Summary:
In non-habitual situations, cognitive control aligns actions with both short- and long-term goals. The capacity for cognitive control is tightly tied to the prefrontal cortex, whose expansion in humans relative to other species is thought to support our superior cognitive control. However, the posterolateral cerebellum has also expanded greatly relatively to non-human primates and has an organizational structure that mirrors the prefrontal cortex. Yet, cerebellar contributions to cognitive control are poorly understood. Here, we sought to explore whether a functional hierarchical processing framework, applied to the cerebellum, could elucidate cerebellar contributions to cognitive control. Using functional magnetic resonance imaging, we show that a gradient within the posterolateral cerebellum supports cognitive control with motor-adjacent cerebellar sub-regions supporting control of concrete, proximal actions, and motor-distal, cerebellar sub-regions supporting abstract, future processing. This gradient was functionally hierarchical, with regions higher in the hierarchy influencing the relationship between regions lower in the hierarchy. This functional hierarchy provides the infrastructure by which context can inform current actions and prepare for future goals. Crucially, this mirrors the hierarchical organization of cognitive control within the prefrontal cortex. Based on these findings, we propose that the cerebellum contains within itself a parallel, but separate hierarchical organization that, along with the prefrontal cortex, supports complex cognition.
Keywords: Cerebellum, Cognitive control, fMRI, hierarchical processing, gradients
eTOC blurb
D’Mello et al. present evidence of a functional processing hierarchy for cognitive control in the human cerebellum. Specific cerebellar subregions process distinct aspects of cognitive control from concrete to abstract. Subregions interact hierarchically to support both proximal actions and future planning.
Introduction:
Humans have an extraordinary capacity for cognitive control – the ability to imagine future goals, determine which actions are necessary to achieve them, and organize these actions effectively. It has been hypothesized that this cognitive capacity emerges in part from the expansion of the prefrontal cortex in humans relative to non-human primates. However, the expansion of the prefrontal cortex in humans has been mirrored by the enormous expansion of the posterolateral cerebellar hemispheres [1–3]. In humans, the cerebellum constitutes over 10% of total brain volume, and contains over four times the number of cells than the cerebral cortex [4]. Although historically considered a motor structure, a vast majority of the cerebellum is actually devoted to cognitive processing and is functionally and anatomically connected to supratentorial association cortices [5,6]. Neuroimaging research finds that the posterolateral cerebellum is active during complex cognitive behaviors including cognitive control [7], and lesions to the posterolateral cerebellum can impair cognition in the absence of motor symptoms [8,9]. Despite this, most neuroimaging research focuses on the role of the cerebral cortex in cognitive behaviors and cerebellar contributions to cognition are less understood.
Cortical research has benefited from unifying frameworks for understanding cerebral cortical organization and how this organization may allow for interactions between cortical areas to support cognitive behaviors. For example, the prefrontal cortex is organized in a gradient-like manner, with somatomotor-proximal regions representing goal-oriented action, and rostral somatomotor-distal regions representing abstract, future oriented processing (Figure 1A) [10–13]. In addition, clinical and neuroimaging research suggests that these regions are hierarchically organized, with increasing hierarchical rank exemplified by increased influence over processing in other regions [10,11,13–17]. A similar framework has, until this point, been lacking in studies of cerebellar contributions to cognition. Here, we propose one such unified framework to elucidate cerebellar contributions to cognitive control: like the prefrontal cortex, the posterolateral cerebellum is organized in a gradient-like manner to support cognitive control, and this organization allows for hierarchical relationships between cerebellar sub-regions. We experimentally show the existence of a processing gradient of activation for cognitive control in the posterior cerebellum, and find that sub-regions are functionally organized in a hierarchical manner, parallel to the hierarchical functional interactions of the prefrontal cortex [14,15,17]. Based on our results, we suggest a parallel organizational processing structure in cerebral and cerebellar regions that supports complex cognitive behaviors.
Figure 1. Laying the groundwork for cognitive control in the cerebellum.
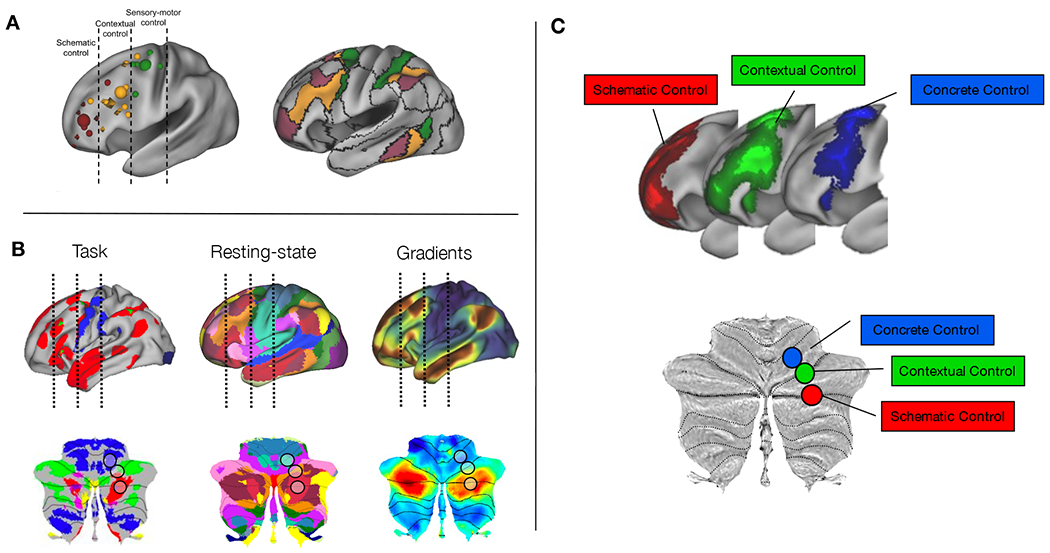
A) Organization of prefrontal cortex intro three rostral-caudal zones (schematic control = abstract, future oriented processing important for organization of lower order actions; contextual control = control of current actions depending on context; sensory-motor or concrete control = control over present actions) for cognitive control and mapping of task-related foci to resting state networks from 17-network parcellation (figure adapted with permission from [17]). B) Task, resting-state, and gradients plotted on the cortex (top) and the cerebellum (bottom). Lines in cortex and black circles in cerebellum represent zones from abstract to concrete. In task-based fMRI, the cerebral cortex show a rostral-caudal progression from language tasks (red), to working memory tasks (green), to motor tasks (blue) [64]. In the 17-network parcellation of resting-state networks, both cerebral cortex [65] and cerebellum [6] show a progression from sensorimotor networks (blue), to frontoparietal networks (orange), to the default mode network (red). In gradient space, the primary gradient of cerebral [34] and cerebellar [21] organization moves from unimodal (blue) to transmodal regions (red). C) The prefrontal cortex shows a rostral-caudal gradient for cognitive control moving from concrete (blue), to contextual (green), to schematic control (red) and these regions interaction in a hierarchical manner [14,15,17]. Bottom: Hypothesized cognitive control gradient organization within the cerebellum based on what is known about cerebellar and prefrontal organization for task-based functional studies, resting state networks, and gradient analyses.
Like the prefrontal cortex, the posterolateral cerebellum is comprised of multiple sub-regions. Clinical and neuroimaging research has elucidated a functional topography within the cerebellum, whereby anterior regions and lobule VIII contain somatotopic representations of the body (including face, hands, and feet), while the large posterolateral hemispheres are associated with non-motor, cognitive behaviors [7]. Importantly, within the posterior “cognitive” cerebellum, discrete tasks can activate particular regions of the cerebellum with subregion specificity [18–20]. Although never explicitly examined, posterior cerebellar engagement in these discrete cognitive tasks is organized in a gradient-like manner with increasing abstraction represented in progressively posterolateral regions of the cerebellum. For example, cognitive tasks that require control over concrete, current processes (e.g. mental arithmetic, active maintenance) activate lobule VI [20], a region of the cerebellum adjacent to the anterior lobe which contains somatotopic representations of motor effectors. Abstract or purely cognitive tasks such as autobiographic recall, mentalizing, or language processing activate the posterolateral hemispheres (Crus I/II) [20]. Regions in-between those recruited for concrete processing and those recruited for abstract processing are engaged by tasks necessitating focused attention such as working memory tasks (Figure 1B) [18,19]. Damage to specific sub-regions of the cognitive cerebellum can affect language ability, visuospatial processing, and/or executive function in a relatively discrete manner [9].
Although the cerebellum is clearly involved in complex cognition, little is known with regards to how sub-regions within the cerebellum interact to support to cognition. Further, given the existence of reciprocal loops between the cerebellum and the cerebral cortex, and lack of long-range intracerebellar connectivity, it is unknown whether putative sub-region interactions within the cerebellum are merely reflective of sub-region interactions in supratentorial regions. We, therefore, sought to explore whether a hierarchical processing framework, applied to the cerebellum, could elucidate cerebellar contributions to cognitive control. Participants completed a cognitive control task which placed demands on progressively abstract levels of cognitive control (from selecting appropriate stimulus features to guide actions, to incorporating context to determine how stimulus features guide actions, to integrating past information with future goals for abstract control over action).
We hypothesized that, like the prefrontal cortex, the posterolateral cerebellum would show a gradientlike functional organization for cognitive control. Specifically, we predicted that motor-adjacent cerebellar sub-regions would support control of concrete, proximal actions, while motor-distal, cerebellar sub-regions would support abstract, future processing (Figure 1C). Given the dual representation of cerebellar resting-state and gradient organization [6,19,21], we also predicted that this functional organization would invert around Crus I/II. Further, we hypothesized that as in the prefrontal cortex, the posterior cerebellum would be organized hierarchically with sub-regions at the top of the hierarchy exerting asymmetrical control over regions at the bottom of the hierarchy. We used fMRI in two independent groups of adult participants to test the hypothesis that progressive abstraction in cognitive control is associated with a functional control gradient in the cerebellum. We correlated cerebellar activation with behavioral performance to assess how different cerebellar sub-regions contributed to processing of current versus future situations. Lastly, we conducted mediation analyses to determine whether activation at the top of the cerebellar cognitive control hierarchy asymmetrically influenced lower-regions.
Results:
Cognitive control in the cerebellum is organized along an abstraction gradient
Participants performed a cognitive control task which placed demands on progressively abstract levels of cognitive control from control of goal-directed actions driven by attention to stimulus features (concrete control), to control over changing contexts (contextual control), and abstract, future-oriented control over actions (schematic control) (Figure 2). These demands produce robust rostral-caudal control gradients in the PFC [14,15,22](Fig 1C). Given known cerebellar functional topography, we hypothesized that factorial manipulations of conditions within the cognitive control task would activate a parallel functional gradient moving from motor-adjacent regions for concrete control (i.e. lobule VI), to transmodal regions for schematic control (i.e. Crus I/II). Regions in-between motor-adjacent and transmodel cerebellar sub-regions would be activated for contextual control. We analyzed cerebellar activation patterns for schematic control, contextual control, and concrete control. The most abstract task processing, requiring individuals to use past information for future planning (schematic control), recruited bilateral Crus I/II in the posterolateral hemispheres of the cerebellum. Less abstract processing, requiring individuals to be aware of changing context to select appropriate actions (contextual control) engaged slightly more medial and anterior bilateral lobules VI/Crus I and Crus II/VIIB. Lastly, processing of low-level, concrete stimulus features (concrete control) engaged right lobules VI and VIIB/VIIIA (Figure 3). Clusters were organized in a sensorimotor-fugal pattern, which inverted around Crus I/II: as task demands became more concrete, activation moved progressively towards unimodal regions of the cerebellar cortex, while more abstract processing activated transmodal regions of cerebellar cortex. Interestingly, activation peaks within the clusters also showed a lateral to medial organization within the cerebellum, with more abstract processing occupying very lateral hemispheric regions, and concrete processing occupying more medial areas close to the vermis. These patterns are consistent with both previous resting-state functional connectivity and task results which find dual representations (or even triple representations) of both resting state networks and task activations within the cerebellum (Fig 1B).
Figure 2. Cognitive control task manipulated temporal and contextual axes.
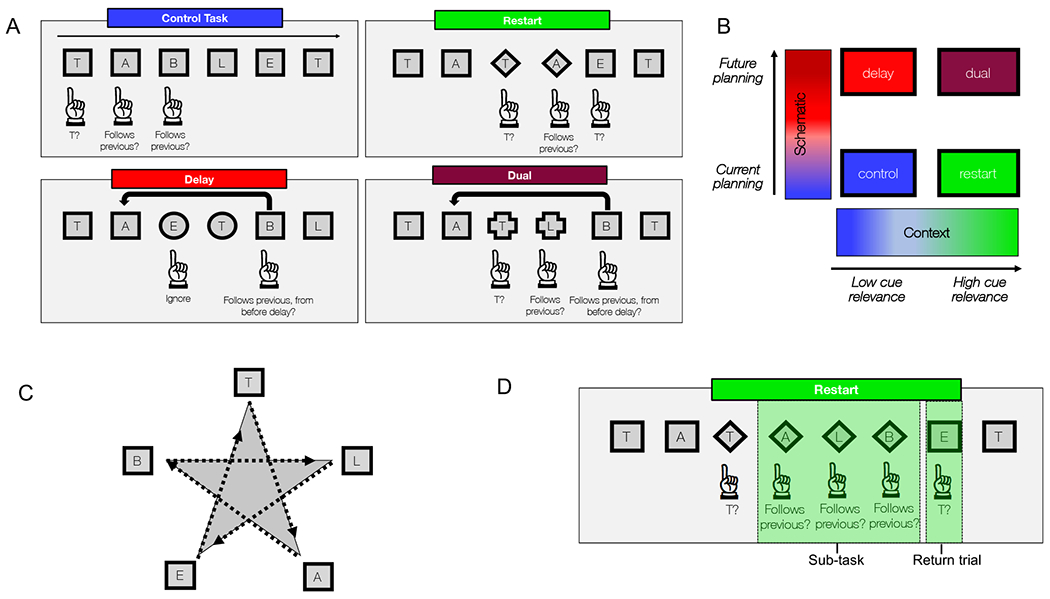
A) Basic task structure. Participants saw letters drawn from the word “TABLET”. Participants were cued to certain sub-tasks by changing shapes. B) The cognitive control task manipulated two main task aspects (high or low schematic control – requiring participants to keep past information in mind to influence future behavior; high or low contextual control – requiring participants to pay attention to changing task cues). C) On verbal trials, the letters were relevant, while on spatial trials, the locations were relevant. Letters appeared along points of a star in spatial trials. (D) Each condition was made up of sub-tasks (restart condition shown here). The trial following a sub-task, when participants returned to the original task, was dubbed a “return trial”.
Figure 3. Cognitive control in the cerebellum is organized along an abstraction gradient.
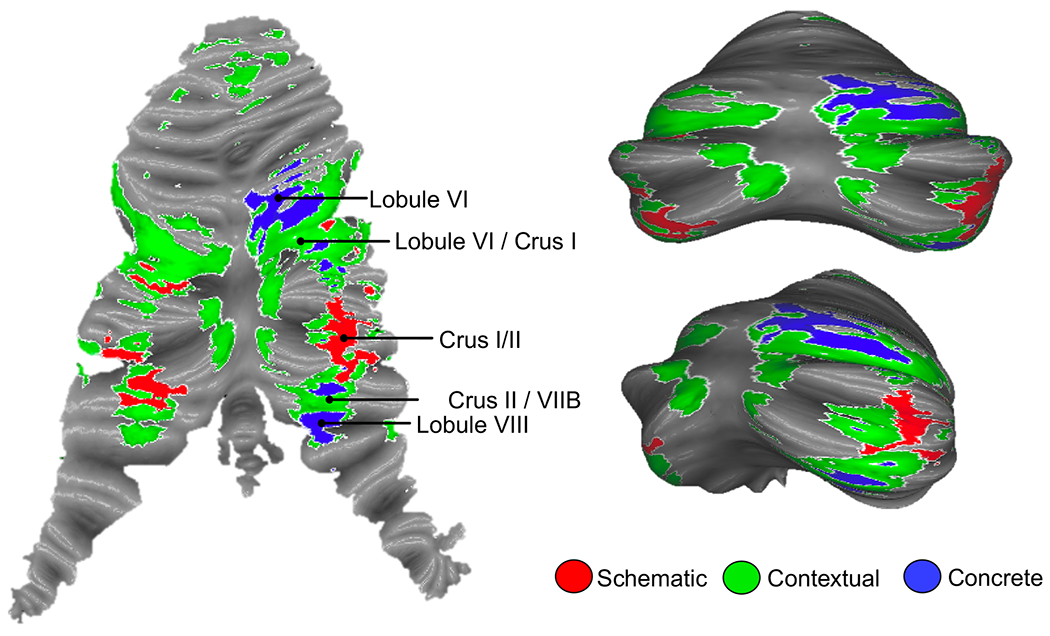
Schematic control (the most abstract condition) engaged bilateral Crus I/II of the cerebellum (red). Concrete control (the most concrete condition) engaged right-lateralized VI and VIIB/VIII (blue), regions proximal to motor areas of the cerebellum. Contextual control (requiring attention to changing contexts to inform action) engaged regions in between schematic and concrete control regions (green). Left: activations mapped to the cerebellar surface and plotted on a flat-map representation of the cerebellum. Right: Activation mapped to the cerebellar surface and plotted on an inflated template of the cerebellum [66]. See Figure S3 for domain-specific effects of verbal versus spatial processing.
The cerebellar cognitive control gradient is related to control over proximal and future actions
In the prefrontal cortex, concrete processing in caudal regions is associated with control over the current situation (e.g. translating features into action). On the other hand, abstract processing in rostral regions of the prefrontal cortex is associated with the ability to integrate past information to optimize future behavior (e.g. planning). We examined the degree to which the gradient organization within the cerebellum was related to current versus future processing demands by assessing how cerebellar activation was associated with reaction times. Current processing demands were defined as the reaction times during the sub-task trials – those trials that formed the estimates for fMRI activations. Future processing demands were defined as the reaction times from trials directly after the fMRI activations were assessed (Figure 2D). Reaction times provide a measure of task engagement, and varied across conditions. Correlations between neural activation and current reaction times would suggest that a particular region was involved in processing current task demands. On the other hand, correlations between neural activation and future reaction times would suggest that a region was involved in preparing for future demands. Repeated measures correlations were conducted between parameter estimates from regions of interest within schematic, contextual, and concrete control contrasts and normalized reaction times. We found that future reaction times were associated with activation in regions of the cerebellum recruited for schematic control (Crus I/II). On the other hand, current reaction times were associated with activation in regions of the cerebellum important for concrete control (Right VI; Right VIIB/VIII). Regions of the cerebellum recruited for contextual control showed correlations with both current and future reaction times (Figure 4). This pattern demonstrates that cerebellar areas activated by increasing abstract task demands are related to increasingly future-oriented behavior.
Figure 4. Cerebellar cognitive control gradient is related to proximal and future control over action.
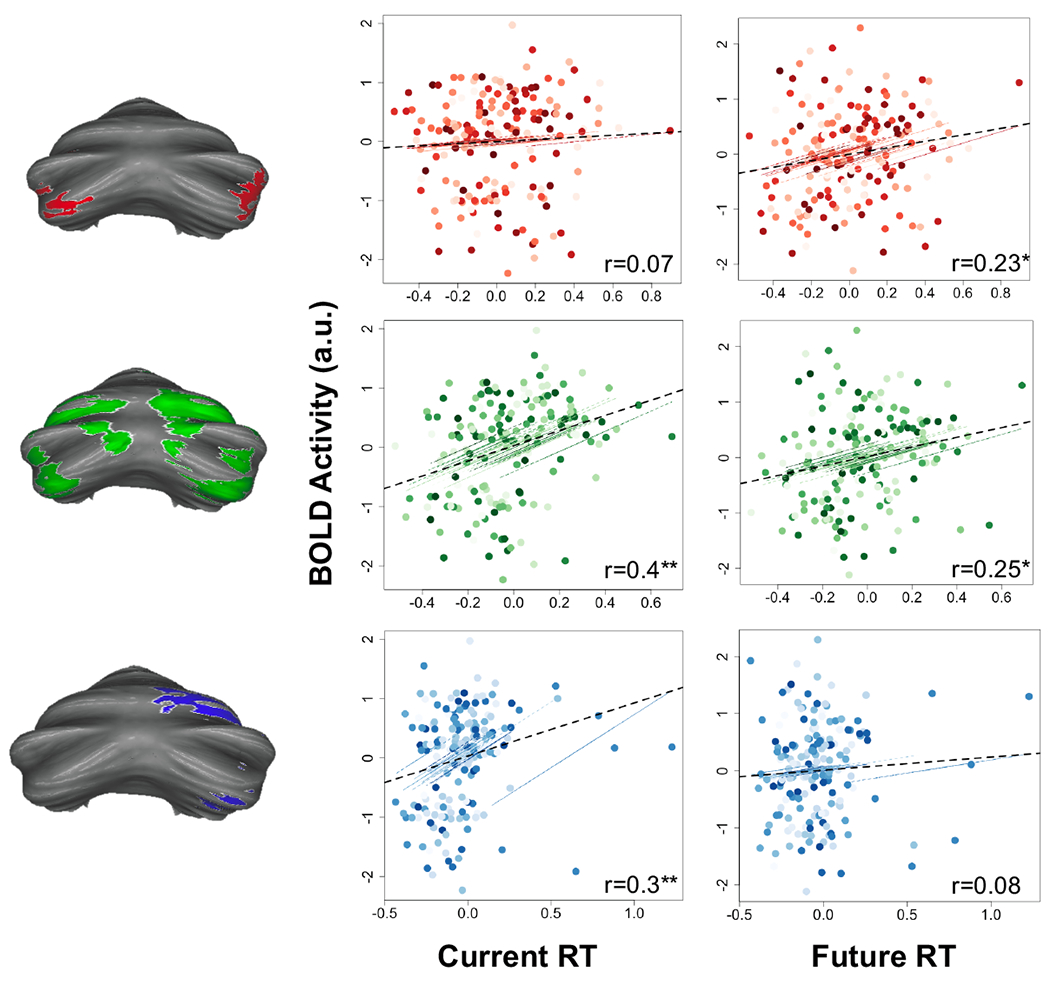
Correlations between activation in specific sub-regions were correlated with specific behaviors. Activation in schematic control regions (red; Crus I/II) were correlated only with future reaction times, but not with current reaction times. Activation in contextual control regions (green; VI/Crus I and Crus II/VIIB) were correlated with both current and future reaction times. Activation in concrete control regions (blue; VI and VIIB) were correlated with current reaction times but not future reaction times. Dotted black line depicts overall correlation, accounting for repeating measures. Colored lines and dots show individual subject data points (clustered by subject) and individual subject correlations. Asterisks depict p-value significant after correcting for multiple comparisons using an FDR correction, *p<0.01; **p<0.001.
Cerebellar sub-regions are organized hierarchically and their interactions are independent of hierarchies in the cerebral cortex
To determine whether this cerebellar gradient was functionally hierarchical – whether relationships between sub-regions lower in the gradient relied on regions higher in the gradient – we conducted a mediation analysis (Figure 5). Hierarchical organization presumes that regions higher in the hierarchy exert asymmetrical influence over regions lower in the hierarchy [13,14,17]. We included the contextual control sub-regions as the mediator, as these were associated with both future and current behavior, and were therefore optimally positioned to integrate information between other regions. Contextual control regions (VI/Crus I; Crus II/VIIB) fully mediated the relationship between regions lower in the hierarchy, meaning that the relationship between regions lower in the hierarchy (direct effect of schematic control regions on concrete control regions) was rendered insignificant when adding contextual control regions into the regression. This implies that the relationship between regions lower in the hierarchy is indirect, and driven by contextual control regions. Given closed-loop circuits between the cerebellum and the cerebral cortex (see [23] for review), it is possible that the hierarchy in cerebellum was simply a reflection of a cerebral cortical hierarchy (for example, within the PFC), and that cerebellar sub-regions were not themselves interacting amongst one another. To account for this potential confound, we partialled out the effect of each reciprocal PFC region from its cerebellar target and performed the mediation again. Despite high correlations between activity in these regions, the cerebellar mediation remained significant and full, suggesting that cerebellar sub-regions are interacting and that cerebellar activation is not merely reflective of hierarchies in supratentorial regions (indirect effect = 0.25, 95% CI [0.12, 0.39]; direct effect, c’, = 0.05, 95% CI [−0.09 0.2]; proportion of effect that is mediated = 85%).We also conducted principal component analyses (PCA) of both frontal and parietal lobe activation maps for schematic, contextual, and concrete control. Relative to ROI analyses, PCA provides a more comprehensive and data driven method by which to select frontal and parietal signals to partial from the cerebellum. The cerebellar mediation analysis was robust, withstanding the regression of multiple PCs (Figure S1). Coupled with simulations (Figure S2), these analyses suggest that it is unlikely that cerebellar mediation is driven entirely by relays from the cortex.
Figure 5. Cerebellar sub-regions are organized hierarchically and their interactions are independent of hierarchies in the cerebral cortex.
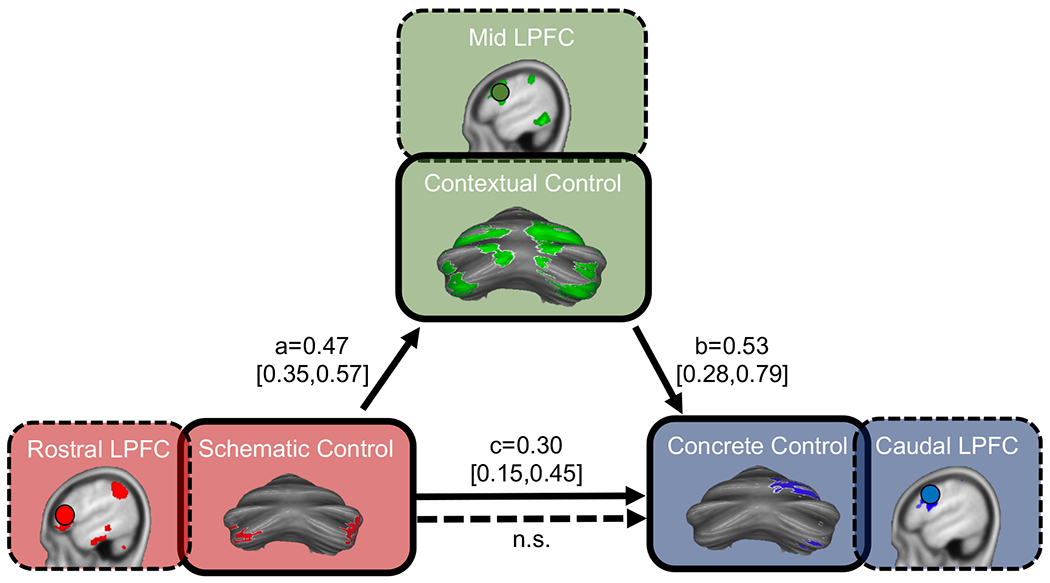
The relationship between schematic control regions and concrete control regions were fully mediated by contextual control regions. This was true even when partialling out the effects of corresponding regions of interest in the prefrontal cortex (dashed-line boxes), and when partialling out the effects of un-biased principle components from the prefrontal cortex and parietal cortex (see Figures S1 and S2). The fixed-effect parameter is presented for each path (a, b, and c) with its associated credible interval (95% mass of the marginal posterior distribution) in brackets. n.s. = not significant (CI included 0).
Cerebellar hierarchical organization supports proximal and future behavior
The analyses above demonstrate a gradient of sensitivity to abstraction in the posterior cerebellum. Progressively motor-adjacent cerebellar regions process concrete stimulus features and current behavioral states, while motor-distal regions plan for the future. Next, we sought to investigate how these sub-regions interact to support cognitive control processes. Given that areas involved in contextual control were related to both current and future demands, and mediated interactions among areas involved in schematic and concrete control, we predicted that contextual control areas would also mediate the relationships between schematic/concrete control areas and future/current behavior, respectively. This would provide strong evidence for hierarchical dependence.
To test for this pattern, we conducted mediation analyses to determine whether the aforementioned relationships between activation patterns and behavior were dependent on activation in contextual control regions. As predicted, we found that activation in contextual control regions of the cerebellum fully mediated the relationship between activation in schematic control regions and future behavior (indirect effect = 1.83, 95% CI [1.12, 2.60]; direct effect = −0.81, 95% CI [−1.66, 0.03]), as well as the relationship between activation in concrete control regions and current behavior (indirect effect = 2.02, 95% CI [1.26, 3.47]; direct effect = 0.3, 95% CI [−0.95, 1.60]). This suggests that contextual control regions of the cerebellum integrate both abstract and concrete information to ultimately influence behavior relevant for a specific context (Figure 6). Importantly, the mediations were not significant when rotating the model (i.e. including either schematic control regions or concrete control regions as the mediator).
Figure 6. Cerebellar hierarchical organization supports proximal and future behavior.
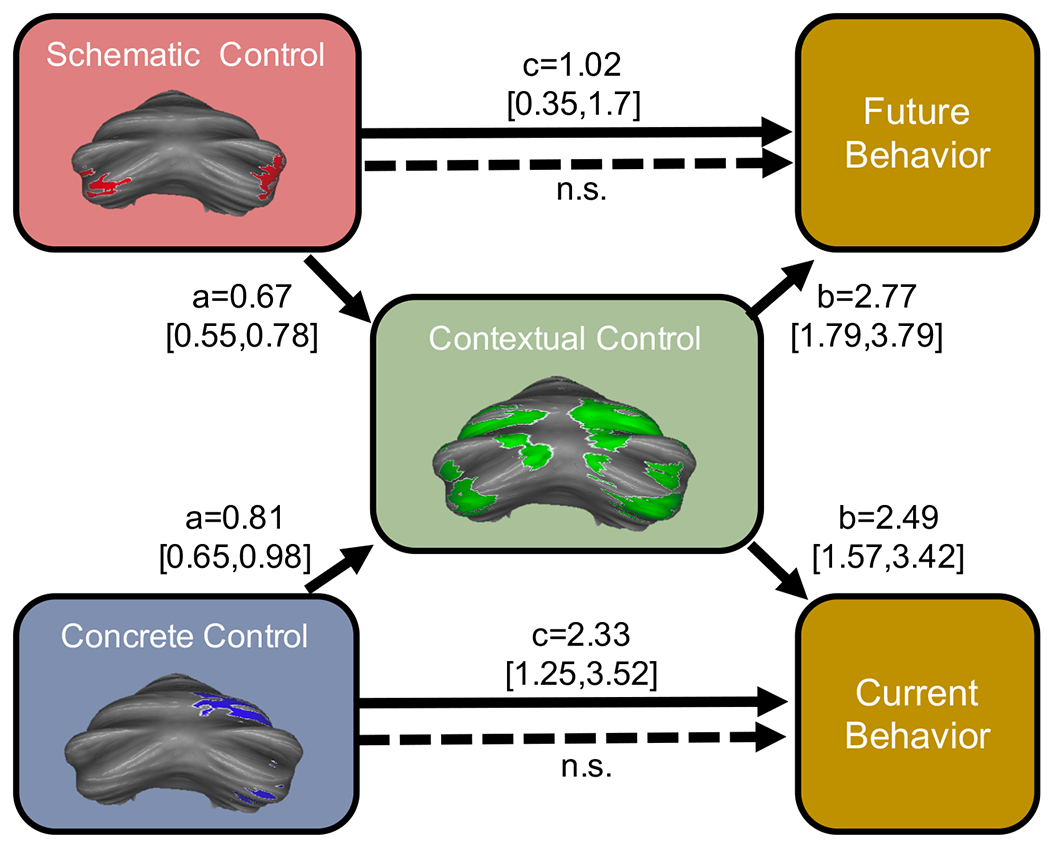
The relationships between schematic control regions and future reaction times, and between concrete control regions and current reaction times were both fully mediated by contextual control regions. n.s.= not significant (CI included 0).
Cerebellar hierarchical organization is robust: replication in an independent sample
We next replicated our analysis in a separate, independent group of participants (n=24) who underwent similar task procedures [15]. We found a robust cerebellar hierarchical organization within this second, independent sample, as in the identification sample (Fig 7A). Once again, activation in abstract processing regions showed associations with future behavior, but not current behavior. Contextual control regions showed associations with both future and current behavior. Lastly, concrete control regions showed only associations with current behavior (Figure 7B). As in our identification sample, contextual control regions fully mediated the relationship between temporal and concrete control regions, even when controlling for PFC activation (indirect effect = 0.15, 95% CI [0.06, 0.24], Figure 7C). Further, contextual control regions fully mediated the relationship between schematic control regions and future reaction times (indirect effect = 0.97, 95% CI [0.58, 1.44]), and concrete control regions and current reaction times (indirect effect = 1.34, 95% CI [0.75, 2.33], Figure 7D).
Figure 7. Cerebellar hierarchical organization is robust: replication in an independent sample.
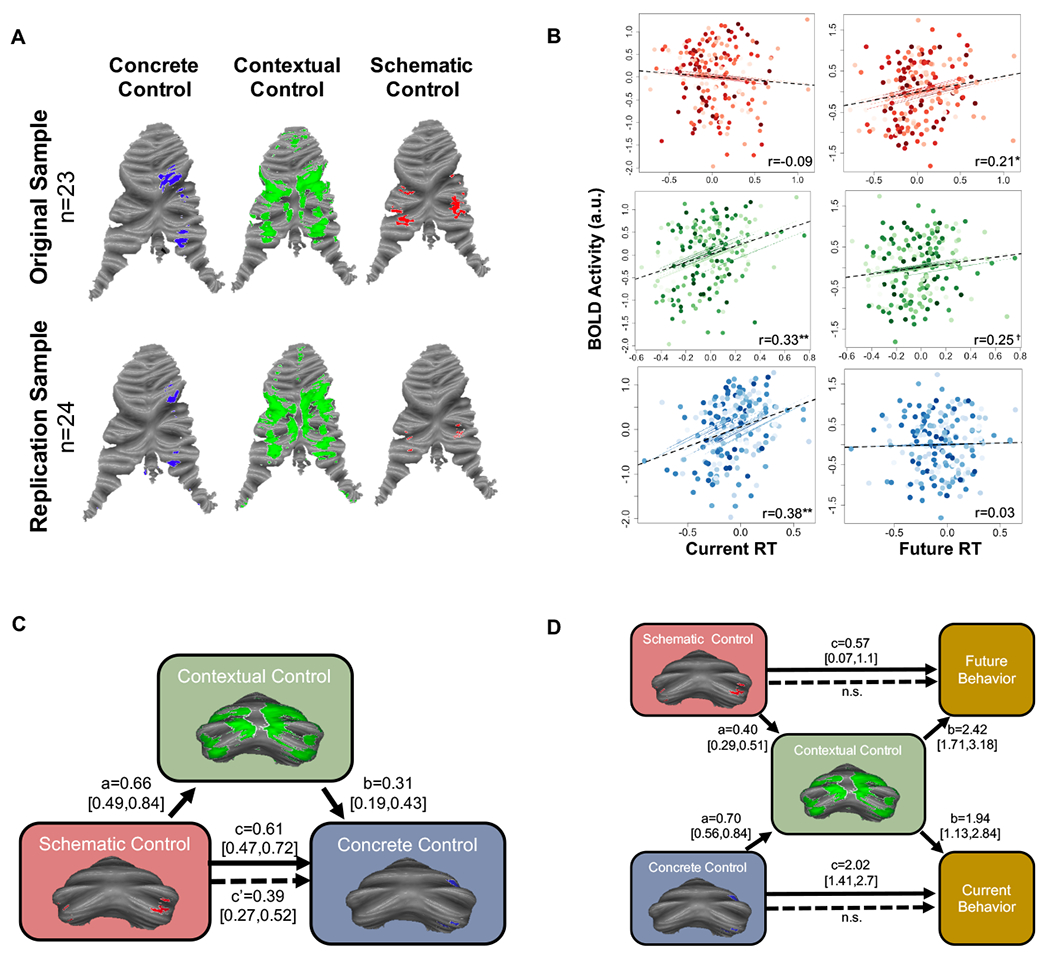
A) An independent cohort (n=24) underwent similar task procedures. A similar gradient was found in this independent replication sample. B) Sub-region correlations with behavior were similar to those found in the original sample. Colored lines and dots show individual subject data points (clustered by subject) and individual subject correlations. Dotted black line depicts overall correlation, accounting for repeating measures. (C) As in the original sample, contextual control regions fully mediated the relationship between schematic and concrete control regions (contributions of corresponding PFC regions have been partialled out). (D) Contextual control regions also fully accounted for the relationship between schematic regions and future behaviors, and concrete regions and current behaviors. Asterisks depict p-value significant after correcting for multiple comparisons using an FDR correction, **p<0.001, *p<0.01, †p=0.051 (0.08 after FDR correction).
Discussion:
Here, we found that cognitive control is organized in a gradient-like manner within the posterior cerebellum. This gradient is functionally relevant, with motor-adjacent cerebellar regions associated with current behaviors, and motor-distal cerebellar regions associated with preparation for future action. Using mediation analyses, we showed that this functional gradient is hierarchical, providing the infrastructure by which context can inform both current actions and prepare for future goals. This hierarchical organization is robust even after controlling for contributions from the cerebral cortex. Crucially, this mirrors the hierarchical organization of cognitive control within the prefrontal cortex. Based on these findings, we propose that the cerebellum contains within itself a parallel, but separate, hierarchical organization that, along with the prefrontal cortex, supports complex cognition. Together, both the prefrontal cortex and cerebellum appear to contribute to the diversity of cognitive capabilities in humans.
Laying the groundwork for cerebellar hierarchies: anatomy, physiology, and cerebro-cerebellar loops
Until this point, task-based fMRI in the cerebellum has focused on discrete tasks to define cerebellar sub-region task specificity. In this way, neuroimaging has elucidated a functional topography within the cerebellum whereby the anterior lobe and bilateral lobule VIII are associated with motor processing, and the posterior lobe is associated broadly with a variety of cognitive behaviors [18]. However, multiple sub-regions of the cerebellum are active in concert during motor and non-motor cognitive tasks [18,19], and specific sub-regions may contribute to discrete demands within a given task [20] rendering it difficult to infer a functional framework by assembling data across studies/tasks. Cognitive control requires the coordination of multiple demands – future goals influence which current actions are necessary, and context determines how actions contribute to goals being achieved. In the current study, we manipulated both abstract and concrete processing in a single task in order to activate the entire posterior cerebellum. Previous findings in the same participants found that this task activated the entire rostrocaudal prefrontal cortex [14,15]. In the most abstract conditions, participants had to hold previous information in memory in order to successfully complete future trials, whereas in the least abstract conditions, participants had to be aware of specific cues to guide current actions. Specific conditions activated different regions of the cerebellum, in a gradient-like manner moving between unimodal regions towards transmodal regions. These results show for the first time a task-based functional gradient within the posterior cerebellum for cognitive control. The localization of particular task demands to discrete regions aligns well with what is already known about the cerebellum from anatomy, physiology, and clinical work.
At the most general level, regions identified here as being engaged by concrete processing (e.g. lobule VI and VIIB) form cerebro-cerebellar loops with premotor areas, and broad attention and executive control networks [6]. In the cerebellum, these regions are motor-adjacent, but are not functionally or anatomically connected with primary motor cortex [5,6,24]. Neuroimaging paradigms of language processing in the cerebellum find that sub-vocal rehearsal relies on regions such as lobule VI, while error-correction and intended phonological output is represented more posterolaterally in Crus I [25]. These regions were also located more medially than abstract regions, consistent with previous research finding that medial cerebellar regions contribute more to action than the lateral hemispheres, and neuromodulation studies finding a greater effect of medial than lateral cerebellar TMS on motor responses [26]. Lesions to medial cerebellar regions are also associated with dysregulation of affect, or inability to properly regulate correct emotional actions in a specific context [8]. Concrete-processing cerebellar regions may therefore be important for the coordination of movements towards particular targets, or goal-directed action. In the current study, these areas, located close to representations of motor effectors in cerebellum, were associated specifically with current behaviors, but not future behaviors. These behavioral patterns, coupled with anatomical evidence regarding the connectivity of this region with caudal PFC, suggest that this region is optimally positioned to use proximal information to guide current action.
On the other hand, schematic control regions of the cerebellum form loops with rostral regions of the PFC, including area 46, and are functionally connected to the default mode network [5,6]. Task-based fMRI of semantic language processing [27,28], learning of rules enabling automaticity [29], social abstraction [30], and theory of mind routinely activate these regions of the cerebellum, and this region is one of the most consistent findings in studies of autism spectrum disorder [31], a disorder defined by impairments in complex social cognition. In one previous study, these regions of cerebellum were found to represent both first and second-order cognitive rules, consistent with the putative role of this region in abstract control over action [32]. Neuromodulation of this region is associated with prediction difficulties – implying that it plays a role in future-oriented processing [27,33]. Physiologically, these regions show low intrinsic spiking rates, consistent with the temporal timescale necessary for abstract processing. In the current study, these regions showed associations with future reaction times, but not with current reaction times. Combined with the physiology and anatomical connections with rostral PFC regions, these behavioral associations are consistent with a role for these regions in preparation for future action.
Cerebellar contextual control regions identified here are often engaged by working memory tasks and are primarily functionally connected with frontoparietal, executive control networks in the brain [6,18,19]. Broad connectivity with frontoparietal regions including the middle dorsolateral PFC [24] coupled with its position between concrete and abstract processing regions of the cerebellum, optimally position this region to integrate concrete and goal-relevant information. Similarly, in the PFC, cognitive control regions are situated between caudal PFC and rostral PFC [14,15]. These regions show connectivity with both sensorimotor prefrontal areas as well as regions involved in affective and cognitive control [24]. Despite being involved in sensorimotor processing, these regions do not show connectivity with primary motor cortex. Importantly, in the current study, we found correlations between these regions and both current and future reaction times. This is consistent with the type of integration necessary to use abstract goals to inform current action in a context specific manner. Although lesions to cerebellar schematic control regions are typically associated with only cognitive symptoms, lesions to these contextual control regions may result in both motor and cognitive impairments and are associated with executive function deficits [9]. Given anatomical connectivity patterns and behavioral associations, we posited that contextual control regions form the apex of a functional processing hierarchy [17].
What’s in a name? The meaning of functional processing hierarchies
Hierarchical processing exists throughout the human brain, but there is often disagreement about what constitutes a hierarchy. Here, we identify a functional processing hierarchy – whereby higher-order regions asymmetrically influence activity in lower-order regions. Functional processing hierarchies may take advantage of the infrastructure provided by anatomical gradients within the brain [34]. For example, the expansion of the prefrontal cortex in humans has been supported by a “sensorimotor-fugal” extension of association cortices away from unimodal sensorimotor areas, giving rise to unimodal to transmodal “gradients” [34]. Theoretical work has long posited a gradient of function in the cerebral cortex, with motor-adjacent regions involved in concrete action and motor-distal regions engaged by abstract thought [10]. Neuroimaging findings in the prefrontal cortex have confirmed that rostral-caudal gradients support a functional processing hierarchy, whereby sub-regions close to motor cortex support concrete actions, and rostral-PFC regions support abstract goals [11,13–15]. Similarly, in the cerebellum, the largest increases in size of the cerebellar cortex in phylogenetically newer species has occurred primarily in regions outside of sensorimotor areas [35]. As in the prefrontal cortex, resting-state fMRI has identified gradients within the cerebellum that span between unimodal and transmodal areas, in a “sensorimotor fugal” manner [21]. These gradients, in parallel with those found in the cerebral cortex, lay the groundwork for regional functional specificity within the cerebellum.
We find that cerebellar regions involved in contextual control drove the relationship between regions involved in abstract and concrete processing. Importantly, regions involved in more abstract, future oriented processing were not located at the putative “top” of the processing hierarchy as predicted by some theories [10,11,13]. Rather, these regions may only be involved in cognitive processing to the degree that abstract, future-oriented, processing is required [17]. In cognitively-demanding situations, concrete, feature-based information is combined with pre-existing internal models in contextual control areas [17]. This basic functional hierarchical structure echoes that seen in the prefrontal cortex, whereby the mid-dorsolateral PFC is situated at the top of the hierarchy over current actions represented in more caudal PFC regions [14,15,17].
Hierarchies for cognitive control in the cerebellum are parallel, but not reliant on those in the prefrontal cortex
Activation in contextual control regions fully mediated the relationship between schematic and feature control regions, suggesting that contextual control regions formed the apex of the hierarchy. Crucially, this relationship held true even when controlling for activation in cerebral areas known to communicate with these cerebellar regions via reciprocal closed-loop circuits. Therefore, cerebellar hierarchical organization was not merely a reflection of hierarchies in the cerebrum. Instead, cerebellar sub-regions interact in a hierarchical manner in parallel to hierarchies in other regions throughout the brain. The hierarchical interactions between sub-regions were also relevant for behavior: contextual control regions fully mediated the relationship between activation in concrete regions and current actions, and between activation in schematic regions and future actions. These data provide further evidence of an apical role of contextual control regions. This is consistent with lesion-based analyses in both the cerebellum and PFC finding that damage to contextual control regions results in impairments in executive function, and behaviors associated with regions lower in the hierarchy [9,13 (but see 36 about minimal permanent cognitive impairments following cerebellar lesions)]. Cerebellar contextual control regions may combine input about future needs from abstract processing regions, with information about current needs from feature processing regions to take into account both current situations and future goals and ultimately choose appropriate actions to fulfil long-term goals.
Hierarchical organization in cerebellum differs from that in the cerebral cortex
Unlike in the PFC, we found multiple bilateral representations of the cerebellar cognitive control hierarchy, inverting around Crus I/II. Resting-state gradient analyses and canonical resting-state networks also show multiple representations of the entire span of cerebral resting state networks within the cerebellum – inverting around Crus I [6,21]. More recent work suggests that there may even be multiple task representations within the cerebellum which invert around Crus I/II [19], and that separate representations might contribute to different aspects of a particular behavior. Although the exact relevance of these multiple representations is not known, clinical evidence suggests that the representations are not equal, as damage to one representation results in different symptoms than that in another [9].
Further, unlike the cerebral cortex, cerebellar cytoarchitecture is uniform throughout the cerebellar cortex. Given this uniform cytoarchitecture, how might regional specificity within the cerebellum emerge? In fact, there are plenty of regional differences in molecular expression patterns, physiological properties, and microcircuitry within the cerebellum [37]. These variations may support slightly different computations within the cerebellum (for review see [20,38]). Importantly, although it is unlikely that there are many long-range association fibers within the cerebellar cortex, there is substantial evidence that the cerebellum communicates intensely within itself allowing for integration of information between disparate cerebellar regions [39]. One such mechanism is via nucleocortical loops - circuits between Purkinje cells (the sole output from the cerebellar cortex) and the deep cerebellar nuclei (DCN, the sole output of the cerebellum to the cerebral cortex). Although many of these loops are closed, some are “open”, or not precisely reciprocal, targeting various areas including contralateral cerebellar regions [39]. This simultaneous open and closed-loop nucleocortical structure could serve to interconnect various cerebellar microzones, and allow for integration across sub-regions [39]. Importantly, the degree of reciprocal nucleocortical connections varies by cerebellar cortical region [40]. Branching in nucleocortical loops allows for innervation and binding of functionally related, but spatially distinct regions of the cerebellar cortex [41]. For example, in the cat DCN, neurons projecting to lobule VI were found to co-localize with neurons projecting to Crus II [41].
Although the cerebellar cortex is often divided into discrete modules made up of input and output cells (see [42] for review), these modules communicate with each other across large swaths of the cerebellar cortex. Beyond nucleocortical loops described above, parallel fibers (ascending ramifications of granule cells – the most abundant cell in the brain) run transversely in the cerebellum for lengths of about 6mm in primates and can modulate the activity of Purkinje cells which project to various different neuron groups in the DCN [43]. The cerebellum is also home to a dense interneuron system made up of multiple cell types (e.g. Golgi cells, Lugaro cells, etc.) which make up a rich network that has strong modulatory influence over main cerebellar cells including granule and Purkinje cells. For example, Golgi cells are connected via gap-junctions (electrical rather than chemical synapses) which may support synchronization of vast regions of the cerebellar cortex [44]. Lugaro interneurons send long-range myelinated axons along the coronal plane but also extend along the sagittal plane of the cerebellar cortex [45]. One study found that these interneurons were most abundant in the curvature between lobules rather than at the lobular apex [45], which may make them excellent source of communication between lobules. Cerebellar interneurons also produce intrinsic (arising from within the cerebellum) oscillatory activity that can be synchronous across entire lobules, and even across hemispheres [46,47]. These oscillations have the power to spatially and temporally coordinate distant neural assemblies, and can rapidly synchronize and desynchronize firing in cell populations [48]. Cerebellar oscillations are also phase-locked with local field potentials (LFPs) in cerebral somatomotor cortices [46,49]. Importantly, animal studies find that certain cerebellar oscillations are strongest at times when the animal is anticipating a reward or waiting for the right time to produce a motor action – which may be especially relevant to cognitive control [46]. Like in the cerebral cortex, oscillations may provide an excellent long-range communication mechanism within the cerebellum. There is some evidence that oscillations may also be region specific: certain types of oscillations entrain firing in different orientations (e.g. primarily sagittal / anterior-to-posterior axis, patchy, or along the transverse axis) [50] and may be be of importance to cognition, as abnormalities in cerebellar oscillatory rhythms have been identified in mouse models of fetal alcohol syndrome [51]. These kinds of intracerebellar communication obviously differ from how sub-regions interact within the prefrontal cortex and other supratentorial structures. These differences may help elucidate the unique role cerebellar hierarchies play in supporting cognitive control. For example, feedback loops between Purkinje cells and the DCN, informed by input from the cerebral cortex, may allow for iterative honing of internal models and predictions that allow for optimization of behavior in a given context. These models may allow for fast adjustment and adaptive control of behavior in other interconnected hierarchies throughout the brain.
It is important to note that the putative mechanisms underlying intracerebellar communication discussed above occur at the level of cells and synapses, rather than at the level of large neuronal populations thought to underlie the BOLD signal. In the cerebellum, there is evidence to suggest that BOLD or cerebral blood flow is reliably associated with LFPs, a measure of synaptic or peri-synaptic activity rather than spiking rate of individual neurons. For example, direct parallel fiber stimulation is associated with increases in LFP and cerebral blood flow (despite the fact that parallel fiber stimulation inhibits spiking activity of Purkinje cells) [52]. Cerebellar granule cells and parallel fibers can also modulate blood flow via release of vasoactive substances [53]. It is likely then, that cerebellar BOLD does not reflect output from or input to the cerebellum, but rather reflects neural processing occurring within the cerebellum [54]. This is not dissimilar from neural underpinnings of BOLD throughout the cerebral cortex, which is also more associated with synaptically evoked field potentials than spike output [54,55]. Nonetheless, it is important to keep in mind that throughout the brain the BOLD signal may actually have quite complex origins. For example, the relationship between BOLD and neural activity can change as a result of cognitive states [56].
What might cerebellar hierarchies contribute to cognitive control?
In the motor realm, the cerebellum is involved in the formation of internal models that allow for prediction of sensory outcomes of a motor action. These internal models are iteratively refined via error-based learning so as to optimize behavior in a given context [42,57]. Via a series of reciprocal closed-loop circuits with nearly every cerebral cortical region, as well as the basal ganglia, the cerebellum is optimally positioned to receive and integrate information to generate internal models and optimize behavior. Due to its relatively uniform cytoarchitecture, it has been proposed that the cerebellum may perform a similar predictive function in the cognitive domain [58]. The ability of the cerebellum to integrate information from various regions and create predictions may allow for automatization and coordinated processing within distributed cortical networks in a manner relevant to cognition [38]. It has been suggested that the cerebellum plays a particular role in detecting and generating sequences in both motor and non-motor domains [59,60]. In the domain of social interaction, one study found that individuals with cerebellar lesions performed poorly only on tasks that required explicit and active sequencing, but not on mechanical tasks in which sequencing was either less necessary or overlearned [60]. In the case of cognitive control, goal-directed behaviors require predictions about likely actions to achieve a desired state [61], and therefore inherently requires some sort of sequencing ability. Cerebellar-based sequence learning may allow individuals to form predictions about sequences of actions, which allow for prediction about future action sequences and rapid error-detection when actions deviate from expected sequences. One study using TMS to disrupt posterolateral cerebellar processing during working memory found that participants had increased errors. Crucially, immediately after cerebellar disruption, participants were more likely to use out of date information to predict upcoming information [62]. Resting-state fMRI analyses find that the cerebellar resting-state signal lags behind that of the cerebral cortex, suggesting that the cerebellum receives input from the cortex to process before relaying it back to the same cortical areas [63]. Importantly, our results find that rather than merely reflecting networks in the rest of the brain, the cerebellum conducts its own parallel, hierarchical processing. In particular, we found evidence for a robust cerebellar hierarchy even when controlling for cerebral signals carried via cerebello-prefrontal loops. This internal hierarchical structure, parallel to functional processing hierarchies throughout the brain, potentially allows for more effective cognitive control using iteratively refined models. In this way, the cerebellum may perform some sort of “quality control” function on information originating in cerebral cortical regions, and adjust signals at odds with predictions based on internal models to ultimately calibrate processing in the rest of the brain.
Conclusions, limitations, and future directions
Here, we identify separate regions of the cerebellum, arranged in a gradient-like pattern, which respond distinctly to different aspects of cognitive control, and show dissociable correlations with behavior. We show for the first time, that cerebellar sub-regions interact to support cognitive control. These interactions are functionally hierarchical. Future work should assess whether there is a finer gradient structure in the path from will to action within the posterolateral cerebellum. At our current resolution and given the highly convoluted foliation of the cerebellum, fine discrimination within the cerebellum is limited. Recent research has unearthed very fine parcellations of task representations within the cerebellum [20]. The univariate task representations here crossed lobular boundaries and overlapped at the border between representations. These overlaps are similarly noted in hierarchies throughout the brain, and may aid integration of information in discrete regions. Alternatively, higher-resolution methods and subtler task manipulations may elucidate greater detail within the posterior cerebellum. In addition, in the current task, participants had to sequence letters verbally or spatially. It is possible, therefore, that this kind of hierarchical organization may be specific to sequencing, and may not be evident for other kinds of tasks (e.g. categorization). Future research should determine whether a similar organization would be present under different task conditions. Lastly, although we controlled for signals from supratentorial cortical regions in our mediation analysis using both ROI and more comprehensive PCA methods, it is nonetheless possible that the hierarchy we propose in cerebellum is not intracerebellar in nature, but reflective of processes happening elsewhere. In particular, given that the cerebellum, frontal, and parietal regions form networks (e.g. all house representations of DMN, frontoparietal networks, sensorimotor networks, etc.), it may be the case that hierarchies occur at the level of networks rather than within individual brain regions. In this sense, it may not be possible using fMRI methods to completely delineate local hierarchies from hierarchies established by relay signals from other regions. Notably however, our PCA analyses and simulations suggest that even the frontal hierarchy was susceptible to influences from the cerebellum. This implies that hierarchical organization identified in many brain regions is to some extent reliant on signals from other brain regions. Our analyses and simulations suggest that although the cerebellum receives signals from the PFC (and the PFC receives signals from the cerebellum), both the cerebellum and frontal regions likely also contain a local hierarchy.
STAR Methods:
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information should be directed to the Lead Contact, Anila M. D’Mello (admello@mit.edu). This study did not generate unique reagents.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Participants:
A total of 24 right-handed, native English speaking participants between the ages of 18-28 years participated in the study (full demographic details previously reported [14]). For the discovery analysis, one participant was excluded because of fewer data-points (only one run), for n=23 participants. An additional 25 participants were used as a replication sample (demographic details previously reported [15]). For the current replication analysis, n=1 participant was excluded due to missing data. This resulted in a total of n=23 participants (12 female, mean age=19.8 years), and n=24 participants (15 female, mean age=20.6 years) for the replication analysis. Informed consent was obtained from all participants and all procedures were approved in accordance with the Committee for Protection of Human Subjects at the University of California, Berkeley.
METHOD DETAILS
Task:
This task was previously described in detail by Nee & D’Esposito [14,15]. Participants performed a cognitive control task in both verbal and spatial domains (Figure 2). For ease of exposition, we will describe the task performed in the verbal domain (Figure 2A), although comparable conditions were also performed in the spatial domain (Figure 2C). On each trial, participants observed one of five letters, presented in one of five locations, surrounded by one of four shapes printed in one of two colors. Colors indicated the relevant domain (verbal or spatial), shapes indicated the relevant task to perform, and letters/location provided inputs for the task rules. In the verbal domain, participants started each block of the task by determining whether the letter they saw was the first letter in the word “TABLET.” On each subsequent trial, participants determined whether each new letter sequentially followed the previous letter in the word “TABLET” (control condition). After a few trials, participants received a sub-task cue that signaled that new task demands were necessary (e.g. squares changed to circles, diamonds, or crosses). Each sub-task cue signaled a different sub-task: circles = ignore stimuli but remember your place in the sequence (delay condition); diamonds = restart the sequence from the beginning (restart condition); crosses = restart the sequence while remembering your place in the previous sequence (dual condition). These sub-tasks allowed us to examine the effect of schematic control and contextual control, both necessary for cognitive control. The schematic axis manipulated demands on the use of past representations to inform future actions. The contextual axis manipulated demands on the use of task context in order to choose the appropriate action to correctly complete the task. This gave rise to various conditions: (1) low schematic control and low contextual control (control), (2) low schematic control and high contextual control (restart), (3) high schematic control and low contextual control (delay), (4) and high temporal and high contextual control (dual). These conditions were examined in both verbal and spatial domains. The control condition required low contextual processing, and low temporal processing. In this condition participants completed the basic task. The restart condition required high levels of contextual processing, and low temporal processing. Here, participants had to be vigilant to changing cues, but did not have to hold any information in memory. The delay condition required low contextual processing, but high temporal processing. Participants were asked to hold information in memory until cued. The dual condition was the most challenging, requiring high contextual processing and high temporal processing. Here, participants were asked to not only maintain information in memory, but simultaneously pay attention to changing contextual cues. A combination of these four conditions allowed for an examination of the continuum of cognitive processing – moving from concrete processing of features (concrete control), to malleable awareness of how features inform contexts in which certain actions are required (contextual control), to planning how current contexts influence future behavior (schematic control).
A week before the scanning session, participants performed a practice session to learn the task under supervision of an experimenter. Once comfortable with the task, participants completed 3 runs of the task on their own outside of the scanner. Prior to data collection in each session, participants also performed a single practice run inside the scanner. In the identification sample, participants completed a total of 12 runs across two separate fMRI sessions. Each session was divided into 6 runs of 16 blocks (160 trials) each. Participants completed 24 blocks of each condition (total of eight conditions: Control, Restart, Delay, and Dual in both spatial and verbal domain). This resulted in a total of 1920 trials (including 96 for each sub-task). In the replication sample, participants completed a total of 6 runs of 16 blocks (144 trials) in a single session. Each Participants completed 12 blocks of each condition resulting in a total of 864 trials (including 48 for each sub-task).
Image acquisition:
All data were collected and previously analyzed by Nee & D’Esposito [14,15]. Image acquisition and protocol are described in detail in Nee & D’Esposito [14,15]. Briefly, images were acquired on a Siemens TIM/Trio 3T MRI equipped with a 32-channel head coil. Functional images were acquired using an EPI sequence with 35 descending slices and 3.44 x 3.44 x 3.75 mm3 voxels (TR = 2000 ms; echo time = 25 ms; flip angle = 70; field of view = 220). Phase and magnitude images were collected to estimate the magnetic inhomogeneity. High-resolution T1-weighted MPRAGE images were collected for spatial normalization (240 x 256 x 160 matrix of 1 mm3 isotropic voxels; TR = 2300 ms; echo time = 2.98 ms; flip angle = 9).
Preprocessing:
Data were despiked using AFNI’s 3dDespike (interpolate method). SPM was used for slice-timing correction, unwarping using SPM’s Field Map toolbox, realignment, coregistration, segmentation, and normalization to MNI space. Data were smoothed with a 6mm FWHM. Motion regressors were included for participants with greater than 3mm/degrees of motion over the course of the session or a single movement of greater than 0.5mm/degrees in TR-to-TR motion. These reflected total displacement, squared total displacement, differential (TR-to-TR) displacement, and squared differential displacement to capture signal artifacts related to motion [67,68].
QUANTIFICATION AND STATISTICAL ANALYSIS
Behavioral analysis:
For each participant, behavioral data were stratified into the eight conditions formed by crossing stimulus domain x contextual control x schematic control and further segmented into task phases. The trials-of-interest for the present study were the sub-task trials and return trials (Fig 2D). Sub-task trials included all trials during the sub-task phase except for the first. Reaction times were calculated on correct trials only. Reaction times faster than 200 ms were discarded as anticipatory and reaction times greater than 2000 ms were discarded as inattentive. Reaction times greater than 2.5 standard deviations of the condition mean were removed as outliers. This resulted in the removal of 0.76% of the trials in the main analysis, and 0.65% of the trials in the replication analysis.
First level modeling:
Analyses were performed in SPM12. Regressors for the Control, Restart, Delay, and Dual conditions crossed with two Stimulus Domain types were included in a first-level model. The Restart, Delay, and Dual conditions spanned the onset of the second trial of the sub-task through the last trial of the sub-task. As the Control condition did not contain an overt sub-task, epochs in the middle of the block were modeled to match the other conditions. In addition to the main conditions, separate impulse regressors were included for the first trial of each block, the first trial of each sub-task, return trials, trials occurring before the sub-task, and trials occurring after the sub-task. Additional nuisance impulse regressors included: left button-press responses, right button-press responses, and error responses. At the first-level, regressors were combined to create three contrasts of interest: Schematic control (Delay + Dual – Control + Restart), Contextual control (Dual + Restart – Delay + Control), and Concrete control (Interaction of Temporal and Contextual control; Dual + Control – Delay + Restart). To assess the presence of a domain-general hierarchical axis of processing within the cerebellum, main effects of each condition collapsed across stimulus domain (Verbal, Spatial). Regressors were also created for effect of stimulus domain (Verbal – Spatial).
Second level modeling:
Each contrast of interest was carried forward to a separate second-level model (schematic control, contextual control, and concrete control), and one-sample T-tests were performed. Results were thresholded at an uncorrected p-value of 0.001 with a FDR-corrected cluster threshold < 0.05. Though only cerebellar results are reported (cerebral cortical results are reported in Nee & D’Esposito [14,15]), thresholds correspond to whole-brain thresholds (no region of interest or masking of the cerebellum) so that cerebellar results were significant in the context of the whole brain. Results were mapped to the surface and plotted on flatmaps or inflated cerebellar maps [66].
Correlations with behavior:
We extracted regions of interest from the main effects described above (Schematic, Contextual, and Concrete contrasts; 4mm radius spheres centered on peak activation within the clusters from the univariate analysis). If the peak voxel coordinates overlapped with other ROIs, subpeaks within the same cluster were used instead. This resulted in a total of eight ROIs: Temporal processing (Right Crus I [44 −72 −38]; Left Crus I [−42 −76 −46]), Contextual processing (Right posterior VI [24 −68 −26]; Left posterior VI [−32 −68 −28]; Right Crus II/VIIB [32 −72 −48]; Left Crus II/VIIB [−34 −72 −48]), and Feature processing (Right anterior VI [6 −76 −16]; R VIIB/VIIIA [22 −70 −44]). Average parameter estimates were extracted from ROIs using REX [69]. We calculated average reaction times for “future” and “current” epochs from each of the 8 conditions of interest. Future epochs were defined as those directly following the sub-task, when participants returned to the condition they were performing prior to being cued to perform the action required by the sub-task. Current epochs corresponded directly to the fMRI signal being measured (during the sub-task, itself). Reaction times were normalized within participant. Partial correlations were calculated between parameter estimates in each ROI and Current RT (controlling for Future RT), and Future RT (controlling for Current RT). To control for repeated measures within each subject, correlations were conducted using the rmcorr package as implemented in R [70]. Results remained significant when conducting an FDR correction for multiple comparisons.
Mediation analysis:
Multi-level mediation analyses were conducted using the bmlm package as implemented in R [71]. Bmlm conducts Bayesian inference using the RStan interface. Parameter estimates from ROI’s described above were extracted. To extract PFC signal from corresponding schematic, contextual, and concrete regions, 4mm spheres were created centered around peak PFC coordinates for each condition as reported by Nee & D’Esposito 2016. To control for PFC contributions to cerebellar signal, we partialled out the effects of PFC activation from corresponding cerebellar ROIS. These values were entered into a mediation analysis (mediator = contextual control activation). To assess significance, credible intervals were used (95% mass of the marginal posterior distribution) [71].
Principle component analysis (PCA) and simulations:
Principal component analyses (PCA) were conducted in MATLAB on statistical maps of schematic, contextual, and concrete control in order to extract the components that explained the most variance in the frontal lobe, parietal lobe, and cerebellum. Frontal and parietal PCs were iteratively regressed out of the cerebellar signals (from 1-183 PCs with 183 being the maximal number of PCs in the smallest map) and mediation analysis were rerun to determine how partialing of frontal or parietal PCs affected the mediation estimates. To assess whether frontal lobe mediations were also affected by regression of cerebellar PCs, the inverse analysis was also conducted (iterative regression of cerebellar PCs from frontal lobe mediation). The ⊗ mediation was calculated by subtracting the mediation effect after regression of n PCs from the original mediation effect. The same analysis was replicated in the secondary independent data set (n=24, described above). Simulation analyses were run to contextualize PCA results. Simulated data was computed for the all regions (frontal, parietal, and cerebellum) in MATLAB. Mediations were conducted using the M3 toolbox (https://github.com/canlab/MediationToolbox). Three different models were tested: (1) No local cerebellar mediation: all inputs for X, Y, and Mediator (M) variables is directly relayed from the frontal lobe. (2) All local cerebellar mediation: X, Y, and M cerebellar regions receive input from the frontal lobe but the cerebellar mediation is entirely local, consisting only of cerebellar signals. (3) X, Y, and M cerebellar regions receive PFC input and this input is intermixed with local cerebellar signals to support parallel mediations.
Replication analysis:
All analyses were replicated in a second, independent sample (n=24; Nee & D’Esposito, 2017). Univariate analysis results were thresholded at p<0.001, FDR cluster correction <0.05. A 4mm sphere was created around the most significant voxel for each condition. ROIS for the replication analysis differed slightly from the discovery sample. Schematic control (Right Crus I [36 −78 −40]), Contextual control (Right Crus II/VIIB [32 −72 −44]), and Concrete control (Right anterior VI [26 −64 −24]). Results remained significant when conducting an FDR correction for multiple comparisons.
DATA AND CODE AVAILABILITY
Group-level data generated during this study is available on Neurovault (https://neurovault.org/collections/6778/). Simulation code is available on the Open Science Framework (OSF; https://osf.io/864mj/). Given human subjects confidentiality concerns and the potential for identification, original raw data supporting the current experiment have not been uploaded to a public repository. Data can be obtained upon request to Derek E. Nee (nee@psy.fsu.edu).
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited Data | ||
| Unthresholded group-level data | https://neurovault.org/collections/6778/ | |
| Software and Algorithms | ||
| MATLAB 2018b | https://www.mathworks.com/products/new_products/release2018b.html | RRID:SCR_001622 |
| Statistical Parametric Mapping (SPM) 12 | https://www.fil.ion.ucl.ac.uk/spm/software/spm12/ | RRID:SCR_007037 |
| Bayesian MultiLevel Mediation (bmlm) Toobox | https://github.com/mvuorre/bmlm | |
| M3 Mediation Toolbox | https://github.com/canlab/MediationToolbox | |
| Other | ||
| Principle Component Analysis Simulation Code (in house) | https://osf.io/864mj/ | |
Highlights.
The posterior cerebellum is organized along a gradient of temporal abstraction.
Distinct cerebellar areas support cognitive control in the moment and the future.
Cerebellar areas interact hierarchically to organize current and future processing.
The cerebellar control hierarchy is not driven entirely by prefrontal signals.
Acknowledgements:
This research was supported by National Institute of Neurological Disorders and Stroke Grants F32 NS0802069 (DEN) and P01 NS040813 (Mark D’Esposito), National Institute of Mental Health Grants R01 MH063901 (Mark D’Esposito), R01 MH121509 (DEN), F32 MH117933 (AMD), and Florida State University COFRS Award 0000034175 (DEN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests:
The authors declare no competing interests.
References:
- 1.Barton RA, and Venditti C (2014). Rapid Evolution of the Cerebellum in Humans and Other Great Apes. Current Biology 24, 2440–2444. [DOI] [PubMed] [Google Scholar]
- 2.Leiner HC, Leiner AL, and Dow RS (1991). The human cerebro-cerebellar system: its computing, cognitive, and language skills. Behavioural Brain Research 44, 113–128. [DOI] [PubMed] [Google Scholar]
- 3.Smaers JB, Turner AH, Gómez-Robles A, and Sherwood CC (2018). A cerebellar substrate for cognition evolved multiple times independently in mammals. eLife 7, e35696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen BB, Korbo L, and Pakkenberg B (1992). A quantitative study of the human cerebellum with unbiased stereological techniques. J. Comp. Neurol 326, 549–560. [DOI] [PubMed] [Google Scholar]
- 5.Kelly RM, and Strick PL (2003). Cerebellar Loops with Motor Cortex and Prefrontal Cortex of a Nonhuman Primate. J. Neurosci 23, 8432–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckner RL, Krienen FM, Castellanos A, Diaz JC, and Yeo BTT (2011). The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106, 2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoodley CJ, and Schmahmann JD (2009). Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage 44, 489–501. [DOI] [PubMed] [Google Scholar]
- 8.Schmahmann JD, and Sherman JC (1998). The cerebellar cognitive affective syndrome. Brain 121 (Pt 4), 561–579. [DOI] [PubMed] [Google Scholar]
- 9.Stoodley CJ, MacMore JP, Makris N, Sherman JC, and Schmahmann JD (2016). Location of lesion determines motor vs. cognitive consequences in patients with cerebellar stroke. Neuroimage: Clinical 12, 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuster JM (2001). The prefrontal cortex--an update: time is of the essence. Neuron 30, 319–333. [DOI] [PubMed] [Google Scholar]
- 11.Koechlin E, Ody C, and Kouneiher F (2003). The Architecture of Cognitive Control in the Human Prefrontal Cortex. Science 302, 1181–1185. [DOI] [PubMed] [Google Scholar]
- 12.Koechlin E, and Summerfield C (2007). An information theoretical approach to prefrontal executive function. Trends in Cognitive Sciences 11, 229–235. [DOI] [PubMed] [Google Scholar]
- 13.Badre D, and D’Esposito M (2009). Is the rostro-caudal axis of the frontal lobe hierarchical? Nat. Rev. Neurosci 10, 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nee DE, and D’Esposito M (2016). The hierarchical organization of the lateral prefrontal cortex. eLife 5, e12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nee DE, and D’Esposito M (2017). Causal evidence for lateral prefrontal cortex dynamics supporting cognitive control. eLife 6, e28040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambon V, Franck N, Koechlin E, Fakra E, Ciuperca G, Azorin J-M, and Farrer C (2008). The architecture of cognitive control in schizophrenia. Brain 131, 962–970. [DOI] [PubMed] [Google Scholar]
- 17.Badre D, and Nee DE (2018). Frontal cortex and the hierarchical control of behavior. Trends Cogn Sci 22, 170–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoodley CJ (2012). The cerebellum and cognition: evidence from functional imaging studies. Cerebellum 11, 352–365. [DOI] [PubMed] [Google Scholar]
- 19.Guell X, Gabrieli JDE, and Schmahmann JD (2018). Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage 172, 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King M, Hernandez-Castillo CR, Poldrack RA, Ivry RB, and Diedrichsen J (2019). Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat Neurosci 22, 1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guell X, Schmahmann JD, Gabrieli JD, and Ghosh SS (2018). Functional gradients of the cerebellum. eLife. Available at: https://elifesciences.org/articles/36652 [Accessed August 23, 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nee DE (2019). fMRI replicability depends upon sufficient individual-level data. Commun Biol 2, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strick PL, Dum RP, and Fiez JA (2009). Cerebellum and Nonmotor Function. Annual Review of Neuroscience 32, 413–434. [DOI] [PubMed] [Google Scholar]
- 24.Kipping JA, Grodd W, Kumar V, Taubert M, Villringer A, and Margulies DS (2013). Overlapping and parallel cerebello-cerebral networks contributing to sensorimotor control: An intrinsic functional connectivity study. Neuroimage 83, 837–848. [DOI] [PubMed] [Google Scholar]
- 25.Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, and Glover GH (1997). Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J. Neurosci 17, 9675–9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Théoret H, Haque J, and Pascual-Leone A (2001). Increased variability of paced finger tapping accuracy following repetitive magnetic stimulation of the cerebellum in humans. Neuroscience Letters 306, 29–32. [DOI] [PubMed] [Google Scholar]
- 27.D’Mello AM, Turkeltaub PE, and Stoodley CJ (2017). Cerebellar tDCS modulates neural circuits during semantic prediction: A combined tDCS-fMRI study. J. Neurosci, 2818–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moberget T, Gullesen EH, Andersson S, Ivry RB, and Endestad T (2014). Generalized Role for the Cerebellum in Encoding Internal Models: Evidence from Semantic Processing. J. Neurosci 34, 2871–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balsters JH, and Ramnani N (2011). Cerebellar Plasticity and the Automation of First-Order Rules. J. Neurosci 31, 2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Overwalle F, Baetens K, Mariën P, and Vandekerckhove M (2014). Social cognition and the cerebellum: A meta-analysis of over 350 fMRI studies. NeuroImage 86, 554–572. [DOI] [PubMed] [Google Scholar]
- 31.D’Mello AM, and Stoodley CJ (2015). Cerebro-cerebellar circuits in autism spectrum disorder. Front Neurosci 9, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balsters JH, Whelan CD, Robertson IH, and Ramnani N (2012). Cerebellum and Cognition: Evidence for the Encoding of Higher Order Rules. Cereb. Cortex, bhs127. [DOI] [PubMed] [Google Scholar]
- 33.Lesage E, Morgan BE, Olson AC, Meyer AS, and Miall RC (2012). Cerebellar rTMS disrupts predictive language processing. Curr. Biol 22, R794–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, Bezgin G, Eickhoff SB, Castellanos FX, Petrides M, et al. (2016). Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci. U.S.A 113, 12574–12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacLeod CE, Zilles K, Schleicher A, Rilling JK, and Gibson KR (2003). Expansion of the neocerebellum in Hominoidea. Journal of Human Evolution 44, 401–429. [DOI] [PubMed] [Google Scholar]
- 36.Alexander MP, Gillingham S, Schweizer T, and Stuss DT (2012). Cognitive impairments due to focal cerebellar injuries in adults. Cortex 48, 980–990. [DOI] [PubMed] [Google Scholar]
- 37.Witter L, and De Zeeuw CI (2015). Regional functionality of the cerebellum. Current Opinion in Neurobiology 33, 150–155. [DOI] [PubMed] [Google Scholar]
- 38.Sokolov AA, Miall RC, and Ivry RB (2017). The Cerebellum: Adaptive Prediction for Movement and Cognition. Trends Cogn. Sci. (Regul. Ed.) 21, 313–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houck BD, and Person AL (2014). Cerebellar Loops: A Review of the Nucleocortical Pathway. Cerebellum 13, 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tolbert DL, and Bantli H (1979). An HRP and autoradiographic study of cerebellar corticonuclear-nucleocortical reciprocity in the monkey. Experimental Brain Research 36 Available at: http://link.springer.com/10.1007/BF00238523 [Accessed August 7, 2019]. [DOI] [PubMed] [Google Scholar]
- 41.Provini L, Marcotti W, Morara S, and Rosina A (1998). Somatotopic nucleocortical projections to the multiple somatosensory cerebellar maps. Neuroscience 83, 1085–1104. [DOI] [PubMed] [Google Scholar]
- 42.Ito M (2006). Cerebellar circuitry as a neuronal machine. Progress in Neurobiology 78, 272–303. [DOI] [PubMed] [Google Scholar]
- 43.Mugnaini E (1983). The length of cerebellar parallel fibers in chicken and rhesus monkey. J. Comp. Neurol 220, 7–15. [DOI] [PubMed] [Google Scholar]
- 44.Galliano E, Mazzarello P, and D’Angelo E (2010). Discovery and rediscoveries of Golgi cells. The Journal of Physiology 588, 3639–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dieudonné S, and Dumoulin A (2000). Serotonin-driven long-range inhibitory connections in the cerebellar cortex. J. Neurosci 20, 1837–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Courtemanche R, Robinson JC, and Aponte DI (2013). Linking oscillations in cerebellar circuits. Front. Neural Circuits 7 Available at: https://www.frontiersin.org/articles/10.3389/fncir.2013.00125/full [Accessed December 11, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartmann MJ, and Bower JM (1998). Oscillatory Activity in the Cerebellar Hemispheres of Unrestrained Rats. Journal of Neurophysiology 80, 1598–1604. [DOI] [PubMed] [Google Scholar]
- 48.Simões de Souza FM, and De Schutter E (2011). Robustness effect of gap junctions between Golgi cells on cerebellar cortex oscillations. Neural Systems & Circuits 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Popa D, Spolidoro M, Proville RD, Guyon N, Belliveau L, and Léna C (2013). Functional Role of the Cerebellum in Gamma-Band Synchronization of the Sensory and Motor Cortices. J. Neurosci 33, 6552–6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Zeeuw CI, Hoebeek FE, and Schonewille M (2008). Causes and Consequences of Oscillations in the Cerebellar Cortex. Neuron 58, 655–658. [DOI] [PubMed] [Google Scholar]
- 51.Cheron G, Servais L, and Dan B (2008). Cerebellar network plasticity: From genes to fast oscillation. Neuroscience 153, 1–19. [DOI] [PubMed] [Google Scholar]
- 52.Iadecola C, Yang G-B, Ebner TJ, and Chen G (1997). Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. Journal of neurophysiology 78, 651–659. [DOI] [PubMed] [Google Scholar]
- 53.Mapelli L, Gagliano G, Soda T, Laforenza U, Moccia F, and D’Angelo EU (2017). Granular Layer Neurons Control Cerebellar Neurovascular Coupling Through an NMDA Receptor/NO-Dependent System. J Neurosci 37, 1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Attwell D, and ladecola C (2002). The neural basis of functional brain imaging signals. Trends in Neurosciences 25, 621–625. [DOI] [PubMed] [Google Scholar]
- 55.Ekstrom A (2010). How and when the fMRI BOLD signal relates to underlying neural activity: The danger in dissociation. Brain Res Rev 62, 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boynton GM (2011). Spikes, BOLD, Attention, and Awareness: A comparison of electrophysiological and fMRI signals in V1. Journal of Vision 11, 12–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marr D (1969). A theory of cerebellar cortex. J Physiol 202, 437–470.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ito M (2008). Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci 9, 304–313. [DOI] [PubMed] [Google Scholar]
- 59.Leggio MG, Tedesco AM, Chiricozzi FR, Clausi S, Orsini A, and Molinari M (2008). Cognitive sequencing impairment in patients with focal or atrophic cerebellar damage. Brain 131, 1332–1343. [DOI] [PubMed] [Google Scholar]
- 60.Van Overwalle F, De Coninck S, Heleven E, Perrotta G, Taib NOB, Manto M, and Mariën P (2019). The role of the cerebellum in reconstructing social action sequences: a pilot study. Soc Cogn Affect Neurosci 14, 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller EK (2000). The prefrontal cortex and cognitive control. Nat. Rev. Neurosci 1, 59–65. [DOI] [PubMed] [Google Scholar]
- 62.Sheu Y-S, Liang Y, and Desmond JE (2019). Disruption of Cerebellar Prediction in Verbal Working Memory. Front. Hum. Neurosci. 13 Available at: https://www.frontiersin.org/articles/10.3389/fnhum.2019.00061/full [Accessed August 9, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marek S, Siegel JS, Gordon EM, Raut RV, Gratton C, Newbold DJ, Ortega M, Laumann TO, Adeyemo B, Miller DB, et al. (2018). Spatial and Temporal Organization of the Individual Human Cerebellum. Neuron 100, 977–993.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, and Ugurbil K (2013). The WU-Minn Human Connectome Project: An Overview. Neuroimage 80, 62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Essen DC (2002). Surface-based atlases of cerebellar cortex in the human, macaque, and mouse. Ann. N. Y. Acad. Sci 978, 468–479. [DOI] [PubMed] [Google Scholar]
- 67.Lund TE, Nørgaard MD, Rostrup E, Rowe JB, and Paulson OB (2005). Motion or activity: their role in intra- and inter-subject variation in fMRI. NeuroImage 26, 960–964. [DOI] [PubMed] [Google Scholar]
- 68.Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, et al. (2013). An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage 64, 240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Whitfield-Gabrieli S, and Nieto-Castanon A (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2, 125–141. [DOI] [PubMed] [Google Scholar]
- 70.Bakdash JZ, and Marusich LR (2017). Repeated Measures Correlation. Front. Psychol. 8 Available at: https://www.frontiersin.org/articles/10.3389/fpsyg.2017.00456/full [Accessed September 20, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vuorre M, and Bolger N (2018). Within-subject mediation analysis for experimental data in cognitive psychology and neuroscience. Behavior Research Methods 50, 2125–2143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Group-level data generated during this study is available on Neurovault (https://neurovault.org/collections/6778/). Simulation code is available on the Open Science Framework (OSF; https://osf.io/864mj/). Given human subjects confidentiality concerns and the potential for identification, original raw data supporting the current experiment have not been uploaded to a public repository. Data can be obtained upon request to Derek E. Nee (nee@psy.fsu.edu).


