Abstract
Breast cancer is a heterogeneous disease. In clinical practice, tumors are classified as hormonal receptor positive, Her2 positive and triple negative tumors. In previous works, our group defined a new hormonal receptor positive subgroup, the TN-like subtype, which had a prognosis and a molecular profile more similar to triple negative tumors. In this study, proteomics and Bayesian networks were used to characterize protein relationships in 96 breast tumor samples. Components obtained by these methods had a clear functional structure. The analysis of these components suggested differences in processes such as mitochondrial function or extracellular matrix between breast cancer subtypes, including our new defined subtype TN-like. In addition, one of the components, mainly related with extracellular matrix processes, had prognostic value in this cohort. Functional approaches allow to build hypotheses about regulatory mechanisms and to establish new relationships among proteins in the breast cancer context.
Introduction
Breast cancer is one of the most prevalent cancers in the world [1]. In clinical practice, breast cancer is classified according to the expression of hormonal receptors (estrogen or progesterone) and Her2, into positive hormonal receptor (ER+), HER2+ and triple negative (TNBC). In previous studies, our group defined a new ER+ molecular subgroup, named TN-like, with a molecular profile and a prognosis more similar to TNBC tumors [2]. We denoted the remaining ER+ tumors as ER-true. We also found significant molecular differences among breast cancer subtypes. For instance, differences related with metabolism of glucose were described between ER-true, TN-like and TNBC tumors [2, 3].
Proteomics supplies complementary information to genomics experiments. Proteomics has been used to find differences between subtypes on a proteomic level in sporadic and hereditary breast tumors [4]. In addition, proteomics coupled with super-SILAC has been also used to define molecular signatures that are differentially expressed between breast cancer subtypes [5].
Proteomics provides useful information about biological process effectors and may quantify thousands of proteins. Undirected probabilistic graphical models (PGM), based on a Bayesian approach, allow characterizing differences between tumor samples at functional level [2, 3, 6, 7]. In this study we explored the utility of Bayesian networks in the molecular characterization of breast cancer. The main feature of targeted Bayesian networks is that they provide a hierarchical structure and targeted relationships between proteins.
Bayesian networks (BN) have been previously used to inference protein signaling networks using phase-reverse protein array data from a breast cancer cell line [8]. In this study, the authors also experimentally tested some of the relationships by an inhibition approach. Baladandayuthapani et al. also applied BN to phase-reverse protein array data, in this case from a panel of ovarian and breast cancer cell lines. Their model was capable to distinguish between both cell line types [9] Most recently, BN has been used to determine genes related to bone metastasis development in breast cancer [10]. Also related to gene expression, BN inference leaded to the identification of TRIB1 as a regulator of cell cycle progression and survival in triple negative cancer cells [11]. BN have been also used to suggest therapeutic targets in breast cancer. In the study of Vundavilli et al., applying BN to gene expression data, the resulting network was used to rank different interventions in order to achieve an apoptosis induction [12]. Beretta et al. used BN to study the inference of signaling downstream of tyrosine kinase receptors, comparing predictions about inhibition of several nodes with experimental data [13]. BN was applied even to rank treatments in triple negative breast cancer datasets [14]. Finally, BN have demonstrated its utility in making associations between clinical data in breast cancer patients [15] or between lifestyle factors in breast cancer survivors [16].
In this work, we aim to explore if BN can be applied to proteomics expression data and if that the results provided by these analyses provide useful biological and clinical information. For this, we used mass-spectrometry proteomics data and Bayesian networks to characterize protein relationships in a cohort of breast cancer tumor paraffin samples. These networks maintained a functional structure and some of them showed prognostic value. This approach also reflected previously described protein-protein interactions and it could be used to propose new hypotheses and mechanisms of regulation of these proteins.
Materials and methods
Ethics statement
Written informed consent had been obtained for the participants on the study. The approval of the study was obtained from Hospital Doce de Octubre and Hospital Universitario La Paz Ethics Committees.
Samples
One hundred and six FFPE samples from patients with breast cancer were recovered from I+12 Biobank and from IdiPAZ Biobank, both integrated in the Spanish Hospital Biobank Network. The histopathological characteristics were reviewed by a pathologist to confirm tumor content. Samples had to include no less than half of tumor cells. The endorsement of the study was obtained by Hospital Doce de Octubre and Hospital Universitario La Paz Ethics Committees. These samples were utilized in previous studies [2, 3, 17].
Protein preparation
Proteins were extracted from formalin-fixed paraffin-embedded (FFPE) samples as previously described [18]. Briefly, FFPE sections were deparaffinized in xylene and washed twice with absolute ethanol. Protein extracts from FFPE samples were set up in 2% SDS buffer using a protocol based on heat-induced antigen retrieval. Protein concentration was quantified using the MicroBCA Protein Assay Kit (Pierce-Thermo Scientific). Protein extracts (10 μg) were processed with trypsin (1:50) and SDS was removed from digested lysates using Detergent Removal Spin Columns (Pierce). Peptide samples were additionally desalted using ZipTips (Millipore), dried, and resolubilized in 15 μL of a 0.1% formic acid and 3% acetonitrile solution before mass-spectrometry (MS) experiments.
Label-free proteomics
Samples were analyzed on a LTQ-Orbitrap Velos hybrid mass spectrometer (Thermo Fischer Scientific, Bremen, Germany) coupled to NanoLC-Ultra system (Eksigent Technologies, Dublin, CA, USA) as described previously [2, 3]. Briefly, after separation, peptides were eluted with a gradient of 5 to 30% acetonitrile in 95 minutes. The mass spectrometer was operated in data-dependent mode (DDA), followed by CID (collision-induced dissociation) fragmentation on the twenty most intense signals per cycle. The acquired raw MS data were processed by MaxQuant (version 1.2.7.4) [19], followed by protein identification using the integrated Andromeda search engine [20]. Briefly, spectra were searched against a forward UniProtKB/Swiss-Prot database for human, concatenated to a reversed decoyed fasta database (NCBI taxonomy ID 9606, release date 2011-12-13). The maximum false discovery rate (FDR) was set to 0.01 for peptides and 0.05 for proteins. Label free quantification was calculated on the basis of the normalized intensities (LFQ intensity). Quantifiable proteins were defined as those detected in at least 75% of samples in at least one type of sample (either ER+ or TNBC samples) showing two or more unique peptides. Only quantifiable proteins were considered for subsequent analyses. Protein expression data were log2 transformed and missing values were replaced using data imputation for label-free data, as explained in [21], using default values. Finally, protein expression values were z-score transformed. All the mass spectrometry raw data files acquired in this study may be downloaded from Chorus (http://chorusproject.org) under the project name Breast Cancer Proteomics.
Network construction
PGM are graph-based representations of joint probability distributions where nodes represent random variables and edges (directed or undirected) represent stochastic dependencies among the variables. In particular, we have used a type of PGM called Bayesian networks (BN) [13]. With these models, the dependences between the variables in our data are specified by a directed acyclic graph (DAG). The obtained networks will indicate causality i.e. if protein A and B are connected and protein A changes its expression value, protein B changes its expression value as well [22].
Firstly, we find the BN that best explains our data [23]. There are different algorithms to learn a DAG from data but we have selected the well-known PC algorithm (named as its inventors Peter Spirtes and Clark Glymour), a constraint-based structure learning algorithm [24] based on conditional independence tests. The PC algorithm was shown to be consistent in high-dimensional settings [25]. Moreover, an order-independent version of the PC algorithm, called PC-stable, was proposed in [26]. All these procedures are implemented in R within packages pcalg [25] and graph [27]. We used protein expression data without other a priori information.
In this way, our data are represented by a large graph that can be partitioned into several connected components. Then, we focused on finding suitable subgraphs that give us a much clearer understanding of the interrelations therein.
STRING v11 (https://string-db.org/) was used to check if some of the protein relations obtained in the DAG analysis were previously described.
Gene ontology analyses
Protein to Gene Symbol conversion was performed using Uniprot (www.uniprot.org) and DAVID (www.david.ncifcrf.gov) [28]. Gene Ontology Analysis was also done in DAVID selecting only “Homo sapiens” background and GOTERM-FAT (http://geneontology.org/), Biocarta (http://doi.org/10.1089/152791601750294344) and KEGG (https://www.genome.jp/kegg/kegg1.html) databases.
Component activity measurements
Component activities were calculated as previously described [2, 3]. Briefly, activity measurement was calculated by the mean expression of all the proteins of each component related with the established major component function.
Statistical analyses
Network visualization was performed using Cytoscape software [29]. Statistical comparison between tumor groups were done in GraphPad Prism v6 using a non-parametric Mann-Whitney test. Prognostic signatures were developed using R v3.2.4 and BRB Array Tools, developed by Dr. Richard Simon and BRB Array Tools Development Team [30]. Briefly, functional node activities were ranked according their p-values in a Kaplan-Meier analysis. Then, a Cox regression including a leave-one-out validation using 1,000 random permutations was used to validate the prognostic capability. P-values under 0.05 were considered statistically significant.
Results
Patient characteristics
Clinical characteristics of this patient cohort have been previously described [2, 3, 31]. Briefly, one hundred and six patients were enrolled into the study. They all had node positive disease, Her2 negative and all had received adjuvant chemotherapy and hormonal therapy in the case of ER+ tumors. Among ER+ tumors, 50 patients were characterized as ER-true and 21 were defined as TN-like (S1 Table) [2].
Mass spectrometry analysis
Proteomics analyses from these samples have been previously described [2]. In summary, one hundred and two FFPE samples had enough protein to perform the MS analyses. After MS workflow, 96 samples provided useful protein expression data. After quality criteria, 1,095 proteins presented at least two unique peptides and detectable expression in at least 75% of the samples in at least one type of sample (either ER+ or TNBC).
Directed networks
Using proteomics data, directed acyclic graphs (DAG) were performed. Altogether, it was possible to establish 789 edges of which 662 were guided and 127 are undetermined. These edges formed 303 components formed by different number of nodes or proteins. An overview of the number of nodes (proteins) included in each component is provided in Table 1.
Table 1. Characteristics of the components obtained from DAG.
| Number of nodes | 1 | 2 | 3 | 4 | 5 | 6 | 8 | 9 | 10 | 11 | 12 | 13 | 15 | 17 | 18 | 23 | 464 |
| Number of components | 188 | 62 | 13 | 18 | 6 | 4 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Number of nodes = number of proteins contained in each component, Number of components = directed components obtained.
We characterized components from DAG analysis. Components including less than 9 nodes were dismissed because they were little informative. All components were named with the number of nodes included by the DAG analysis.
Afterwards, components were interrogated for biological function. Characteristics about all components are supplied in Table 2 and S1 File.
Table 2. Features of components obtained by DAG analysis.
| Component | Number of nodes | Main function |
|---|---|---|
| Component 23 | 23 | Membrane and mitochondria |
| Component 18 | 18 | Cytoskeleton |
| Component 17 | 17 | RNA binding |
| Component 15 | 15 | Extracellular exosome |
| Component 13 | 13 | Extracellular matrix |
| Component 12 | 12 | RNA binding and translation |
| Component 11 | 11 | Proliferation and oxphos |
| Component 10 | 10 | Extracellular exosome |
| Component 9 | 9 | RNA binding and splicing |
Component activity measurements
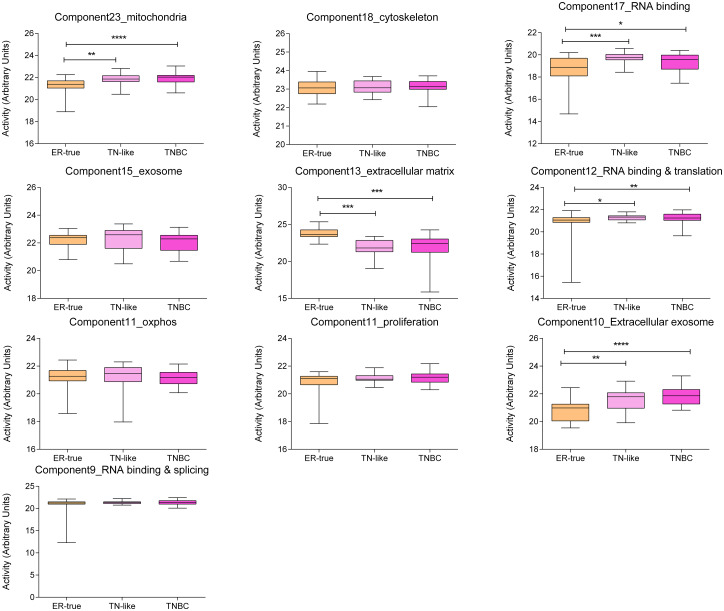
Component activities were calculated for each node. There were significant differences between ER-true, TN-like and TNBC tumors in the component activity for component 23: mitochondria, component 17: RNA binding, component 13: extracellular matrix, and component 10: extracellular exosome (Fig 1).
Fig 1. Component activity measurements for ER-true, TN-like and TNBC respectively.
Component 13: Extracellular matrix
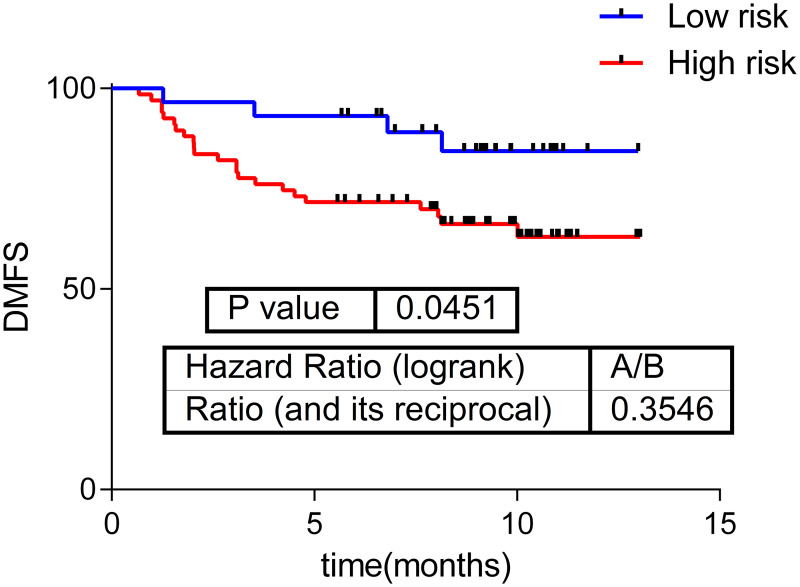
Component 13 activity showed prognosis value in our series, splitting our population into a high and a low risk group and it can be used as a distant-metastasis free survival (DMFS) predictor (p = 0.045, HR = 0.35, 30–70%) (Fig 2). Interestingly, the predictor classified all the TN-like tumors and most of TNBC tumors into the high-risk group (Table 3).
Fig 2. Component 13 activity prognostic value in the whole cohort.
Table 3. Number of patients classified by the DMFS predictor into a low or a high-risk group.
| Subtype | Number in low-risk group | Number in high-risk group |
|---|---|---|
| ER-true | 26 | 24 |
| TN-like | 0 | 21 |
| TNBC | 3 | 22 |
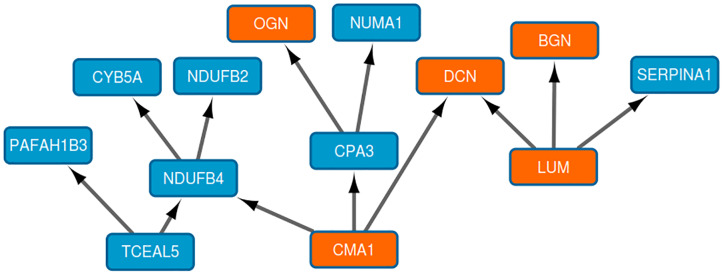
Component 13 contains thirteen proteins mainly related to extracellular matrix (Fig 3). The five proteins related by gene ontology analysis to extracellular matrix were OGN, BGN, LUM, CMA1, and DCN, all of them, with the exception of CMA1, belonged to small leucine-rich proteoglycan family of proteins.
Fig 3. Component 13.
Orange nodes: Proteins related to extracellular matrix ontology.
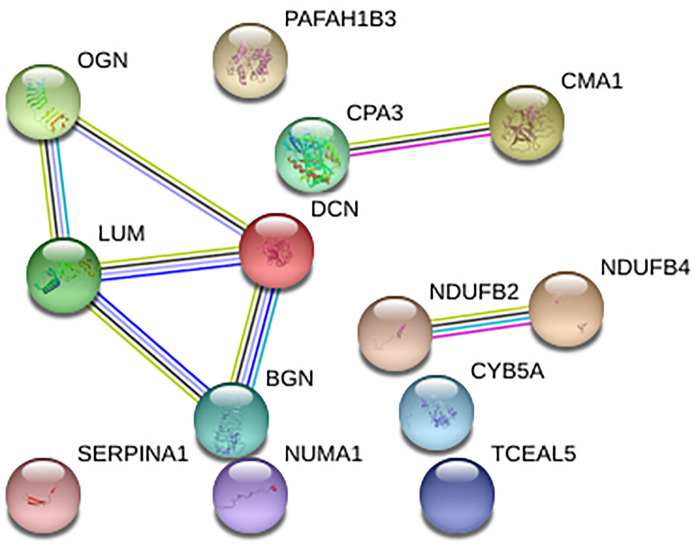
Additionally, the existence of described connections was checked using STRING v11 (Fig 4). Interestingly, some of the connections suggested by BN analysis in Component 13, (NDUFB2 and NDUFB4; CPA3 and CMA1; and BGN, LUM, and DCN) were connected both in the DAG graph and in the STRING network, meaning that these interactions have been previously described.
Fig 4. Network built with the proteins from Component13 using STRING.
Discussion
In this study, we used proteomics and DAG to characterize relationships between proteins in breast cancer tumor samples. Unlike other approaches, such as Genes2FANS [32], our DAG method supplies directed relationships between proteins and a hierarchical structure. Traditionally, protein-protein interaction (PPI) networks, such as STRING, are based in relationships described in the literature. However, we built a directed network, i.e. a graph formed by edges with a direction, using protein expression data without other a priori information, so it was possible to propose new hypotheses about protein interactions. We used probabilistic graphical models (PGM) because they offer a way to relate many random variables with a complex dependency structure.
As it has been previously mentioned, arrows in directed networks indicate causality between two proteins. This approach allows making hypotheses about causal relationships between proteins and proposes a hierarchical structure. In some cases, an experimental relationship between two proteins connected in the directed network had been previously described. For instance, in component 18, it has been widely described that PIP binds AZGP1 in breast cancer [33]. Another example is component 11 which related COX5A, MT-CO2 and COX6C, all proteins of mitochondrial complex IV [34].
We demonstrated in previous works that non-directed graphs provided functional information [2, 3]. Interestingly, a functional structure also appeared in this type of networks. Component activities suggested differences in functions such as extracellular matrix and mitochondria. Differences in mitochondria between these subtypes have been previously described using non-directed PGMs [2, 3].
On the other hand, component 13, composed by thirteen proteins, five of them related to extracellular matrix, had prognostic value in our series. Of these five proteins, four of them belonged to the small leucine-rich proteoglycan (SLRP) family (lumican, biglycan, osteoglycin, and decorin). Biglycan (BGN) could promote migration in breast cancer [35]. It has been widely described the anti-metastatic role of decorin (DCN1) in breast cancer [36–38]. Lumican (LUM) significantly attenuated cell functional processes, including proliferation, migration and invasion [39]. In the same study, it was described that lumican modulates matrix effectors in MCF7 and MDAMB231 cells. Finally, osteoglycin (OGN) has been suggested as a biomarker of ECM in TNBC [40]. On the other hand, chymase 1 (CMA1) is secreted by mast cells and may play a role in angiogenesis [41]. It is also involved in extracellular matrix degradation. Higher levels of this protein were observed in Luminal subtype [42]. DCN, OGN, BGN, and LUM appeared also interconnected in the STRING network, so the DAG graph reflected protein interactions previously described. NDUFB2 and NDUFB4 were also connected in both networks.
Other proteins included in this component related to cancer are NUMA1, SERPINA1, and PAFAH1B3. Nuclear mitotic apparatus (NUMA1) is a structural component of the nuclear matrix. The encoded protein interacts with microtubules and plays a role in formation and organization of the mitotic spindle during cell division. It also modulates p-53 mediated transcription in cancer cells [43]. Serpin family A member 1 (SERPINA1) is a direct estrogen receptor target and a predictor of survival in breast cancer patients [44]. Platelet activating factor acetylhydrolase 1b catalytic subunit 3 (PAFAH1B3) encodes an acetylhydrolase that catalyzes the removal of an acetyl group from the glycerol backbone of platelet-activating factor. A study identified PAFAH1B3 as a key metabolic driver of breast cancer pathogenicity that is upregulated in primary human breast tumors and correlated with poor prognosis [45]. This enzyme may be dysregulated across many cancer types [46].
In previous studies we have used functional node activities from non-directed network to develop prognostic predictors [7]. Now, this approach is also validated in directed networks.
We used a mathematical method as DAG analysis and applied them to proteomics data of breast cancer tumors in order to infer causal relationships between these proteins. This method supplied some known relationships but also proposed new ones. Additionally, it associated proteins with a similar function. Therefore, it seems that it is a good approach to propose new hypotheses about mechanisms of action. Moreover, it was possible to associate the results obtained by DAG analysis with prognosis and built a prognostic signature. As far we know, this is the first time that this type of analysis is applied to clinical data and is associated with clinical outcome.
Our study has some limitations. Proteomics provides complementary information to other techniques such as genomics. However, an improvement in the number of detected proteins is still necessary. On the other hand, breast cancer clinical scenario is far more complex, and stratified analyses (by molecular or clinical subtypes, for example) could provide more complex and insightful information. Finally, all these mathematical approaches (and others), despite being useful by themselves, should be combined to obtain more information about the clinical scenario analyzed, as long as it seems that different analyses provide different and complementary information from the same data.
To sum up, in this study, we used proteomics and directed networks to characterize relationships between proteins in breast cancer tumors. This approach reflected some previously described interactions and it could be used to propose new hypotheses and mechanisms of action.
Supporting information
(DOCX)
(DOCX)
Data Availability
All the mass spectrometry raw data files acquired in this study may be downloaded from Chorus (http://chorusproject.org) under the project name Breast Cancer Proteomics.
Funding Statement
This study was supported by Instituto de Salud Carlos III, Spanish Economy and Competitiveness Ministry, Spain and co-funded by the FEDER program, “Una forma de hacer Europa” (PI07/1302). LT-F is supported by the Spanish Economy and Competitiveness Ministry (DI-15-07614). AZ-M is supported by Consejería de Educación e Investigación de la Comunidad de Madrid (IND2018/BMD-9262). GP-V is supported by Consejería de Educación, Juventud y Deporte of Comunidad de Madrid (IND2017/BMD7783). EL-C is supported by the Spanish Economy and Competitiveness Ministry (PTQ2018-009760). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. Epub 2014/10/09. 10.1002/ijc.29210 . [DOI] [PubMed] [Google Scholar]
- 2.Gámez-Pozo A, Trilla-Fuertes L, Berges-Soria J, Selevsek N, López-Vacas R, Díaz-Almirón M, et al. Functional proteomics outlines the complexity of breast cancer molecular subtypes. Scientific Reports. 2017;7(1):10100 10.1038/s41598-017-10493-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gámez-Pozo A, Berges-Soria J, Arevalillo JM, Nanni P, López-Vacas R, Navarro H, et al. Combined label-free quantitative proteomics and microRNA expression analysis of breast cancer unravel molecular differences with clinical implications. Cancer Res; 2015. p. 2243–53. 10.1158/0008-5472.CAN-14-1937 [DOI] [PubMed] [Google Scholar]
- 4.Waldemarson S, Kurbasic E, Krogh M, Cifani P, Berggård T, Borg Å, et al. Proteomic analysis of breast tumors confirms the mRNA intrinsic molecular subtypes using different classifiers: a large-scale analysis of fresh frozen tissue samples. Breast Cancer Res. 2016;18(1):69 Epub 2016/06/29. 10.1186/s13058-016-0732-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyanova S, Temu T, Sinitcyn P, Carlson A, Hein MY, Geiger T, et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat Methods. 2016;13(9):731–40. Epub 2016/06/27. 10.1038/nmeth.3901 . [DOI] [PubMed] [Google Scholar]
- 6.Trilla-Fuertes L, Gámez-Pozo A, Arevalillo JM, Díaz-Almirón M, Prado-Vázquez G, Zapater-Moros A, et al. Molecular characterization of breast cancer cell response to metabolic drugs. Oncotarget. 2018;9(11):9645–60. Epub 2018/01/08. 10.18632/oncotarget.24047 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Velasco G, Trilla-Fuertes L, Gamez-Pozo A, Urbanowicz M, Ruiz-Ares G, Sepúlveda JM, et al. Urothelial cancer proteomics provides both prognostic and functional information. Sci Rep. 2017;7(1):15819 Epub 2017/11/17. 10.1038/s41598-017-15920-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill SM, Lu Y, Molina J, Heiser LM, Spellman PT, Speed TP, et al. Bayesian inference of signaling network topology in a cancer cell line. Bioinformatics. 2012;28(21):2804–10. Epub 2012/08/24. 10.1093/bioinformatics/bts514 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baladandayuthapani V, Talluri R, Ji Y, Coombes KR, Lu Y, Hennessy BT, et al. BAYESIAN SPARSE GRAPHICAL MODELS FOR CLASSIFICATION WITH APPLICATION TO PROTEIN EXPRESSION DATA. Ann Appl Stat. 2014;8(3):1443–68. 10.1214/14-AOAS722 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SB, Chung CK, Gonzalez E, Yoo C. Causal Inference Network of Genes Related with Bone Metastasis of Breast Cancer and Osteoblasts Using Causal Bayesian Networks. J Bone Metab. 2018;25(4):251–66. Epub 2018/11/30. 10.11005/jbm.2018.25.4.251 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gendelman R, Xing H, Mirzoeva OK, Sarde P, Curtis C, Feiler HS, et al. Bayesian Network Inference Modeling Identifies TRIB1 as a Novel Regulator of Cell-Cycle Progression and Survival in Cancer Cells. Cancer Res. 2017;77(7):1575–85. Epub 2017/01/13. 10.1158/0008-5472.CAN-16-0512 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vundavilli H, Datta A, Sima C, Hua J, Lopes R, Bittner M. Bayesian Inference Identifies Combination Therapeutic Targets in Breast Cancer. IEEE Trans Biomed Eng. 2019;66(9):2684–92. Epub 2019/01/23. 10.1109/TBME.2019.2894980 . [DOI] [PubMed] [Google Scholar]
- 13.Beretta S, Castelli M, Gonçalves I, Henriques R, Ramazotti D. Learning the structure of Bayesian networks: A quantitative assessment of the effect of different algorithmic schemes Hindawi 2018.
- 14.Chen H, Lu W, Zhang Y, Zhu X, Zhou J, Chen Y. A Bayesian network meta-analysis of the efficacy of targeted therapies and chemotherapy for treatment of triple-negative breast cancer. Cancer Med. 2019;8(1):383–99. Epub 2018/12/07. 10.1002/cam4.1892 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soto-Ferrari M, Prieto D, Munene G. A Bayesian network and heuristic approach for systematic characterization of radiotherapy receipt after breast-conservation surgery. BMC Med Inform Decis Mak. 2017;17(1):93 Epub 2017/06/28. 10.1186/s12911-017-0479-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu S, Thompson W, Kerr J, Godbole S, Sears DD, Patterson R, et al. Modeling interrelationships between health behaviors in overweight breast cancer survivors: Applying Bayesian networks. PLoS One. 2018;13(9):e0202923 Epub 2018/09/04. 10.1371/journal.pone.0202923 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gámez-Pozo A, Trilla-Fuertes L, Prado-Vázquez G, Chiva C, López-Vacas R, Nanni P, et al. Prediction of adjuvant chemotherapy response in triple negative breast cancer with discovery and targeted proteomics. PLoS One. 2017;12(6):e0178296 Epub 2017/06/08. 10.1371/journal.pone.0178296 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gámez-Pozo A, Ferrer NI, Ciruelos E, López-Vacas R, Martínez FG, Espinosa E, et al. Shotgun proteomics of archival triple-negative breast cancer samples. Proteomics Clin Appl. 2013;7(3–4):283–91. 10.1002/prca.201200048 . [DOI] [PubMed] [Google Scholar]
- 19.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26(12):1367–72. Epub 2008/11/30. 10.1038/nbt.1511 . [DOI] [PubMed] [Google Scholar]
- 20.Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011;10(4):1794–805. Epub 2011/02/22. 10.1021/pr101065j . [DOI] [PubMed] [Google Scholar]
- 21.Deeb SJ, D’Souza RC, Cox J, Schmidt-Supprian M, Mann M. Super-SILAC allows classification of diffuse large B-cell lymphoma subtypes by their protein expression profiles. Mol Cell Proteomics. 2012;11(5):77–89. Epub 2012/03/21. 10.1074/mcp.M111.015362 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Needham CJ, Bradford JR, Bulpitt AJ, Westhead DR. A primer on learning in Bayesian networks for computational biology. PLoS Comput Biol. 2007;3(8):e129 10.1371/journal.pcbi.0030129 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neapolitan R, Xue D, Jiang X. Modeling the altered expression levels of genes on signaling pathways in tumors as causal bayesian networks. Cancer Inform. 2014;13:77–84. Epub 2014/05/25. 10.4137/CIN.S13578 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spirtes P, Glymour C, Scheines R. Causation, Prediction, and Search. Adaptive Computation and Machine Learning. 2nd ed The MIT Press; 2000. [Google Scholar]
- 25.Kalisch M, Maechler M, Colombo D, Maathius MH, Bluehlmann P. Causal Inference Using Graphical Models with the R Package pcalg. Journal of Statistical Software 2012. p. 1–26. [Google Scholar]
- 26.Colombo D, Maathuis M. Order-independent constraint-based causal structure. arXiv:1211.3295v2; 2013.
- 27.Gentleman R, Whalen E, Huber W, Falcon S. graph: A package to handle graph data structures. R package version 1.54.0.
- 28.Huang dW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. 10.1038/nprot.2008.211 . [DOI] [PubMed] [Google Scholar]
- 29.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504. 10.1101/gr.1239303 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon R. Roadmap for developing and validating therapeutically relevant genomic classifiers. J Clin Oncol. 2005;23(29):7332–41. Epub 2005/09/06. 10.1200/JCO.2005.02.8712 . [DOI] [PubMed] [Google Scholar]
- 31.Trilla-Fuertes L, Gámez-Pozo A, Díaz-Almirón M, Prado-Vázquez G, Zapater-Moros A, López-Vacas R, et al. Computational metabolism predicts risk of distant relapse-free survival in breast cancer patients. Future Oncology. 2019;30:3483–90. 10.2217/fon-2018-0698 [DOI] [PubMed] [Google Scholar]
- 32.Dannenfelser R, Clark NR, Ma’ayan A. Genes2FANs: connecting genes through functional association networks. BMC Bioinformatics. 2012;13:156 10.1186/1471-2105-13-156 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Debily MA, Marhomy SE, Boulanger V, Eveno E, Mariage-Samson R, Camarca A, et al. A functional and regulatory network associated with PIP expression in human breast cancer. PLoS One. 2009;4(3):e4696 Epub 2009/03/05. 10.1371/journal.pone.0004696 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazarou M, Smith SM, Thorburn DR, Ryan MT, McKenzie M. Assembly of nuclear DNA-encoded subunits into mitochondrial complex IV, and their preferential integration into supercomplex forms in patient mitochondria. FEBS J. 2009;276(22):6701–13. Epub 2009/10/16. 10.1111/j.1742-4658.2009.07384.x . [DOI] [PubMed] [Google Scholar]
- 35.Chen A, Wang L, Liu S, Wang Y, Liu Y, Wang M, et al. Attraction and Compaction of Migratory Breast Cancer Cells by Bone Matrix Proteins through Tumor-Osteocyte Interactions. Sci Rep. 2018;8(1):5420 Epub 2018/04/03. 10.1038/s41598-018-23833-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed CC, Waterhouse A, Kirby S, Kay P, Owens RT, McQuillan DJ, et al. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005;24(6):1104–10. 10.1038/sj.onc.1208329 . [DOI] [PubMed] [Google Scholar]
- 37.Oda G, Sato T, Ishikawa T, Kawachi H, Nakagawa T, Kuwayama T, et al. Significance of stromal decorin expression during the progression of breast cancer. Oncol Rep. 2012;28(6):2003–8. Epub 2012/09/17. 10.3892/or.2012.2040 . [DOI] [PubMed] [Google Scholar]
- 38.Goldoni S, Seidler DG, Heath J, Fassan M, Baffa R, Thakur ML, et al. An antimetastatic role for decorin in breast cancer. Am J Pathol. 2008;173(3):844–55. Epub 2008/08/07. 10.2353/ajpath.2008.080275 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karamanou K, Franchi M, Piperigkou Z, Perreau C, Maquart FX, Vynios DH, et al. Lumican effectively regulates the estrogen receptors-associated functional properties of breast cancer cells, expression of matrix effectors and epithelial-to-mesenchymal transition. Sci Rep. 2017;7:45138 Epub 2017/03/23. 10.1038/srep45138 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moriggi M, Giussani M, Torretta E, Capitanio D, Sandri M, Leone R, et al. ECM Remodeling in Breast Cancer with Different Grade: Contribution of 2D-DIGE Proteomics. Proteomics. 2018;18(24):e1800278 10.1002/pmic.201800278 . [DOI] [PubMed] [Google Scholar]
- 41.de Souza DA Junior, Santana AC, da Silva EZ, Oliver C, Jamur MC. The Role of Mast Cell Specific Chymases and Tryptases in Tumor Angiogenesis. Biomed Res Int. 2015;2015:142359 Epub 2015/06/04. 10.1155/2015/142359 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glajcar A, Szpor J, Pacek A, Tyrak KE, Chan F, Streb J, et al. The relationship between breast cancer molecular subtypes and mast cell populations in tumor microenvironment. Virchows Arch. 2017;470(5):505–15. Epub 2017/03/18. 10.1007/s00428-017-2103-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endo A, Moyori A, Kobayashi A, Wong RW. Nuclear mitotic apparatus protein, NuMA, modulates p53-mediated transcription in cancer cells. Cell Death Dis. 2013;4:e713 Epub 2013/07/04. 10.1038/cddis.2013.239 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan HJ, Li H, Liu Z, Yuan YC, Mortimer J, Chen S. SERPINA1 is a direct estrogen receptor target gene and a predictor of survival in breast cancer patients. Oncotarget. 2015;6(28):25815–27. 10.18632/oncotarget.4441 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulvihill MM, Benjamin DI, Ji X, Le Scolan E, Louie SM, Shieh A, et al. Metabolic profiling reveals PAFAH1B3 as a critical driver of breast cancer pathogenicity. Chem Biol. 2014;21(7):831–40. Epub 2014/06/19. 10.1016/j.chembiol.2014.05.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohnz RA, Mulvihill MM, Chang JW, Hsu KL, Sorrentino A, Cravatt BF, et al. Activity-Based Protein Profiling of Oncogene-Driven Changes in Metabolism Reveals Broad Dysregulation of PAFAH1B2 and 1B3 in Cancer. ACS Chem Biol. 2015;10(7):1624–30. Epub 2015/05/07. 10.1021/acschembio.5b00053 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All the mass spectrometry raw data files acquired in this study may be downloaded from Chorus (http://chorusproject.org) under the project name Breast Cancer Proteomics.