Abstract
Background
Neurons are an integral component of the immune system that functions to coordinate responses to bacterial pathogens. Sensory nociceptive neurons that can detect bacterial pathogens are found throughout the body with dense innervation of the intestinal tract.
Methods
In this study, we assessed the role of these nerves in the coordination of host defenses to Citrobacter rodentium. Selective ablation of nociceptive neurons significantly increased bacterial burden 10 days postinfection and delayed pathogen clearance.
Results
Because the sensory neuropeptide CGRP (calcitonin gene-related peptide) regulates host responses during infection of the skin, lung, and small intestine, we assessed the role of CGRP receptor signaling during C rodentium infection. Although CGRP receptor blockade reduced certain proinflammatory gene expression, bacterial burden and Il-22 expression was unaffected.
Conclusions
Our data highlight that sensory nociceptive neurons exert a significant host protective role during C rodentium infection, independent of CGRP receptor signaling.
Keywords: Citrobacter rodentium, CGRP, nociceptors, TRPV1
Sensory neurons are important in coordinating the host mucosal immune response to the enteric bacterial pathogen Citrobacter rodentium.
Host responses to bacterial pathogens are complex and coordinated processes. Although the focus on cellular immunology has elucidated numerous pathways that reduce infection, there is an integrative physiological response to pathogens mediated in part by the nervous system, which serves as an active participant during the host immune response against bacterial pathogens [1, 2]. In the colonic mucosa, this functionality of the nervous system is at least partially conferred through the dense innervation of sensory nociceptive neurons [3, 4]. These types of sensory neurons induce physiological changes upon exposure to noxious stimuli, including heat, ATP, mechanical injury, inflammation, and bacterial products, due to expression of the transient receptor potential cation channel subfamily V member 1 (TRPV1) [5, 6]. Typically, activation of this receptor induces activation of sensory neurons, resulting in the relaying of neuronal signals to the dorsal root ganglia in the spinal cord and from the gastrointestinal tract to the nodose ganglion (NG) via vagal afferent fibers [4, 7, 8]. Activation of nociceptive neurons causes the release of neuropeptides including calcitonin gene-related peptide (CGRP), neurokinin A, and substance P (SP). Localized release of neuropeptides at the site of activation exert potent immune-stimulatory or inhibitory effects [9, 10], which is the molecular underpinning of neurogenic inflammation [11, 12].
In recent studies, these sensory neurons have been demonstrated to play a critical role during infection of the lung, skin, and small intestine [13–15]. These studies revealed that activation of nociceptive neurons by the bacterial pathogen, or their products, induce CGRP-dependent maladaptive host responses that increase susceptibility to infection [13]. Despite these findings, the role of TRPV1 + sensory neurons during infection with a noninvasive enteric bacterial pathogen is unknown. To determine their role, we used the murine pathogen Citrobacter rodentium; a model self-limiting attaching and effacing pathogen. Citrobacter rodentium infection of mice has been well characterized to cause colonic intestinal epithelial cell (IEC) hyperplasia, with bacterial clearance approximately 30 days postinfection (p.i.) [16]. Critical to the control of infection is the successive activation of innate lymphoid cells (ILCs) followed by recruitment of CD4+ T cells that are required for clearance and host survival [17–19]. As specialized innate immune cells, ILCs produce interleukin (IL)-22 early during the course of infection while CD4+ T-cell responses are developing [18, 20], to enhance IEC production of antimicrobial peptides (AMP) that can kill bacteria [21–23]. Homing and extravasation into the colonic mucosa of IL-17A and IL-22 producing Th17 and Th22 T cells occurs during the peak of infection (approximately 10 p.i.) and is governed by the expression of chemotactic factors and receptors expressed by immune cells [17]. Extravasation into the infected colon depends on the expression of adhesion molecules such as intercellular adhesion molecule (ICAM)-1 or vascular cell adhesion molecule (VCAM)-1 by endothelial cells. Mucosal homing of T cells is conferred through expression of mucosal addressin cell adhesion molecule 1 (MAdCAM-1) expression that significantly increases in intestinal venules during inflammation [24–26]. Activation of naive T cells in the lymph nodes draining mucosal sites induces α 4β 7 integrin expression, the receptor for MAdCAM-1, resulting in preferential trafficking into the intestine [27–29]. Although recruited Th17 cells amplify inflammation through increased expression of cytokines such as IL-1β [30], Th22 cells serve to increase AMP production.
To determine whether nociceptive sensory neurons have a role in orchestrating immune responses in the intestine, we used targeted ablation of these neurons with resiniferatoxin (RTX) [31] before C rodentium infection. Using this approach, we show for the first time that sensory nerve ablation significantly increased C rodentium bacterial burden and delayed clearance. We further demonstrate that host protection requires TRPV1, since TRPV1−/− mice had increased bacterial burden at the peak of the infection along with delayed clearance. These changes were associated with reduced colonic IL-22 expression and T-cell recruitment in mice with nociceptive neuronal ablation compared with controls. We further identified significantly reduced expression of Madcam1 10 days p.i. with significant increases 29 days p.i., mirroring the recruitment of T cells. This deficiency in T-cell recruitment was not due to reduced chemokines required for Th17/Th22 recruitment.
Given the importance of CGRP in host responses in other bacterial infections, we assessed its role during C rodentium infection using a selective receptor antagonist. Unlike RTX-mediated nociceptor ablation, we show that blocking of CGRP receptors did not increase bacterial burden despite reducing select aspects of immune function. Taken together, our findings highlight a unique role for sensory nociceptors in the host response to the enteric bacterial pathogen C rodentium, particularly during the late phase of bacterial clearance.
METHODS
Mice
C57BL/6 and TRPV1−/− mice were originally purchased from the Jackson Laboratory (Bar Harbor, ME) to establish a breeding colony. All procedures were approved by the institutional animal care committee at UC Davis in accordance with the Guide for the Care and use of Laboratory Animals. Mice were euthanized by CO2 asphyxiation followed by cervical dislocation according to American Veterinary Medical Association guidelines. Resiniferatoxin (Tocris, Minneapolis, MN) was injected into adult C57BL/6 (6–7 weeks old) mice in 3 escalating doses (30 µg/kg, 70 µg/kg, 100 µg/kg i.p.) every other day. Control mice received injections with vehicle (Dulbecco’s modified Eagle’s medium diluted in phosphate-buffered saline). Mice were infected 10 days after the last injection of RTX with C rodentium. BIBN 4096 1 mg/kg (Tocris), a potent and selective CGRP receptor antagonist, was injected i.p. on the day of C rodentium infection and every other day onwards. Gastrointestinal motility was assessed in RTX or vehicle-treated mice and wild-type (WT) and TRPV1−/− mice, where the number and weight of fecal pellets for each mouse was determined over a 20-minute period.
Citrobacter rodentium Infection and Bacterial Burden Quantification
Citrobacter rodentium (strain DBS100) was kindly provided by Dr. Andreas Baumler (Department of medical microbiology and immunology, School of Medicine, UC Davis, Davis, CA). Mice were infected (108 colony-forming units [CFUs]; 100 µL) by oral gavage, and Luria-Bertani was used as vehicle control. For quantification of bacterial burden, feces or distal colonic tissue (1 cm) were weighed and homogenized using a stainless steel bead (QIAGEN, Germantown, MD) and a bead beater (QIAGEN), followed by serial dilution and plating on MacConkey agar. Colonies were counted after 16 hours of incubation at 37°C, and CFUs were calculated per gram of sample.
Quantitative Polymerase Chain Reaction
Total ribonucleic acid was extracted from distal colon using TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions, using 5-mm stainless steel bead in a bead beater (QIAGEN). Ribonucleic acid samples were reverse transcribed to complementary deoxyribonucleic acid using an iSCRIPT kit (Bio-Rad, Hercules, CA). Real-time quantitative polymerase chain reaction was performed using primer pairs obtained from Primerbank [32] (Supplementary Table S1).
Histology
At euthanasia, distal colon (1 cm) was collected and fixed in 10% buffered formalin. Samples were paraffin-embedded on end to allow for cross-sectioning of tissue. Sections (6 µm) were cut, and slides were deparaffinized, rehydrated, and stained with hematoxylin and eosin using a standard histological protocol. Tissue morphology was evaluated using bright-field microscopy at ×20 objective. Crypt length was measured as previously described, whereby at least 20 well oriented crypts were measured per animal with FIJI (Fiji Is Just ImageJ) [33].
Immunofluorescence and Confocal Microscopy
Slides of colon and NG tissues were prepared from formalin-fixed paraffin-embedded samples, deparaffinized and rehydrated for immunofluorescence. Slides were subjected to heat-induced epitope retrieval in citrate buffer (10 mM, pH = 6.0) for 30 minutes at 95°C. After cooling to room temperature (RT), slides were incubated in blocking solution consisting of 5% (w/v) bovine serum albumin with 5% (v/v) normal serum (antibody species specific) in Tris-buffered saline Tween 20 (.5% v/v) for 1 hour at RT (Supplementary Table S2). Slides were incubated in primary antibodies diluted in blocking solution (4°C, 16 hours) followed by extensive washing in wash buffer (3 × 5 minutes). Slides were incubated with appropriate secondary antibodies for 1 hour at RT, washed extensively (3 × 5 minutes), followed by counterstaining of nuclei with 4’,6-diamidino-2-phenylindole (DAPI). Coverslips were mounted using prolong gold antifade reagent (Thermo Fisher Scientific, Waltham, MA). Staining of βIII Tubulin was performed using a mouse-on-mouse kit according to the manufacturer’s instructions (Vector Laboratories, Burlingame, CA). Imaging of slides was performed on a Leica SP8 confocal microscope. Image areas larger than the field of view were acquired as overlapping tiles with a 10% overlap and stitched using Imaris Stitcher 9.0. Images were then viewed and analyzed using Imaris (Bitplane Scientific, Belfast, United Kingdom) or FIJI.
RESULTS
Depletion of Nociceptive Sensory Afferent Nerves Reduces Host Defenses to Citrobacter rodentium
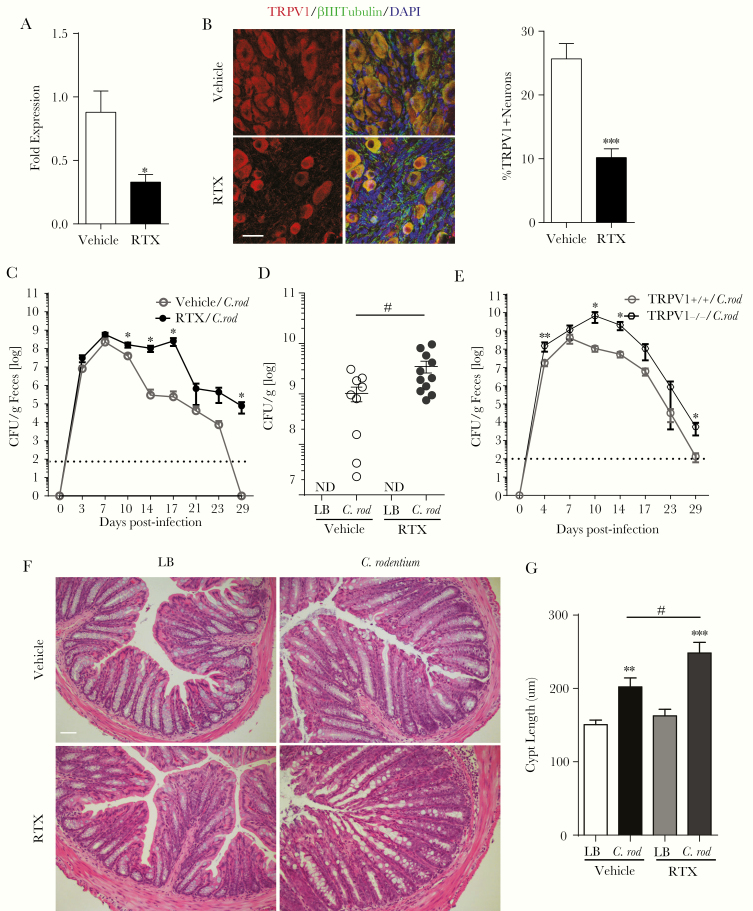
The role of nociceptive neurons in the host response to C rodentium infection was assessed by selective ablation of TRPV1-expressing sensory nociceptors. Administration of the excitotoxic TRPV1 agonist RTX [34] significantly reduced Trpv1 expression in full-thickness colonic tissues (Figure 1A) and TRPV1 + neurons (βIII tubulin+) in the NG (Figure 1B) compared with vehicle controls. Citrobacter rodentium infection of RTX-treated mice significantly increased fecal and colonic adherent bacteria beginning on day 10 p.i. compared with vehicle controls (Figure 1C and D). This increased the number of bacteria continued until day 29 p.i., a time point when no C rodentium was detected in the infected vehicle control group (Figure 1C). Delayed clearance and increased C rodentium bacterial burden in feces was also observed in TRPV1−/− compared with WT (TRPV1+/+) control mice (Figure 1E). Histological analysis of the colon 10 days p.i. revealed significantly increased crypt hyperplasia in RTX-treated compared with vehicle-treated infected mice (Figure 1F and G). We noted no significant difference in colonic motility as assessed by output of fecal pellets in uninfected WT vehicle and RTX-treated mice or WT and TRPV1 mice (Supplementary Figure S1). These results highlight that TRPV1 + neurons exert a host protective role during infection with an enteric bacterial pathogen.
Figure 1.
Ablation of nociceptive neurons increases the severity of infection and delays clearance of Citrobacter rodentium. Trpv1 message in the colon (10–12 mice/group) (A) and transient receptor potential cation channel subfamily V member 1 (TRPV1) immunoreactive neurons in the nodose ganglion (B) were assessed 20 days after administration of resiniferatoxin (RTX) or vehicle (5–6 mice/group; scale bar, 30 µm). Mice subjected to sensory nociceptive ablation were assessed for host response to C rodentium infection. The bacterial burden was assessed in the feces every 3–4 days over the course of the infection (C) and adherent to the colon at 10 days postinfection (p.i.) (15–16 animals/group, 3 independent experiments, dotted line: limit of detection) (D). TRPV1+/+ and TRPV1−/− were infected with C rodentium, and the bacterial burden in the feces were measured every 3–4 days (E). The effect of this infection on the colonic histopathology at 10 days p.i. (F and G) was determined by crypt length in ≥20 well oriented crypts per animal (7–9 mice/group, from 3 independent experiments). Scale bar, 50 µm. Data are presented as mean ± standard error of the mean: *, P < .05, **, P < .01, and ***, P < .0001 vs uninfected mice; #, P < .05 compared with vehicle C rodentium-infected mice; Student’s t test (A and B) and two-way analysis of variance (ANOVA) (C) and one-way ANOVA (D and G) with Tukey posttest. CFU, colony-forming units; DAPI, 4’,6-diamidino-2-phenylindole; LB, Luria-Bertani.
Ablation of Nociceptive Neurons Reduces Expression of Select Host Protective Genes
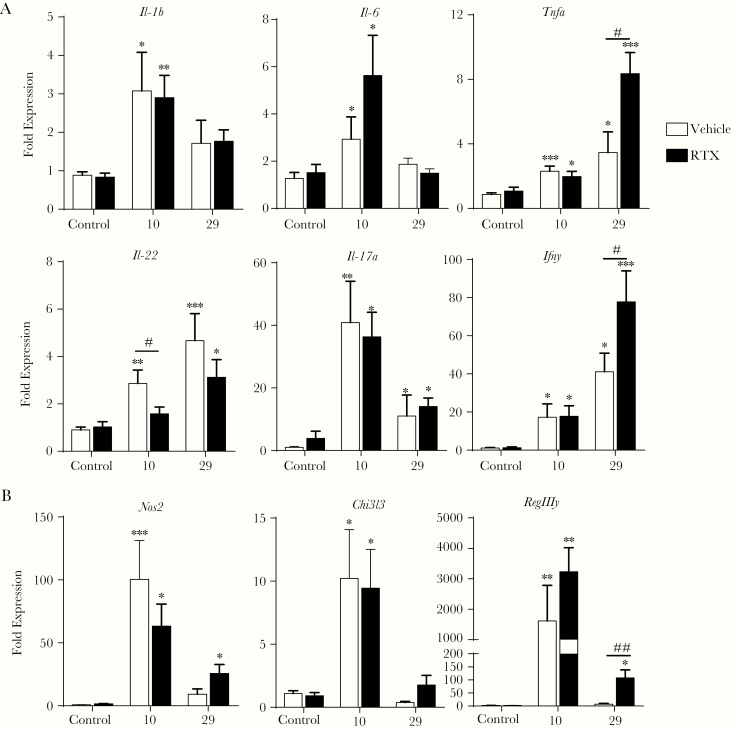
To understand how nociceptive sensory neurons regulate host defenses against C rodentium, we assessed the expression of genes involved in the innate and adaptive immune response to this enteric bacterial pathogen (Figure 2A). Infection with C rodentium significantly increased Il-1b, Il-6, and Il-17a expression on day 10 p.i., returning to baseline by day 29 p.i., as previously reported [35, 36]. Although Tnf and Ifng expression were also significantly increased by day 10 p.i., increased expression continued until 29 days p.i. and was further increased in infected RTX-treated mice compared with infected vehicle-treated controls. Both of these cytokines can be produced as part of the host response to C rodentium infection by T cells recruited to the colon [36, 37]. In striking contrast, ablation of sensory neurons before infection reduced Il-22 expression at day 10 p.i. compared with vehicle-treated infected controls. These results suggest that only select aspects of the host immune response is impinged upon by the lack of sensory neurons. In support of this contention, expression of genes characteristic of inflammatory (Nos2) and alternatively activated macrophages (Chi3l3) or the AMP RegIIIy were not significantly different in infected mice ± RTX 10 days p.i. (Figure 2B). However, expression of Nos2 and RegIIIy were significantly increased in infected RTX-treated mice after 29 days p.i. This inability of RTX-treated mice to increase Il-22 expression 10 days p.i. suggests that sensory innervation could aid the recruitment of immune cells that produce this cytokine during infection.
Figure 2.
Reduced Il-22 expression in Citrobacter rodentium-infected mice lacking nociceptive neurons. The host immune response to C rodentium infection was determined by using quantitative polymerase chain reaction from mice with intact sensory nociceptor (vehicle) and ablated nociceptor (resiniferatoxin [RTX]) at 10 and 29 days postinfection. These included the expression of prototypical proinflammatory cytokines produced by innate and adaptive immune cells (A), M1 and M2 macrophages, and antimicrobial peptide (B) with 10–14 mice/group. Data are presented as mean ± standard error of the mean: *, P < .05, **, P < .01, and ***, P < .0001 versus uninfected mice; #, P < .05 and ##, P < .001 compared with vehicle C rodentium-infected mice; one-way analysis of variance with Tukey posttest.
Delayed T-Cell Recruitment After Loss of Nociceptive Neurons
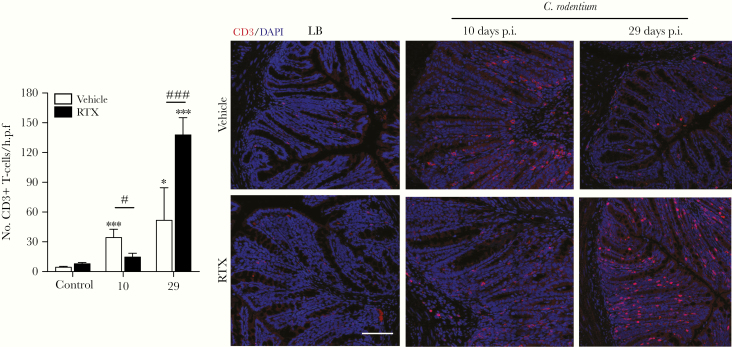
To determine whether mice with ablation of sensory nociceptors regulate T-cell recruitment during C rodentium infection, we assessed the number of these cells in the colonic mucosa. Confocal microcopy of colonic sections revealed that C rodentium infection significantly increased the number of T cells (CD3+ DAPI+) at day 10 and 29 p.i. The number of T cells was significantly reduced in RTX-treated C rodentium-infected mice compared with vehicle-treated infected controls at 10 days p.i. However, at day 29 p.i., the number of T cells was significantly increased in RTX-treated infected mice compared with infected controls (Figure 3). These data indicate that sensory nociceptive neurons aid recruitment of CD3+ T cells to the colon during C rodentium infection.
Figure 3.
Delayed T-cell recruitment during Citrobacter rodentium infection after nociceptor ablation. Colonic tissue sections were assessed for recruitment of T cells (CD3+ DAPI+) in vehicle ± C rodentium and resiniferatoxin (RTX) ± C rodentium-treated mice and quantified. Scale bar, 50 µm. Data are presented as mean ± standard error of the mean: *, P < .05 and ***, P < .001 compared with uninfected mice; #, P < .05 and ###, P < .001 compared with vehicle C rodentium-infected mice; one-way analysis of variance with Tukey posttest, with 8–12 animals per group. DAPI, 4’,6-diamidino-2-phenylindole; LB, Luria-Bertani; p.i., postinfection.
Control of T-Cell Migration to the Colonic Mucosa by Nociceptive Neurons During Citrobacter rodentium Infection
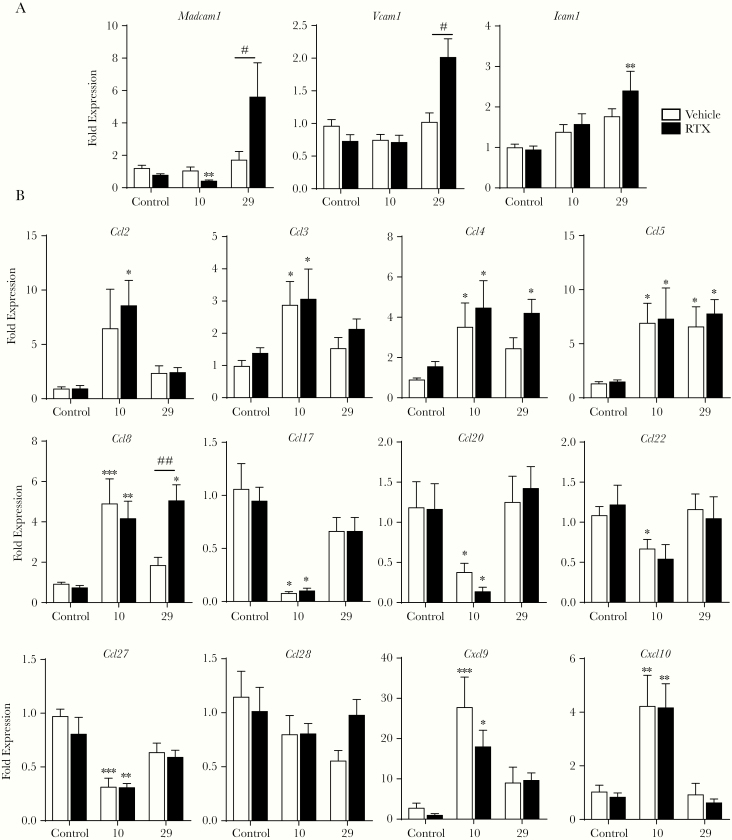
To evaluate mechanisms that could lead to reduced T-cell recruitment in the colonic mucosa during C rodentium infection in RTX-treated mice, expression of adhesion molecules and chemokines was assessed (Figure 4A). Expression of Madcam1, required for T-cell entry into the colon, was significantly reduced 10 days p.i. in RTX-treated mice infected with C rodentium versus controls. At 29 days p.i., expression of this addressin molecule was significantly increased in the RTX-treated C rodentium-infected mice compared with controls. No change in Madcam1 expression was observed in uninfected RTX-treated controls, suggesting that this was not a general effect of RTX. Expression of the Icam1 and Vcam1 were not significantly different in any treatment group 10 days p.i., with significant increases observed only at day 29 p.i. in RTX + C rodentium mice compared with controls.
Figure 4.
Loss of sensory innervation alters adhesion molecule expression in the colon during Citrobacter rodentium infection. Expression of adhesion molecules (A) and chemokines (B) involved in the homing and migration of adaptive immune cells into the colonic mucosa were evaluated by quantitative polymerase chain reaction at 10 and 29 days postinfection (p.i.). Data are presented as mean ± standard error of the mean: *, P < .05, **, P < .01, and ***, P < .001 versus uninfected mice; #, P < .05 and ##, P < .01 compared with vehicle C rodentium-infected mice; one-way analysis of variance with Tukey posttest, with 8–12 animals per group.
Because immune cell recruitment is partially controlled by chemotactic signals from the tissues, we evaluated chemokine expression in naive and infected mice with intact or ablated sensory neurons (Figure 4B). Infection with C rodentium ± RTX treatment significantly increased expression of Ccl2 (monocyte chemoattractant protein [MCP]-1), Ccl3 (macrophage-inflammatory protein [MIP]1a), Ccl4 (MIP-1β), Ccl5 (RANTES), and Ccl8 (MCP-2) 10 days p.i. Although Ccl2, Ccl3, and Ccl4 were not increased at day 29 p.i., Ccl5 remained elevated at this time point. In contrast, Ccl8 expression remained significantly increased at day 29 p.i. only in the C rodentium + RTX-treated mice. Because the cell surface receptors CCR4 and CCR6 are expressed by Th17 and Th22 cells, expression of the cognate ligands for these receptors was assessed. Expression of ligands for CCR4 (Ccl2, Ccl4, Ccl5, Ccl17, Ccl22), CCR6 (Ccl20), and CCR10 (Ccl27, Ccl28) were either not affected or significantly reduced during C rodentium infection in vehicle and RTX-treated mice compared with controls (Figure 4B). Infection with C rodentium significantly increased expression of Cxcl9 and Cxcl10, chemoattractants that are also bactericidal proteins against C rodentium [38, 39] at day 10 p.i. irrespective of RTX treatment (Figure 4B). These data suggest that reduced colonic T-cell recruitment in mice with prior sensory nociceptor ablation is due to loss of mucosal addressin and not reduced expression of chemokines that serve to recruit Th17 and Th22 T cells.
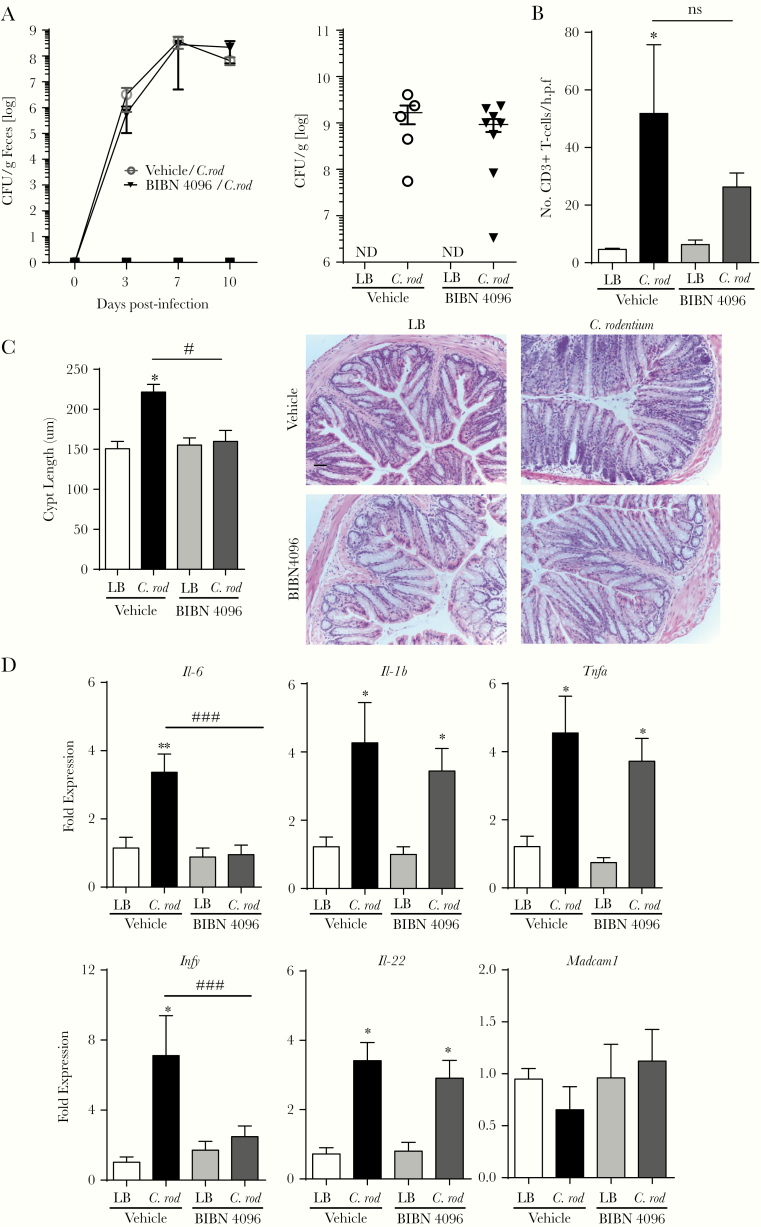
Calcitonin Gene-Related Peptide Receptor Antagonism Does Not Alter the Citrobacter rodentium Infection
Because the sensory neuropeptide CGRP exerts a significant effect on host immune functions in the lung, skin, and small intestine [13–15], the role of CGRP was assessed using a potent and selective CGRP receptor antagonist [40]. Administration of BIBN 4096 beginning on the day of infection and every other day thereafter did not affect fecal (Figure 5A, left) and colonic adherent (Figure 5A, right) C rodentium 10 days p.i. compared with vehicle-treated mice. Confocal microscopy revealed that colonic T-cell recruitment in C rodentium-infected mice + BIBN was not significantly reduced compared with infected vehicle controls (Figure 5B). Despite this lack of effect on bacterial burden and T-cell recruitment, mice in the C rodentium + BIBN 4096 treatment group had significantly reduced (1) colonic crypt hyperplasia (Figure 5C) and (2) colonic expression of Il-6 and Ifng compared with infected vehicle controls (Figure 5D). In contrast, expression of Il-1b, Tnf, and Il-22 was significantly increased 10 days p.i. infection in vehicle and BIBN 4096-treated mice. Unlike our data with RTX, Madcam1 expression in the C rodentium + BIBN 4096 treatment group was not significantly reduced compared with the C rodentium + vehicle control group. These data, taken together, indicate that although CGRP signaling controls select aspects of host immune responses, other non-CGRP-dependent host-protective effects are provided by sensory afferent neurons during enteric bacterial infection.
Figure 5.
Calcitonin gene-related peptide (CGRP) receptor blockade alters select aspects of host immune function but does not affect Citrobacter rodentium bacterial burden. The role of CGRP signaling during C rodentium infection was assessed by administration of vehicle or BIBN 4096 starting on the day of the infection with consecutive injections every other day. The effect on host response was assessed by fecal bacterial burden and adherent bacterial burden at days 10 postinfection (A). T-cell recruitment, (B) colonic crypt hyperplasia, (C) scale bar, 50 µm, and (D) colonic expression of proinflammatory cytokines and Madcam1. Data are presented as mean ± standard error of the mean: *, P < .05 and **, P < .01 versus uninfected mice; #, P < .05 and ###, P < .001 compared with vehicle C rodentium-infected mice; one-way analysis of variance with Tukey posttest, with 6–10 animals per group. CFU, colony-forming units; LB, Luria-Bertani; ns, no significant difference.
DISCUSSION
Although there is a wealth of literature describing the effect of sensory nociceptive peptides on inducing neurogenic inflammation [10, 41] and enhancement of immunopathology [42], little was known about the host responses to pathogens [43]. Although recent studies identified the involvement of sensory nociceptive neurons in the host response to infection of the skin, lung, and small intestine [13–15], the role of these neurons during C rodentium infection was unknown. Our studies highlight a previously unappreciated role for nociceptive neurons in coordinating the host response to C rodentium infection. Immune responses to this noninvasive, nontoxin-producing pathogen involves complex interactions between the bacteria, epithelial cells, and successive waves of immune cells. Early in the course of infection ILC dominate, serving as a major source of IL-22, followed by CD4+ T cells that assume a critical host-protective role at the height of infection [17]. The resulting IL-22 from these cells induces IEC production of AMP, with T-cell derived IL-17A, and interferon-γ further reinforcing inflammation by inducing cytokine production and macrophage activation [44]. Our data indicate that TRPV1 + nociceptive neurons are a critical component in the development of host immune responses against C rodentium that serve to induce recruitment of IL-22- producing T cells to the colon.
The development of T-cell responses in the intestine is a multistep process allowing naive antigen-specific T cells to become activated, home to, and enter the mucosa. During activation in the draining lymph nodes, preferential homing to the intestine is achieved by imprinting due to dendritic cells and lymph node stromal cells that produce retinoic acid to increase expression of chemokine receptors and the α 4β 7 integrin [28, 29]. During extravasation into the colon, α 4β 7-expressing T cells interact with the mucosal addressin molecule MAdCAM-1 on the luminal surface of endothelial cells [24]. Thus, the expression of chemokines, chemokine receptors, integrins, and addressin proteins are critical in mounting host-protective immune responses in the intestine. Because ablation of sensory afferent neurons reduced T-cell infiltration into the colonic mucosa at the peak of infection, we assessed whether these pathways were altered by RTX treatment. We noted no significant reductions in chemokines broadly associated with the homing immune cells or chemokines selective for Th17 or Th22 T cells. These data suggested that lack of T-cell recruitment to the colon during C rodentium infection in RTX-treated mice was not due to a deficiency in chemokine expression.
Because expression of adhesion molecules and vasodilation can occur during neurogenic inflammation, we assessed expression of ICAM1, VCAM1, and MadCAM-1. We noted no significant difference in ICAM1 or VCAM1 expression in RTX ± C rodentium-infected mice 10 days p.i. In contrast, colonic Madcam1 expression was significantly reduced 10 days p.i. followed by increased expression 29 days p.i., mirroring colonic CD3+ T-cell recruitment in C rodentium-infected mice with prior sensory nerve ablation. Increased Madcam1 expression at this late time point, despite ablation of sensory innervation, is likely due to persistence of C rodentium and consequently increased inflammatory cytokines such as tumor necrosis factor (TNF)α driving expression at this time point [45, 46]. This contention is in keeping with nociceptive neuropeptides acting synergistically with stimuli, such as TNFα [47], to enhance expression of NF-κB-regulated genes including Madcam1 in endothelial cells [46]. These data suggest that sensory nociceptive neurons and their neurotransmitters induce or act synergistically to increase the expression of select endothelial adhesion molecules during enteric bacterial infection. Although there are redundant mechanisms that allow for increased expression of Madcam1 and delayed colonic T-cell homing, this delay could significantly impinge on the development of protective immunity.
Although sensory nociceptors can release multiple neuropeptides, CGRP has been the predominant focus in host defenses against bacterial pathogens [15, 41, 43]. Because ablation of sensory nociceptors in our studies identified a host-protective function, we investigated the role of CGRP using the highly selective CGRP receptor antagonist BIBN 4096 concurrent with infection. Our results indicate that CGRP receptor blockade significantly reduced crypt hyperplasia and expression of specific cytokines. These data indicate that as a nociceptive neurotransmitter, CGRP exerts a host-protective role by inducing select aspects of host immune responses during C rodentium infection. It is likely that other nociceptive neuropeptides contribute to different aspects of host immune function. In support of this, SP can be proinflammatory, exacerbating models of colonic inflammation, and is host protective during Salmonella typhimurium infection [48]. Recent studies indicate that sensory innervation-derived CGRP can control the small intestinal microbiota and induce differentiation of microfold “M” cells, an entry point for invasion by S typhimurium. The consequences of CGRP release in the small intestine is enhanced susceptibility to S typhimurium infection [15]. These data highlight the unique roles that sensory innervation could exert depending on the site of infection in the intestinal tract and pathogen.
CONCLUSIONS
Our findings, taken together, demonstrate a unique host-protective role of sensory nociceptors during enteric bacterial infection with C rodentium infection. This innervation appears to aid in the coordination of the host immune system and the production of inflammatory cytokines. Modulation of these unique nociceptive neurons or their target cells could provide new therapeutic avenues for control of these pathogens. It is also important to consider that RTX-mediated nociceptor ablation is also being used in preclinical [49] and clinical trials (Clinical trial registration: NCT00804154) in management of pain [49, 50]. Our data suggest that increased susceptibility to certain bacterial infections could occur in these patient groups, highlighting the importance of understanding neuroimmune circuits in health and during infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementry Figure S1. Fecal pellet output is not altered by ablation of sensory nociceptors or TRPV1 deficiency. The effect of RTX or TRPV1−/− on colonic motility was assessed by monitoring fecal output over a 20-minute period. Pellets were counted (left panel) and weighed (right panel). One-way ANOVA with Tukey posttest, 6–7 animals per group.
Fecal pellet output is not altered by ablation of sensory nociceptors or TRPV1 deficiency. The effect of RTX or TRPV1-/- on colonic motility was assessed by monitoring fecal output over a 20-minute period. Pellets were counted (left panel) and weighed (right panel). One-way ANOVA with Tukey post-test, 6-7 animals per group.
Notes
Financial support. This study was funded by the National Institutes of Health (Grant AI133116 ; to C. R.) and NIGMS Pharmacology Training Program (Grant T32GM099608; to K. M.). V. T. R. is a recipient of a postdoctoral fellowship from the National Committee of Science and Technology of Chile (CONICYT, Becas Chile).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Reardon C, Murray K, Lomax AE. Neuroimmune communication in health and disease. Physiol Rev 2018; 98:2287–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pinho-Ribeiro FA, Verri WA Jr, Chiu IM. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol 2017; 38:5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feng B, Gebhart GF. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am J Physiol Gastrointest Liver Physiol 2011; 300:G170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brierley SM, Hibberd TJ, Spencer NJ. Spinal afferent innervation of the colon and rectum. Front Cell Neurosci 2018; 12:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alawi K, Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther 2010; 125:181–95. [DOI] [PubMed] [Google Scholar]
- 6. Zheng J. Molecular mechanism of TRP channels. Compr Physiol 2013; 3:221–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang L, Jones S, Brody K, Costa M, Brookes SJ. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am J Physiol Gastrointest Liver Physiol 2004; 286:G983–91. [DOI] [PubMed] [Google Scholar]
- 8. Zhao H, Sprunger LK, Simasko SM. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am J Physiol Gastrointest Liver Physiol 2010; 298:G212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mashaghi A, Marmalidou A, Tehrani M, Grace PM, Pothoulakis C, Dana R. Neuropeptide substance P and the immune response. Cell Mol Life Sci 2016; 73:4249–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suvas S. Role of Substance P neuropeptide in inflammation, wound healing, and tissue homeostasis. J Immunol 2017; 199:1543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun J, Ramnath RD, Bhatia M. Neuropeptide substance P upregulates chemokine and chemokine receptor expression in primary mouse neutrophils. Am J Physiol Cell Physiol 2007; 293:C696–704. [DOI] [PubMed] [Google Scholar]
- 12. Xanthos DN, Sandkühler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci 2014; 15. [DOI] [PubMed] [Google Scholar]
- 13. Pinho-Ribeiro FA, Baddal B, Haarsma R, et al. . Blocking neuronal signaling to immune cells treats streptococcal invasive infection. Cell 2018; 173:1083–97.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baral P, Umans BD, Li L, et al. . Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia. Nat Med 2018; 24:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lai NY, Musser MA, Pinho-Ribeiro FA, et al. . Gut-innervating nociceptor neurons regulate peyer’s patch microfold cells and SFB levels to mediate salmonella host defense. Cell 2020; 180:33–49.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borenshtein D, McBee ME, Schauer DB. Utility of the Citrobacter rodentium infection model in laboratory mice. Curr Opin Gastroenterol 2008; 24:32–7. [DOI] [PubMed] [Google Scholar]
- 17. Basu R, O’Quinn DB, Silberger DJ, et al. . Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 2012; 37:1061–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rankin LC, Girard-Madoux MJ, Seillet C, et al. . Complementarity and redundancy of IL-22-producing innate lymphoid cells. Nat Immunol 2016; 17:179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simmons CP, Clare S, Ghaem-Maghami M, et al. . Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect Immun 2003; 71:5077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tumanov Alexei V, Koroleva Ekaterina P, Guo X, et al. . Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe 2011; 10:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liang SC, Tan XY, Luxenberg DP, et al. . Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 2006; 203:2271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 2008; 29:947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol 2011; 12:383–90. [DOI] [PubMed] [Google Scholar]
- 24. Berlin C, Berg EL, Briskin MJ, et al. . Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 1993; 74:185–95. [DOI] [PubMed] [Google Scholar]
- 25. Briskin M, Winsor-Hines D, Shyjan A, et al. . Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol 1997; 151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 26. Habtezion A, Nguyen LP, Hadeiba H, Butcher EC. Leukocyte trafficking to the small intestine and colon. Gastroenterology 2016; 150:340–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity 2004; 21:527–38. [DOI] [PubMed] [Google Scholar]
- 28. Johansson-Lindbom B, Svensson M, Pabst O, et al. . Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med 2005; 202:1063–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hammerschmidt SI, Ahrendt M, Bode U, et al. . Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med 2008; 205:2483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity 2011; 34:149–62. [DOI] [PubMed] [Google Scholar]
- 31. Karai L, Brown DC, Mannes AJ, et al. . Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest 2004; 113:1344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res 2010; 38:D792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramirez VT, Godinez DR, Brust-Mascher I, et al. . T-cell derived acetylcholine aids host defenses during enteric bacterial infection with Citrobacter rodentium. PLoS Pathog 2019; 15:e1007719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Szolcsanyi J, Szallasi A, Szallasi Z, Joo F, Blumberg PM. Resiniferatoxin: an ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Ther 1990; 255:923–8. [PubMed] [Google Scholar]
- 35. Dann SM, Spehlmann ME, Hammond DC, et al. . IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J Immunol 2008; 180:6816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Symonds EL, Riedel CU, O’Mahony D, Lapthorne S, O’Mahony L, Shanahan F. Involvement of T helper type 17 and regulatory T cell activity in Citrobacter rodentium invasion and inflammatory damage. Clin Exp Immunol 2009; 157:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shiomi H, Masuda A, Nishiumi S, et al. . Gamma interferon produced by antigen-specific CD4+ T cells regulates the mucosal immune responses to Citrobacter rodentium infection. Infect Immun 2010; 78:2653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reid-Yu SA, Tuinema BR, Small CN, Xing L, Coombes BK. CXCL9 contributes to antimicrobial protection of the gut during citrobacter rodentium infection independent of chemokine-receptor signaling. PLoS Pathog 2015; 11:e1004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Margulieux KR, Fox JW, Nakamoto RK, Hughes MA. CXCL10 acts as a bifunctional antimicrobial molecule against Bacillus anthracis. MBio 2016; 7:e00334–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Glowka TR, Steinebach A, Stein K, et al. . The novel CGRP receptor antagonist BIBN4096BS alleviates a postoperative intestinal inflammation and prevents postoperative ileus. Neurogastroenterol Motil 2015; 27:1038–49. [DOI] [PubMed] [Google Scholar]
- 41. Brain SD. Sensory neuropeptides: their role in inflammation and wound healing. Immunopharmacology 1997; 37:133–52. [DOI] [PubMed] [Google Scholar]
- 42. O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol 2004; 201:167–80. [DOI] [PubMed] [Google Scholar]
- 43. Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci 2012; 15:1063–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perez-Lopez A, Behnsen J, Nuccio SP, Raffatellu M. Mucosal immunity to pathogenic intestinal bacteria. Nat Rev Immunol 2016; 16:135–48. [DOI] [PubMed] [Google Scholar]
- 45. Takeuchi M, Baichwal VR. Induction of the gene encoding mucosal vascular addressin cell adhesion molecule 1 by tumor necrosis factor alpha is mediated by NF-kappa B proteins. Proc Natl Acad Sci U S A 1995; 92:3561–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oshima T, Pavlick KP, Laroux FS, et al. . Regulation and distribution of MAdCAM-1 in endothelial cells in vitro. Am J Physiol Cell Physiol 2001; 281:C1096–105. [DOI] [PubMed] [Google Scholar]
- 47. Quinlan KL, Naik SM, Cannon G, et al. . Substance P activates coincident NF-AT- and NF-κB-dependent adhesion molecule gene expression in microvascular endothelial cells through intracellular calcium mobilization. J Immunol 1999; 163:5656–65. [PubMed] [Google Scholar]
- 48. Kincy-Cain T, Bost KL. Increased susceptibility of mice to Salmonella infection following in vivo treatment with the substance P antagonist, spantide II. J Immunol 1996; 157:255–64. [PubMed] [Google Scholar]
- 49. Iadarola MJ, Sapio MR, Raithel SJ, Mannes AJ, Brown DC. Long-term pain relief in canine osteoarthritis by a single intra-articular injection of resiniferatoxin, a potent TRPV1 agonist. Pain 2018; 159:2105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iadarola MJ, Gonnella GL. Resiniferatoxin for pain treatment: an interventional approach to personalized pain medicine. Open Pain J 2013; 6:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fecal pellet output is not altered by ablation of sensory nociceptors or TRPV1 deficiency. The effect of RTX or TRPV1-/- on colonic motility was assessed by monitoring fecal output over a 20-minute period. Pellets were counted (left panel) and weighed (right panel). One-way ANOVA with Tukey post-test, 6-7 animals per group.