Abstract
Few studies have addressed gene expression of hemostasis-related factors during acute thrombo-hemorrhagic diseases. Bites by the lanced-headed viper Bothrops jaracaca induce rapid hemostatic disturbances in victims, leading to systemic bleedings, thrombocytopenia and consumption coagulopathy. Although circulating levels of coagulation factors recover rapidly after administration of specific antivenom therapy, it is unclear if B. jararaca venom (BjV) upregulates the mRNA synthesis of hepatic hemostasis-related factors, or if the recovery occurs under basal conditions after the neutralization of venom components by antivenom. Thus, we aimed to investigate if BjV regulates gene expression of important hemostasis-related factors synthetized by the liver. On that account, Swiss mice were injected with saline or BjV (1.6 mg/kg b.w, s.c.), and after 3, 6 and 24 h blood samples and liver fragments were collected to analyze mRNA expression by real-time qPCR. Increased gene expression of fibrinogen chains, haptoglobin and STAT3 was observed during envenomation, particularly at 3 and 6 h. At 24h, mRNA levels of F10 were raised, while those of Serpinc1, Proc and Adamts13 were diminished. Surprisingly, F3 mRNA levels were steadily decreased at 3 h. Gene expression of Thpo, F7, F5 Tfpi, Mug1 was unaltered. mRNA levels of Vwf, P4hb, F8, F2, Plg, and Serpinf2 were minimally altered, but showed important associations with Nfkb1 gene expression. In conclusion, snakebite envenomation upregulates hepatic mRNA synthesis particularly of fibrinogen chains, and acute-phase markers. This response explains the fast recovery of fibrinogen levels after antivenom administration to patients bitten by B. jararaca snakes.
Author summary
Bothrops jararaca snakebites induce hemostatic disturbances in man and animals, but no information is available about how the liver, the main organ involved in the synthesis of hemostasis-related factors, copes with this condition. In an effort to understand how these factors recover after B. jararaca snakebites, we evaluated the hepatic gene expression of hemostatic-related factors after experimental envenomation in mice. We noticed that a marked increase in gene expression of fibrinogen chains occurred 3 and 6 h after subcutaneous administration of B. jararaca venom, explaining thereby the rapid recovery of the coagulation disturbance after administration of specific antivenom to patients. Interestingly, gene expression of other coagulation factors consumed during envenomation, e.g. factors V, VIII, and prothrombin, was scarcely affected over time, while the expression of tissue factor mRNA was promptly decreased at 3 h. Moreover, gene expression of acute-phase proteins was modified by envenomation, as well as of the transcription factor STAT3. Gene expression of NF-κB1 was not altered significantly over time, but it was associated with the regulation of mRNA levels of various hemostasis-related genes. Our results showed that B. jararaca envenomation induces a rapid change in gene expression of some coagulation factors and acute phase proteins.
Introduction
In 1.8–2.7 million patients bitten by various venomous snakes worldwide annually, hemostatic disorders are commonly observed [1, 2]. Although regarded as a secondary cause of acquired hemostatic disorders in hemostasis textbooks, bleedings evoked by snakebite envenomation are particularly common in patients from low-income tropical countries [3], leading to severe or fatal outcomes, e.g. life-threatening hemorrhage [4, 5], intracranial hemorrhage [6, 7], and spontaneous abortion in women [8].
Snakes from Bothrops genus–popularly known as jararacas in Brazil–inhabit most regions in Central and South America, where they are an important public health problem [5, 9, 10]. The venom from Bothrops jararaca (BjV), a snake species found in the southeastern region of Brazil, contains various enzymatic and non-enzymatic proteins–e.g., phospholipases A2, snake venom serine proteinases (SVSP), snake venom metalloproteinases (SVMP), L-amino acid oxidases, disintegrins and C-type lectins, among others [11]. These proteins interfere with hemostasis and disrupt the structure of vessel walls [12–14], leading to bleeding manifestations (e.g. gingival bleeding, hematuria, gastrointestinal bleeding, petechiae, ecchymosis, etc) in patients envenomed by these snakes [15, 16]. Besides, BjV also induces a phlogistic reaction at the site of venom inoculation, which may deteriorate progressively, leading to local necrosis as an ultimate outcome [17]. This exacerbated inflammatory reaction is considered essential to the burst of local and systemic inflammatory mediators, and synthesis of acute-phase proteins (APP) observed in patients and mice inoculated with BjV [18, 19].
Hemostatic abnormalities evoked by B. jararaca snakebites include direct proteolytic activity of snake venom enzymes on fibrinogen, prothrombin, and factors X, VIII and V, in a mechanistic process that is distinct from the extrinsic and intrinsic coagulation pathways, and by a feedback mechanism of these activated factors, especially meizothrombin or thrombin, which ultimately leads to activation and consumption of coagulation factors in circulation, as well as exacerbated secondary fibrinolysis [12, 20–23]. Thrombocytopenia and platelet dysfunction are also observed during B. jararaca envenomation [16, 22, 24], but they are independent of coagulation activation [21, 25]. Gradual fibrinogen consumption during snakebite envenomation causes hypofibrinogenemia or afibrinogenemia, leading to a prolongation of whole blood coagulation time (WBCT)–which is a sign of snake envenomation [26]. In health centers that treat snakebites, the modified WBCT is the most frequently used laboratory test to evaluate how intensely snake venoms have impaired the blood coagulation cascade [24, 26, 27].
Due to the effectiveness of specific serotherapy to treat coagulopathy and thrombocytopenia evoked by B. jararaca envenomation [15], restoration of blood coagulability and platelet counts occurs rapidly in patients, between 6 and 18 h after antivenom administration. However, it is not known how this process occurs, whether hemostasis-related factors are regenerated under basal hepatic conditions by simple neutralization of circulating antivenom, or whether pathophysiological events that take place during envenomation upregulate gene expression. Indeed, the current international consensus [28], based on previous clinical studies [29, 30], suggests that an additional dose of antivenom should be administered six hours after the first dose if the WBCT remains prolonged. However, this indication needs further scientific evidence, as the rate of coagulation factor synthesis is unknown under conditions of envenomation. Under basal conditions, it is known that fibrinogen has a half-life of ca. 90 h and is synthetized at a rate of ca. 34 mg/kg/day [31]. Thus, in a completely defibrinogenated 70-kg patient, taking an average plasma volume of 50 mL/kg, plasma fibrinogen concentration would reach mean levels around 68 mg/dL after 24 hours under basal fibrinogen synthesis condition, if venom had been completely neutralized by antivenom. In fact, this value is below the inferior hemostatic level of 100 mg/dL for fibrinogen [32], not explaining thereby the rapid restoration of fibrinogen levels observed in patients bitten by B. jararaca [15]. We hypothesized that the expression of hemostasis-related genes was somehow stimulated after envenomation in the liver, the main organ involved in the synthesis of hemostatic factors, even in the absence of antivenom administration. To address this hypothesis, taking into account both the inflammatory and hemostatic events occurring during envenomation that may influence hepatic gene expression, we evaluated mRNA expression levels in livers of mice injected with BjV [33], a model that replicates the hemostatic manifestations observed in bitten patients.
Methods
Materials
Lyophilized venom from adult specimens of B. jararaca snakes was obtained from the Laboratory of Herpetology, Butantan Institute (Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado, SisGen AF375C2). Bothrops antivenom was kindly donated by Butantan Institute (lot: 1305077). All reagents were of analytical grade or better.
Ethics statement
Male Swiss mice (n = 30), weighing 30–35 g, were obtained from the Animal Facility of Butantan Institute, and were maintained with free access to food and water. The experimental procedures involving mice were in accordance with National Guidelines, and were approved by the Institutional Animal Care and Use Committee from Butantan Institute (CEUAIB 4388061115) and from the School of Medicine, University of São Paulo (protocol 188/15). All procedures involving animals were in accordance with the National Guidelines from Conselho Nacional de Controle de Experimentação Animal (CONCEA) [34]. Blood and liver samples were collected from the same mice used previously [33].
Envenomation
Mice were randomly assigned to two experimental groups (n = 5 animals/group/time interval). Mice from the saline control group were injected subcutaneously (s.c.) with sterile saline alone (vehicle), and mice from the BjV group were injected with freshly diluted BjV (1.6 mg/kg body weight, s.c.). The dose of BjV was based on a previous report [33], and it evoked characteristic hemostatic disturbances.
After 3, 6 or 24 h, mice were anesthetized with isoflurane (induction and maintenance at 2.5%) for the collection of blood and tissues. Blood was collected from the caudal vena cava into plastic syringes, and added to plastic flasks containing 269 mM Na2EDTA for complete blood cell counts. Blood was also added to plastic flasks containing the anticoagulant CTAD (75 mM trisodium citrate, 42 mM citric acid, 139 mM dextrose, 15 mM theophylline, 3.7 mM adenosine, 0.2 mM dipyridamole, and 2 μM imipramine), and plasma was obtained by centrifugation at 2500 g for 15 min at 4°C. Bothrops antivenom (1 vol. to 100 vol. of blood) was added to all flasks to halt the in vitro activity of BjV after blood collection. Liver fragments from the central region of the left lateral lobe (50–100 mg) were collected, immersed in 500 μL of RNAlater (Thermo, USA) and stored at -80°C until used.
Hemostatic parameters
Platelet counts were obtained using an automated cell counter (BC-2800 Vet, Mindray, China) and plasma fibrinogen levels were measured with a colorimetric assay [35].
RNA isolation and cDNA synthesis
Liver fragments were removed from RNALater, immersed in 1 mL of TRIzol reagent, incubated for 5 min at room temperature, and disrupted using FastPrep-24 (#6004–500, MP Biomedicals) for 60 s at 6.5 m/s. Total RNA extraction and purification were performed with TRIzol Plus RNA Purification Kit (ThermoFisher, USA), following the manufacturer’s instructions. RNA concentration was quantified with Qubit 2.0 Fluorometer and Qubit RNA Assay Kit, Broad Range (Invitrogen, USA), following the manufacturer’s instructions. RNA quality was determined by micro-fluidic capillary electrophoresis in the Agilent 2100 Bioanalyzer (Agilent Technologies, USA) using the RNA Nano6000 kit, and all RNA samples showed a RNA Integrity Number (RIN) ≥ 7.9 [36]. A pool of mRNA from livers of 6 control mice was prepared as described above, and served as a reference sample.
Total RNA (1 μg) was reversed transcribed using the iScript cDNA Syntesis Kit (Bio-Rad, USA), according to the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction (qPCR)
Genes and primers are listed in Table 1. Primers were designed using Primer-Blast (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) or obtained from PrimerBank (https://pga.mgh.harvard.edu/primerbank/) [37]. Primer specificity was confirmed by analyzing melting curves from amplicons, and by performing agarose gel electrophoresis to discriminate PCR products. Amplified PCR fragments were visualized by ethidium bromide staining following electrophoresis in 1.5% agarose gels.
Table 1. Genes and primers used for gene expression analysis by qPCR.
| Gene (NCBI access) |
Forward primer (5’→3’) Reverse primer (5’→ 3’) |
Amplicon length (bp) |
|---|---|---|
|
Rplp0 (Ribosomal protein, large, P0) (NM_007475.5) |
AGATTCGGGATATGCTGTTGGC TCGGGTCCTAGACCAGTGTTC |
109 |
|
Vwf (von Willebrand Factor) (NM_011708.4) |
CTTCTGTACGCCTCAGCTATG GCCGTTGTAATTCCCACACAAG |
125 |
|
Adamts13 (ADAMTS13) (NM_001001322.2; NM_001290463.1) |
AACACAGTGGTGGTGAAGCA ACAATTTCACCCCGAGGTCC |
134 |
|
P4hb (Protein disulfide isomerase A1, PDI A1) (NM_011032.3) |
GCCGCAAAACTGAAGGCAG GGTAGCCACGGACACCATAC |
100 |
|
F3 (Tissue factor) (NM_010171.3) |
AACCCACCAACTATACCTACACT GTCTGTGAGGTCGCACTCG |
101 |
|
F7 (Factor VII) (NM_010172.4) |
GCTGGACGCCAGATGGATAG AGTCATGTTCACCCATCACCA |
92 |
|
F8 (Factor VIII) (NM_007977.2; NM_001161373.1; NM_001161374.1) |
AGAATCAAGCAAGCCGACCA ACGTGCTTTATACCTCTTGGCA |
97 |
|
F5 (Factor V) (NM_007976.3) |
CCTGGTCAGCGCAACATCTA GCCTGCATCCCAGCTTGATA |
105 |
|
F10 (Factor X) (NM_001242368.1) |
AGGCTGAGATAAGCAGAAGGC GGTTAATAAACACACCTTTCCCAGG |
179 |
|
F2 (Factor II) (NM_010168.3) |
CCGAAAGGGCAACCTAGAGC GGCCCAGAACACGTCTGTG |
103 |
|
Thpo (Thrombopoietin) (NM_009379.3; NM_001289894.1, NM_001173505.1) |
CACAGCTGTCCCAAGCAGTA GGCTGTGACACTGAAGTTCG |
97 |
|
Tfpi (Tissue factor pathway inhibitor) (NM_011576.1; NM_001177319.1; NM_001177320.1; NM_001355271.1; NM_001355273.1) |
TGGAGCAGAAAGGCCAGATT TCAAAGTTGTTGCGGTTGCC |
147 |
|
Serpinc1 (Antithrombin III) (NM_080844.4) |
GGCTGCTGGTGAGAGGAAG GGATTCACGGGGATGTCTCG |
129 |
|
Proc (Protein C) (NM_008934.4; NM_001042767.3; NM_001042768.3; NM_001313938.1) |
CCACCTGGGGAATATCTAGCA GAAGCTGTTGGCACGTCTG |
101 |
|
Plg (Plasminogen) (NM_008877.3) |
TGCAGTGGAGAAAAGTATGAGGG AGGGATGTATCCATGAGCATGT |
102 |
|
Serpinf2 (α2-antiplasmin) (NM_008878.2) |
TTCTCCTCAACGCCATCCA GGTGAGGCTCGGGTCAAAC |
62 |
|
Fga (Fibrinogen—α chain, variant 1) (NM_001111048.2) |
GCCCAACGAGAGACTGTGAT TCTTGCCAGGTCCGGTTAAA |
193 |
|
Fgb (Fibrinogen—β chain) (NM_181849.3) |
ACGATGAACCGACGGATAGC CCGTAGGACACAACACTCCC |
225 |
|
Fgg 1 (Fibrinogen—γ chain, variant 1) (NM_133862.2) |
GACGGCATTATTTGGGCGAC AACGTCTCCAGCCTGTTTGG |
141 |
|
Fgg 2 (Fibrinogen—γ chain, variant 2) (NM_001317105.1) |
TCACCAAGGTGGTACTTACTCAAA TGATCCACGCTGACCTGTTT |
192 |
|
Hp (Haptoglobin) (NM_017370.2; NM_001329965.1) |
GCTATGTGGAGCACTTGGTTC CACCCATTGCTTCTCGTCGTT |
101 |
|
Mug1 (Murinoglobulin 1) (NM_008645.3) |
GGCAGAGTTCTCCATAGATACCA TGCTTTGCACTTGCATGTCTT |
126 |
|
Nfkb1 (Nuclear factor of kappa light polypeptide gene enhancer in B cells 1) (NM_008689.2) |
ATGGCAGACGATGATCCCTAC TGTTGACAGTGGTATTTCTGGTG |
111 |
|
Stat3 (Signal transducer and activator of transcription 3) (NM_213659.3; NM_213660.3) |
CAATACCATTGACCTGCCGAT GAGCGACTCAAACTGCCCT |
109 |
For qPCR reactions, cDNA samples were diluted to obtain 1–100 ng template per reaction, depending on the analyzed gene: 100 ng template per reaction for the genes Vwf, Adamts13, F3, F7, F8, F5, F10, Tfpi, and Thpo; 10 ng template per reaction for the genes Rplp0, P4hb, F2, Serpinc1, Proc, Plg, Serpinf2, Fga, Fgg 2, Mug1, Nfkb1 and Stat3; 1 ng template per reaction for the genes Fgb, Fgg 1 and Hp. The reaction mixture contained cDNA templates (in 1–2 μL), 5 μL of 2X Fast SYBR Green Master Mix (ThermoFisher, USA), and 1 μL of gene-specific primers (0.2 μM final concentration; Invitrogen or Sigma, USA), in a final volume of 10 μL. PCR reactions were carried out in triplicate in 96-well format plates (ThermoFisher, USA) using a StepOnePlus Real-Time PCR System (Applied Biosystems, USA), in a total of 40 cycles, following manufacture instructions.
For the normalization of data, amplification efficiencies of six reference genes were tested (Gapdh, Actb, Hprt, Rps29, B2m and Rplp0), and analyzed with geNorm [38]. Rplp0 showed the best stability expression (M value = 0.281), and was chosen as the reference gene for the normalization of qPCR data. The relative gene expression in mice from saline control and BjV groups was analyzed by the 2-ΔΔCT method, using the pool of mRNA from six normal mice, loaded in each experiment, as the reference sample [39]. Results are shown as relative mRNA levels based on 2-ΔΔCT values. For statistical analyses, ΔΔCT values were used to compare experimental groups using ANOVA and correlation tests (see underneath).
Statistical analyses
Sample size, i.e., the number of mice allocated in each experimental group, was based on previous data of experimental envenomation that resulted in falls in platelet counts and fibrinogen levels around 50–75% at 3 h after injection [33]. Normal distribution and homoscedasticity of results were analyzed using the software STATATM, version 10, and data were transformed whenever necessary. For statistical comparisons among groups, two-way ANOVA was used, followed by Bonferroni post-hoc test. Results were considered statistically significant when p< 0.05. Data were expressed as mean ± standard error of mean (S.E.M.).
Scatterplots and Pearson’s correlation matrices were created to examine the correlation coefficients and linear regression between variables, taking into account data from control and experimental groups together; whenever statistically significant correlation coefficients were observed (p< 0.05 and correlation coefficients> 0.700), the data were split into control and experimental groups and re-analyzed independently to check if the significance was due to false correlations between both groups. Confidence intervals (95%) for correlation values were estimated using a percentile bootstrap, using 1000 replicates, and reported inside square brackets. Correlation coefficients, linear regressions and ANOVA analyses were performed in SPSS (version 22).
Results
Envenomation induces hemostatic disturbances
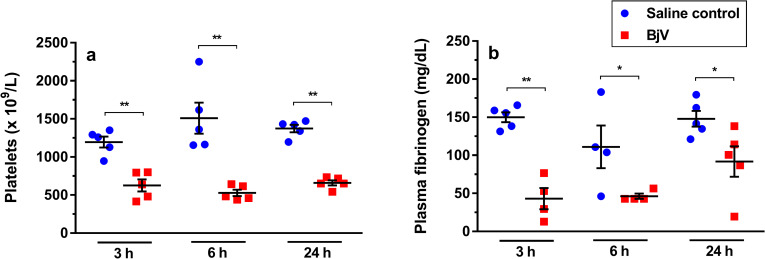
As expected, platelet counts (Fig 1A) and fibrinogen levels (Fig 1B) steadily decreased after BjV injection. Platelet counts decreased around 50% at 3 and 24 h (p< 0.001), and showed a more evident decrease, around 65%, at 6 h (p< 0.001) in the BjV group. The drop (around 70%) in plasma fibrinogen levels was more evident at 3 h (p< 0.001). Although there was a tendency for recovery of fibrinogen levels over time, envenomed animals still showed hypofibrinogenemia at 6 and 24 h (p< 0.05).
Fig 1.
Platelet counts (a) and plasma fibrinogen levels (b) at 3, 6 and 24 h after injection of saline (saline control) or Bothrops jararaca venom (BjV). Two-way ANOVA, followed by Bonferroni post-hoc test were used to analyze data; * p< 0.05 and ** p< 0.001. Data were expressed as mean ± S.E.M. (n = 4-5/group).
BjV induces expression of APP genes and the transcription factor Stat3
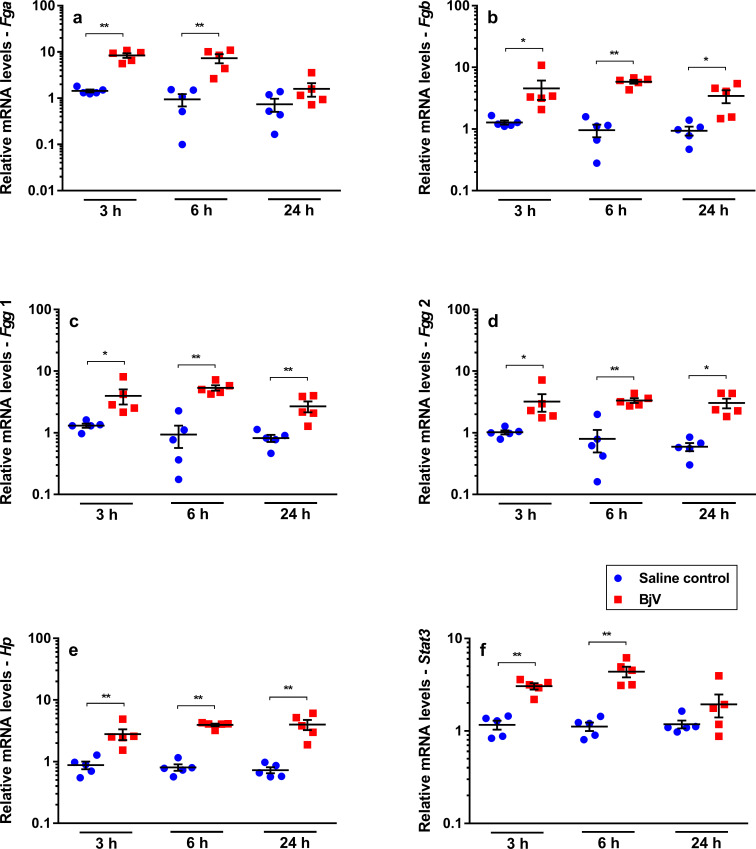
Considering that BjV induces the synthesis of APP [18, 19], we studied fibrinogen gene expression [40], and observed that mRNA levels of fibrinogen chains were the most increased after venom injection. Expression of Fga (Fig 2A) only increased in the acute phase of envenomation (at 3 and 6 h, p< 0.001), whilst those of Fgb (Fig 2B), Fgg 1 (Fig 2C) and Fgg 2 (Fig 2D) were elevated at all time periods analyzed (p< 0.05), most evidently at 6 h (a 6-fold increase, p< 0.001). BjV also enhanced the expression of another APP gene, namely haptoglobin (Hp), which showed a 4 to 6-fold increase at all time intervals (p< 0.001, Fig 2E). On the other hand, the negative APP gene Mug1 (Fig 3K), encoding murinoglobulin–which is a key serpin inhibitor in rodents [41] and that inhibits various venom activities in vivo [42]–showed no statistically significant differences between groups over time.
Fig 2.
Relative mRNA levels of Fga (a), Fgb (b), Fgg 1 (c), Fgg 2 (d), Hp (e) and Stat3 (f) genes highly altered in mouse livers at 3, 6 and 24 h after injection of saline (saline control) or Bothrops jararaca venom (BjV). mRNA levels were calculated using 2-ΔΔCT method, using Rplp0 as the reference gene. A pool of mRNA from six normal mouse livers was employed as the reference sample. Two-way ANOVA, followed by Bonferroni post-hoc test, were used to analyze data; * p< 0.05 and ** p< 0.001. Data were expressed as mean ± S.E.M. (n = 5/group, triplicates).
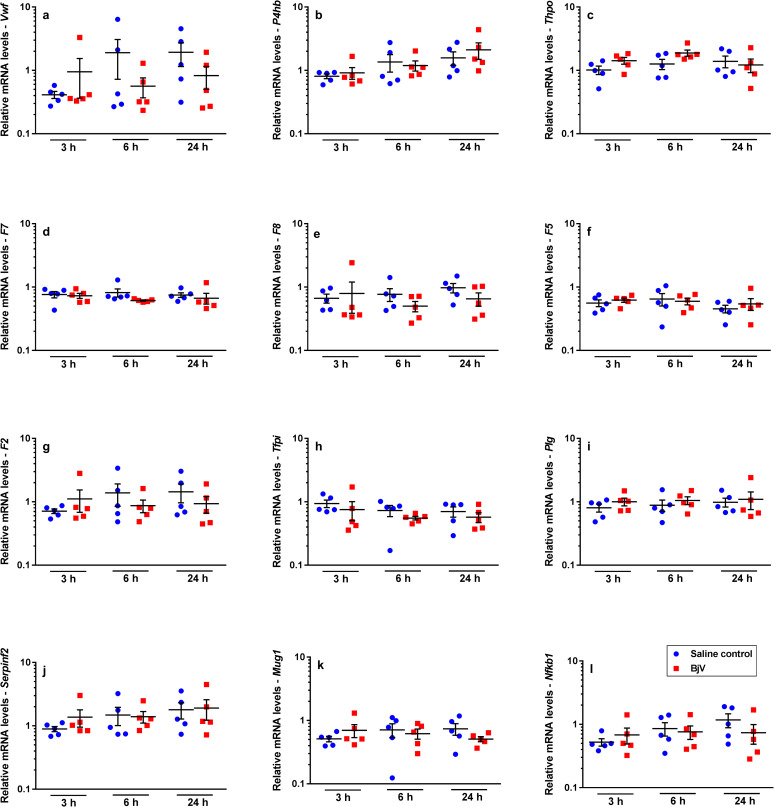
Fig 3.
Relative mRNA levels of Vwf (a), P4hb (b), Thpo (c), F7 (d), F8 (e), F5 (f), F2 (g), Tfpi (h), Plg (i), Serpinf2 (j), Mug1 (k) and Nfkb1 (l), genes that were marginally altered in mouse livers at 3, 6 and 24 h after injection of saline (saline control) or Bothrops jararaca venom (BjV). mRNA levels were calculated using 2-ΔΔCT method, using Rplp0 as the reference gene. A pool of mRNA from six normal mouse livers was employed as the reference sample. Two-way ANOVA, followed by Bonferroni post-hoc test, were used to analyze data; * p< 0.05 and ** p< 0.001. Data were expressed as mean ± S.E.M. (n = 5/group, triplicates).
The cross-talk between the transcription factors STAT3 and NF-κB has an important role in the regulation of APP gene expression [43]. Interestingly, Stat3 mRNA levels (Fig 2F) were increased at 3 and 6 h (p< 0.001) after envenomation, whilst Nfkb1 mRNA levels (Fig 3L) showed no statistically significant differences between groups over time.
STAT3 and thrombopoietin have also been involved in the regulation of platelet signaling, activation and production [44, 45]. In addition, the JAK2-STAT3 pathway was shown to be involved in the increased hepatic Thpo mRNA levels and control of thrombopoiesis [45]. Even though thrombocytopenia occurred rapidly after envenomation, no change in Thpo mRNA levels (Fig 3C) was noticed over time. Besides, no statistically significant correlation (p> 0.05) was observed between gene expression of Thpo, Stat3 and platelet counts.
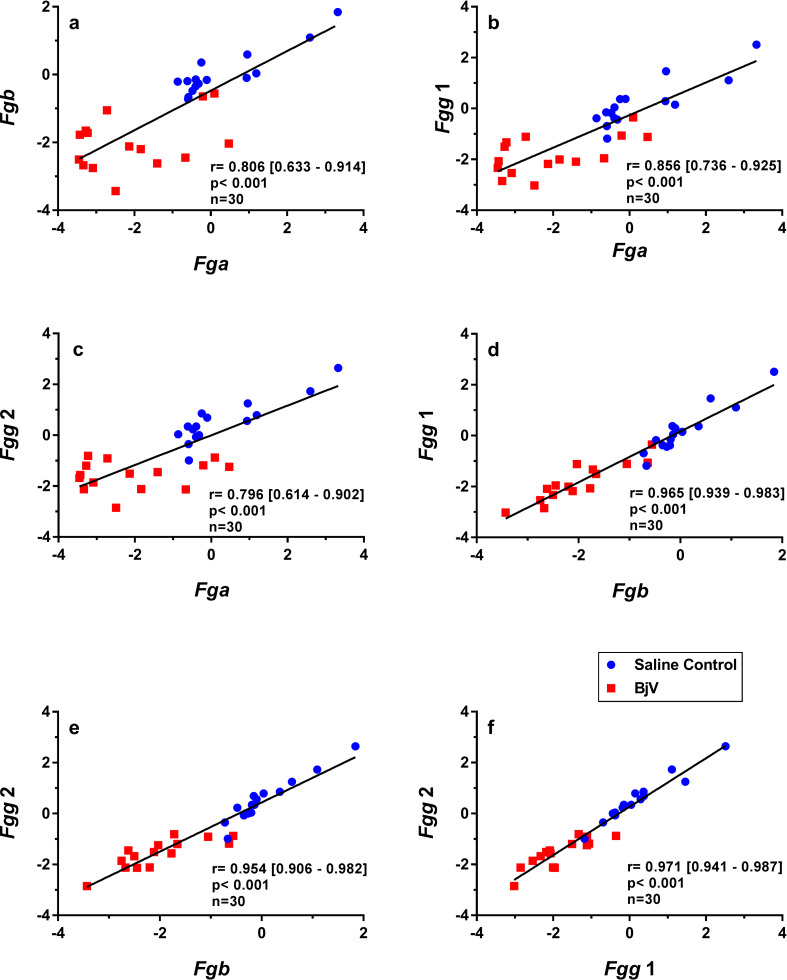
Gene expression of fibrinogen chain genes are correlated
Fibrinogen synthesis seemed a coordinated process, once mRNA levels of fibrinogen chains correlated significantly among each other (Fig 4A–4F) (r = 0.796–0.971, p< 0.001, n = 30). However, when correlation analyses were performed with split data between control and experimental groups, different results were obtained for Fga. At basal conditions of the control groups, the statistical significance of correlations was maintained between Fga and Fgb (r = 0.897 [95% confidence interval: 0.510–0.969], p< 0.001, n = 15), Fgg1 (r = 0.859 [0.594–0.951], p< 0.001, n = 15) and Fgg2 (r = 0.880 [0.564–0.963], p< 0.001, n = 15). In contrast, regarding the BjV group, Fga expression only predicted 3–30% of the variance of Fgb and Fgg, unveiled by weak correlation coefficients (r = 0.192–0.570, p = 0.026–0.493, n = 15) among these genes. These findings indicate that the regulatory mechanisms of Fga transcription may differ from those of other fibrinogen chains during envenomation.
Fig 4.
Scatterplots and linear regressions of paired observations between relative mRNA levels of the fibrinogen chain genes Fgb and Fga (a), Fgg 1 and Fga (b), Fgg 2 and Fga (c), Fgg 1 and Fgb (d), Fgg 2 and Fgb (e), Fgg 2 and Fgg 1 (f) in mouse livers at 3, 6 and 24 h after injection of saline (saline control) or Bothrops jararaca venom (BjV). mRNA levels were calculated using 2-ΔΔCT method, using Rplp0 as the reference gene. A pool of mRNA from six normal mouse livers was employed as the reference sample. Data were expressed as relative mRNA levels based on ΔΔCT values (n = 30). Pearson’s correlation was used, and results were expressed as correlation coefficient (r) and 95% confidence interval between square brackets.
Hemostasis-related genes are differently expressed during envenomation
Few authors have studied hepatic gene expression of hemostasis-related factors after acute experimental procedures or acquired conditions. In our model, using a snake venom that induces profound and rapid hemostatic changes in victims, hepatic expression of hemostasis-related genes (Figs 5 and 3) showed variable results. Interestingly, most of the studied genes did not display a statistically significant difference among groups over time (Fig 3).
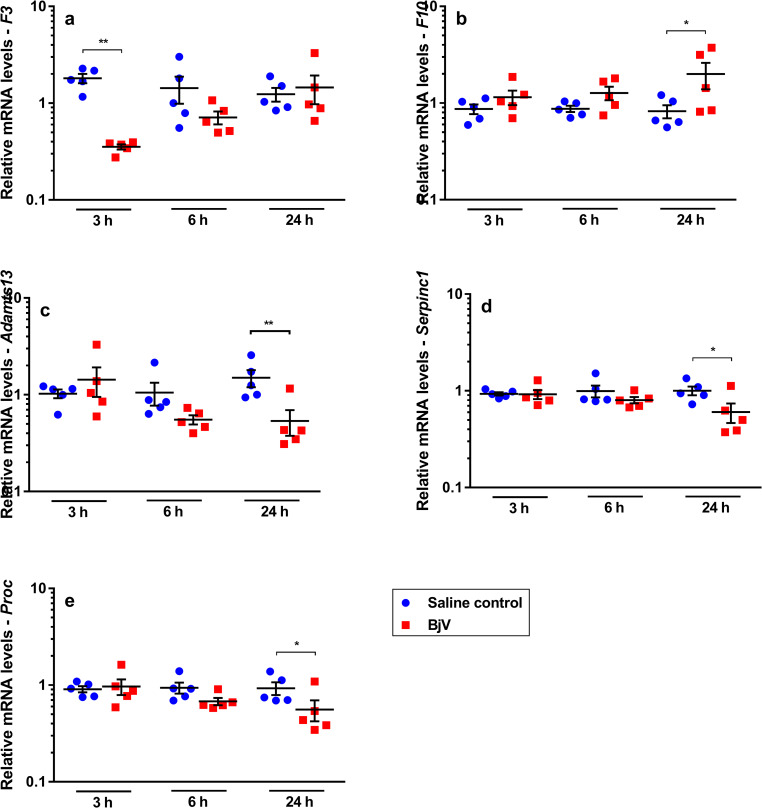
Fig 5.
Relative mRNA levels of F3 (a), F10 (b), Adamts13 (c), Serpinc1 (d) and Proc (e), genes that showed alterations in mouse livers at 3, 6 and 24 h after injection of saline (saline control) or Bothrops jararaca venom (BjV). mRNA levels were calculated using 2-ΔΔCT method, using Rplp0 as the reference gene. A pool of mRNA from six normal mouse livers was employed as the reference sample. Two-way ANOVA, followed by Bonferroni post-hoc test, were used to analyze data; * p< 0.05 and ** p< 0.001. Data were expressed as mean ± S.E.M. (n = 5/group, triplicates).
Bothrops snake venoms induce protein expression and increased activity of tissue factor (TF) [25, 46, 47], but surprisingly a rapid and gross decrease in F3 gene expression was noticed in the liver. In fact, F3 (Fig 5A) was the only coagulation factor whose expression decreased significantly (p< 0.001) in the BjV group at 3 h, returning gradually to levels similar to controls only at 24 h.
In regard to other coagulation factors, envenomation also induces alterations in coagulation factors X, II, V and VIII, but, interestingly, only F10 mRNA levels (p< 0.05, Fig 5B) increased at 24h after envenomation. On the other hand, gene expression of F7 (Fig 3D), F8 (Fig 3E), F5 (Fig 3F) and F2 (Fig 3G) did not change over time. Although plasma levels of antithrombin III remain constant during envenomation [20, 48], at 24 h an unexpected decrease in the mRNA levels of anticoagulant factors antithrombin III (Serpinc1, p< 0.05, Fig 5D) and protein C (Proc, p< 0.05, Fig 5E) was noticed, as well as a decrease in gene expression of the VWF-cleaving protease ADAMTS13 (Adamts13) (p< 0.001, Fig 5C).
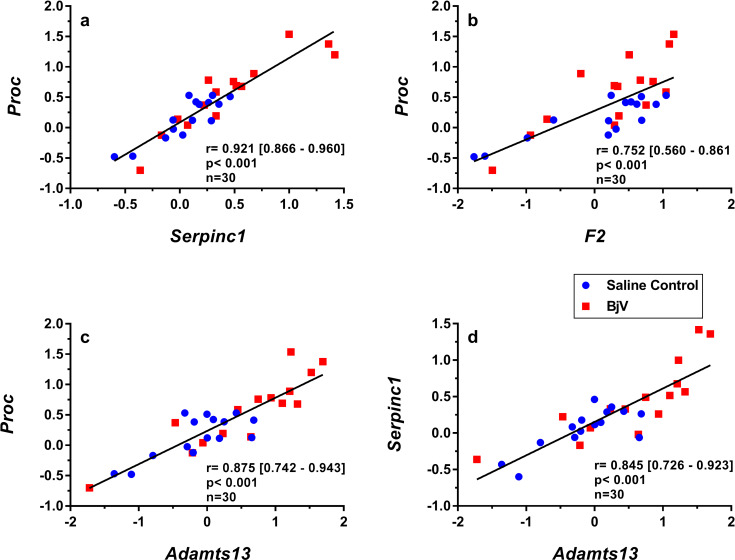
Although different patterns of gene expression were noticed over time, significant correlations were observed between relative mRNA levels (Figs 6 and 7). mRNA levels of the anticoagulants Proc and Serpinc1 had strong correlations (Fig 6A). Proc expression also correlated with F2 (Fig 6B) and Adamts13 (Fig 6C), and Serpinc1 correlated with Adamts13 (Fig 6D).
Fig 6.
Scatterplots and linear regressions of paired observations between relative mRNA levels of hemostasis-related genes Proc and Serpinc1 (a), Proc and F2 (b), Proc and Adamts13 (c) and Serpinc1 and Adamts13 (d) in mouse livers at 3, 6 and 24 h after injection of saline (saline control) or Bothrops jararaca venom (BjV). mRNA levels were calculated using 2-ΔΔCT method, using Rplp0 as the reference gene. A pool of mRNA from six normal mouse livers was employed as the reference sample. Data were expressed as relative mRNA levels based on ΔΔCT values (n = 30). Pearson’s correlation was used, and results were expressed as correlation coefficient (r) and 95% confidence interval between square brackets.
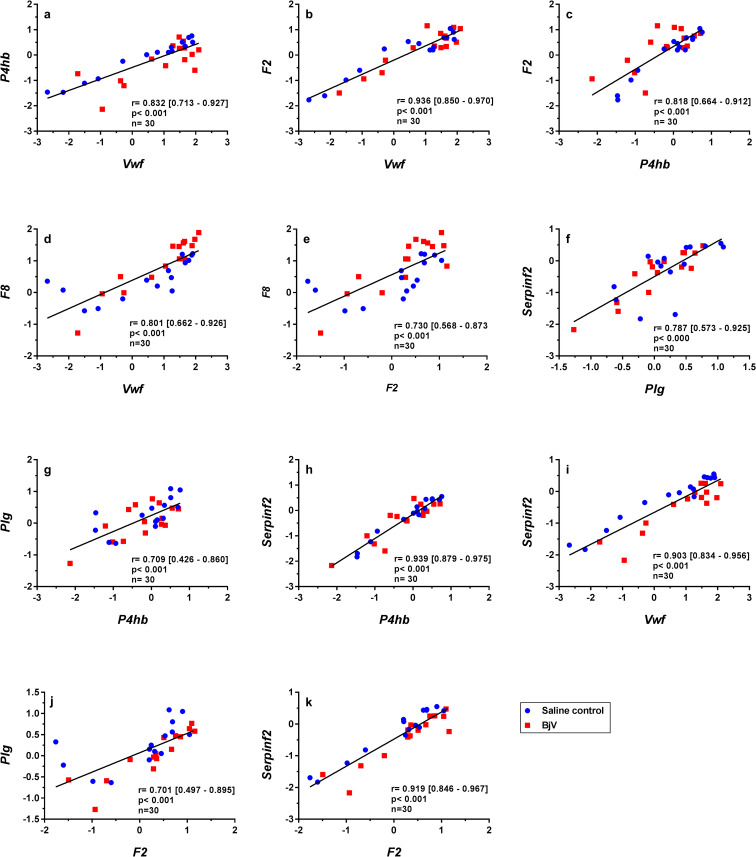
Fig 7.
Scatterplots and linear regressions of paired observations between relative mRNA levels of hemostatic genes P4hb and Vwf (a), F2 and Vwf (b), F2 and P4hb (c), Vwf and F8 (d), F2 and F8 (e), Serpinf2 and Plg (f), Plg and P4hb (g), Serpinf2 and P4hb (h), Serpinf2 and Vwf (i), Plg and F2 (j) and Serpinf2 and F2 (k) in mouse livers at 3, 6 and 24 h after injection of saline (saline control) or Bothrops jararaca venom (BjV). mRNA levels were calculated using 2-ΔΔCT method, using Rplp0 as the reference gene. A pool of mRNA from six normal mouse livers was employed as the reference sample. Data were expressed as relative mRNA levels based on ΔΔCT values (n = 30). Pearson’s correlation was used, and results were expressed as correlation coefficient (r) and 95% confidence interval between square brackets.
The functional association between VWF and factor VIII is well-established at the protein level since VWF, the plasma carrier of factor VIII, is also involved in the regulation of FVIII activity [49]. In addition, PDIA1 (encoded by P4hb) was shown to be involved in the dimerization of VWF, a key step for VWF regulation [50]. Interestingly, these and other associations were also observed at a gene level herein. Thus, strong correlations between gene expression of Vwf and P4hb (Fig 7A), Vwf and F2 (Fig 7B), P4hb and F2 (Fig 7C), Vwf and F8 (Fig 7D), and F2 and F8 (Fig 7E) were noticed.
Expression of two genes encoding major proteins of the fibrinolytic system–α2-antiplasmin (gene Serpinf2) and plasminogen (gene Plg)–was significantly correlated (Fig 7F), as well as with other genes: Plg and P4hb (Fig 7G), Serpinf2 and P4hb (Fig 7H), Serpinf2 and Vwf (Fig 7I), Plg and F2 (Fig 7J), and Serpinf2 and F2 (Fig 7K).
Hemostasis-related factors in the liver show different responses to envenomation. However, regulation of some genes seems to be associated and share common pathways of regulation.
Expression of transcription and hemostatic-related factors is correlated
To better understand the correlation in gene expression between the aforementioned genes, scatterplots from data of mRNA levels of hemostasis-related genes and the transcription factors Nfkb1 and Stat3 were also analyzed.
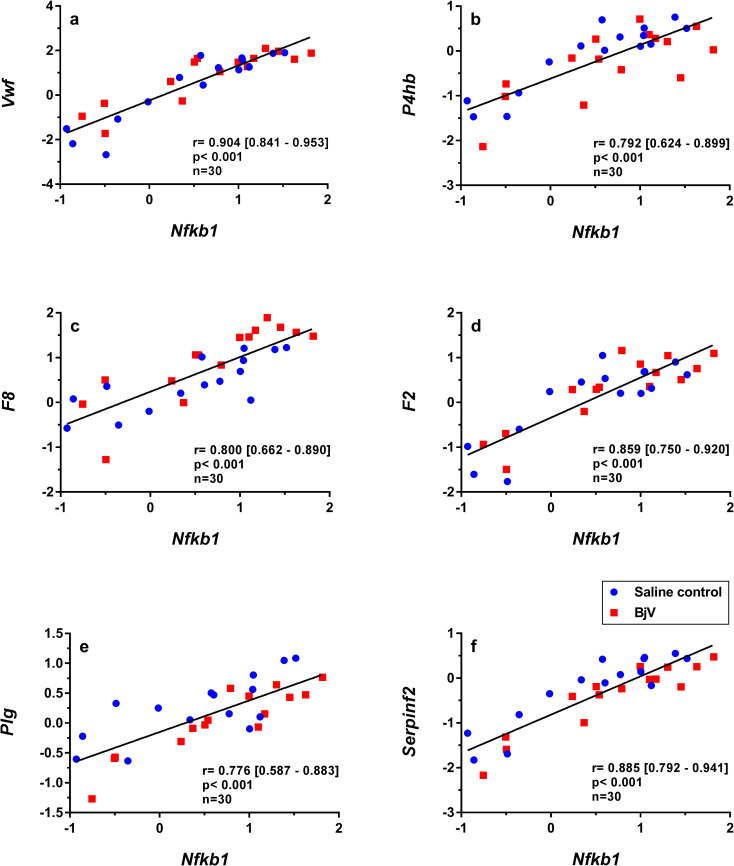
NF-κB1 is a transcription factor that plays an important role in immunity and inflammation [43]. NF-κB1 may be activated by several stimuli, such as pro-inflammatory cytokines and oxidative stress and regulates genes related to hemostasis and inflammation [51]. Interestingly, important correlations were noted for Nfkb1 mRNA levels and hemostasis-related genes, e.g., Vwf (Fig 8A), P4hb (Fig 8B), F8 (Fig 8C) and F2 (Fig 8D). Moreover, correlations were observed between gene expression of Nfkb1 and the fibrinolytic proteins Plg (Fig 8E) and Serpinf2 (Fig 8F). Using the coefficient of determination (r2) as index to predict this variation, these data suggest that Nfkb1 accounted for 60–80% of the expression variance of important hemostatic genes, indicating that Nfkb1 could be a relevant gene involved in the regulation of hemostasis-related genes.
Fig 8.
Scatterplots and linear regressions of paired observations between relative mRNA levels of hemostasis-related genes and Nfkb1, Vwf and Nfkb1 (a), P4hb and Nfkb1 (b), F8 and Nfkb1 (c), F2 and Nfkb1 (d), Plg and Nfkb1 (e), Serpinf2 and Nfkb1 (f) in mouse livers at 3, 6 and 24 h after injection of saline (saline control) or Bothrops jararaca venom (BjV). mRNA levels were calculated using 2-ΔΔCT method, using Rplp0 as the reference gene. A pool of mRNA from six normal mouse livers was employed as the reference sample. Data were expressed as relative mRNA levels based on ΔΔCT values (n = 30). Pearson’s correlation was used, and results were expressed as correlation coefficient (r) and 95% confidence interval between square brackets.
Discussion
Envenomation caused by Bothrops jararaca snakebites induces clinical alterations in bitten patients, as hematological and hemostatic disturbances [16, 20]. The recovery of these parameters over time is not completely elucidated, as well as the venom-induced hepatic response. Herein we present new insights into the complex regulation of gene expression of hemostasis-related factors.
In order to validate our experiment model, we first analyzed the occurrence of thrombocytopenia and hypofibrinogenemia, two major characteristic hemostatic disorders resulting from B. jararaca envenomation. Thrombocytopenia is an important hematological alteration that is related to the severity of envenomation, as well as to the development of systemic bleedings and edema. In agreement with previous results, thrombocytopenia and hypofibrinogenemia are not correlated, however the association of these disturbances increases the risk of bleedings during envenomation [46, 52, 53].
In models of acute immune thrombocytopenia [45] or non-immune thrombocytopenia [54], platelet counts are inversely related to plasma thrombopoietin levels, which are elevated independently of hepatic Thpo mRNA levels. Based on our results, the restoration of platelet counts during B. jararaca envenomation does not seem to be regulated by an increase in Thpo gene expression in the liver. In agreement with these results, rabbits had a minor increase in reticulated platelets in circulating blood following injection of BjV [48].
Hypofibrinogenemia is the result of the action of BjV toxins, such as SVSP and SVMP, that activate coagulation factors and lead to the formation of intravascular thrombin. The slow and constant activation of the coagulation cascade induces the consumption of coagulation factors, mainly fibrinogen, generating intravascular fibrin, which in turn, is degraded by the fibrinolytic system, generating a marked increase in fibrinogen/fibrin degradation products (FDP) [15, 21, 52, 55]. As expected in our model after 24 h of envenomation, the levels of fibrinogen tended to increase without the use of antivenom, which is also observed in patients bitten by B. jararaca snakes and treated with antivenom [55, 56]. The increment in the rate of fibrinogen synthesis after defibrinogenation induced by B. jararaca envenomation may be explained by the augmented synthesis of fibrinogen chains, as demonstrated by the elevated mRNA levels of fibrinogen chains in hepatocytes. Augmented and coordinated gene expression of fibrinogen chains was also observed in rat livers following infusion of the thrombin-like enzyme from Calloselasma rhodostoma snake venom [57]. The coordinated hepatic synthesis of three fibrinogen chains is well-described to be stimulated by the presence of FDP fragments in circulation [58–60], and due to the increase in circulating cytokines, mainly interleukin-6, during inflammation models [40, 61]. During Bothrops envenomation, cytokines and immune modulators are overexpressed [18], and FDP levels raise steadily, and in concert they might upregulate fibrinogen synthesis. That is, hepatocytes may increase synthesis rates when stimulated by components endogenously generated as a response to envenomation.
Furthermore, we showed that not only was that the gene expression of fibrinogen chains upregulated by envenomation, but also that of haptoglobin. Indeed, the stimulation of fibrinogen and haptoglobin genes is a well-known positive acute-phase response (APR) [62]. APR is an important systemic reaction evoked by diverse inflammatory conditions and tissue damage, like those occurring during envenomation [63, 64]. The increase in cytokine levels in such conditions may in turn lead to alterations in the transcription of APR genes in the liver. Our results are in agreement with the main APR features, i.e., the upregulation of positive APP genes, such as fibrinogen and haptoglobin, as well as the downregulation of negative APPs genes, e.g. protein C, antithrombin III, and murinoglobulin 1 [65–67].
During B. jararaca envenomation, the contribution of the inflammatory reaction or raised FDP levels to the upregulation of gene expression of fibrinogen chains and other APP in liver is difficult to separate. Infusion of the C. rhodostoma thrombin-like enzyme induced a rapid increase in Fga, Fgb and Fgg, peaking at 12–16 h [57]. On the other hand, asseptic inflammatory stimuli, such as burn injury [61] or turpentine injection [68], induced a peak in fibrinogen chains and Hp around 24 h post-stimuli. Our findings showed that the relative mRNA levels of Fga, Fgb, Fgg1, Fgg2, and Hp were already elevated at 3 h, and tended to peak at 6 h. Thus, both the inflammatory reaction and the rapid rise in FDP levels tended to accelerate the synthesis of these genes during B. jararaca envenomation. Moreover, secondary infections, which occur in some patients bitten by B. jararaca at later periods [69], might upregulate the mRNA synthesis of fibrinogen more strinkingly.
Reasoning about important transcription factors in hepatocytes, which could be linked to APR, we evaluated gene expression of STAT3 and NF-κB1. Hepatic STAT3 functions as a mechanism for the control of systemic inflammation, and upon stimuli IL-6 activates STAT3, which in turn induces the transcription of APPs genes [62, 70]. In fact, Stat3 expression was indeed increased in the acute-phase of B. jararaca envenomation. IL-6 signaling not only activates STAT3, but also induces Stat3 expression, via IL-6 responsive element in the gene promoter, explaining thereby the Stat3 mRNA increase in hepatocytes during envenomation and the generation of an APR [71, 72].
Our results showed that neither Fga mRNA expression nor plasma fibrinogen levels were strongly associated with the gene expression levels of other fibrinogen chains after venom injection. In rats, gene expression of Fga differs from that of Fgb or Fgg, since the response of Fga to IL-6 stimuli is not mediated by STAT3 [70, 73]. Even though expression of fibrinogen chain genes was increased as soon as 3 h after venom injection, restoration of the circulating fibrinogen levels began only after 24 h in mice not treated with antivenom administration. It is important to emphasize that the circulating fibrinogen levels are regulated by its production and degradation, at both transcriptional and post-transcriptional levels [62, 70], but during envenomation venom enzymes that lead to fibrinogen consumption retard the restoration of fibrinogen levels in circulating blood.
Despite the coagulopathy observed following Bothrops envenomation, the hemostasis-related genes Vwf, P4hb, Thpo, F7, F8, F5, F2, Tfpi, Plg, Serpinf2 and Mug1 showed no quantitative difference in gene expression, but their expression was associated among each other and with Nfkb1. Interaction between transcription factors STAT3 and NF-κB is important for the transcriptional regulation of several genes related to modulation of inflammatory responses [43, 51]. However Nfkb1 may also exert suppressor activity on inflammatory pathways [74] and our results suggest that Nfkb1 may also be associated to the regulation of hemostasis-related genes. Moreover, our findings indicate that the restoration of prothrombin and factors V, VIII and X, which are decreased during envenomation [20, 21], seems to occur under basal hepatic conditions. In addition, it was demonstrated that hepatic expression of several genes may have common regulatory mechanisms and that the physiological synthesis rates of most factors remain unaltered even in the presence of anti-hemostatic and pro-inflammatory conditions, like snake envenomation-induced coagulopathy.
Circulating levels of factor VII were not altered during B. jararaca envenomation [25], and herein we showed that F7 mRNA levels are neither altered. However, we showed for the first time a decrease in hepatic F3 mRNA, which occurred as soon as 3 h after BjV administration, returning to control levels only at 24 h. TF expression is well known to differ depending on the tissue being analyzed, and to be regulated by several transcription factors, pathways and stimuli [75]. Interestingly, higher levels of constitutive TF expression seem to confer an additional hemostatic protection to specific tissues, such as lungs and heart. However, under physiological conditions, TF levels in the liver are considered comparatively low in regard to other organs, and in low-TF transgenic mice (which have only ≈ 1% of TF) the liver-specific hemostasis is considered normal, and those mice show no bleedings [75, 76]. Further studies are currently being carried out to understand the importance of TF alterations during envenomation.
Differently from the early pattern of F3 gene expression during envenomation, gene expression of Adamts13, Serpinc1 and Proc decreased, whilst that of F10 increased at a later period of envenomation (24 h). Similar results were reported in an experimental model of direct liver hyperplasia and were associated to liver regeneration [77]. This may suggest that the impairment of anticoagulants during envenomation may be aggravated by the decrease in their hepatic synthesis. Interestingly, gene interactions as those of Proc and Serpinc1, and Plg and Serpinf2 were already reported to be associated with liver function and liver regeneration [78, 79].
Although we could not evaluate all proteins whose genes have been studied due to limitations in the blood volume obtained from mice, it is important to emphasize that few studies have addressed how gene expression of various hemostatic and inflammatory factors synthetized by the liver occurs under steady state conditions and under acute systemic injuries. Understanding these mechanisms, using models that disturb both systems simultaneously, is important to increase our knowledge about how coagulation and inflammation are regulated under protein and gene levels.
In conclusion, our results demonstrate that envenomation by BjV induces different expression patterns of hemostasis-related genes. The association of gene expression of hemostasis-related factors, inflammatory proteins and transcription factors reaffirms the complex interactions that occur during envenomation. Moreover, the rapid induction of mRNA synthesis of fibrinogen chains explains the fast recovery of fibrinogen levels observed in bitten patients after antivenom therapy.
Supporting information
(PDF)
Data Availability
Raw data are available within the supporting information.
Funding Statement
This work was supported by the São Paulo Research Foundation (FAPESP, grant # 2013/25177-0 and 2018/26015-8, www.fapesp.br), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant # 312469/2018-7, www.cnpq.br), and Fundação Butantan. ATAS was a former recipient of a fellowship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Prevalence of snakebite envenoming 2019. Available from: https://www.who.int/snakebites/epidemiology/en/. [Google Scholar]
- 2.White J. Snake venoms and coagulopathy. Toxicon. 2005; 45: 951–67. 10.1016/j.toxicon.2005.02.030 [DOI] [PubMed] [Google Scholar]
- 3.Slagboom J, Kool J, Harrison RA, Casewell NR. Haemotoxic snake venoms: their functional activity, impact on snakebite victims and pharmaceutical promise. Br J Haematol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ariaratnam CA, Sheriff MH, Arambepola C, Theakston RD, Warrell DA. Syndromic approach to treatment of snake bite in Sri Lanka based on results of a prospective national hospital-based survey of patients envenomed by identified snakes. American Journal of Tropical Medicine & Hygiene. 2009; 81: 725–31. [DOI] [PubMed] [Google Scholar]
- 5.Souza AS, Sachett JAG, Alcântara JA, Freire M, Alecrim MGC, Lacerda M, et al. Snakebites as cause of deaths in the Western Brazilian Amazon: why and who dies? Deaths from snakebites in the Amazon. Toxicon. 2018; 145: 15–24. 10.1016/j.toxicon.2018.02.041 [DOI] [PubMed] [Google Scholar]
- 6.Delgado ABT, Gondim C, Reichert LP, da Silva PHV, Souza R, Fernandes TMP, et al. Hemorrhagic stroke secondary to Bothrops spp. venom: A case report. Toxicon. 2017; 132: 6–8. 10.1016/j.toxicon.2017.03.015 [DOI] [PubMed] [Google Scholar]
- 7.Fonseka CL, Jeevagan V, Gnanathasan CA. Life threatening intracerebral haemorrhage following saw-scaled viper (Echis carinatus) envenoming—authenticated case report from Sri Lanka. BMC Emerg Med. 2013; 13: 5 10.1186/1471-227X-13-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore EC, Porter LM, Ruha AM. Rattlesnake venom-induced recurrent coagulopathy in first trimester pregnant women—Two Cases. Toxicon. 2019; 163: 8–11. 10.1016/j.toxicon.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez JM. Envenenamientos por mordeduras de serpientes en América Latina y el Caribe: una visión integral de carácter regional. Boletín de Malariología y Salud Ambiental. 2011; 51: 1–16. [Google Scholar]
- 10.Otero R, Leon G, Gutierrez JM, Rojas G, Toro MF, Barona J, et al. Efficacy and safety of two whole IgG polyvalent antivenoms, refined by caprylic acid fractionation with or without b-propiolactone, in the treatment of Bothrops asper bites in Colombia. Transactions of the Royal Society of Tropical Medicine & Hygiene. 2006; 100: 1173–82. [DOI] [PubMed] [Google Scholar]
- 11.Nicolau CA, Carvalho PC, Junqueira-de-Azevedo IL, Teixeira-Ferreira A, Junqueira M, Perales J, et al. An in-depth snake venom proteopeptidome characterization: Benchmarking Bothrops jararaca. J Proteomics. 2017; 151: 214–31. 10.1016/j.jprot.2016.06.029 [DOI] [PubMed] [Google Scholar]
- 12.Sano-Martins IS, Santoro ML. Distúrbios hemostáticos em envenenamentos por animais peçonhentos no Brasil In: Cardoso JLC, França FOS, Fan HW, Málaque CMS, Haddad V Jr., editors. Animais Peçonhentos no Brasil. 1 ed. São Paulo: Sarvier; 2009. p. 289–309. [Google Scholar]
- 13.Antunes TC, Yamashita KM, Barbaro KC, Saiki M, Santoro ML. Comparative analysis of newborn and adult Bothrops jararaca snake venoms. Toxicon. 2010; 56: 1443–58. 10.1016/j.toxicon.2010.08.011 [DOI] [PubMed] [Google Scholar]
- 14.Gutiérrez JM, Escalante T, Rucavado A, Herrera C. Hemorrhage caused by snake venom metalloproteinases: a journey of discovery and understanding. Toxins. 2016; 8: 93 10.3390/toxins8040093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardoso JLC, Fan HW, França FOS, Jorge MT, Leite RP, Nishioka SA, et al. Randomized comparative trial of three antivenoms in the treatment of envenoming by lance-headed vipers (Bothrops jararaca) in São Paulo, Brazil. Q J Med. 1993; 86: 315–25. [PubMed] [Google Scholar]
- 16.Santoro ML, Sano-Martins IS, Fan HW, Cardoso JLC, Theakston RDG, Warrell DA, et al. Haematological evaluation of patients bitten by the jararaca, Bothrops jararaca, in Brazil. Toxicon. 2008; 51: 1440–8. 10.1016/j.toxicon.2008.03.018 [DOI] [PubMed] [Google Scholar]
- 17.Nicoleti AF, de Medeiros CR, Duarte MR, Franca FO. Comparison of Bothropoides jararaca bites with and without envenoming treated at the Vital Brazil Hospital of the Butantan Institute, State of Sao Paulo, Brazil. Rev Soc Bras Med Trop. 2010; 43: 657–61. 10.1590/s0037-86822010000600011 [DOI] [PubMed] [Google Scholar]
- 18.Petricevich VL, Teixeira CF, Tambourgi DV, Gutiérrez JM. Increments in serum cytokine and nitric oxide levels in mice injected with Bothrops asper and Bothrops jararaca snake venoms. Toxicon. 2000; 38: 1253–66. 10.1016/s0041-0101(99)00227-5 [DOI] [PubMed] [Google Scholar]
- 19.Barraviera B, Lomonte B, Tarkowski A, Hanson LA, Meira DA. Acute-phase reactions including cytokines in patients bitten by Bothrops and Crotalus snakes in Brazil. J Venomous Animals and Toxins. 1995; 1: 11–22. [Google Scholar]
- 20.Maruyama M, Kamiguti AS, Cardoso JLC, Sano-Martins IS, Chudzinski AM, Santoro ML, et al. Studies on blood coagulation and fibrinolysis in patients bitten by Bothrops jararaca (jararaca). Thrombosis & Haemostasis. 1990; 63: 449–53. [PubMed] [Google Scholar]
- 21.Senise LV, Yamashita KM, Santoro ML. Bothrops jararaca envenomation: Pathogenesis of hemostatic disturbances and intravascular hemolysis. Experimental Biology & Medicine (Maywood, NJ). 2015; 240: 1528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Málaque CMS, Duayer IF, Santoro ML. Acute kidney injury induced by thrombotic microangiopathy in two cases of Bothrops envenomation. Clin Toxicol (Phila). 2019; 57: 213–6. [DOI] [PubMed] [Google Scholar]
- 23.Sachetto ATA, Mackman N. Modulation of the mammalian coagulation system by venoms and other proteins from snakes, arthropods, nematodes and insects. Thromb Res. 2019; 178: 145–54. 10.1016/j.thromres.2019.04.019 [DOI] [PubMed] [Google Scholar]
- 24.Bucaretchi F, Pimenta MMB, Borrasca-Fernandes CF, Prado CC, Capitani EM, Hyslop S. Thrombotic microangiopathy following Bothrops jararaca snakebite: case report. Clin Toxicol (Phila). 2019; 57: 294–9. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita KM, Alves AF, Barbaro KC, Santoro ML. Bothrops jararaca venom metalloproteinases are essential for coagulopathy and increase plasma tissue factor levels during envenomation. PLoS Negl Trop Dis. 2014; 8: e2814 10.1371/journal.pntd.0002814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sano-Martins IS, Fan HW, Castro SCB, Tomy SC, França FOS, Jorge MT, et al. Reliability of the simple 20 minute whole blood clotting test (WBCT20) as an indicator of low plasma fibrinogen concentration in patients envenomed by Bothrops snakes. Toxicon. 1994; 32: 1045–50. 10.1016/0041-0101(94)90388-3 [DOI] [PubMed] [Google Scholar]
- 27.Ratnayake I, Shihana F, Dissanayake DM, Buckley NA, Maduwage K, Isbister GK. Performance of the 20-minute whole blood clotting test in detecting venom induced consumption coagulopathy from Russell's viper (Daboia russelii) bites. Thrombosis & Haemostasis. 2017; 117: 500–7. [DOI] [PubMed] [Google Scholar]
- 28.Gutiérrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA. Snakebite envenoming. Nat Rev Dis Primers. 2017; 3: 17063 10.1038/nrdp.2017.63 [DOI] [PubMed] [Google Scholar]
- 29.Warrell DA, Davidson NM, Greenwood BM, Ormerod LD, Pope HM, Watkins BJ, et al. Poisoning by bites of the saw-scaled or carpet viper (Echis carinatus) in Nigeria. Q J Med. 1977; 46: 33–62. [PubMed] [Google Scholar]
- 30.Warrell DA, Davidson NM, Omerod LD, Pope HM, Watkins BJ, Greenwood BM, et al. Bites by the saw-scaled or carpet viper (Echis carinatus): trial of two specific antivenoms. Br Med J. 1974; 4: 437–40. 10.1136/bmj.4.5942.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regoeczi E. Iodine-labelled fibrinogen: a review. Br J Haematol. 1971; 20: 649–63. 10.1111/j.1365-2141.1971.tb00804.x [DOI] [PubMed] [Google Scholar]
- 32.Hayakawa M. Dynamics of fibrinogen in acute phases of trauma. Journal of Intensive Care. 2017; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachetto ATA, Rosa JG, Santoro ML. Rutin (quercetin-3-rutinoside) modulates the hemostatic disturbances and redox imbalance induced by Bothrops jararaca snake venom in mice. PLoS Negl Trop Dis. 2018; 12: e0006774 10.1371/journal.pntd.0006774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conselho Nacional de Controle de Experimentação Animal. Normativas do Concea para produção, manutenção ou utilização de animais em atividades de ensino ou pesquisa científica 2 ed. Brasília: Ministério da Ciência, Tecnologia e Inovação.; 2015. [Google Scholar]
- 35.Ratnoff OD, Menzie C. A new method for the determination of fibrinogen in small samples of plasma. J Lab Clin Med. 1951; 37: 316–20. [PubMed] [Google Scholar]
- 36.Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 2006; 27: 126–39. 10.1016/j.mam.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 37.Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010; 38: D792–9. 10.1093/nar/gkp1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandesompele J, Preter K, Pattyn F, Poppe B, Van Roy N, Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology. 2002; 3: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nature Protocols. 2008; 3: 1101–8. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 40.Rokita H, Neta R, Sipe J. Increased fibrinogen synthesis in mice during the acute phase response: co-operative interaction of interleukin 1, interleukin 6, and interleukin 1 receptor antagonist. Cytokine. 1993; 5: 454–8. 10.1016/1043-4666(93)90035-4 [DOI] [PubMed] [Google Scholar]
- 41.Overbergh L, Torrekens S, Van Leuven F, Van den Berghe H. Molecular characterization of the murinoglobulins. J Biol Chem. 1991; 266: 16903–10. [PubMed] [Google Scholar]
- 42.Ribeiro Filho W, Sugiki M, Yoshida E, Maruyama M. Inhibition of hemorrhagic and edematogenic activities of snake venoms by a broad-spectrum protease inhibitor, murinoglobulin; the effect on venoms from five different genera in Viperidae family. Toxicon. 2003; 42: 173–81. 10.1016/s0041-0101(03)00130-2 [DOI] [PubMed] [Google Scholar]
- 43.Bode JG, Albrecht U, Haussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins—regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-kB-dependent signaling. Eur J Cell Biol. 2012; 91: 496–505. 10.1016/j.ejcb.2011.09.008 [DOI] [PubMed] [Google Scholar]
- 44.Lu WJ, Lin KC, Huang SY, Thomas PA, Wu YH, Wu HC, et al. Role of a Janus kinase 2-dependent signaling pathway in platelet activation. Thromb Res. 2014; 133: 1088–96. 10.1016/j.thromres.2014.03.042 [DOI] [PubMed] [Google Scholar]
- 45.Grozovsky R, Begonja AJ, Liu K, Visner G, Hartwig JH, Falet H, et al. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat Med. 2015; 21: 47–54. 10.1038/nm.3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sachetto ATA, Rosa JG, Santoro ML. Rutin (quercetin-3-rutinoside) modulates the hemostatic disturbances and redox imbalance induced by Bothrops jararaca snake venom in mice. PLoS Negl Trop Dis. 2018; 12: e0006774 10.1371/journal.pntd.0006774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sartim MA, Costa TR, Laure HJ, Espindola MS, Frantz FG, Sorgi CA, et al. Moojenactivase, a novel pro-coagulant PIIId metalloprotease isolated from Bothrops moojeni snake venom, activates coagulation factors II and X and induces tissue factor up-regulation in leukocytes. Arch Toxicol. 2016; 90: 1261–78. 10.1007/s00204-015-1533-6 [DOI] [PubMed] [Google Scholar]
- 48.Santoro ML, Sano-Martins IS. Platelet dysfunction during Bothrops jararaca snake envenomation in rabbits. Thrombosis & Haemostasis. 2004; 92: 369–83. [DOI] [PubMed] [Google Scholar]
- 49.Federici A. The Factor VIII/von Willebrand Factor Complex: Basic and Clinical Issues. Haematologica. 2003; 88: EREP02 [PubMed] [Google Scholar]
- 50.Lippok S, Kolsek K, Lof A, Eggert D, Vanderlinden W, Muller JP, et al. von Willebrand factor is dimerized by protein disulfide isomerase. Blood. 2016; 127: 1183–91. 10.1182/blood-2015-04-641902 [DOI] [PubMed] [Google Scholar]
- 51.Fan Y, Mao R, Yang J. NF-kappaB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013; 4: 176–85. 10.1007/s13238-013-2084-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamashita KM, Alves AF, Barbaro KC, Santoro ML. Bothrops jararaca venom metalloproteinases are essential for coagulopathy and increase plasma tissue factor levels during envenomation. PLoS Negl Trop Dis. 2014; 8: e2814 10.1371/journal.pntd.0002814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamiguti AS, Cardoso JL, Theakston RD, Sano-Martins IS, Hutton RA, Rugman FP, et al. Coagulopathy and haemorrhage in human victims of Bothrops jararaca envenoming in Brazil. Toxicon. 1991; 29: 961–72. 10.1016/0041-0101(91)90079-7 [DOI] [PubMed] [Google Scholar]
- 54.Yang C, Li Y, Kuter D. The physiological response of thrombopoietin (c-Mpl ligand) to thrombocytopenia in the rat. British Journal of Haematology,. 1999; 105: 478–85. [PubMed] [Google Scholar]
- 55.Kamiguti AS, Matsunaga S, Spir M, Sano-Martins IS, Nahas L. Alterations of the blood coagulation system after accidental human inoculation by Bothrops jararaca venom. Braz J Med Biol Res. 1986; 19: 199–204. [PubMed] [Google Scholar]
- 56.Cardoso JL, Fan HW, França FO, Jorge MT, Leite RP, Nishioka SA, et al. Randomized comparative trial of three antivenoms in the treatment of envenoming by lance-headed vipers (Bothrops jararaca) in São Paulo, Brazil. Q J Med. 1993; 86: 315–25. [PubMed] [Google Scholar]
- 57.Crabtree GR, Kant JA. Coordinate accumulation of the mRNAs for the a, b and g chains of rat fibrinogen following defibrination. J Biol Chem. 1982; 257: 7277–9. [PubMed] [Google Scholar]
- 58.Qureshi GD, Guzelian PS, Vennart RM, Evans HJ. Stimulation of fibrinogen synthesis in cultured rat hepatocytes by fibrinogen fragment E. Biochim Biophys Acta. 1985; 844: 288–95. 10.1016/0167-4889(85)90129-6 [DOI] [PubMed] [Google Scholar]
- 59.LaDuca FM, Tinsley LA, Dang CV, Bell WR. Stimulation of fibrinogen synthesis in cultured rat hepatocytes by fibrinogen degradation product fragment D. Proc Natl Acad Sci U S A. 1989; 86: 8788–92. 10.1073/pnas.86.22.8788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaffney PJ. Fibrin degradation products: a review of structures found in vitro and in vivo. Ann N Y Acad Sci. 2001; 936: 594–610. [PubMed] [Google Scholar]
- 61.Bauza G, Miller G, Kaseje N, Wang Z, Sherburne A, Agarwal S, et al. Injury-induced changes in liver specific transcription factors HNF-1a and HNF-4a. J Surg Res. 2012; 175: 298–304. 10.1016/j.jss.2011.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fish RJ, Neerman-Arbez M. Fibrinogen gene regulation. Thrombosis & Haemostasis. 2012; 108: 419–26. [DOI] [PubMed] [Google Scholar]
- 63.Petricevich VL, Teixeira CFP, Tambourgi DV, Gutiérrez JM. Increments in serum cytokine and nitric oxide. Toxicon. 2000; 38: 1253–66. 10.1016/s0041-0101(99)00227-5 [DOI] [PubMed] [Google Scholar]
- 64.Barravieira B, Lomonte B, Tarkowski A, Hanson L, Meira D. Acute-phase reactions, including cytokines, in patients bitten by Bothrops and Crotalus snakes in Brazil. J Venom Anim Toxins. 1995; 1: 1–10. [Google Scholar]
- 65.Dhainaut J, Marin N, Mignon A, Vinsonneau C. Hepatic response to sepsis: interaction between coagulation and inflammatory processes. Crit Care Med. 2001; 29: S42–S7. 10.1097/00003246-200107001-00016 [DOI] [PubMed] [Google Scholar]
- 66.Gruys E, Toussaint MJ, Niewold TA, Koopmans SJ. Acute phase reaction and acute phase proteins. J Zhejiang Univ Sci B. 2005; 6: 1045–56. 10.1631/jzus.2005.B1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Birch H, Schreiber G. Transcriptional regulation of plasma protein synthesis during inflammation. J Biol Chem. 1986; 261: 8077–80. [PubMed] [Google Scholar]
- 68.Milland J, Tsykin A, Thomas T, Aldred AR, Cole T, Schreiber G. Gene expression in regenerating and acute-phase rat liver. Am J Physiol. 1990; 259: G340–7. 10.1152/ajpgi.1990.259.3.G340 [DOI] [PubMed] [Google Scholar]
- 69.Jorge MT, Ribeiro LA, da Silva ML, Kusano EJ, de Mendonca JS. Microbiological studies of abscesses complicating Bothrops snakebite in humans: a prospective study. Toxicon. 1994; 32: 743–8. 10.1016/0041-0101(94)90343-3 [DOI] [PubMed] [Google Scholar]
- 70.Fuller G, Zhang Z. Transcriptional control mechanism of fibrinogen gene expression. Ann N Y Acad Sci. 2001; 936: 469–79. 10.1111/j.1749-6632.2001.tb03534.x [DOI] [PubMed] [Google Scholar]
- 71.Akira S, Nishio Y, Inoue M, Wang X, Wei S, Matsusaka T, et al. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994; 77: 63–71. 10.1016/0092-8674(94)90235-6 [DOI] [PubMed] [Google Scholar]
- 72.Ichiba M, Nakajima K, Yamanaka Y, Kiuchi N, Hirano T. Autoregulation of the Stat3 gene through cooperation with a cAMP-responsive element-binding protein. J Biol Chem. 1998; 273: 6132–8. 10.1074/jbc.273.11.6132 [DOI] [PubMed] [Google Scholar]
- 73.Liu Z, Fuller G. Detection of a novel transcription factor for the Aα fibrinogen gene in response to interleukin-6. Journal of Biological Chemistry. 1995; 270: 7580–6. 10.1074/jbc.270.13.7580 [DOI] [PubMed] [Google Scholar]
- 74.Cartwright T, Perkins ND, C LW. NFKB1: a suppressor of inflammation, ageing and cancer. FEBS J. 2016; 283: 1812–22. 10.1111/febs.13627 [DOI] [PubMed] [Google Scholar]
- 75.Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018; 38: 709–25. 10.1161/ATVBAHA.117.309846 [DOI] [PubMed] [Google Scholar]
- 76.Mackman N. Tissue-specific hemostasis in mice. Arterioscler Thromb Vasc Biol. 2005; 25: 2273–81. 10.1161/01.ATV.0000183884.06371.52 [DOI] [PubMed] [Google Scholar]
- 77.Tatsumi K, Ohashi K, Taminishi S, Takagi S, Utoh R, Yoshioka A, et al. Effects on coagulation factor production following primary hepatomitogen-induced direct hyperplasia. World J Gastroenterol. 2009; 15: 5307–15. 10.3748/wjg.15.5307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodeghiero F, Mannucci P, Vigano S, Barbui T, Gugliotta L, Cortellaro M, et al. Liver dysfunction rather than intravascular coagulation as the main cause of low protein C and antithrombin III in acute leukemia. Blood. 1984; 63: 965–9. [PubMed] [Google Scholar]
- 79.Okada K, Ueshima S, Imano M, Kataoka K, Matsuo O. The regulation of liver regeneration by the plasmin/alpha 2-antiplasmin system. J Hepatol. 2004; 40: 110–6. 10.1016/j.jhep.2003.09.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
Raw data are available within the supporting information.