Abstract
Roots enable the plant to survive in the natural environment by providing anchorage and acquisition of water and nutrients. In this study, root architectural traits of 153 mungbean genotypes were compared under optimum and low phosphorus (P) conditions. Significant variations and medium to high heritability were observed for the root traits. Total root length was positively and significantly correlated with total root surface area, total root volume, total root tips and root forks under both optimum P (r = 0.95, r = 0.85, r = 0.68 and r = 0.82 respectively) and low P (r = 0.95, r = 0.82, r = 0.71 and r = 0.81 respectively). The magnitudes of the coefficient of variations were relatively higher for root forks, total root tips and total root volume. Total root length, total root surface area and total root volume were major contributors of variation and can be utilized for screening of P efficiency at the seedling stage. Released Indian mungbean varieties were found to be superior for root traits than other genotypic groups. Based on comprehensive P efficiency measurement, IPM-288, TM 96–25, TM 96–2, M 1477, PUSA 1342 were found to be the best highly efficient genotypes, whereas M 1131, PS-16, Pusa Vishal, M 831, IC 325828 were highly inefficient. Highly efficient genotypes identified would be valuable genetic resources for P efficiency for utilizing in the mungbean breeding programme.
Introduction
Mungbean is an important warm season grain legume grown in more than 6 million hectares area [1] for its protein rich seeds. Mungbean seeds are rich source of iron [2], vitamin C and folates [3]. Cultivation of mungbean improves soil fertility through biological nitrogen fixation [4]. Mungbean is cultivated on marginal lands resulting in poor growth, development and yield. Therefore, fertilizer management is important for realizing the potential yield of the crop [5]. Nitrogen (N) and phosphorus (P) are the important macronutrients required for the crop. In mungbean, 80–90% N requirement is met through biological N2 fixation [6] however, it requires 48.1 kg P2O5 for producing one ton of grains [7]. Under tropical and subtropical conditions, P is the main yield limiting factor [8].
Globally, by the year 2020, P fertilizer requirement is expected to reach 64.68 million tonnes, whereas, the estimated supply is 53.08 million tonnes and the demand for P fertilizer requirement is increasing annually by 2.2% on an average from 2015–2020 [9]. Countries like US, China and Morocco are the leading producers of phosphatic fertilizer [10]. In anticipation of future domestic demands, the US and China have stopped the export of rock phosphate to other countries [11]. The deficiency of P leads to a higher root/shoot ratio as shoot growth is relatively more affected in comparison to root growth [12]. It also causes stunted growth and foliage turns dark green color with reddish-purple tips and leaf margins due to the accumulation of starch and anthocyanin in the leaves [13]. Deficiency in leaves disturbs the photosynthetic machinery and electron transport chain through repression of orthophosphate concentration in chloroplast stroma inhibiting ATP synthase activity [14]. P is a key component of nucleic acids, membrane lipids and participates in energy transfer reactions, and thus determines the yield and quality of a crop [15, 16]. Its deficiency in soil can be overcome by P fertilizer application, but excess application leads to the delayed formation of reproductive organs [17]. Acquisition of P from the soil is a complex process as it is bound to calcium in alkaline soils and iron and aluminium in acid soils [18].
Change in root architecture explores the soil space and thus enhanced root-soil contact increases P efficiency [19–21]. The rate of nutrient acquisition by plant roots depends upon the particular nutrient concentration at the root surface, root properties and plant requirements [22]. The root is an indispensable organ of the plant for the absorption of nutrients and water by expanding its surface area and enhancement of explored soil volume [23]. Under low P conditions, plants modify their root architectural traits [23, 24] which include reduced primary root growth, increase in number and length of lateral roots and root hairs [25–27], increase in root surface area and volume [28], shallower root growth angle [29] and enhancement of root biomass [30] for enhancement of phosphorus uptake.
Genetic variation in plant root architecture can be exploited to improve the nutrient and water use efficiency under difficult growing conditions [31]. Root surface area, volume, biomass and root carboxylate exudation capacity were reported to be significantly higher in P efficient mungbean genotype compared to inefficient genotype [32]. The significant contribution of root length, root volume, surface area and the number of lateral roots towards P uptake at 45 days after sowing was observed in blackgram [28]. P deficiency causes a significant increase in primary root length, total root length and number of lateral roots after eight days of treatment in lentil [33]. High adventitious and lateral root densities were associated with high P uptake per unit length in soybean and common bean [34, 35]. Genotypes with a large root system with deep lateral roots exhibited high shoot and root P use efficiency compared to genotypes with medium and small root system in lupin [36]. In rice, root hair length and density significantly increased in all tested genotypes under low P conditions [37]. Shen et al. [38] stressed on maintaining root biomass and root length to cope with the deficiency of P in wheat. Under low P condition, decrease in root length was more in fibrous root species (wheat, rapeseed than in legumes (broad bean, soybean, chickpea and lupin). Maize and wheat had a higher root to shoot ratio and rapeseed had higher specific root length than legumes [39].
Although P deficiency can affect crop growth throughout the season, phenotypic evaluation at the seedling stage is an attractive approach, as it is high throughput and low-cost, which saves time and space [40]. Stress gradient hypothesis [41, 42] proposes that the fate of seedlings determines the structure and dynamics of the plant population. Current digital image analysis enables accurate analysis of plant root system and is time and labour-saving technology [43, 44]. Considering the role of root architecture in P efficiency, the present study was designed to (i) characterize the phenotypic variation for morphological root traits in 153 mungbean genotypes, (ii) identify the root related traits accounting for most of the variation among the tested mungbean genotypes, and (iii) evaluate the efficiency of mungbean genotypes under optimum and low P conditions.
Materials and methods
Plant materials and plant growth conditions
One hundred and fifty-three mungbean genotypes including 41 Indian released varieties (IRV), 44 Advanced Breeding lines (ABL)and 68 Germplasm lines (GL) were studied for root architecture characteristics under optimum P (OP) and low P(LP) conditions (S1 Table). The experiment was conducted in a National Initiative on Climate Resilient Agriculture (NICRA)-controlled environment facility of the Indian Agricultural Research Institute, New Delhi, India from December 2017 to September 2018. The growth conditions in the greenhouse were maintained as 30/18°C day/night temperature, photoperiod of 12 h and relative humidity at 90%. For screening under hydroponics, mungbean seeds were surface sterilized with 0.1% (w/v) HgCl2 for 3 minutes followed by rinsing with double distilled water and wrapped in germination paper. Upon the emergence of cotyledonary leaves, seedlings of uniform size and without visible root injuries were transferred to the modified Hoagland solution. Composition of basal nutrient solution used was MgSO4 (1mM), K2SO4(0.92 mM), CaCl2.2H2O (0.75 mM), Fe-EDTA (0.04mM), Urea (5 mM), and micronutrients [H3BO3(2.4μM), MnSO4 (0.9μM), ZnSO4 (0.6μM), CuSO4 (0.62μM), and Na2MoO4 (0.6μM)] [45]. Two levels of P were maintained using KH2PO4 as optimum P (250 μM) and low P (3μM). A preliminary experiment was conducted with a series of P concentrations to select the optimum and low P levels. Analysis of observations on biomass, chlorophyll content and visual symptoms led to the selection of optimum (250μM) and low (3 μM) P concentration (data not presented) (S1 Fig). The chlorophyll concentration was measured by using MC-100 chlorophyll concentration meter (Apogee Instrumnets, Inc., USA). The biomass was calculated by drying the plants at 60°C until obtaining the constant mass of dry weight. The pH of the nutrient solution was maintained at 6.0 using 1 M KOH or 1 M HCL. Seedlings were supported on a 2” thick thermocol sheet with holes made at 5 × 5 cm plant-to-plant and row-to-row distance. This sheet was fitted into plastic containers (30 × 45 × 15 cm) with 10 L of basal nutrient solution. Forty-five seedlings were raised in one such container and fifteen genotypes were screened at a time with three replicates for each genotype. The solution was aerated regularly by aquarium air pump and replaced on alternate days.
Root measurements
The data on root traits were recorded on twenty-one days old seedlings raised under low and optimum P conditions. The complete root system was isolated from each plant and spread out in a tray with no overlapping of roots. Roots were scanned using root scanner (Epson professional scanner) and greyscale images obtained in TIFF format were analyzed using WinRhizo (Pro version 2016a; Regent Instrument Inc., Quebec, Canada). The settings were used as follows: image resolution, 400 dpi; calibration, intrinsic for the scanner; manual—dark root on white background; bit depth (8 –bit); focal length (0 mm); image dimensions (4395 × 6125 pixels). Roots were placed in a 30 × 40 × 2 cm size acrylic tray with 700 ml water. During root scanning, the debris consisting of occasional broken root segments were manually separated from the root sample by floating them in trays containing water. The recovered clean roots were used for scanning. The following root parameters were obtained: primary root length (PRL), total root length (TRL), total root surface area (TSA), total root volume (TRV), root average diameter (RAD), total root tips (TRT), and root forks (RF). PRL was measured manually using the scale. TRL represents the sum of primary, seminal, crown, basal and lateral roots.
WinRhizo also generated additional output that allowed us to categorize root traits root length (RL), root surface area (RSA), root volume (RV) and number of root tips (RT) into five classes based on root diameter intervals of 0–0.5 mm, 0.5–1.0 mm, 1.0–1.5 mm, 1.5–2.0 mm and >2.0 mm [46–48].
Statistical analysis
The P efficiency coefficient (PEC) was calculated as the ratio of the data derived from the low P (LP) and optimum P (OP) treatment of the same genotype for each trait using the following equation.
Where PECij is the P efficiency coefficient of the trait (j) for the cultivar (i); XijLP and XijOP are the value of the root trait (j) for the cultivar (i) evaluated under low P (LP) and optimum P (OP) treatments, respectively.
The data were subjected to descriptive statistics including mean, standard deviation, coefficient of variation, analysis of variation, heritability and Pearson’s correlation were calculated for tested traits under OP and LP conditions using STAR (Statistical Tool for Agricultural Research) 2.1.0 software [49]. For performing analysis of variance of root traits, the model used was a fixed factor model with both genotype and P as fixed factors. The additive linear model used was:
where Yijk is the observation from kth replicate of ijth experimental unit, μ is the overall mean, Gi is the main effect of ith genotype, Pj is the main effect of jth P level, GPij is the interaction effect between genotype and P, Eijk is the random effect error confounded in the experiment.
The proportion of root traits in each diameter class was calculated as the percentage of the total trait under OP and LP conditions in different groups [48]. Mungbean genotypes (153) were classified into three different categories based on their performance: (i) low performing genotypes (), (ii) medium performing genotypes () to ), and (iii) high performing genotypes (), where and SD are mean and standard deviation of respected root trait [50, 51]. A polymorphic diversity index, Shannon-Weaver diversity index (H’), was calculated for each trait [52–54] using the formula:
Where pi is the proportion of individuals belonging to the ith class and s is the total number of genotypes.
The principal component analysis was performed to identify traits contributing most of the variation in tested mungbean genotypes using STAR 2.1.0 software. A comprehensive P efficiency measurement value (CPEM value) was used to estimate the efficiency capability of all tested mungbean genotypes. The CPEM value was calculated across traits to evaluate mungbean P efficiency by using the formulas described below [46, 55].
Fuzzy subordination method could be used to analyze the P efficiency completely and avoid the shortage of single index. The membership function of a fuzzy set is a generalization of the indicator function in classical sets; it represents the degree of truth as an extension of valuation [46, 56 and 57]. Uij stands for the membership function value of P efficiency (MFVP) that indicates a positive correlation between the trait and P efficiency.
Where Uij is the membership function value of the trait (j) for the cultivar (i) for P efficiency; PECjmax is the maximum value of the P efficiency coefficient for the trait (j); PECjmin is the minimum value of PECj.
Comprehensive P efficiency measurement was made using the formula:
Where CPEM is the comprehensive P efficiency measurement of each mungbean genotype under LP condition. Based CPEM value all mungbean genotypes were classified into five groups, highly efficient, efficient, moderately efficient, inefficient and highly inefficient.
Results
Response of root traits to phosphorus stress
The study of 153 mungbean genotypes for root traits under optimum and low P conditions revealed a high variation in the mean values for the studied traits (Table 1). Independent t-test at the level of significance 0.05 indicated that the mean values of PRL (p-value 0.04) and RAD (p-value <0.01) were significantly high in LP as compared to OP condition. For P efficiency coefficient (PEC), RAD showed high mean value followed by TRV and PRL. The mean values of RL, RSA, RV and RT in five root diameter classes: 0–0.5 mm, 0.5–1.0 mm, 1.0–1.5 mm, 1.5–2.0 mm and >2.0 mm exhibited variation under OP and LP conditions. RL and RSA revealed PEC value above 1 for all root diameters except 0–0.5 mm. The PEC was above 1.0 for RV at all root diameters indicating an increase in root volume in LP condition. The RT was higher under LP in the root diameter class of 0.5–1.0 mm.
Table 1. Mean value, Standard Deviation (SD) of traits investigated under two phosphorus regimes and the Phosphorus Efficiency-Coefficient (PEC) of each trait.
| Trait | Mean ± SD (LP) | Mean ± SD (OP) | Mean ± SD (PEC) |
|---|---|---|---|
| PRL | 36.04 ± 6.38 | 34.78 ± 6.40 | 1.06 ± 0.22 |
| TRL | 809.34 ± 180.07 | 897.67 ± 209.74 | 0.92 ± 0.17 |
| TSA | 85.58 ± 19.32 | 89.48 ± 22.54 | 0.98 ± 0.19 |
| TRV | 0.74 ± 0.19 | 0.71 ± 0.21 | 1.07 ± 0.24 |
| RAD | 0.34 ± 0.02 | 0.31 ± 0.02 | 1.08 ± 0.06 |
| TRT | 788.62 ± 248.04 | 964.77 ± 291.77 | 0.85 ± 0.26 |
| RF | 1817.32 ± 583.50 | 2495.68 ± 735.22 | 0.74 ± 0.20 |
| RL1 | 719.50 ± 158.99 | 815.93 ± 175.23 | 0.89 ± 0.17 |
| RL2 | 64.31 ± 23.85 | 59.96 ± 24.05 | 1.12 ± 0.29 |
| RL3 | 6.31 ± 1.74 | 6.23 ± 1.96 | 1.06 ± 0.29 |
| RL4 | 2.51 ± 0.78 | 2.42 ± 0.87 | 1.14 ± 0.48 |
| RL5 | 0.35 ± 0.18 | 0.41 ± 0.21 | 1.08 ± 1.48 |
| RSA1 | 57.46 ± 12.14 | 59.90 ± 13.63 | 0.98 ± 0.19 |
| RSA2 | 12.68 ± 4.58 | 11.86 ± 4.63 | 1.11 ± 0.29 |
| RSA3 | 2.37 ± 0.65 | 2.34 ± 0.73 | 1.06 ± 0.29 |
| RSA4 | 1.35 ± 0.42 | 1.30 ± 0.47 | 1.14 ± 0.48 |
| RSA5 | 0.29 ± 0.17 | 0.36 ± 0.19 | 1.11 ± 1.77 |
| RV1 | 0.41 ± 0.09 | 0.40 ± 0.10 | 1.05 ± 0.22 |
| RV2 | 0.21 ± 0.07 | 0.19 ± 0.07 | 1.11 ± 0.29 |
| RV3 | 0.07 ± 0.02 | 0.07 ± 0.02 | 1.07 ± 0.29 |
| RV4 | 0.06 ± 0.02 | 0.06 ± 0.02 | 1.14 ± 0.48 |
| RV5 | 0.02 ± 0.01 | 0.03 ± 0.02 | 1.20 ± 2.26 |
| RT1 | 772.15 ± 232.56 | 951.37 ± 277.88 | 0.84 ± 0.25 |
| RT2 | 6.64 ± 2.70 | 5.28 ± 2.20 | 1.48 ± 0.98 |
| RT3 | 0.49 ± 0.49 | 0.46 ± 0.50 | 0.89 ± 1.17 |
| RT4 | 0.27 ± 0.35 | 0.18 ± 0.30 | 0.39 ± 0.52 |
| RT5 | 0.05 ± 0.05 | 0.06 ± 0.06 | 0.37 ± 0.49 |
Independent t-test indicated that the mean values of PRL and RAD were significantly high in LP condition compared to OP condition. LP, low phosphorus; OP, optimum phosphorus; PRL, primary root length; TRL, total root length; TSA, total root surface area; TRV, total root volume; TRT, total root tips; RAD, root average diameter; RF, root forks; RL1-5, RSA1-5, RV1-5, RT1-5 indicate average root length, root surface area, root volume and root tips in diameter between 0.0 and 0.5 mm, 0.5 and 1.0 mm, 1.0 and 1.5 mm, 1.5 and 2 mm and greater than 2.0 mm respectively.
Genetic variation and broad sense heritability studies
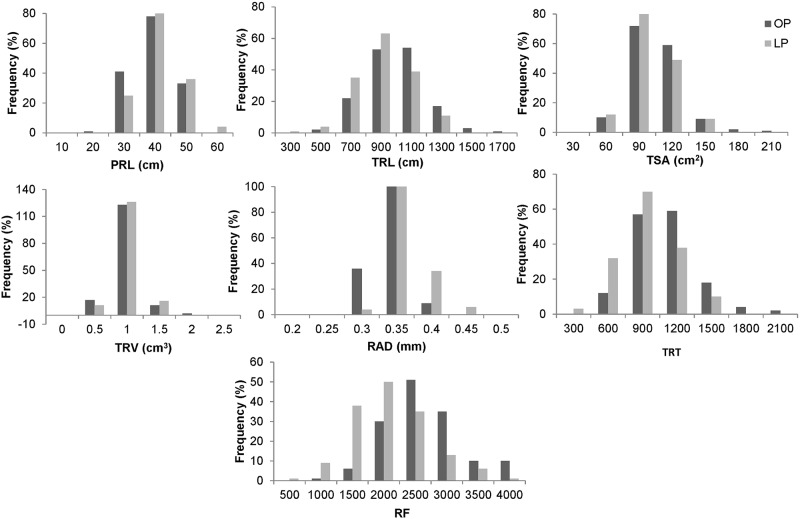
ANOVA analysis revealed highly significant variation among the genotypes for seven traits (PRL, TRL, TSA, TRV, RAD, TRT and RF) evaluated under two P regimes (Table 2). The study revealed highly significant variation among the evaluated traits at two P conditions. The highly significant interaction between genotype and P treatment indicates that genotypes were significantly affected for studied root traits at different P regimes. The level of variation for studied seven P efficiency traits is presented as Fig 1. Histogram of frequency distribution revealed near normal distribution of root traits evaluated in the study. The coefficient of variation for seven investigated traits ranged from 4.64% (RAD) to 16.01% (RF). The broad sense heritability for the studied traits ranged from 0.59 to 0.79. The highest broad sense heritability was observed in RAD (0.79) followed by TRV (0.78) and lowest was observed in PRL (0.59).
Table 2. Analysis of variance for the tested traits under two phosphorus regimes.
| Variables | Mean squares | CV (%) | Heritability | |||
|---|---|---|---|---|---|---|
| Replication (R) | Genotype (G) | Phosphorus (P) | G × P | |||
| df | 2 | 152 | 1 | 152 | ||
| PRL | 39.48 (0.032) | 174.33 (<0.001) | 376.63 (<0.001) | 70.89 (<0.001) | 9.55 | 0.59 |
| TRL | 83366.00 (<0.001) | 183719.63 (<0.001) | 1789765.97 (<0.001) | 45566.49 (<0.001) | 11.96 | 0.75 |
| TSA | 874.45 (<0.001) | 2151.76 (<0.001) | 3503.19 (<0.001) | 491.44 (<0.001) | 12.40 | 0.77 |
| TRV | 0.08 (0.001) | 0.20 (<0.001) | 0.18 (0.001) | 0.04 (<0.001) | 15.26 | 0.78 |
| RAD | 0 (0.93) | 0.00 (<0.001) | 0.14 (<0.001) | 0.00 (<0.001) | 4.64 | 0.79 |
| TRT | 21779.66 (0.311) | 321101.87 (<0.001) | 7121141.19 (<0.001) | 118860.09 (<0.001) | 15.58 | 0.63 |
| RF | 364416.28 (0.047) | 2094494.45 (<0.001) | 105608699.30 (<0.001) | 548698.57 (<0.001) | 16.01 | 0.74 |
| RL1 | 65020.02 (0.017) | 138491.6 (<0.001) | 1796062.03 (<0.001) | 43826.93 (<0.001) | 16.11 | 0.68 |
| RL2 | 628.35 (0.021) | 3405.73 (<0.001) | 4450.00 (<0.001) | 634.44 (<0.001) | 20.32 | 0.81 |
| RL3 | 0.98 (0.577) | 15.63 (<0.001) | 0.55 (0.58) | 5.87 (<0.001) | 21.35 | 0.62 |
| RL4 | 0.93 (0.236) | 2.80 (<0.001) | 2.97 (0.031) | 1.17 (<0.001) | 33.03 | 0.58 |
| RL5 | 0.01 (0.697) | 0.17 (<0.001) | 1.20 (<0.001) | 0.09 (<0.001) | 38.06 | 0.47 |
| RSA1 | 264.11 (0.076) | 813.99 (<0.001) | 2472.15 (<0.001) | 274.26 (<0.001) | 17.08 | 0.66 |
| RSA2 | 20.82 (0.034) | 133.11 (<0.001) | 148.84 (<0.001) | 22.01 (<0.001) | 19.90 | 0.83 |
| RSA3 | 0.23 (0.546) | 2.15 (<0.001) | 0.56 (0.223) | 0.78 (<0.001) | 25.95 | 0.64 |
| RSA4 | 0.31 (0.227) | 0.82 (<0.001) | 0.12 (0.441) | 0.35 (<0.001) | 33.76 | 0.57 |
| RSA5 | 0.00 (0.994) | 0.15 (<0.001) | 1.47 (<0.001) | 0.08 (<0.001) | 50.23 | 0.47 |
| RV1 | 0.02 (0.038) | 0.05 (<0.001) | 0.03 (0.035) | 0.01 (<0.001) | 20.03 | 0.80 |
| RV2 | 0.01 (0.043) | 0.03 (<0.001) | 0.03 (0.001) | 0.01 (<0.001) | 27.59 | 0.67 |
| RV3 | 0.000 (0.628) | 0.002 (<0.001) | 0.000 (0.598) | 0.001 (<0.001) | 22.77 | 0.50 |
| RV4 | 0.000 (0.286) | 0.002 (<0.001) | 0.000 (0.244) | 0.001 (<0.001) | 30.92 | 0.50 |
| RV5 | 0 (0.945) | 0.001 (<0.001) | 0.009 (<0.001) | 0.001 (<0.001) | 55.85 | 0.00 |
| RT1 | 4075.20 (0.871) | 328543.55 (<0.001) | 8363414.91 (<0.001) | 127486.04 (<0.001) | 19.43 | 0.61 |
| RT2 | 1.24 (0.761) | 23.51 (<0.001) | 453.89 (<0.001) | 17.45 (<0.001) | 35.10 | 0.26 |
| RT3 | 0.26 (0.395) | 1.18 (<0.001) | 0.06 (0.648) | 0.92(<0.001) | 78.67 | 0.22 |
| RT4 | 0.11 (0.2) | 0.56 (<0.001) | 7.86 (<0.001) | 0.71 (<0.001) | 69.10 | 0.00 |
| RT5 | 0.03 (0.001) | 0.01 (<0.001) | 0.02 (0.014) | 0.01 (<0.001) | 76.48 | 0.08 |
df, degree of freedom; PRL, primary root length; TRL, total root length; TSA, total root surface area; TRV, total root volume; RAD, root average diameter; TRT, total root tips; RF, root forks; RL1-5, RSA1-5, RV1-5, RT1-5 indicate average root length, root surface area, root volume and root tips in diameter between 0.0 and 0.5 mm, 0.5 and 1.0 mm, 1.0 and 1.5 mm, 1.5 and 2 mm and greater than 2.0 mm respectively; p-values were given in parentheses behind mean squares.
Fig 1. Frequency distribution of variation for seven root traits in 153 mungbean lines.
PRL, primary root length; TRL, total root length; TSA, total root surface area; RAD, root average diameter; TRV, total root volume; TRT, total root tips; RF, root forks; OP, optimum phosphorus condition; LP, low phosphorus condition.
Genetic correlations among tested traits
Pearson correlations coefficients among all the traits under two P regimes were analyzed and significant correlations (p<0.01 and p<0.001) were observed between pairs of traits (Table 3). Under OP condition, highly significant and positive correlation were obtained between TRL and TSA (r = 0.953), TSA and TRV (r = 0.953) followed by TRL and TRV (r = 0.855). The RF exhibited a highly significant correlation with TRL, TSA and TRV whereas under LP condition, a highly significant and positive correlation was observed for TRL and TSA (r = 0.951) followed by TSA and TRV (r = 0.929). Under both OP and LP conditions, TRV showed a highly significant and positive correlation with all other tested traits. The PRL and TRL showed significant and positive correlations with all other tested traits except RAD under both P regimes. RAD showed a significant negative correlation with TRT and RF under low P condition. The results showed that the relationship between PRL and TRL, PRL and TSA and TRV and TRT were the same under both P conditions, but RAD and TRT and RAD and RF were much smaller in OP than LP condition. The relationship between PRL and RAD, and RAD and TSA were also very different between OP and LP conditions.
Table 3. Pearson correlations among tested traits under optimumand low phosphorus conditions (sample size = 153).
| OP | PRL | TRL | TSA | TRV | RAD | TRT | RF |
|---|---|---|---|---|---|---|---|
| PRL | 1 | ||||||
| TRL | 0.417*** | 1 | |||||
| TSA | 0.398*** | 0.953*** | 1 | ||||
| TRV | 0.350*** | 0.855*** | 0.953*** | 1 | |||
| RAD | 0.058 | 0.149 | 0.379*** | 0.576*** | 1 | ||
| TRT | 0.398*** | 0.685*** | 0.599*** | 0.496*** | -0.089 | 1 | |
| RF | 0.249** | 0.824*** | 0.807*** | 0.717*** | 0.097 | 0.526 | 1 |
| LP | |||||||
| PRL | 1 | ||||||
| TRL | 0.415*** | 1 | |||||
| TSA | 0.399*** | 0.951*** | 1 | ||||
| TRV | 0.335*** | 0.819*** | 0.929*** | 1 | |||
| RAD | -0.102 | -0.146 | 0.121 | 0.397*** | 1 | ||
| TRT | 0.329*** | 0.708*** | 0.611*** | 0.492*** | -0.288** | 1 | |
| RF | 0.262** | 0.805*** | 0.723*** | 0.560*** | -0.284** | 0.577*** | 1 |
** and *** significant at p<0.01 and p<0.001.
OP, optimum phosphorus; LP, low phosphorus; PRL, primary root length; TRL, total root length; TSA, total root surface area; TRV, total root volume; RAD, root average diameter; TRT, total root tips; RF, root forks; ** and *** significant at p<0.01 and p<0.001 respectively.
Comparison of root traits in different diameter classes under two phosphorus regimes
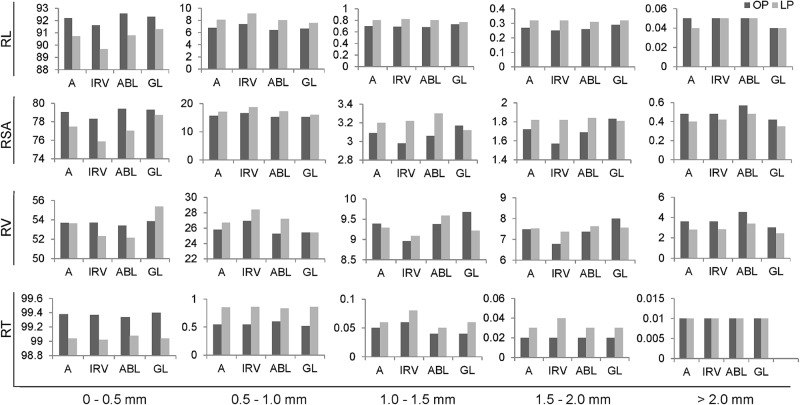
Root traits TRL, TSA, TRV and TRT were classified into different classes based on root diameter intervals i.e. 0–0.5 mm, 0.5–1.0 mm, 1.0–1.5 mm, 1.5–2.0 mm and >2.0 mm and named them as RL1-5, RSA1-5, RV1-5 and RT1-5. This was done to compare the fine root distribution across different diameter intervals under both P conditions. The proportion of roots in each diameter class was calculated as a percentage of the total for each across different genotype groups under two P regimes (Table 4). The higher percentage distribution for studied root traits (RL, RSA, RV and RT) was recorded in 0–0.5 mm diameter class as compared to other diameter classes across different genotype groups under two P regimes. For root diameter classes 0.5–1.0 mm, 1.0–1.5 mm and 1.5–2.0 mm RL, RSA and RT percentage were higher in LP in comparison to OP condition (Fig 2). For diameter class in 0–0.5 mm and >2.0 mm, RL, RSA and RV recorded a higher percentage in OP condition as compared to LP condition.
Table 4. Percentage distribution of root traits across five root diameter classes under optimum and low phosphorus conditions in different groups.
| Traits | Treatment | Root diameter class (mm) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–0.5 | 0.5–1.0 | 1.0–1.5 | 1.5–2.0 | >2.0 | |||||||||||||||||
| A | IRV | ABL | GL | A | IRV | ABL | GL | A | IRV | ABL | GL | A | IRV | ABL | GL | A | IRV | ABL | GL | ||
| RL (%) | OP | 92.20 | 91.61 | 92.58 | 92.32 | 6.78 | 7.39 | 6.43 | 6.62 | 0.70 | 0.69 | 0.68 | 0.73 | 0.27 | 0.25 | 0.26 | 0.29 | 0.05 | 0.05 | 0.05 | 0.04 |
| LP | 90.73 | 89.68 | 90.80 | 91.30 | 8.11 | 9.13 | 8.03 | 7.57 | 0.80 | 0.82 | 0.80 | 0.77 | 0.32 | 0.32 | 0.31 | 0.32 | 0.04 | 0.05 | 0.05 | 0.04 | |
| RSA (%) | OP | 79.06 | 78.32 | 79.41 | 79.30 | 15.66 | 16.64 | 15.27 | 15.28 | 3.09 | 2.98 | 3.06 | 3.17 | 1.72 | 1.57 | 1.69 | 1.83 | 0.48 | 0.48 | 0.57 | 0.42 |
| LP | 77.48 | 75.85 | 77.04 | 78.73 | 17.10 | 18.70 | 17.34 | 16.00 | 3.20 | 3.22 | 3.30 | 3.12 | 1.82 | 1.82 | 1.84 | 1.81 | 0.40 | 0.42 | 0.48 | 0.35 | |
| RV (%) | OP | 53.69 | 53.72 | 53.41 | 53.85 | 25.82 | 26.94 | 25.29 | 25.43 | 9.39 | 8.96 | 9.38 | 9.68 | 7.48 | 6.78 | 7.37 | 8.00 | 3.62 | 3.60 | 4.55 | 3.04 |
| LP | 53.63 | 52.32 | 52.15 | 55.37 | 26.74 | 28.40 | 27.21 | 25.42 | 9.29 | 9.09 | 9.59 | 9.22 | 7.53 | 7.37 | 7.63 | 7.56 | 2.82 | 2.83 | 3.41 | 2.43 | |
| RT (%) | OP | 99.38 | 99.37 | 99.34 | 99.40 | 0.55 | 0.55 | 0.60 | 0.52 | 0.05 | 0.06 | 0.04 | 0.04 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 |
| LP | 99.04 | 99.02 | 99.08 | 99.04 | 0.85 | 0.86 | 0.83 | 0.86 | 0.06 | 0.08 | 0.05 | 0.06 | 0.03 | 0.04 | 0.03 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | |
OP, optimum phosphorus; LP, low phosphorus; A, all 153 mungbean genotypes: IRV, indianreleased varieties: ABL, advanced breeding lines: GL, germplasm lines; RL, root length; RSA, root surface area; RV, root volume; RT, root tips.
Fig 2. Percentage of root traits across five diameter classes assessed under two phosphorus regimes.
RL, root length; RSA, root surface area; RV, root volume; RT, root tips; OP, optimum phosphorus condition; LP, low phosphorus condition; A, all 153 mungbean genotypes; IRV, Indian released varieties; ABL, advanced breeding lines; GL, germplasm lines.
Diversity pattern with respect to different groups
A comparison of the root morphology of the different groups showed clear variation for all studied root traits. The mungbean genotypes in the IRV, ABL and GL groups were classified into three categories, namely low, medium and high performance groups based on mean and standard deviation of each trait under both OP and LP conditions (Table 5). This classification mainly shows the frequency distribution of genotypes for studied root traits under both OP and LP conditions. For all studied traits, a larger number of genotypes were classified into the medium group. A critical review of performance under different P conditions revealed that among 153 genotypes studied, the greater number of genotypes were grouped in the high group in LP condition for PRL, TRL, TRV, RAD and TRT. Under LP condition, 8 (20%), 7 (16%) and 10 (15%) genotypes from IRV, ABL and GL groups showed larger TRL. For all traits except TSA, the GL group had a lower proportion of genotypes with high performance () than either the IRV or ABL groups under two P regimes. Except for the trait TRL, ABL group had a lower proportion of genotypes with high performance (() than the IRV group under two P regimes. This indicates that more genotypes with P responsive root traits are present in the order IRV>ABL>GL groups under the two P regimes.
Table 5. The Shannon-Weaver diversity index (H’) and performance categories under two different phosphorus conditions.
| Traits | Treatment | All 153 mungbean genotypes | Indian Released varieties | Advanced breeding lines | Germplasm lines | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | H’ | Low | Medium | High | H’ | Low | Medium | High | H’ | Low | Medium | High | H’ | ||
| PRL | OP | 25 | 102 | 26 | 0.87 | 5 | 28 | 8 | 0.85 | 8 | 30 | 6 | 0.84 | 14 | 44 | 10 | 0.89 |
| LP | 22 | 103 | 28 | 0.83 | 4 | 29 | 8 | 0.79 | 5 | 29 | 10 | 0.86 | 11 | 48 | 9 | 0.81 | |
| TRL | OP | 21 | 112 | 20 | 0.77 | 5 | 29 | 7 | 0.80 | 6 | 33 | 5 | 0.73 | 11 | 50 | 7 | 0.75 |
| LP | 23 | 106 | 24 | 0.86 | 5 | 28 | 8 | 0.84 | 4 | 33 | 7 | 0.73 | 11 | 47 | 10 | 0.83 | |
| TSA | OP | 22 | 109 | 22 | 0.80 | 6 | 30 | 5 | 0.77 | 5 | 33 | 6 | 0.74 | 9 | 49 | 10 | 0.79 |
| LP | 21 | 110 | 22 | 0.79 | 5 | 29 | 7 | 0.80 | 4 | 35 | 5 | 0.65 | 13 | 46 | 9 | 0.85 | |
| TRV | OP | 17 | 114 | 22 | 0.74 | 5 | 31 | 5 | 0.73 | 4 | 35 | 5 | 0.65 | 9 | 48 | 11 | 0.81 |
| LP | 21 | 107 | 25 | 0.82 | 6 | 27 | 8 | 0.87 | 3 | 35 | 6 | 0.64 | 11 | 48 | 9 | 0.81 | |
| RAD | OP | 17 | 113 | 23 | 0.75 | 9 | 25 | 7 | 0.94 | 4 | 35 | 5 | 0.65 | 12 | 45 | 11 | 0.87 |
| LP | 28 | 101 | 24 | 0.88 | 5 | 29 | 7 | 0.80 | 6 | 29 | 9 | 0.87 | 10 | 52 | 6 | 0.70 | |
| TRT | OP | 18 | 118 | 17 | 0.69 | 3 | 33 | 5 | 0.62 | 7 | 29 | 8 | 0.88 | 8 | 51 | 9 | 0.73 |
| LP | 25 | 105 | 23 | 0.84 | 8 | 25 | 8 | 0.94 | 6 | 34 | 4 | 0.69 | 11 | 47 | 10 | 0.83 | |
| RF | OP | 18 | 110 | 25 | 0.78 | 5 | 27 | 9 | 0.86 | 7 | 32 | 5 | 0.77 | 6 | 51 | 11 | 0.72 |
| LP | 22 | 109 | 22 | 0.79 | 5 | 29 | 7 | 0.80 | 8 | 29 | 7 | 0.88 | 9 | 50 | 9 | 0.76 | |
OP, optimum phosphorus; LP, low phosphorus; PRL, primary root length; TRL, total root length; TSA, total root surface area; TRV, total root volume; RAD, root average diameter; TRT, total root tips; RF, root forks.
The Shannon-Weaver diversity index (H’) was calculated to study the diversity among the tested traits in different genotypic groups (Table 5). The H’ values varied for traits PRL, TRL, TSA, TRV, RAD, TRT and RF with an average of 0.80 in mungbean genotypes. Among the studied groups H’ was maximum for Indian Released Varieties. Under OP condition, PRL and TSA exhibited higher H’ value in studied mungbean genotypes whereas in LP condition TRL, TRV, RAD, TRT and RF revealed higher H’ value. Under both Pregimes, RAD and PRL showed a relatively higher level of variation while TRV and RF were less variable across different genotypic groups. All root traits except PRL and TSA showed higher H’ values indicate higher diversity under the LP condition than the OP condition. For three traits, TRL, RAD and RF under OP condition and three traits, TRL, TRV and TRT under LP condition showed higher diversity in the IRV group than ABL and GL group.
Principal component analysis
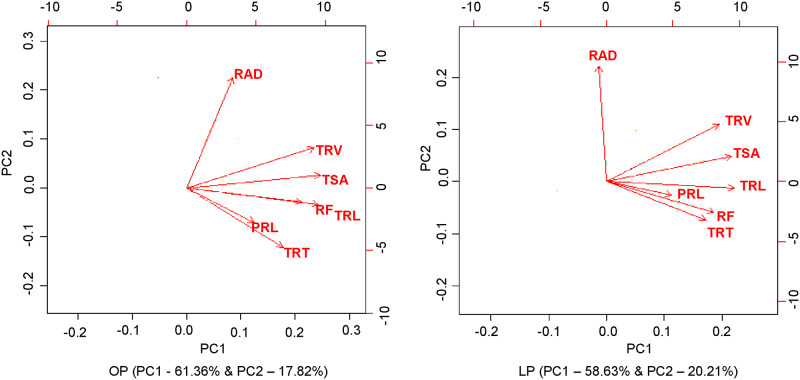
The principal component analysis was carried out to know the most contributing traits under two P regimes. The first two principal components (PCs) explained 79.19% and 78.84% of the total variation among the tested mungbean genotypes under OP and LP conditions (Table 6 and Fig 3). The first principal component explained the 61% and 59% of total variation under OP and LP condition, revealed that TRL and TSA, and their highly correlated traits TRV and RF are the most important contributing traits. The most important contributing trait in the second principal component is RAD, which contributed nearly 20% of the total variation.
Table 6. Principle component analysis of seven traits under two phosphorus conditions.
| Characters | OP | LP | ||
|---|---|---|---|---|
| PC1 | PC2 | PC1 | PC2 | |
| PRL | 0.24 | -0.25 | 0.25 | -0.09 |
| TRL | 0.46 | -0.12 | 0.48 | -0.05 |
| TSA | 0.47 | 0.09 | 0.47 | 0.18 |
| TRV | 0.45 | 0.29 | 0.42 | 0.41 |
| RAD | 0.16 | 0.80 | -0.03 | 0.82 |
| TRT | 0.34 | -0.43 | 0.37 | -0.27 |
| RF | 0.41 | -0.10 | 0.40 | -0.22 |
| EigenValues | 4.29 | 1.25 | 4.10 | 1.41 |
| % Variance | 0.61 | 0.18 | 0.59 | 0.20 |
| Cumulative % Variance | 0.61 | 0.79 | 0.59 | 0.79 |
| Most contributing traits | TSA, TRL, TRV | RAD | TRL, TSA, TRV | RAD |
OP, optimum phosphorus; LP, low phosphorus; PC1, principal component 1; PC2, principal component 2; PRL, primary root length; TRL, total root length; TSA, total root surface area; TRV, total root volume; RAD, root average diameter; TRT, total root tips; RF, root forks.
Fig 3. Biplots of first two principal components (PC) showing variation among seven root traits under optimum P and Low P condition.
PRL, primary root length; TRL, total root length, TSA, total root surface area; RAD, root average diameter; TRV, total root volume; TRT, total root tips; RF, root forks; OP, optimum phosphorus condition; LP, low phosphorus condition.
Comprehensive phosphorus efficiency measurement
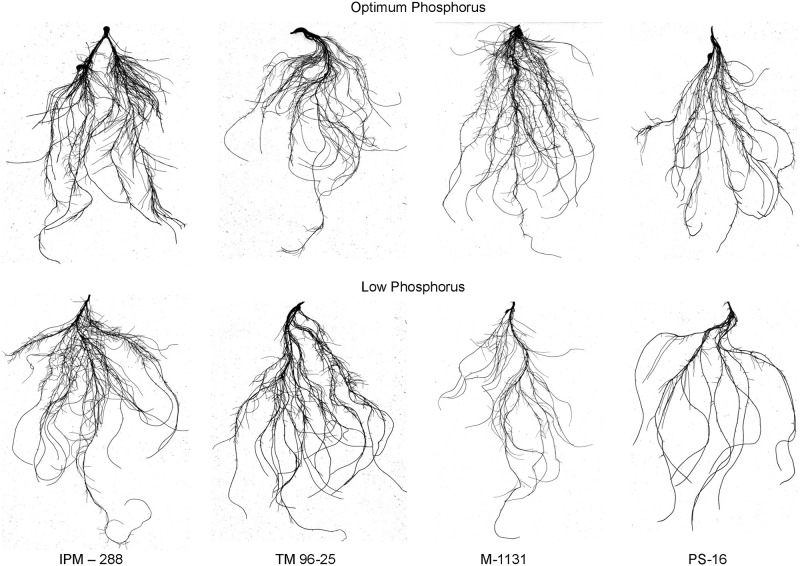
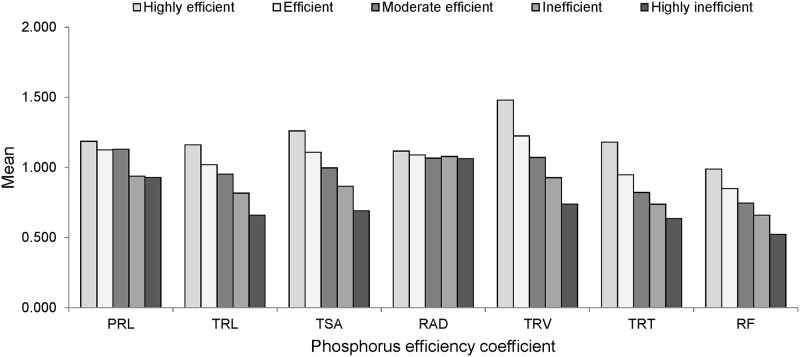
By using subordination function value analysis, the CPEM value, as a comprehensive synthetic index was derived to study the efficiency of root morphology among mungbean genotypes under P deficiency (S2 Table). Based on CPEM values, all mungbean genotypes were classified into five groups. Group 1 includes 21 genotypes showed high P efficiency with CPEM values greater than 0.9. Group 2 with 25 genotypes showed P efficiency with CPEM values between 0.7 and 0.9. Group 3 with 48 genotypes showed moderate efficiency with CPEM values between 0.5 and 0.7. Group 4 with 41 genotypes showed inefficiency with CPEM values between 0.3 and 0.5 while group 5 including 18 genotypes were highly inefficiency with CPEM values less than 0.3. The genotype which comes under respective groups is listed in S2 Table. Based on CPEM values, variation in root architectural traits of contrasting genotypes is presented in Table 7 and Fig 4. Genotype IPM-288 with the highest CPEM value recorded higher values for TRL, TSA, TRV and TRT under LP condition compared to OP condition. Genotypes with low CPEM values, M1131 and PS-16 were found to be poor performers for TRL, TSA and TRV under LP condition compared to OP condition. The mean values of the P efficiency coefficient for each trait in five groups with different levels of P efficiency are shown in Fig 5. The mean values of P efficiency coefficient for all root traits were highest in group 1, moderate in group 2, 3 and 4, and lowest in group 5 except for RAD. This result indicates that P efficient mungbean genotypes with higher CPEM values also had higher P efficiency coefficients.
Table 7. Variation in root architectural traits of ten contrasting mungbean genotypes under optimum and low phosphorus conditions.
| Genotypes | CP EM | Root traits | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRL | TRL | TSA | TRV | RAD | TRT | RF | |||||||||
| OP | LP | OP | LP | OP | LP | OP | LP | OP | LP | OP | LP | OP | LP | ||
| Top five genotypes | |||||||||||||||
| IPM-288 | 1.77 | 25.00 | 32.67 | 839.70 | 1198.07 | 82.17 | 129.37 | 0.62 | 1.11 | 0.30 | 0.35 | 618.70 | 1067.33 | 2669.00 | 2301.33 |
| TM 96–25 | 1.55 | 32.00 | 31.00 | 525.00 | 740.40 | 65.87 | 84.27 | 0.44 | 0.76 | 0.32 | 0.36 | 474.67 | 849.33 | 1517.33 | 1566.67 |
| TM 96–2 | 1.48 | 44.33 | 41.00 | 777.53 | 932.53 | 70.67 | 100.20 | 0.51 | 0.86 | 0.29 | 0.34 | 728.33 | 1138.67 | 1817.33 | 2028.67 |
| M 1477 | 1.37 | 28.83 | 32.33 | 631.75 | 740.74 | 60.34 | 78.67 | 0.44 | 0.72 | 0.30 | 0.35 | 594.00 | 653.33 | 1252.33 | 1644.33 |
| PUSA 1342 | 1.27 | 29.33 | 44.00 | 734.35 | 928.65 | 73.73 | 96.42 | 0.52 | 0.80 | 0.31 | 0.33 | 898.33 | 742.00 | 1765.00 | 2017.33 |
| Bottom five genotypes | |||||||||||||||
| M 1131 | 0.18 | 38.00 | 33.00 | 843.25 | 509.77 | 85.62 | 49.83 | 0.61 | 0.37 | 0.31 | 0.31 | 900.67 | 703.67 | 2402.00 | 1388.33 |
| PS—16 | 0.19 | 26.67 | 23.00 | 591.66 | 277.07 | 67.35 | 35.60 | 0.61 | 0.36 | 0.37 | 0.41 | 697.33 | 233.67 | 1107.33 | 448.67 |
| PUSA VISHAL | 0.19 | 38.00 | 32.33 | 1449.93 | 726.73 | 175.81 | 88.14 | 1.68 | 0.94 | 0.37 | 0.41 | 1222.00 | 475.00 | 3755.67 | 1204.00 |
| M 831 | 0.22 | 41.00 | 32.17 | 945.84 | 662.59 | 95.35 | 63.94 | 0.65 | 0.49 | 0.30 | 0.31 | 1209.33 | 785.67 | 3234.00 | 1556.00 |
| IC 325828 | 0.23 | 41.00 | 36.67 | 1061.36 | 709.37 | 113.83 | 77.45 | 0.95 | 0.67 | 0.35 | 0.35 | 960.67 | 793.00 | 3559.33 | 1502.33 |
Contrasting genotypes identified using Comprehensive phosphorus efficiency measurement value. PRL, primary root length; TRL, total root length; TSA, total root surface area; TRV, total root volume; TRT, total root tips; RAD, root average diameter; RF, root forks; OP, optimum phosphorus; LP, low phosphorus.
Fig 4. Variation in root system architecture in four contrasting mungbean genotypes grown under optimum and low phosphorus conditions.
Fig 5. The mean values of phosphorus efficiency coefficient for seven traits in five groups classified for phosphorus efficiency.
Groups 1, 2, 3, 4 and 5 represent mungbean genotypes identified with highly efficiency, efficiency, moderate efficiency, inefficiency and highly inefficiency. N = 21, 25, 48, 41 and 18 for groups 1, 2, 3, 4 and 5 respectively. PRL, primary root length; TRL, total root length; TSA, total root surface area; RAD, root average diameter; TRV, total root volume; TRT, total root tips; RF, root forks.
Discussion
Characterization of mungbean genotypes for stress tolerance traits and screening for P efficient genotypes are indispensable for the success of the breeding programme. Conventionally, a higher root to shoot ratio has been considered as an index for P efficiency due to the increase in root biomass and large deep root system able to extract more nutrients [58, 59]. Total root length represents the sum of primary, seminal, crown, basal and lateral roots. The various components of the root system have also been selected as important traits for the screening of genotypes under P deficiency. In this study, we examined the influence of low P on root morphology of 153 mungbean genotypes and investigated the various root traits including PRL, TRL, TSA, TRV, TRT and RF. We found significant variation, medium to high heritability, approximately normal distribution and significant correlations for these root traits. Low coefficient of variation and high heritability of these traits indicates the genetic stability of the traits in the genotypes. The observed results were in agreement with reports of previous researchers [60–62]. The widely used indicator, RAD, was highly heritable suggesting that it is a reliable parameter for P efficiency. Frano et al [63] reported that root traits especially root diameter increases (hypertrophy) in response to abiotic stress conditions. The root diameter mainly controls the root length and surface area and results in bigger root dry weight [64].
Although the genetic variation of root system varies from plant to plant, the presence of very fine roots (<0.5 mm diameter) and fine roots (0.5 to 2.0 mm) determines the most percentage of root traits, is important for nutrient and water uptake [48, 65 and 66]. In this study, we identified the high percentage of fine roots in diameter class from 0.5 to 2.0 mm in LP compared to OP condition, while the percentage of very fine roots with <0.5 mm diameter was more in OP condition. This indicates the effect of P availability on the percentage of fine root distribution at different diameter classes in studied mungbean genotypes. Under low P, plants may increase the development of root cortical aerenchyma which enables the plant to maintain greater root diameter but reduce overall total root cost and root respiration [67, 68]. Greater fine root production results in increasing the overall adsorption area as an adaptive mechanism of stress tolerance [69].
The principal component analysis showed that TRL, TSA, TRV and RAD were responsible for most of the phenotypic variation at the seedling stage in the tested mungbean genotypes. TRL was significantly and positively correlated with TSA, TRV, TRT and RF under both OP and LP conditions. In combination with principal component analysis, we identified that TRL, TSA and TRV were sufficient to explain most of the variation and these were proved to be ideal traits for P efficiency screening at the seedling stage. Under the LP condition, RAD was significantly and negatively correlated with TRT and RF. This indicates that RAD is a key trait to differentiate P availability among the tested root traits. Moreover, these traits showed high P efficiency coefficient values in P efficient mungbean genotypes. This result is in agreement with previous reports. Pandey et al. [32] reported significantly higher root surface area and root volume in P efficient mungbean genotype under P stress. Root surface area has been found to be in close association with the nutrient absorption rate [70, 71]. Vigorous root growth with high root length and surface area ensures the efficient absorption of macro and micronutrients at the early growth stage of the plant [72]. Furthermore, root architectural traits mainly total root length and root number were significantly and positively correlated with biomass and grain yield [73, 74]. Therefore, the vigorous root system of the plant not only supports good crop establishment but also ensures the plants’ survival under stressful conditions.
Diversity in root architecture enables us to improve nutrient and water use efficiency under stressful conditions. A combination of availability of diverse mungbean genotypes and stress tolerance ability will be key criteria for the success of crop improvement programme. Considering the mean performance and standard deviation of root traits as selection criteria, genotypes were categorized into high, medium and low performance groups [46, 51]. Furthermore, the Shannon–Weaver diversity index (H’) was calculated based on the categorization of genotypes to compare the phenotypic diversity among the traits. Among all traits, TRL and RAD (>0.8) showed a relatively high level of H’ under LP condition and PRL showed the relatively highest value of H’ (>0.8) under both P regimes. Independently of root length, greater diversity in diameter is due to the changes in the fine root distribution for each root diameter in response to the nutritional environment [75]. The high value of H’ indicates greater phenotypic diversity and balanced frequency distribution [76], while low H’ indicates an extremely unbalanced frequency distribution with a lack of diversity [77]. The Shannon-Weaver diversity index has been used previously to describe root traits diversity in rice [78], maize [79], wheat [80] and cowpea [81].
In this study, a comparison of root morphological traits across different genotypic groups indicated that the IRV group showed greater diversity for root traits than ABL and GL groups. The presence of high H’ values in the IRV group for TRL, TSA, TRV and TRT with medium to high heritability and number of genotypes in the high group in LP condition indicates that these genotypes used in the study are a rich source to improve the plant performance under LP. Results from broad-sense heritability, Shannon-weaver diversity index and principal component analysis facilitate the root architectural traits suitable for target genotype selection in cowpea [81]. Presence of more variable root architectural traits with high heritability suggests that genotypes are more P efficient in soybean under P limiting condition [82]. Among tested traits, RF was found to be less variable root trait conferring P efficiency in this study. In maize, RF was less variable due to less H’ value in all studied lines in response to water stress condition at the seedling stage [46]. Root traits, PRL and RAD were observed to be more variable across different genotypic groups under two P regimes. Further mean values of both these traits were significantly high in LP compared to OP condition and can be used as indicators of P deficiency. Plasticity of root traits including root length, root average diameter and percentage of lateral roots confers the improved plant performance under P stress condition [83]. Reduction of root diameter was reported under low P compared sufficient P condition in maize [62] and Aegilops tauschii [84]. Term ‘root etiolation’ has been suggested for the reduction in root diameter under P stress in common bean genotypes to increase soil exploration and to reduce metabolic cost [85]. In response to P deficiency growth of PRL was observed to be inhibited in Arabidopsis [86]. Whereas, enhanced induction of primary root was observed in rice [87]. Reduction in growth of primary root of P deficient plants correlates with inhibition of cell differentiation in primary root meristem and the reduction of cell proliferation in the root elongation part [88]. However, in present study, a comprehensive index CPEM has been estimated considering all the tested traits with irrespective of the nature of trait. For example, genotype with shorter or longer PRL, CPEM will consider ratio of trait value derived from LP and OP conditions. Further, CPEM is based on relative trait values i.e. PEC of traits and degree of membership between trait value and P efficiency i.e. MFVP [89]. In combination with correlation and principal component analysis, TRL with high H’ value and significant and positive correlation with TSA and TRV indicates that these traits are sufficient to explain the variation and could be used as selection criteria for P efficiency at the seedling stage. In maize, total root length and root dry weight were able to provide the most contribution to total phenotypic variation and sufficient to improve other root traits [46, 79]. This result provides valuable information to improve both agronomic traits as well as nutrient use efficiency traits in the mungbean breeding programme. In the 21st century, due to environmental concerns and the high cost of inorganic fertilizers, nutrient efficient crop plants play an important role in improving crop yields compared to 20th century [90].
Based on CPEM values, mungbean genotypes were classified as highly efficient, efficient, moderately efficient, inefficient and highly inefficient groups. Among these, IPM-288, TM 96–25, TM 96–2, M-1477, PUSA 1342 were identified as best five highly efficient genotypes whereas M-1131, PS-16, Pusa Vishal, M 831, IC 325828 were highly inefficient genotypes. Except for RAD, P efficiency coefficients for all traits were highest in group 1, intermediate in group 2, 3 and 4 while lowest in group 5. This type of classification is required for screening and selection of genotypes for desirable root traits under varied P conditions. Further, these genotypes with contrasting traits can be exploited in recombination breeding programme to develop P efficient cultivars [91, 92]. In this study, 21 highly efficient genotypes with a well developed root system were identified and these could be used in the mungbean breeding programme for further improvement of tolerance to abiotic stresses.
In conclusion, the veracity of the root system was maintained by growing the tested mungbean lines in hydroponic culture. The in vitro screening method of hydroponics proves to be the ideal method to screen large set genotypes with the least effect of environmental influence [93]. Previous reports examined the root traits using the simple hydroponic system based on the aerated nutrient solution with different levels of P with the replacement of solution at a fixed interval in maize [94], soybean [95], mungbean [32] and wheat [96]. In the present study, we identified a range of responses to P deficiency in mungbean genotypes for root system traits at the seedling stage. We found that TRL, TSA and TRV are the ideal selection criteria at the seedling stage for predicting the nutrient use efficiency in the field. Thus, the root system response to P deficiency can be studied without root damage in hydroponic culture by controlling access to water and nutrients. Further, the tested mungbean genotypes need to be evaluated at the adult stage under OP and LP conditions. In addition, the association of seedling stage root traits with adult stage traits needs to be further examined.
Supporting information
(PDF)
(PDF)
(PDF)
Acknowledgments
Authors are thankful to Dr. A. K. Singh, Head, Division of Genetics and Joint Director Research, IARI, New Delhi, Dr. A. K. Singh Director, IARI, New Delhi, Dr. Vinod, Professor Division of Genetics, IARI, New Delhi and Dr. C. Viswanathan, Head, Division of Plant Physiology, IARI, New Delhi for providing the necessary facilities for smooth conductance of research. V.R.P.R. also thankful to Acharya N G Ranga Agricultural University, Andhra Pradesh for according deputation to pursue doctoral degree program at IARI, New Delhi.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The funds for this study were provided by Department of Science and Technology, Ministry of Science and Technology, Government of India vide grant number CRG/2018/002642 to Dr. Harsh Kumar Dikshit.
References
- 1.Schafleitner R, Nair R, Rathore A, Wang YW, Lin CY, Chu SH, et al. The world vegetable center mungbean (Vigna radiata) core and minicore collection. BMC Genom. 2015; 16:344 10.1186/s12864-015-1556-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vairam N, Lavanya SA, Muthamilan M, Vanniarajan C. Screening of M3 mutant for yellow vein mosaic virus resistance in green gram (Vigna radiata L.). Int. J. Plant Sci. 2016;11(2): 265–269. 10.15740/HAS/IJPS/11.2/265-269 [DOI] [Google Scholar]
- 3.Nair RK, Yang RY, Eastdown W, Thavarajah D, Thavarajah P, Hughes JD, et al. Biofertification of mungbean (Vigna radiata) as a whole food to enhance human health. J Sci Food Agric. 2013; 93(8): 1805–1813. 10.1002/jsfa.6110. [DOI] [PubMed] [Google Scholar]
- 4.Nadeem MA, Ahmad R, Ahmad MS. Effect of seed inoculation and different fertilizer levels on the growth and yield of mungbean (Vigna radiata L.). J. Agron. 2004; 3: 40–42. 10.3923/ja.2004.40.42 [DOI] [Google Scholar]
- 5.Asaduzzaman M, Karim F, Ullah J, Hasanuzzaman M. Response of mungbean (Vigna radiata L.) to nitrogen and irrigation management. Am-Eurasian J. Sci. Res. 2008; 3: 40–43. [Google Scholar]
- 6.Thiyagarajan TM, Backiyavathy MR, Savithri P. Nutrient management for pulses—A Review. Agric.Re. 2003; 24(1): 40–48. [Google Scholar]
- 7.FAI 2011. Fertilizer statistics. The Fertilizer Association of India, New Delhi.
- 8.Ramaekers L, Remans R, Rao IM, Blair MW, Vanderleyden J. Strategies for improving phosphorous acquisition efficiency of crop plants. Field Crop Res. 2010;117: 169–176. 10.1016/j.fcr.2010.03.001 [DOI] [Google Scholar]
- 9.FAO 2017. World fertilizer trends and outlook to 2020 –Summary report. Food and agriculture organization of the united nations, Rome.
- 10.GPRI 2009. Declaration on global phosphorus security. Global Phosphorus Research Initiative. www.phosphorusfutures.net.
- 11.Wiel CCM, Linden CG, Scholten OE. Improving phosphorous use efficiency in agriculture: opportunities for breeding. Euphytica. 2016; 207: 1–22. 10.1007/s10681-015-1572-3 [DOI] [Google Scholar]
- 12.Pandey R. Mineral nutrition of plants. Plant biology and biotechnology. 2015; 499–538. 10.1007/978-81-322-2286-6-20 [DOI] [Google Scholar]
- 13.Chen L, Lin L, Cai G, Sun Y, Huang T, Wang K, et al. Identification of nitrogem phosphorus and potassium deficiencies in rice based on static scanning technology and hierarchial identification method. PLoSONE. 2014; 9(11):e113200 10.1371/journal.pone.0113200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carstensen A, Andrei H, Schmidt SB, Sharma A, Spetea C, Pribil M, et al. The Impacts of Phosphorus Deficiency on the Photosynthetic Electron Transport Chain. Plant Physiol. 2018; 177: 271–284. 10.1104/pp.17.01624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yun SJ, Kaeppler SM. Induction of maize acid phosphatase activities under phosphorus starvation. Plant and Soil. 2001; 237(1): 109–115. 10.1023/A:1013329430212 [DOI] [Google Scholar]
- 16.Qiu H, Mei X, Liu C, Wang J, Wang G, Wang X, et al. Fine mapping of quantitative trait loci for acid phosphatase activity in maize leaf under low phosphorus stress. Mol Breed. 2013; 32(3): 629–639. 10.1007/s11032-013-9895-z [DOI] [Google Scholar]
- 17.Maharajan T, Ceasar SA, Krishna TPA, Ramakrishnan M, Duraipandiyan V, Abdulla AN, et al. Utilization of molecular markers for improving the phosphorous efficiency in crop plants. Plant Breed. 2017; 1–17. 10.1111/pbr.12537 [DOI] [Google Scholar]
- 18.Heuer S, Gaxiola R, Schilling R, Estrella LH, Arredondo DL, Wissuwa M, et al. Improving phosphorous use efficiency: a complex trait with emerging opportunities. The Plant J. 2017; 90: 868–885. 10.1111/tpj.13423. [DOI] [PubMed] [Google Scholar]
- 19.Panigrahy M, Rao DN, Sarla N. Molecular mechanisms in response to phosphate starvation in rice. Biotechnol. Adv. 2009;27: 389–397. 10.1016/j.biotechadv.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Sarker BC, Karmoker J. Effects of phosphorus deficiency on the root growth of lentil seedlings grown in rhizobox. Bangladesh J Bot. 2009; 38: 215–218. 10.3329/bjb.v38i2.5153 [DOI] [Google Scholar]
- 21.Lynch JP. Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol. 2011; 156: 1041–1049. 10.1104/pp.111.175414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adler PR, Cumming JR, Arora R. Agricultural sciences–Vol. I, Nature of Mineral Nutrient Uptake by Plants. Encyclopedia of Life Support Systems. 2004.
- 23.Hodge A, Berta G, Doussan C, Merchan F, Crespi M. Plant root growth, architecture and function. Plant Soil. 2009; 321: 153–187. 10.1007/s11104-009-9929-9 [DOI] [Google Scholar]
- 24.Hodge A. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phyt. 2004; 162: 9–24. 10.1111/j.1469-8137.2004.01015.x [DOI] [Google Scholar]
- 25.Lopez-Bucio J, Cruz-Ramírez A, Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003; 6: 280–287. 10.1016/s1369-5266(03)00035-9 [DOI] [PubMed] [Google Scholar]
- 26.Perez-Torres C, López-Bucio J, Cruz-Ramírez A, Ibarra-Laclette E, Dharmasiri S, Estelle M, et al. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell. 2008; 20: 3258–3272. 10.1105/tpc.108.058719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akthar MS, Oki Y, Adachi T. Genetic variability in phosphorus acquisition and utilization efficiency from sparingly soluble P-sources by Brassica cultivars under P-stress environment. J. Agric. Crop Sci. 2008; 194: 380–392. 10.1111/j.1439-037X.2008.00326.x [DOI] [Google Scholar]
- 28.Jakkeral SA, Kajjidoni ST, Koti RV. Genotypic variation for root traits to phosphorus deficiency in blackgram (Vigna mungo L. Hepper). Karnataka J. Agric. Sci. 2009; 22: 946–950. [Google Scholar]
- 29.Zhu JM, Kaeppler S, Lynch J. Topsoil foraging and phosphorus acquisition efficiency in maize. Funct. Plant Biol. 2005; 32: 749–762. 10.1071/FP05005 [DOI] [PubMed] [Google Scholar]
- 30.Hermans C, Hammond JP, White PJ, Verbruggen N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006; 11: 610–617. 10.1016/j.tplants.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 31.Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997; 124: 33–44. [DOI] [PubMed] [Google Scholar]
- 32.Pandey R, Meena SK, Krishnapriya V, Ahmad A, Kishora N. Root carboxylate exudation capacity under phosphorus stress does not improve grain yield in green gram. Plant Cell Rep. 2014; 33: 919–928. 10.1007/s00299-014-1570-2 [DOI] [PubMed] [Google Scholar]
- 33.Sarkar BC, Karmokar JL. Effects of phosphorus deficiency on the root growth of lentil seedlings [Lens culinaris Medik] growth in RhizoBox. Bangladesh J. Bot. 2009; 38(2): 215–218. 10.3329/bjb.v38i2.5153 [DOI] [Google Scholar]
- 34.He J, Jin Y, Du YL, Wang T, Tumer NC, Yang RP, et al. Genotypic variation in yield, yield components, root morphology and architecture in soybean in relation to water and phosphorus supply. Front Plant Sci. 2017; 8:1499 10.3389/fpls.2017.01499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borch K, Bouma TJ, Lynch JP, Brown KM. Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant Cell Environ. 1999; 22: 425–431. 10.1046/j.1365-3040.1999.00405.x [DOI] [Google Scholar]
- 36.Chen YL, Dunbabin VM, Diggle AJ, Siddique KHM, Renegel Z. Phosphorus starvation boosts carboxylate secretion in P- deficient genotypes of Lupinus angustifolius with contrasting root structure. Crop Pasture Sci. 2013; 64: 588–599. 10.1071/cp13012 [DOI] [Google Scholar]
- 37.Vejchasarn P, Lynch JP, Brown KM. Genetic variability in phosphorus responses of rice root phenotypes. Rice. 2016; 9: 29 10.1186/s12284-016-0102-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen Q, Wen Z, Dong Y, Li H, Miao Y, Shen J. The responses of root morphology and phosphorus-mobilizing exudations in wheat to increasing shoot phosphorus concentration. AoB Plants. 2018; 10(5): 1–11. 10.1093/aobpla/ply054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyu Y, Tang H, Li H, Zhang F, Rengel Z, Whalley WR, et al. Major crop species show differential balance between root morphological and physiological responses to variable phosphorus supply. Front Plant Sci. 2016; 7:1939 10.3389/fpls.2016.01939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meeks M, Murray SC, Hague S, Hays D. Measuring maize seedling drought response in search of tolerant germplasm. Agronomy. 2013; 3: 135–147. 10.3390/agronomy3010135 [DOI] [Google Scholar]
- 41.Kitajima K, Fenner M. Ecology of seedling regeneration In: Fenner M. (ed.) Seeds: the ecology of regeneration in plant communities, CABI Publishing, Wallingford, pp. 2000; 331–359. 10.1079/9780851994321.0331 [DOI] [Google Scholar]
- 42.De La Cruz M, Romao RL, Escudero A, Maestre FT. Where do seedlings go? A spatio-temporal analysis of seedling mortality in a semi-arid gypsophyte. Ecography. 2008, 31: 720–730. 10.1111/j.0906-7590.2008.05299.x [DOI] [Google Scholar]
- 43.Bouma TJ, Nielsen KL, Koutstaal B. Sample preparation and scanning protocol for computerised analysis of root length and diameter. Plant and Soil 2000; 218: 185–196. 10.1023/A:1014905104017 [DOI] [Google Scholar]
- 44.Himmelbauer ML, Loiskandl W, Kastanek F. Estimating length, average diameter and surface area of roots using two different image analyses systems. Plant and Soil 2004; 260: 111–120. 10.1023/B:PLSO.0000030171.28821.55 [DOI] [Google Scholar]
- 45.Sivasakthi K, Tharanya M, Kholová J, Muriuki RW, Thirunalasundari T, Vadez V. Chickpea genotypes contrasting for vigor and canopy conductance also differ in their dependence on different water transport pathways. Front Plant Sci. 2017; 8: 1663–1679. 10.3389/fpls.2017.01663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li R, Zeng Y, Xu J, Wang Q, Wu F, Cao M, et al. Genetic variation for maize root architecture in response to drought stress at the seedling stage. Breed. Sci. 2015; 65: 298–307. 10.1270/jsbbs.65.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorim LY, Vandenberg A. Root Traits, Nodulation and Root Distribution in Soil for Five Wild Lentil Species and Lens culinaris (Medik.) Grown under Well-Watered Conditions. Front. Plant Sci. 2017; 8: 1632 10.3389/fpls.2017.01632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Wang G, Yu K, Li P, Xiao L, Liu G. A new method to optimize root order classification based on the diameter interval of fine root. Sci. Rep. 2018; 8: 29–60. 10.1038/s41598-018-21248-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gulles AA, Bartolome VI, Morantte RIZA, Nora LA. Randomization and analysis of data using STAR (Statistical Tool for Agricultural Research). Philipp J Crop Sci. 2014; 39 (1): 137. [Google Scholar]
- 50.Abdel-Ghani AH, Kumar B, Reyes-Matamoros J, Gonzalez-Portilla PJ, Jansen C, Martin JPS, et al. Genotypic variation and relationships between seedling and adult plant traits in maize (Zea mays L.) inbred lines grown under contrasting nitrogen levels. Euphytica. 2012; 189: 123–133. 10.1007/s10681-012-0759-0 [DOI] [Google Scholar]
- 51.Zar JH. Biostatistical analysis, 5th edn Practice Hall, New Jersey; 2010. p. 944. [Google Scholar]
- 52.Shannon CE, Weaver W. The Mathematical Theory of Communication. Urbana, IL: University of Illinois Press: 1963. [Google Scholar]
- 53.Hutcheson K. A test for comparing diversities based on the Shannon formula. J. Theor. Biol. 1970; 29: 151–154. 10.1016/0022-5193(70)90124-4 [DOI] [PubMed] [Google Scholar]
- 54.Wu H, Guo J, Wang C, Li K, Zhang X, Yang Z, et al. An effective screening method and a reliable screening trait for salt tolerance of Brassica napus at the germination stage. Front Plant Sci. 2019; 10:530 10.3389/fpls.2019.00530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu GF, Zhang ZY, Xiang ZX. Comprehensive evealuation of cold resistance on four Lysimachia plants by subordinate function value analysis. J. Northwest For. Univ. 2009; 24: 24–26. [Google Scholar]
- 56.Zadeh LA. "Fuzzy sets". Information and Control. 1965; 8: 338–353. [Google Scholar]
- 57.Chen X, Min D, Yasir TA, Hu YG. Evaluation of 14 morphological, yield-related and physiological traits as indicators of drought tolerance in Chinese winter bread wheat revealed by analysis of the membership function value of drought tolerance (MFVD). Field Crops Res. 2012; 137:195–201. 10.1016/j.fcr.2012.09.008 [DOI] [Google Scholar]
- 58.Swinnen J. Rhizodeposition and turnover of root-derived organic material in barley and wheat under conventional and integrated management. Agric. Ecosyst. Environ. 1994; 51: 115–128. 10.1016/0167-8809(94)90038-8 [DOI] [Google Scholar]
- 59.Nielsen KI, Eshel A, Lynch JP. The effect of phosphorus availability on the carbon economy of contrasting common bean genotypes. J. Exp. Bot. 2001; 52 (355): 329–339. [PubMed] [Google Scholar]
- 60.Da Silva DA, Esteves JADeF, Goncalves JGR, Azevedo CVG, Ribeiro T, Chiorato AF, et al. Evaluation of common bean genotypes for phosphorus use efficiency in eutrophic oxisol. Bragantia. 2016; 75(2): 152–163. 10.1590/1678-4499.454 [DOI] [Google Scholar]
- 61.Wang J, Dun X, Shi J, Wang X, Liu G, Wang H. Genetic dissection of root morphological traits related to nitrogen use efficiency in Brassica napusL. under two contrasting nitrogen conditions. Front Plant Sci. 2017; 8:1709 10.3389/fpls.2017.01709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang QJ, Yuan Y, Liao Z, Jiang Y, Wang Q, Zhang L, et al. Genome wide association of 13 traits in maize seedlings under low phosphorus stress. The Plant Genome. 2019; 12(3): 1–13. 10.3835/plantgenome2019.06.0039 [DOI] [PubMed] [Google Scholar]
- 63.Frano JA, Banon S, Vicente MJ, Miralles J, Martinez-Sanchaz JJ. Root development in horticultural plants grown under abiotic stress condition. J. Hortic. Sci. Biotechnol. 2011; 86:543–556. 10.1080/14620316.2011.11512802 [DOI] [Google Scholar]
- 64.Wasaya A, Zhang X, Fang Q, Yan Z. Root phenotyping for drought tolerance: A Review. Agronomy. 2018; 8:241 10.3390/agronomy8110241 [DOI] [Google Scholar]
- 65.Zobel RW, Waisel Y. A plant root system architectural taxonomy: a framework for root nomenclature. Plant Biosyst. 2010; 144: 507–512. 10.1080/11263501003764483 [DOI] [Google Scholar]
- 66.Liu L, Gan Y, Buekert R, Rees KV, Warkentin T. Fine root distributions in oilseed and pulse crops. Crop Sci. 2010; 50: 222–226. 10.2135/cropsci2009.03.0156 [DOI] [Google Scholar]
- 67.Postma JA, Lynch JP. Theoretical evidence for the functional benefit of root cortical aerenchyma in soils with low phosphorus availability. Ann. Bot. 2011; 107: 829–841. 10.1093/aob/mcq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCormack ML, Guo D. Impacts of environmental factors on fine root lifespan. Front. Plant Sci. 2014; 16(5): 205 10.3389/fpls.2014.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arif MR, Islam MT, Robin AHK. Salinity stress alters root morphology and root hairs in Brassica napus. Plants. 2019; 8:192 10.3390/plants8070192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imada S, Yamanaka N, Tamai S. Water table depth affects Populus alba fine root growth and whole plant biomass. Funct Ecol. 2008; 22: 1018–1026. 10.1111/j.1365-2435.2008.01454.x [DOI] [Google Scholar]
- 71.Zhang LT, Li J, Rong TZ, Gao SB, Wu FK, Xu J, et al. Large-scale screening maize germplasm for low-phosphorus tolerance using multiple selection criteria. Euphytica. 2014; 197: 435–446. 10.1007/s10681-014-1079-3 [DOI] [Google Scholar]
- 72.Wang Y, Kristensen KT, Jensen LS, Magid J. Vigorous Root Growth Is a Better Indicator of Early Nutrient Uptake than Root Hair Traits in Spring Wheat Grown under Low Fertility. Front Plant Sci. 2016; 7: 865 10.3389/fpls.2016.00865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie Q, Fernando KMC, Mayes S, Sparkes DL. Identifying seedling root architectural traits associated with yield and yield components in wheat. Ann. Bot. 2017; 119(7): 1115–1129. 10.1093/aob/mcx001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ke Liu, He A, Ye C, Liu S, Lu J, Gao M, et al. Root Morphological Traits and Spatial Distribution under Diferent Nitrogen Treatments and Their Relationship with Grain Yield in Super Hybrid Rice. Sci. Rep.2018; 8:131 10.1038/s41598-017-18576-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zobel RW, Kinraide TB, Baligar VC. Fine root diameters can change in response to change in nutrient concentrations. Plant Soil. 2007; 297:243–254. 10.1007/s11104-007-9341-2 [DOI] [Google Scholar]
- 76.Yadav RK, Gautam S, Palikhey E, Joshi BK, Ghimire KH, Gurung R, et al. Agro-morphological diversity of Nepalese naked barley landraces. Agriculture & Food Security. 2018; 7:86 10.1186/s40066-018-0238-5 [DOI] [Google Scholar]
- 77.Rathinavel K. Exploration of Genetic Diversity for Qualitative Traits among the Extant Upland Cotton (Gossypium hirsutum L.) Varieties and Parental Lines. Int.J.Curr.Microbiol.App.Sci. 2017; 6(8): 2407–2421. 10.20546/ijcmas.2017.608.285 [DOI] [Google Scholar]
- 78.Raju BR, Narayanaswamy BR, Mohankumar MV, Sumanth KK, Rajanna MP, Mohanraju B, et al. Root traits and cellular level tolerance hold the key in maintaining higher spikelet fertility of rice under water limited conditions. Funct Plant Biol. 2014; 41(9): 930–939. 10.1071/FP13291 [DOI] [PubMed] [Google Scholar]
- 79.Kumar B, Abdel Ghani AH, Reyes Matamoros J, Hochholdinger F, Lubberstedt T. Genotypic variation for root architecture traits in seedlings of maize (Zea mays L.) inbred lines. Plant Breed. 2012; 131: 465–478. 10.1111/j.1439-0523.2012.01980.x [DOI] [Google Scholar]
- 80.Lin Y, Yi X, Tang S, Chen W, Wu F, Yang X, et al. Dissection of phenotypic and genetic variation of drought related traits in diverse Chinese wheat landraces. Plant Genome. 2019; 12:3 10.3835/plantgenome2019.03.0025 [DOI] [PubMed] [Google Scholar]
- 81.Adu MO, Asare PA, Yawson DO, Dzidzienyo DK, Nyadanu D, Asare-Bediako EI, et al. Identifying key contributing root system traits to genetic diversity in field grown cowpea (Vigna unguiculata L. Walp) genotypes. Field Crops Res. 2019; 232:106–118. 10.1016/j.fcr.2018.12.015 [DOI] [Google Scholar]
- 82.Ao J, Fu J, Tian J, Yan X, Liao H. Genetic variability for root morph-architecture traits and root growth dynamics as related to phosphorus efficiency in soybean. Funct Plant Biol. 2010; 37: 304–312. 10.1071/FP09215 [DOI] [Google Scholar]
- 83.Sandhu N, Raman KA, Torres RO, Audebert A, Dardou A, Kumar A, et al. Rice root architectural plasticity traits and genetic regions for adaptability to variable cultivation and stress conditions. Plant Physiol. 2016; 171: 2562–2576. 10.1104/pp.16.00705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Y, Wang L, Deng M, Li Z, Lu Y, Wang J, et al. Genome wide association study of phosphorus deficiency tolerance traits in Aegilops tauschii. TheorAppl Genet. 2015; 128 (11): 2203–2212. 10.1007/s00122-015-2578-x. [DOI] [PubMed] [Google Scholar]
- 85.Morrow de la Riva L. Root etiolation as a strategy for phosphorus acquisition in common bean. MSc. Thesis. 2010. The Pennsylvania state university, University Park, PA. https://etda.libraries.psu.edu/files/final_submissions/3727
- 86.Zheng H, Pan X, Deng Y, Wu H, Liu P, Li X. AtOPR3 specifically inhibits primary root growth in Arabidopsis under phosphate deficiency. Sci Rep. 2016; 6: 24778 10.1038/srep24778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dai X, Wang Y, Yang A, Zhang WH. OsMYB2P-1, an R2R3 MYB transcription factor, is involved in the regulation of phosphate-starvationresponses and root architecture in rice. Plant Physiol. 2012; 159: 169–183. 10.1104/pp.112.194217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niu YF, Chai RS, Jin GL, Wang H, Tang CX, Zhang YS. Responses of root architecture development to low phosphorus availability: a review. Ann Bot. 2013; 112: 391–408. 10.1093/aob/mcs285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Y, Bowman BC, Hu YG, Liang X, Zhao W, Wheeler J, et al. Evaluation of agronomic traits and drought tolerance of winter wheat accessions from the USDA-ARS national small grains collections. Agronomy. 2017; 7: 51 10.3390/agronomy7030051 [DOI] [Google Scholar]
- 90.Fageria NK, Baligar VC, Li Y. The Role of Nutrient Efficient Plants in Improving Crop Yields in the Twenty First Century. J. Plant Nutr. 2008; 31(6): 1121–1157. 10.1080/01904160802116068 [DOI] [Google Scholar]
- 91.Gill HS, Singh A, Sethi SK, Behl RK. Phosphorus uptake and use efficiency in different varieties of bread wheat (Triticum aestivum L). Arch Agron Soil Sci. 2004; 50:563–572. 10.1080/03650340410001729708 [DOI] [Google Scholar]
- 92.Aziz T, Rahmatullah, Maqsood MA, Sabir M, Kanwal S. Categorization of Brassica cultivars for phosphorus acquisition from phosphate rock on basis of growth and ionic parameters. J. Plant Nutr. 2011; 34:522–533. 10.1080/01904167.2011.538114 [DOI] [Google Scholar]
- 93.Rajkumar S, Ibrahim SM. In vitro Hydroponic Studies on Root Characters for Drought Resistance Assessment in Rice. Am. Eurasian J. Agric Environ. Sci. 2014; 14 (5): 396–400. 10.5829/idosi.aejaes.2014.14.05.12324 [DOI] [Google Scholar]
- 94.Gong Y, Guo Z, He L, Li J. Identification of maize genotypes with high tolerance or sensitivity to phosphorus deficiency. J. Plant Nutr. 2011; 34: 1290–1302. 10.1080/01904167.2011.580816 [DOI] [Google Scholar]
- 95.Ochigbo AE, Bello LL. Screening of soybean varieties for phosphorus use efficiency in nutrient solution. Agric. Biol. J. N. Am. 2014; 2151–7525. 10.5251/abjna.2014.5.2.68.77 [DOI] [Google Scholar]
- 96.Liu C, Guo T, Du Y, Hu YG. Nitrogen and phosphorus use efficiency of 43 wheat alien chromosome addition lines evaluated by hydroponic culture. J. Plant Nutr. 2016; 41: 2470–2481. 10.1080/01904167.2018.1476539 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.