans. Thus, a better understanding of endometrial LE and GE at the molecular level could advance diagnosis and treatment of women infertility. A better understanding of the epithelial transcriptome should advance discovery of novel biomarkers of uterine competency for pregnancy useful to diagnose and treat infertility in humans and domestic animals.
Supplementary Material
Acknowledgment
We thank Derek Pouchnik of Molecular Biology and Genomics Core at the Washington State University in Pullman for performing microarray gene expression analyses.
References
- 1. Brody JR,Cunha GR.. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: I. Normal development.Am J Anat 1989;186:1–20. [DOI] [PubMed] [Google Scholar]
- 2. Bartol FF,Wiley AA,Floyd JG,Ott TL,Bazer FW,Gray CA,Spencer TE.. Uterine differentiation as a foundation for subsequent fertility.J Reprod Fertil Suppl 1999;54:287–302. [PubMed] [Google Scholar]
- 3. Hu J,Gray CA,Spencer TE.. Gene expression profiling of neonatal mouse uterine development.Biol Reprod 2004;70:1870–1876. [DOI] [PubMed] [Google Scholar]
- 4. Branham WS,Sheehan DM,Zehr DR,Ridlon E,Nelson CJ.. The postnatal ontogeny of rat uterine glands and age-related effects of 17 beta-estradiol.Endocrinology 1985;117:2229–2237. [DOI] [PubMed] [Google Scholar]
- 5. Spencer TE,Dunlap KA,Filant J.. Comparative developmental biology of the uterus: insights into mechanisms and developmental disruption.Mol Cell Endocrinol 2012;354:34–53. [DOI] [PubMed] [Google Scholar]
- 6. Garry R,Hart R,Karthigasu KA,Burke C.. Structural changes in endometrial basal glands during menstruation.BJOG 2010;117:1175–1185. [DOI] [PubMed] [Google Scholar]
- 7. Huang CC,Orvis GD,Wang Y,Behringer RR.. Stromal-to-epithelial transition during postpartum endometrial regeneration.PLoS One 2012;7:e44285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooke PS,Spencer TE,Bartol FF,Hayashi K.. Uterine glands: development, function and experimental model systems.Mol Hum Reprod 2013;19:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang S,Lin H,Kong S,Wang S,Wang H,Wang H,Armant DR.. Physiological and molecular determinants of embryo implantation.Mol Aspects Med 2013;34:939–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cha J,Sun X,Dey SK.. Mechanisms of implantation: strategies for successful pregnancy.Nat Med 2012;18:1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gonzalez IM,Martin PM,Burdsal C,Sloan JL,Mager S,Harris T,Sutherland AE.. Leucine and arginine regulate trophoblast motility through mTOR-dependent and independent pathways in the preimplantation mouse embryo.Dev Biol 2012;361:286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Filant J,Spencer TE.. Endometrial glands are essential for blastocyst implantation and decidualization in the mouse uterus.Biol Reprod 2013;88:93. [DOI] [PubMed] [Google Scholar]
- 13. Gray CA,Burghardt RC,Johnson GA,Bazer FW,Spencer TE.. Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation.Reproduction 2002;124:289–300. [PubMed] [Google Scholar]
- 14. Large MJ,DeMayo FJ.. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling.Mol Cell Endocrinol 2012;358:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeong JW,Kwak I,Lee KY,Kim TH,Large MJ,Stewart CL,Kaestner KH,Lydon JP,DeMayo FJ.. Foxa2 is essential for mouse endometrial gland development and fertility.Biol Reprod 2010;83:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stewart CL. The role of leukemia inhibitory factor (LIF) and other cytokines in regulating implantation in mammals.Ann N Y Acad Sci 1994;734:157–165. [DOI] [PubMed] [Google Scholar]
- 17. Campbell EA,O'Hara L,Catalano RD,Sharkey AM,Freeman TC,Johnson MH.. Temporal expression profiling of the uterine luminal epithelium of the pseudo-pregnant mouse suggests receptivity to the fertilized egg is associated with complex transcriptional changes.Hum Reprod 2006;21:2495–2513. [DOI] [PubMed] [Google Scholar]
- 18. Chen Y,Ni H,Ma XH,Hu SJ,Luan LM,Ren G,Zhao YC,Li SJ,Diao HL,Xu X,Zhao ZA,Yang ZM.. Global analysis of differential luminal epithelial gene expression at mouse implantation sites.J Mol Endocrinol 2006;37:147–161. [DOI] [PubMed] [Google Scholar]
- 19. Ma XH,Hu SJ,Ni H,Zhao YC,Tian Z,Liu JL,Ren G,Liang XH,Yu H,Wan P,Yang ZM.. Serial analysis of gene expression in mouse uterus at the implantation site.J Biol Chem 2006;281:9351–9360. [DOI] [PubMed] [Google Scholar]
- 20. Niklaus AL,Pollard JW.. Mining the mouse transcriptome of receptive endometrium reveals distinct molecular signatures for the luminal and glandular epithelium.Endocrinology 2006;147:3375–3390. [DOI] [PubMed] [Google Scholar]
- 21. Pan H,Zhu L,Deng Y,Pollard JW.. Microarray analysis of uterine epithelial gene expression during the implantation window in the mouse.Endocrinology 2006;147:4904–4916. [DOI] [PubMed] [Google Scholar]
- 22. Stewart CL,Kaspar P,Brunet LJ,Bhatt H,Gadi I,Kontgen F,Abbondanzo SJ.. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor.Nature 1992;359:76–79. [DOI] [PubMed] [Google Scholar]
- 23. Zhu LJ,Bagchi MK,Bagchi IC.. Attenuation of calcitonin gene expression in pregnant rat uterus leads to a block in embryonic implantation.Endocrinology 1998;139:330–339. [DOI] [PubMed] [Google Scholar]
- 24. Lee K,Jeong J,Kwak I,Yu CT,Lanske B,Soegiarto DW,Toftgard R,Tsai MJ,Tsai S,Lydon JP,DeMayo FJ.. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus.Nat Genet 2006;38:1204–1209. [DOI] [PubMed] [Google Scholar]
- 25. Wang H,Dey SK.. Roadmap to embryo implantation: clues from mouse models.Nat Rev Genet 2006;7:185–199. [DOI] [PubMed] [Google Scholar]
- 26. Daikoku T,Cha J,Sun X,Tranguch S,Xie H,Fujita T,Hirota Y,Lydon J,DeMayo F,Maxson R,Dey SK.. Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity.Developmental Cell 2011;21:1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lim H,Paria BC,Das SK,Dinchuk JE,Langenbach R,Trzaskos JM,Dey SK.. Multiple female reproductive failures in cyclooxygenase 2-deficient mice.Cell 1997;91:197–208. [DOI] [PubMed] [Google Scholar]
- 28. Filant J,Zhou H,Spencer TE.. Progesterone inhibits uterine gland development in the neonatal mouse uterus.Biol Reprod 2012;86(146):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cummings M,McGinley CV,Wilkinson N,Field SL,Duffy SR,Orsi NM.. A robust RNA integrity-preserving staining protocol for laser capture microdissection of endometrial cancer tissue.Anal Biochem 2011;416:123–125. [DOI] [PubMed] [Google Scholar]
- 30. Irizarry RA,Hobbs B,Collin F,Beazer-Barclay YD,Antonellis KJ,Scherf U,Speed TP.. Exploration, normalization, and summaries of high density oligonucleotide array probe level data.Biostatistics 2003;4:249–264. [DOI] [PubMed] [Google Scholar]
- 31. Huang da W,Sherman BT,Lempicki RA.. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources.Nat Protoc 2009;4:44–57. [DOI] [PubMed] [Google Scholar]
- 32. Wang J,Duncan D,Shi Z,Zhang B.. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013.Nucleic Acids Res 2013;41:W77–W83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deb K,Reese J,Paria BC.. Placenta and Trophoblast: Methods and Protocols.Totowa, NJ:Humana Press Inc.;2005. [Google Scholar]
- 34. Miller C,Pavlova A,Sassoon DA.. Differential expression patterns of Wnt genes in the murine female reproductive tract during development and the estrous cycle.Mech Dev 1998;76:91–99. [DOI] [PubMed] [Google Scholar]
- 35. Cheon YP,Xu X,Bagchi MK,Bagchi IC.. Immune-responsive gene 1 is a novel target of progesterone receptor and plays a critical role during implantation in the mouse.Endocrinology 2003;144:5623–5630. [DOI] [PubMed] [Google Scholar]
- 36. Ye X,Hama K,Contos JJ,Anliker B,Inoue A,Skinner MK,Suzuki H,Amano T,Kennedy G,Arai H,Aoki J,Chun J.. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing.Nature 2005;435:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Diao H,Xiao S,Zhao F,Ye X.. Uterine luminal epithelium-specific proline-rich acidic protein 1 (PRAP1) as a marker for successful embryo implantation.Fertil Steril 2010;94:2808–2811. [DOI] [PubMed] [Google Scholar]
- 38. Ruan YC,Guo JH,Liu X,Zhang R,Tsang LL,Dong JD,Chen H,Yu MK,Jiang X,Zhang XH,Fok KL,Chung YW et al. Activation of the epithelial Na+ channel triggers prostaglandin E(2) release and production required for embryo implantation.Nat Med 2012;18:1112–1117. [DOI] [PubMed] [Google Scholar]
- 39. Schmitz JM,McCracken VJ,Dimmitt RA,Lorenz RG.. Expression of CXCL15 (Lungkine) in murine gastrointestinal, urogenital, and endocrine organs.J Histochem Cytochem 2007;55:515–524. [DOI] [PubMed] [Google Scholar]
- 40. Shelton DN,Fornalik H,Neff T,Park SY,Bender D,DeGeest K,Liu X,Xie W,Meyerholz DK,Engelhardt JF,Goodheart MJ.. The role of LEF1 in endometrial gland formation and carcinogenesis.PLoS One 2012;7:e40312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Episkopou V,Maeda S,Nishiguchi S,Shimada K,Gaitanaris GA,Gottesman ME,Robertson EJ.. Disruption of the transthyretin gene results in mice with depressed levels of plasma retinol and thyroid hormone.Proc Natl Acad Sci U S A 1993;90:2375–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen W,Han BC,Wang RC,Xiong GF,Peng JP.. Role of secretory protease inhibitor SPINK3 in mouse uterus during early pregnancy.Cell Tissue Res 2010;341:441–451. [DOI] [PubMed] [Google Scholar]
- 43. Cooke PS,Ekman GC,Kaur J,Davila J,Bagchi IC,Clark SG,Dziuk PJ,Hayashi K,Bartol FF.. Brief exposure to progesterone during a critical neonatal window prevents uterine gland formation in mice.Biol Reprod 2012;86:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miller C,Sassoon DA.. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract.Development 1998;125:3201–3211. [DOI] [PubMed] [Google Scholar]
- 45. Hayashi K,Yoshioka S,Reardon SN,Rucker EB III,,Spencer TE,DeMayo FJ,Lydon JP,MacLean JA II.. WNTs in the neonatal mouse uterus: potential regulation of endometrial gland development.Biol Reprod 2011;84:308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parr BA,McMahon AP.. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a.Nature 1998;395:707–710. [DOI] [PubMed] [Google Scholar]
- 47. Hayashi K,Erikson DW,Tilford SA,Bany BM,Maclean JA II,,Rucker EB III,,Johnson GA,Spencer TE.. Wnt genes in the mouse uterus: potential regulation of implantation.Biol Reprod 2009;80:989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jeong JW,Lee HS,Franco HL,Broaddus RR,Taketo MM,Tsai SY,Lydon JP. DeMayo FJ. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus.Oncogene 2009;28:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reardon SN,King ML,MacLean JA II,,Mann JL,DeMayo FJ,Lydon JP,Hayashi K.. CDH1 is essential for endometrial differentiation, gland development, and adult function in the mouse uterus.Biol Reprod 2012;86(141):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen SC,Mehrad B,Deng JC,Vassileva G,Manfra DJ,Cook DN,Wiekowski MT,Zlotnik A,Standiford TJ,Lira SA.. Impaired pulmonary host defense in mice lacking expression of the CXC chemokine lungkine.J Immunol 2001;166:3362–3368. [DOI] [PubMed] [Google Scholar]
- 51. Mark M,Ghyselinck NB,Chambon P.. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis.Annu Rev Pharmacol Toxicol 2006;46:451–480. [DOI] [PubMed] [Google Scholar]
- 52. Matt N,Dupe V,Garnier JM,Dennefeld C,Chambon P,Mark M,Ghyselinck NB.. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells.Development 2005;132:4789–4800. [DOI] [PubMed] [Google Scholar]
- 53. Zhang M,Hu P,Krois CR,Kane MA,Napoli JL.. Altered vitamin A homeostasis and increased size and adiposity in the rdh1-null mouse.FASEB J 2007;21:2886–2896. [DOI] [PubMed] [Google Scholar]
- 54. Dupe V,Matt N,Garnier JM,Chambon P,Mark M,Ghyselinck NB.. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment.Proc Natl Acad Sci U S A 2003;100:14036–14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sandell LL,Lynn ML,Inman KE,McDowell W,Trainor PA.. RDH10 oxidation of vitamin A is a critical control step in synthesis of retinoic acid during mouse embryogenesis.PLoS One 2012;7:e30698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mendelsohn C,Lohnes D,Decimo D,Lufkin T,LeMeur M,Chambon P,Mark M.. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants.Development 1994;120:2749–2771. [DOI] [PubMed] [Google Scholar]
- 57. Soyal SM,Mukherjee A,Lee KY,Li J,Li H,DeMayo FJ,Lydon JP.. Cre-mediated recombination in cell lineages that express the progesterone receptor.Genesis 2005;41:58–66. [DOI] [PubMed] [Google Scholar]
- 58. Lepesheva GI,Waterman MR.. Sterol 14alpha-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms.Biochim Biophys Acta 2007;1770:467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Song X,Tai P,Yan J,Xu B,Chen X,Ouyang H,Zhang M,Xia G.. Expression and regulation of lanosterol 14alpha-demethylase in mouse embryo and uterus during the peri-implantation period.Reprod Fertil Dev 2008;20:964–972. [DOI] [PubMed] [Google Scholar]
- 60. Das A,Mantena SR,Kannan A,Evans DB,Bagchi MK,Bagchi IC.. De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis.Proc Natl Acad Sci U S A 2009;106:12542–12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Haigh J,McVeigh J,Greer P.. The fps/fes tyrosine kinase is expressed in myeloid, vascular endothelial, epithelial, and neuronal cells and is localized in the trans-Golgi network.Cell Growth Differ 1996;7:931–944. [PubMed] [Google Scholar]
- 62. Franco HL,Lee KY,Broaddus RR,White LD,Lanske B,Lydon JP,Jeong JW,DeMayo FJ.. Ablation of Indian hedgehog in the murine uterus results in decreased cell cycle progression, aberrant epidermal growth factor signaling, and increased estrogen signaling.Biol Reprod 2010;82:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sun X,Zhang L,Xie H,Wan H,Magella B,Whitsett JA,Dey SK.. Kruppel-like factor 5 (KLF5) is critical for conferring uterine receptivity to implantation.Proc Natl Acad Sci U S A 2012;109:1145–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Frolova AI,Moley KH.. Glucose transporters in the uterus: an analysis of tissue distribution and proposed physiological roles.Reproduction 2011;142:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhou J,Bondy CA.. Placental glucose transporter gene expression and metabolism in the rat.J Clin Invest 1993;91:845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim ST,Moley KH.. Regulation of facilitative glucose transporters and AKT/MAPK/PRKAA signaling via estradiol and progesterone in the mouse uterine epithelium.Biol Reprod 2009;81:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Niwa H,Burdon T,Chambers I,Smith A.. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3.Genes Dev 1998;12:2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kimber SJ. Leukaemia inhibitory factor in implantation and uterine biology.Reproduction 2005;130:131–145. [DOI] [PubMed] [Google Scholar]
- 69. Lee JH,Kim TH,Oh SJ,Yoo JY,Akira S,Ku BJ,Lydon JP,Jeong JW.. Signal transducer and activator of transcription-3 (Stat3) plays a critical role in implantation via progesterone receptor in uterus.FASEB J 2013;27:2553–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. O'Sullivan CM,Liu SY,Rancourt SL,Rancourt DE.. Regulation of the strypsin-related proteinase ISP2 by progesterone in endometrial gland epithelium during implantation in mice.Reproduction 2001;122:235–244. [DOI] [PubMed] [Google Scholar]
- 71. O'Sullivan CM,Liu SY,Karpinka JB,Rancourt DE.. Embryonic hatching enzyme strypsin/ISP1 is expressed with ISP2 in endometrial glands during implantation.Mol Reprod Dev 2002;62:328–334. [DOI] [PubMed] [Google Scholar]
- 72. Johansen FE,Pekna M,Norderhaug IN,Haneberg B,Hietala MA,Krajci P,Betsholtz C,Brandtzaeg P.. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice.J Exp Med 1999;190:915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pucci ML,Bao Y,Chan B,Itoh S,Lu R,Copeland NG,Gilbert DJ,Jenkins NA,Schuster VL.. Cloning of mouse prostaglandin transporter PGT cDNA: species-specific substrate affinities.Am J Physiol 1999;277:R734–R741. [DOI] [PubMed] [Google Scholar]
- 74. Lu R,Kanai N,Bao Y,Schuster VL.. Cloning, in vitro expression, and tissue distribution of a human prostaglandin transporter cDNA(hPGT).J Clin Invest 1996;98:1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Airik R,Bussen M,Singh MK,Petry M,Kispert A.. Tbx18 regulates the development of the ureteral mesenchyme.J Clin Invest 2006;116:663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stasko SE,DiMattia GE,Wagner GF.. Dynamic changes in stanniocalcin gene expression in the mouse uterus during early implantation.Mol Cell Endocrinol 2001;174:145–149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.