Abstract
The speed and scale of the global COVID-19 pandemic has resulted in unprecedented pressures on health services worldwide, requiring new methods of service delivery during the health crisis. In the setting of severe resource constraint and high risk of infection to patients and clinicians, there is an urgent need to identify consensus statements on head and neck surgical oncology practice. We completed a modified Delphi consensus process of three rounds with 40 international experts in head and neck cancer surgical, radiation, and medical oncology, representing 35 international professional societies and national clinical trial groups. Endorsed by 39 societies and professional bodies, these consensus practice recommendations aim to decrease inconsistency of practice, reduce uncertainty in care, and provide reassurance for clinicians worldwide for head and neck surgical oncology in the context of the COVID-19 pandemic and in the setting of acute severe resource constraint and high risk of infection to patients and staff.
Introduction
WHO declared the COVID-19 outbreak to be a global pandemic on March 11, 2020.1 The speed and scale of the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in unprecedented pressures on health services worldwide.2, 3, 4 The need for hospitalisation and mechanical ventilation in intensive care for a considerable proportion of patients, in addition to staff shortages due to illness and concerns of viral transmission to health-care workers and other patients, have led to a severe curtailment of health-care capacity and resources.5, 6, 7, 8 System constraints preventing the delivery of timely and comprehensive care, the fear of viral transmission, and poor clinical outcomes of patients with head and neck cancer developing COVID-19 during the perioperative period have greatly impacted surgical practice and decision making in this cancer setting.9, 10, 11, 12, 13, 14 Many clinical services have had to substantially reduce, or even cease, their routine clinical activity.15, 16, 17 Furthermore, services have had to adapt by adopting new strategies for care delivery, with the aim of releasing capacity and reducing the risk of nosocomial infection to patients and staff due to travel and face-to-face contact in hospital.18, 19
In the setting of the COVID-19 pandemic, adherence to previously established standards of care have proven difficult.20 Patient evaluation (including use of flexible nasendoscopy and diagnostic imaging), achieving target wait times for surgery, and oncological surveillance have all been affected.21 In this climate of constrained resources, consideration should be given to prioritisation of surgical cases and innovative methods of patient evaluation and surveillance.14 Individual institutions and national professional organisations have produced guidelines and clinical protocols during the pandemic.22, 23, 24, 25, 26, 27 By necessity, such guidelines have been developed hastily, usually by individuals or small groups of clinicians, and have often not been subjected to the usual processes of peer review that were implemented before the COVID-19 pandemic. As a result, there has been some confusion among head and neck cancer surgeons as to which advice should be followed.
The Head and Neck Cancer International Group (HNCIG), a collaboration of 20 national clinical trial groups for head and neck cancer across three continents, identified an urgent need for consensus practice recommendations for head and neck surgical oncology that could be applied globally in the setting of severely constrained resources. To address this need, we rapidly developed expert consensus recommendations for the management of surgical patients with head and neck cancer during the COVID-19 pandemic using a modified online Delphi process with representation from the relevant multidisciplinary bodies worldwide.
Data collection
Participant selection
In total, 35 international and national head and neck oncology organisations were invited to participate by the steering committee, which was composed of the members of the HNCIG Surgical Committee. The invited organisations included all 20 clinical trial groups of the HNCIG, who were invited to nominate a surgical representative to be part of the consensus panel. Member groups were the Canadian Cancer Trials Group, Cancer Trials Ireland, the Danish Head and Neck Cancer Group, the Dutch Head and Neck Society, the Eastern Cooperative Oncology Group and the American College of Radiology Imaging Network, the European Organisation for Research and Treatment of Cancer, the French Head and Neck Cancer Group, Fudan University Shanghai Cancer Center, the German Interdisciplinary Working Group for Head and Neck Tumors, the Hellenic Cooperative Oncology Group, Hong Kong Nasopharyngeal Carcinoma Study Group, the Japanese Clinical Oncology Group and The Head and Neck Cancer Study Group, the National Cancer Centre Singapore, the National Cancer Research Institute UK, the North West Italian Oncology Group, NRG Oncology-Head and Neck Cancer Committee, the Spanish Head and Neck Cancer Cooperative Group, Taiwan Cooperative Oncology Group, Tata Memorial Centre, and Trans-Tasman Radiation Oncology Group.
To ensure adequate representation from all continents and geographical regions, the following international and national head and neck surgical societies were also invited to nominate surgical representatives: the European Head and Neck Society, the African Head and Neck Society, the Latin American Cooperative Oncology Group, the International Committee of the American Head and Neck Society, the International Federation of Head and Neck Oncological Societies, the International Association of Oral Oncology, the Korean Society of Head and Neck Surgery, the National Cancer Centre–Chinese Academy of Medical Sciences and Peking Union Medical College Cancer Hospital, the United Arab Emirates Otorhinolaryngological and Head and Neck Society (Middle East), the British Association of Head and Neck Oncologists, the Head and Neck Cancer Society of Turkey, and the Australian and New Zealand Head and Neck Cancer Society.
To ensure multidisciplinary representation, the following international organisations were also invited to nominate medical and radiation oncologists: the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the European Society for Radiotherapy and Oncology. These leading radiotherapy and medical oncology societies all have global membership.
All invited organisations agreed to participate and each organisation's governing executive made their respective nomination. Nominees had to be currently practising as a head and neck cancer clinician, considered to be a national or international expert, and be willing to complete all three rounds of the online Delphi process within 14 days. All participants were included in the manuscript authorship.
Consensus formation
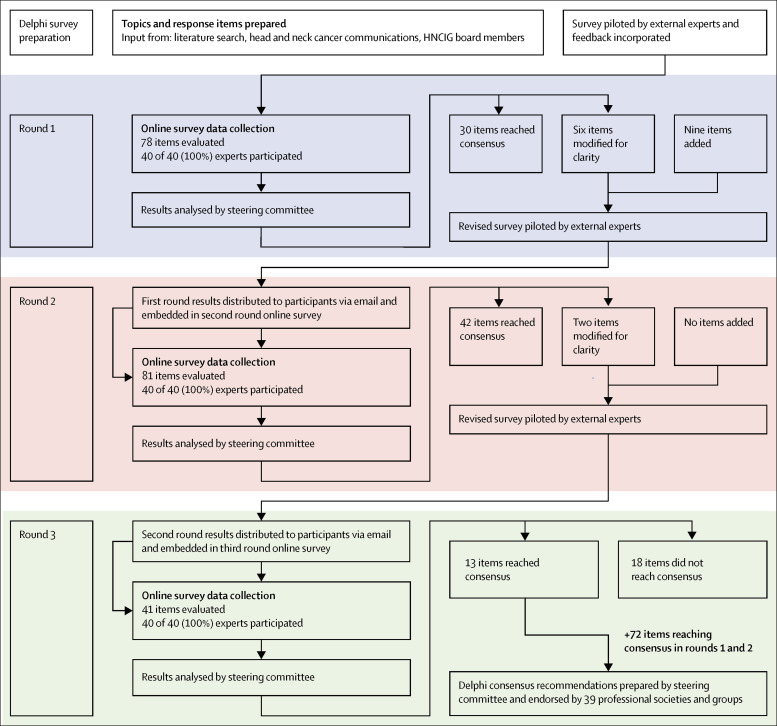
To achieve consensus, an online modified Delphi process was done over three rounds. Nominated experts were invited to complete an anonymous online questionnaire, delivered by the Qualtrics online survey platform (Qualtrics, Salt Lake City, UT, USA). The survey included three main sections: clinical protocols, treatment protocols, and prioritisation of treatment, all within the context of severe resource constraint and risk of transmission caused by the prevalence of SARS-CoV-2 in the patient population. An overview of the modified Delphi process is shown in the figure .
Figure.
HNCIG modified Delphi process
HNCIG=Head and Neck Cancer International Group.
Participants were invited by email to complete each round of the survey, which was open for a period of 24 h. A reminder email was sent at 2 h and 1 h before the deadline to prompt participants who had not yet completed their responses.
When making their recommendations, participants were instructed to consider an extremely constrained setting in terms of capacity and resources (including a severe reduction in staffing, operating room capacity, inpatient and intensive care bed capacity) compared with baseline before the COVID-19 pandemic.
After each round, data were analysed by the multidisciplinary steering group and predetermined criteria for agreement were applied. These criteria were set a priori in the protocol before the start of the project and were extrapolated from the RAND method.28 A threshold of 80% and above was used to signify strong agreement for a statement; whereas, 20% and below indicated strong disagreement. For each statement, the Delphi process was stopped either when strong agreement was reached or after completion of three rounds, whichever occurred first. Items that reached strong agreement were dropped from subsequent rounds. Additionally, after the third round, statements that did not reach the strong agreement threshold but reached a threshold of 67% and over were considered to have reached agreement.13
Results from the first and second rounds were emailed to participants to allow them to be reviewed before the next round opened. Participants were repeatedly reminded that they could change their responses in the subsequent round for questions that had not yet reached strong agreement. Results from the first and second rounds were also presented immediately above the relevant question on Qualtrics. When necessary, questions were iteratively revised between rounds before being repiloted, and a few new questions were introduced to provide more granularity to the topic or to reduce ambiguity on the phrasing of a previous question. The resulting recommendations were then circulated to the participating bodies and societies for endorsement. Furthermore, we received requests from other professional bodies of head and neck surgical oncology to endorse the recommendations.
The project was given a waiver by the Research Ethics Department at the University of Birmingham (Birmingham, UK) and by the Institutional Review Board at Stanford University (Paolo Alto, CA, USA).
Findings
Invitations were sent to 40 nominees representing 35 societies and groups. These nominees included 33 surgeons, three radiation oncologists, two medical oncologists, and two maxillofacial surgeons. The full list is provided in the appendix (p 1). All 40 experts participated in each of the three rounds of the survey, with no dropouts or substitutions between rounds. The recommendations were endorsed by 39 societies and professional bodies (panel 1 ).
Panel 1. Bodies and societies endorsing the recommendations.
-
•
Head and Neck Cancer International Group
-
•
African Head and Neck Society
-
•
American Society for Radiation Oncology
-
•
Associazione Italiana Oncologia Cervico-Cefalica
-
•
Australian and New Zealand Head and Neck Cancer Society
-
•
Australian Society of Otolaryngology
-
•
British Association of Head and Neck Oncologists
-
•
Canadian Cancer Trials Group
-
•
Cancer Trials Ireland
-
•
Danish Head and Neck Cancer Group
-
•
Dutch Head and Neck Society
-
•
European Head and Neck Society
-
•
European Organization for Research and Treatment of Cancer
-
•
European Society for Radiotherapy and Oncology
-
•
French Head and Neck Cancer Group
-
•
German Interdisciplinary Working Group for Head and Neck Tumors
-
•
Head and Neck Cancer Society, Singapore
-
•
Head and Neck Cancer Society of Turkey
-
•
Head and Neck Cancer Study Group of the Japan Clinical Oncology Group
-
•
Hellenic Cooperative Oncology Group
-
•
Korean Society of Head and Neck Surgery
-
•
Hong Kong Nasopharyngeal Carcinoma Study Group
-
•
Hong Kong Head and Neck Society
-
•
International Committee of the American Head and Neck Society
-
•
International Federation of Head and Neck Oncological Societies
-
•
Latin American Clinical Oncology Group
-
•
National Cancer Center–Chinese Academy of Medical Sciences and Peking Union Medical College Cancer Hospital
-
•
National Cancer Centre Singapore
-
•
UK National Cancer Research Institute Head and Neck Group
-
•
North West Italian Oncology Group
-
•
NRG Oncology Head and Neck Cancer Committee
-
•
Spanish Head and Neck Cancer Cooperative Group
-
•
Spanish Otorhinolaryngology and Head and Neck Surgery Society
-
•
Spanish Oral, Maxillofacial and Head and Neck Surgery Society
-
•
Taiwan Cooperative Oncology Group
-
•
Taiwan Head and Neck Society
-
•
Tata Medical Center
-
•
Trans-Tasman Radiation Oncology Group
In total, 78 questions were asked in the first round with an average completion time of 80·0 min, 81 questions in the second round with an average completion time of 33·2 min, and 41 questions in the third round with an average completion time of 19·3 min.
The rates of agreement reported reflect when the item first reached one of the agreement thresholds that were outlined in the data collection section. These rates might have been after one, two, or three rounds of questioning. Full results of each round are provided in the appendix (pp 2–5).
Clinical protocols and procedures
A summary of the consensus findings for clinical management is available in table 1 . Experts reached a strong agreement that flexible nasendoscopy is appropriate only if adequate personal protection equipment (PPE) is available for clinicians in patients with either symptoms or examination findings suggestive of a new primary head and neck cancer or recurrence (92·5%), and in patients with concern for critical airway obstruction (97·5%).
Table 1.
Consensus recommendations for clinical procedures and treatment protocols in a setting of acute severe resource constraint resulting from the COVID-19 pandemic
| Agreement level | ||
|---|---|---|
| Clinical and diagnostic procedures | ||
| Use of flexible nasendoscopy | ||
| For patients with symptoms or signs suggestive of a new primary cancer or recurrence: use flexible nasendoscopy only if adequate PPE is available and do not use flexible nasendoscopy in absence of adequate PPE | Strong agreement | |
| For patients with concern for critical airway obstruction: use flexible nasendoscopy only if adequate PPE is available and no not use flexible nasendoscopy in absence of adequate PPE | Strong agreement | |
| For asymptomatic patients with a previous history of head and neck cancer attending clinic for routine follow-up: do not use flexible nasendoscopy in absence of adequate PPE | Strong agreement | |
| For patients with no history of head and neck cancer presenting with low-risk symptoms (eg, globus pharyngeus): do not use flexible nasendoscopy | Strong agreement | |
| To confirm a diagnosis of head and neck cancer | ||
| Positive fine needle aspiration or core biopsy of a suspicious lymph node and suspicious imaging together are acceptable | Strong agreement | |
| Suspicious findings on imaging, whether CT, MRI, or PET-CT scans alone, without biopsy, are not acceptable | Strong agreement | |
| If a biopsy under local anaesthesia can be done, no panendoscopy is needed | Strong agreement | |
| If a biopsy under general anaesthesia is needed, a full panendoscopy should be done at the same time | Agreement | |
| Follow-up of patients with head and neck cancer ≥3 months after surgery | ||
| Use video or phone consultations, with face-to-face reviews only in the case of suspicious findings | Strong agreement | |
| Use a combination of routine scheduled face-to-face and video or phone consultations | Agreement | |
| Do not stop follow-up completely | Strong agreement | |
| Maintain the normal frequency of follow-up | Agreement | |
| Minimum criteria required for diagnosing a patient with COVID-19 before head and neck cancer surgery | ||
| COVID-19 status should be considered before surgery | Strong agreement | |
| Positive laboratory test alone is sufficient | Strong agreement | |
| Positive clinical history and positive laboratory test together are sufficient | Agreement | |
| Positive clinical history (including symptoms) alone is not sufficient | Agreement | |
| Positive chest imaging alone is not sufficient | Strong agreement | |
| Delay of surgery in patients with confirmed or highly suspected COVID-19, with no indication for emergency intervention | ||
| Delay operation until patient symptoms resolve and negative COVID-19 repeat laboratory testing | Strong agreement | |
| Treatment protocols | ||
| For T1–T2 N0 oral cancer | ||
| Operate within 8 weeks from diagnosis | Strong agreement | |
| Do not delay surgery for up to 12 weeks from diagnosis | Strong agreement | |
| If surgery delay of 4–8 weeks is anticipated, do not treat immediately with alternative treatments such as radiotherapy | Strong agreement | |
| If surgery delay of 4–8 weeks is anticipated, use serial monitoring with surgery or alternative treatment (eg, radiotherapy) only if tumour progresses clinically significantly | Strong agreement | |
| If surgery delay of >8 weeks is anticipated, use serial monitoring, with surgery or alternative treatment (eg, radiotherapy) only if tumour progresses clinically significantly | Agreement | |
| If surgery delay of any duration is anticipated, do not treat with palliation as primary treatment | Strong agreement | |
| For early T1 N0 laryngeal cancer | ||
| Can delay surgery for >4 weeks, if necessary | Agreement | |
| Do not delay surgery beyond 8 weeks | Strong agreement | |
| Treat immediately with radiotherapy as an alternative to surgery | Agreement | |
| If surgery delay of 4–8 weeks is anticipated, recommend radiotherapy immediately instead of surgery | Agreement | |
| If surgery delay of >8 weeks is anticipated, recommend radiotherapy immediately instead of surgery | Strong agreement | |
| Do not use serial monitoring with treatment only if tumour progresses | Agreement | |
| Do not treat with palliation as primary treatment | Strong agreement | |
| For advanced head and neck cancer | ||
| Do not delay surgery; operate within 4 weeks of diagnosis | Strong agreement | |
| Do not use serial monitoring or give palliation as only treatment | Strong agreement | |
| Give alternative treatment (eg, radiotherapy or chemoradiation) immediately if surgery cannot occur within 4 weeks | Strong agreement | |
| For differentiated thyroid cancer (T1–T3 or N0–N1b) with no adverse features | ||
| Can delay surgery for up to 12 weeks from diagnosis, if necessary | Strong agreement | |
| Do not delay surgery for up to 18 weeks from diagnosis | Agreement | |
| If surgery is not possible within 12 weeks, use serial monitoring and only consider surgery if the tumour progresses clinically significantly | Strong agreement | |
| If surgery is not possible within 12 weeks, do not treat with radioactive iodine or radiotherapy or palliative treatment as the primary treatment option | Strong agreement | |
| Surgery delay | ||
| Use serial monitoring to assess tumour progression while waiting | Strong agreement | |
| Promptly re-evaluate treatment options if any evidence of tumour progression | Strong agreement | |
| Actions to optimise resources and reduce risk to patients and staff | ||
| Only experienced surgeons should operate on patients | Strong agreement | |
| Avoid a tracheostomy in an oropharyngeal cancer undergoing transoral surgery | Strong agreement | |
| Do not avoid primary free flap reconstruction in favour of delayed reconstruction at a later date | Strong agreement | |
| Avoid primary free flap reconstruction and instead do local or pedicled flap, if appropriate | Agreement | |
| Do not avoid neck dissection or sentinel node biopsy in a radiologically N0 neck cancer at risk of occult metastasis in a T1–T2 or T3–T4 oral or oropharyngeal cancer | Strong agreement | |
| Do not avoid salvage surgery | Strong agreement | |
| Do not avoid a tracheostomy in an advanced T2–T3 oral cancer requiring free flap | Agreement | |
| Palliative care as primary treatment in severly constrained settings | ||
| Offer primary palliation to patients with poor functional status (eg, spends >50% of the day in bed or Eastern Cooperative Oncology Group performance status 3) who have advanced disease | Strong agreement | |
| Offer primary palliation to patients with advanced biological age (eg, >85 years) who have advanced stage disease | Strong agreement | |
PPE=personal protective equipment. Strong agreement indicates a threshold of 80% and above. Agreement indicates a threshold of 67% and above after the third round for statements not considered to have reached a strong agreement.
Experts also reached strong agreement (80·0%) that flexible nasendoscopy is not appropriate in patients with no history of head and neck cancer who present with low-risk symptoms (eg, globus pharyngeus). In asymptomatic patients with a previous history of head and neck cancer attending clinic for routine follow-up, there was strong agreement (97·5%) that flexible nasendoscopy is not appropriate without adequate PPE. However, experts could not agree on whether flexible nasendoscopy in these patients is appropriate if PPE is available. A majority of respondents (60·0%) believed that it was not appropriate to use flexible nasendoscopy at all in this cohort.
When confirming a diagnosis of head and neck cancer, there was strong agreement (92·5%) that a positive cytohistological result from fine needle aspiration or core biopsy of a suspicious node and suspicious imaging together are acceptable confirmations of a cancer diagnosis, even in the absence of a biopsy of the primary tumour site. There was also strong agreement that suspicious findings on CT or MRI (80·0%), or PET-CT (82·5%) scans alone, are not acceptable to confirm a cancer diagnosis.
There was also strong agreement (85·0%) that if a tumour can be biopsied under local anaesthesia, then a full panendoscopy (laryngoscopy, hypopharyngoscopy, and upper oesophagoscopy) under general anaesthesia is not required. However, if general anaesthesia is needed to biopsy the tumour, there was agreement (67·5%) that a full panendoscopy should be done at the same time.
In routine patients with head and neck cancer 3 months or more after surgery, there was strong agreement (80·0%) on monitoring follow-up through video or phone consultations, with face-to-face review only in the case of suspicious findings. There was also agreement (70·0%) that it is appropriate to combine routine face-to-face and video or phone consultations. However, there was no agreement (47·5%) that video or phone consultations alone is an appropriate method of follow-up for these patients. There was strong agreement (100·0%) that it is not appropriate to stop follow-up altogether. There was also agreement (67·5%) that the normal frequency of follow-up should be maintained.
There was strong agreement (100·0%) that the COVID-19 status of a patient should be considered before surgery. To make a positive diagnosis of COVID-19, there was strong agreement (80·0%) that a positive laboratory test alone would be sufficient as the minimum criterion. There was also agreement (72·5%) that a positive clinical history and a positive laboratory test together are acceptable minimum criteria. Furthermore, experts agreed (70·0%) that it is not sufficient to use a positive clinical history (including symptoms) alone to make a COVID-19 diagnosis, and strongly agreed (100·0%) that positive chest imaging alone is also not sufficient for diagnosis. There was no agreement regarding use of positive clinical history and positive imaging together (57·5%) or use of clinical history, positive laboratory test, and positive imaging altogether (52·5%) as the minimum criteria for a diagnosis of COVID-19.
When operating on a patient with confirmed or highly suspected COVID-19 and who does not have indications for emergency intervention (eg, no impending airway obstruction), there was strong agreement (95·0%) that the operation should be postponed until both the patient's symptoms resolve and a negative COVID-19 result is obtained on repeat laboratory testing.
Treatment protocols
Early head and neck cancer
In the case of an early T1–T2 N0 oral cancer, there was strong consensus (95·0%) that it is not acceptable to delay surgery for more than 8 weeks from diagnosis. Among this group of respondents, 47·5% would not accept delaying surgery for more than 4 weeks from diagnosis; whereas, 55·0% would accept delaying surgery for up to 8 weeks. There was also strong agreement (100·0%) against palliation as primary treatment in the setting of anticipated delay to surgery. If delay to surgery is anticipated to be between 4 weeks and 8 weeks, there was strong agreement that immediate treatment with alternative therapies to surgery (eg, radiotherapy) was not acceptable (82·5%), and that serial monitoring is acceptable (87·5%), with consideration of surgery or alternative therapies if the tumour progresses clinically significantly. If surgery is not anticipated to occur within 8 weeks, there was preferential agreement (67·5%) to use serial monitoring, with no agreement (45·0%) on treating with primary radiotherapy in such a case.
In the case of an early T1 N0 laryngeal cancer, there was agreement (72·5%) that surgery could be delayed for more than 4 weeks, but only 47·5% of respondents agreed that surgery could be delayed for up to 8 weeks. There was strong agreement (92·5%) not to delay surgery beyond 8 weeks. There was agreement (70·0%) that it is acceptable to treat immediately with radiotherapy as an alternative to surgery. If a delay to surgery of 4–8 weeks is anticipated, there was agreement (67·5%) that radiotherapy should be recommended immediately instead of surgery; whereas, if a delay of more than 8 weeks is anticipated, there was strong agreement (92·5%) that radiotherapy should be recommended immediately over surgery. There was agreement (75·0%) that serial monitoring should not be used and strong agreement (100%) that palliative treatment as the only treatment was not appropriate in this setting.
Advanced head and neck cancer
In the case of an advanced head and neck cancer that would require extended operative time and extended hospital stay, intensive care, or both (eg, T4 N1 laryngeal cancer, N2b oral cancer, or a patient requiring bone resection such as maxillectomy), there was strong agreement (87·5%) that it is not acceptable to delay surgery beyond 4 weeks of diagnosis, as per previous standards of care. If surgery could not occur within this timeframe, there was strong agreement (90%) that alternative treatment such as radiotherapy or chemoradiotherapy should be given immediately. There was no consensus regarding the provision of induction (metronomic) chemotherapy until surgery is possible, with only 50·0% of respondents supporting this form of treatment if surgery is not anticipated within an acceptable timeframe. There was strong agreement (86·2%) against use of serial monitoring or palliation as the only treatment in these situations.
Differentiated thyroid cancer
In the case of a differentiated thyroid cancer (eg, T1–T3 or N0–N1b) with no adverse features (no extension into strap muscles, trachea or oesophageal musculature, no critical airway compression, and no imminent risk to or involvement of the recurrent laryngeal nerve), there was strong agreement (82·5%) that it was acceptable to delay surgery for up to 12 weeks from diagnosis. There was agreement that delaying surgery for up to 18 weeks (70·0%) or more (77·5%) is not appropriate. There was also agreement (77·5%) against delaying surgery indefinitely, with serial monitoring until progression. However, if surgery was not anticipated to occur within the acceptable timeframe, then there was strong consensus (96·8%) to use serial monitoring and only consider surgery if the tumour progresses. There was also strong consensus against treating with radioactive iodine or radiotherapy (96·8%), or considering palliative treatment (100·0%) as the primary treatment option.
In the case of a T1–T2 differentiated thyroid cancer of less than 4 cm, there was strong agreement (82·5%) that nodules directly abutting the airway but not invading it should be considered as an indication to operate within 4 weeks. There was strong agreement that gross extrathyroidal extension invading strap muscles only (85·0%), or regional lymph node metastasis (92·5%) were not indications for expediting surgery within 4 weeks. There was no agreement (60·0% in support) on a posterior nodule in the tracheoesophageal groove.
Monitoring during delay to surgery
When surgery is delayed because of system constraints, there was strong agreement (92·5%) to use serial monitoring to assess tumour progression while waiting for definitive treatment, and any evidence of tumour progression should prompt re-evaluation of treatment options or reprioritisation (100·0%).
Actions to optimise resources and reduce risk to patients and staff
Experts were asked about the acceptability of avoiding particular surgical procedures during a time of system constraints to optimise available resources (eg, to release bed resources, to decrease PPE use, to decrease operative time use) and to reduce the risk to patients and staff of nosocomial transmission of SARS-CoV-2.
There was strong agreement that only experienced senior surgeons should operate on patients (80·0%) and that a tracheostomy should be avoided in a patient with oropharyngeal cancer undergoing transoral surgery (87·5%). There was agreement to accept less monitoring after surgery (eg, no intensive care bed or step-down unit) than would usually be used for such a case (65·0%), and to avoid primary free flap reconstruction and instead use local or pedicled flap reconstruction (72·5%).
There was strong agreement that the following would not be appropriate: avoiding primary free flap reconstruction and instead operating delayed reconstruction at a later date (80·0%); avoiding salvage surgery (87·5%); and avoiding neck dissection or sentinel node biopsy in a radiologically N0 neck at risk of occult metastasis in a T1–T2 (85·0%) and T3–T4 (92·5%) oral or oropharyngeal cancer. There was agreement to not avoid a tracheostomy in an advanced T2–T3 oral cancer requiring free flap (67·5%).
There was no agreement on doing a sentinel node biopsy instead of elective neck dissection for T1–T2 oral cancer or melanoma (45·0%) or avoiding a neck dissection or sentinel node biopsy in a radiologically N0 neck in a case of cutaneous melanoma (35·0%).
Palliative care as primary treatment
There was no consensus on whether indications for palliative care as the primary treatment should be changed in severely constrained settings (47·5%). There was strong consensus that patients with poor functional status (eg, patients who spend >50% of the day in bed or have a Eastern Cooperative Oncology Group performance status of 3 [82·5%]) and individuals with advanced biological age (>85 years) who have advanced-stage disease (92·5%) should also be offered palliative care. There was no consensus (60·0%) with respect to offering palliative care for patients who have a low cure rate (<20% 5-year survival).
Prioritisation in the context of severely constrained resources
Experts were asked to rank a group of representative surgical cases in order of priority for time to surgery. Ranking of the cases was the same in the first and second rounds, with the top five choices receiving higher mean priority ranking in the second round than in the first round (table 2 ).
Table 2.
Prioritisation of surgery by ranking for patients with head and neck cancer in a setting of acute severe resource constraint
| Average aggregated scores (Round 1) | Average aggregated scores (Round 2) | Head and neck surgical scenarios | |
|---|---|---|---|
| 1 | 10·5 | 11·7 | T3 N2 oral cancer |
| 2 | 10·0 | 10·9 | T4 N1 laryngeal cancer |
| 3 | 8·8 | 9·8 | T4 N0 maxillary cancer |
| 4 | 8·0 | 8·7 | T4a N1 papillary thyroid cancer with tracheal invasion |
| 5 | 7·9 | 8·0 | T3 N1 carcinoma ex-pleomorphic parotid cancer |
| 6 | 6·9 | 6·9 | T1 or T2 N0 oral cancer |
| 7 | 6·7 | 6·1 | T2 N1 oropharyngeal cancer p16-negative |
| 8 | 4·6 | 4·8 | T2 N1 oropharyngeal cancer p16-positive |
| 9 | 4·2 | 3·8 | T0 N1 unknown primary |
| 10 | 4·1 | 3·5 | T2 N0 adenoid cystic oral cavity |
| 11 | 3·4 | 2·4 | T1 N0 laryngeal cancer |
| 12 | 3·1 | 1·4 | T2 N0 papillary thyroid cancer with a posterior nodule |
Head and neck surgical scenarios are ranked in order of priority, from highest to lowest. Rankings did not change between the first round and second round, so the question was not asked again in the third round.
Factors that participants considered to be the most important when prioritising patients in the setting of constrained resources can be found in panel 2 . The order of selections did not change in the second round.
Panel 2. Top five factors for the prioritisation of surgery in patients with head and neck cancer in a setting of acute severe resource constraint.
-
•
Chance of tumour progression with delay (risk to patient)
-
•
COVID-19 status of patient (risk to patient, other patients, and staff)
-
•
Prognosis (risk to patient)
-
•
Availability of infrastructure to operate on patients with COVID-19, including personal protective equipment and trained staff (risk to patients and staff)
-
•
Effectiveness and availability of alternative treatments (risk to patient)
Factors are listed in order of considered importance, from most commonly selected to least commonly selected.
Discussion
These recommendations, the result of a global consensus process, aim to provide urgent guidance to frontline head and neck cancer surgeons who are overworked and stressed by the COVID-19 pandemic. Endorsed by 39 national and international organisations spanning the globe, these recommendations should be interpreted and implemented in the context of national frameworks and local circumstances, which vary from region to region and from hospital to hospital, and can change rapidly. Additionally, these guidelines should be implemented with multidisciplinary discussion and consider individual patients' informed wishes. We have purposefully incorporated flexibility in the recommendations to enable its adaptation to the different stages of the pandemic, including the recovery phase, during which capacity might be constrained to varying degrees for many months to come. Importantly, these are recommendations for times of acute and severe resource limitation, such as the COVID-19 pandemic and natural disasters, and should not be continued when circumstances return to a typical state.
In settings with severe resource limitations, reduced surgical and perioperative care capacities often lead to a delay in the time to surgery. Therefore, new methods of service delivery are needed to address these challenges, which has been acknowledged by the experts in their recommendations for acceptable delay to surgery. For early head and neck cancer and low-risk thyroid cancer, when surgery is likely to be considerably delayed, serial monitoring might be a viable alternative with intervention only on progression. Consideration of non-surgical treatments is another alternative, but this option depends on the type of cancer and is much more acceptable for early cancer of the larynx than for other head and neck subsites. With serial monitoring, it is important to ensure that it is sufficiently frequent and in a format that allows early identification of progression. If these conditions are not possible because of resource limitation, then alternative treatment options should be explored instead.
The situation is different when considering advanced head and neck cancer. Delay or serial monitoring was considered far less acceptable than in the case of early head and neck cancer. In this setting, early consideration of alternative treatments, such as radiotherapy or chemoradiotherapy, should be considered when optimal primary surgery cannot be delivered in acceptable timeframes.
Experts strongly agreed on the need to protect staff and patients from SARS-CoV-2 exposure. The statements reflect this agreement in their recommendations to use flexible nasendoscopy only in patients who are deemed to be at high risk and, even then, only when adequate PPE is available. Experts also supported new modalities of follow-up, favouring remote (eg, telehealth) consultations where possible, with face-to-face follow-up reserved for patients with concerning symptoms. Cessation of consultations altogether was not considered acceptable.
The COVID-19 pandemic has severely strained typical doctor–patient relationships.29 Clinicians are used to the act of prioritisation as resources are not infinite. However, the extent and scale to which this prioritisation has been necessary during the pandemic is unprecedented for most practitioners. The resultant considerable uncertainty and ethical concerns about the implications for patients in terms of stage migration, or worse, pose the risk of so-called moral injury to clinicians.30 These recommendations might help to reduce uncertainty in care, decrease inconsistencies, and provide reassurance as to what is acceptable to most experts in the field, thus reducing the risks of stress and moral injury.
These recommendations have limitations. They address what we believe are the most important issues for head and neck surgeons in a setting of severely constrained resources, but they cannot cover every eventuality, especially as the nature of the pandemic changes from acute to endemic. Therefore, clinical networks are needed (locally, nationally, and internationally) to help to provide ongoing support to frontline clinicians and to address difficult issues as they arise. The HNCIG, with its global reach and network of partners established during this consensus process, is considering ways of addressing this need moving forward.
The original Delphi process allowed participants to change their past responses after being presented with the results of the previous round.28 In view of the urgency and to simplify and speed up the process, we presented participants with the results of the previous round, then invited them to change their responses in the next round, because there were only 2 days between each round. The proportions of agreement that are reported within the body of the recommendations are those that were reached at the point of first consensus. An agreement of 85% at the first round might have improved to 95% in the second or third round, but reaching the highest degree of consensus is not the purpose of this process. Therefore, the rates of strong agreement that are reported should not be directly compared with each other, as they might have been reached at different rounds.
Agreement is always a reflection of the participants and their backgrounds. In this process, most of the participants were surgeons as these were recommendations for surgical practice and this should be taken into account when considering this statement. Recommendations for head and neck cancer radiotherapy during the COVID-19 pandemic have recently been published by the American Society for Therapeutic Radiology and Oncology and the European Society for Radiotherapy and Oncology.13 These recommendations also used an online modified Delphi process, which we used when designing our method. Taken together, these two statements will hopefully provide multidisciplinary services for head and neck oncology with comprehensive recommendations for practice during the COVID-19 pandemic.
Conclusion
Sadly, this pandemic is not likely to be the last disaster faced by our global community. Although these recommendations for head and neck surgical practice have been developed during the COVID-19 pandemic, we believe that the methods used and the context of the questions posed means that they could be used in other settings of acute and severely constrained resources, as well as in settings with a high risk of injury to patients and staff. These environments could include epidemics and other natural disasters, such as large-scale flooding or earthquakes. Furthermore, we believe that the methods used to develop these recommendations, the speed with which they were developed, and the scale of cooperation and collaboration between the international and national bodies involved is a strong example of how global collaboration can help to address urgent challenges facing clinicians in times of acute stress and disaster. We plan to publish the detailed methods and provide open-access templates on the HNCIG website, so that they can be easily applied should the need arise again in the future.
Search strategy and selection criteria
To identify areas of uncertainty in clinical practice caused by acute resource constraint and the COVID-19 pandemic, we did a literature search of PubMED, Embase, Medline, and Google that included grey literature, individual association correspondence, and guidelines on COVID-19 and head and neck cancer. We used the main search terms ‘head neck cancer’ and ‘COVID-19’ and searched for articles published between Nov 1, 2019, to April 1, 2020. We limited our search to papers published in English and prioritised peer-reviewed papers, large series papers, and guidelines published by national or international bodies. Discussions with HNCIG board members highlighted areas of uncertainty identified in the literature, along with challenges to clinical practice. All issues were then collated into initial domains and questions formulated by the multidisciplinary steering group. All questions were piloted by three independent experts (SVP, VP, CS) for readability, as well as face and content validity,. Survey questions and the results of all three rounds are provided in the appendix (pp 2–5).
Acknowledgments
Acknowledgments
The Policy Review was funded by the National Institute of Health Research, UK.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
HM and FCH conceived the study concept and initiated the study design. HM, JCH, JAS, AKA-F, MCT, SP, JRDA, SC, and FCH were involved in study development, data analysis, and data interpretation of this Policy Review. All authors participated in data collection, manuscript preparation, and approved the final manuscript. HM is a senior investigator for the National Institute for Health Research (NIHR). The views expressed in this Policy Review are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Declaration of interests
HM is the director and a shareholder of Warwickshire Head and Neck Clinic; chair of the Head and Neck Cancer International Group (HNCIG); and past president of the British Association of Head and Neck Oncologists. HM also reports personal fees and grants from AstraZeneca and Sanofi Pasteur; grants from GlaxoSmithKline Biologicals; non-financial support from Merck; and personal fees, grants, and non-financial support from Merck Sharp and Dohme, all outside of the Policy Review. JYKC reports personal fees from Intuitive Surgical and non-financial support from Aptorum Group, outside of the Policy Review. SL reports personal fees from Cardinal Health, outside of the Policy Review. CRL reports personal fees from Merck and Rakuten Medical, outside of the Policy Review. LL reports personal fees from Bayer, Swedish Orphan Biovitrum, Ipsen, GlaxoSmithKline, Doxa Pharma, Incyte Biosciences Italy, Amgen, and Nanobiotics; grants from Celgene International, Exelixis, Hoffmann-La Roche, IRX Therapeutics, Medpace, and Pfizer; and grants and personal fees from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Debiopharm International, Eisai, Merck Serono, Merck Sharp and Dohme, Novartis, and Roche, outside of the Policy Review. SSY reports grants from Genentech, Bristol-Myers Squibb, Merck, and BioMimetix; and personal fees from Springer, and UpToDate, outside of the Policy Review. SP is secretary of HNCIG and reports personal fees from MerckSerono, UpToDate, Merck Sharpe and Dohme, and Regeneron, outside of the Policy Review. CS serves on the trial steering committee for Pfizer and is a consultant for Merck and Merck Sharp and Dohme, outside of the Policy Review. FCH is a scientific advisor for Surgical Vision Technologies, outside of the Policy Review. All other authors declare no competing interests.
Supplementary Material
References
- 1.WHO WHO Director-General's opening remarks at the media briefing on COVID-19–11 March 2020. March 11, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020
- 2.Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395:931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heymann DL, Shindo N. COVID-19: what is next for public health? Lancet. 2020;395:542–545. doi: 10.1016/S0140-6736(20)30374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao Y, Torok ME. Taking the right measures to control COVID-19. Lancet Infect Dis. 2020;20:523–524. doi: 10.1016/S1473-3099(20)30152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams JG, Walls RM. Supporting the health care workforce during the COVID-19 global epidemic. JAMA. 2020 doi: 10.1001/jama.2020.3972. published online March 12. [DOI] [PubMed] [Google Scholar]
- 7.Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz J, King C-C, Yen M-Y. Protecting healthcare workers during the coronavirus disease 2019 (COVID-19) outbreak: lessons from Taiwan's severe acute respiratory syndrome response. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa255. published online March 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100331. published online April 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day AT, Sher DJ, Lee RC, et al. Head and neck oncology during the COVID-19 pandemic: reconsidering traditional treatment paradigms in light of new surgical and other multilevel risks. Oral Oncol. 2020;105 doi: 10.1016/j.oraloncology.2020.104684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Givi B, Schiff BA, Chinn SB, et al. Safety recommendations for evaluation and surgery of the head and neck during the COVID-19 pandemic. JAMA Otolaryngol Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.0780. published online March 31. [DOI] [PubMed] [Google Scholar]
- 12.Kowalski LP, Sanabria A, Ridge JA, et al. COVID-19 pandemic: effects and evidence-based recommendations for otolaryngology and head and neck surgery practice. Head Neck. 2020 doi: 10.1002/hed.26164. published online April 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomson DJ, Palma D, Guckenberger M, et al. Practice recommendations for risk-adapted head and neck cancer radiotherapy during the COVID-19 pandemic: an ASTRO-ESTRO consensus statement. Int J Radiat Oncol Biol Phys. 2020 doi: 10.1016/j.ijrobp.2020.04.016. published online April 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topf MC, Shenson JA, Holsinger FC, et al. A framework for prioritizing head and neck surgery during the COVID-19 pandemic. Head Neck. 2020 doi: 10.1002/hed.26184. published online April 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Felice F, Polimeni A, Valentini V. The impact of Coronavirus (COVID-19) on head and neck cancer patients' care. Radiother Oncol. 2020;147:84–85. doi: 10.1016/j.radonc.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Muharraqi MA. Testing recommendation for COVID-19 (SARS-CoV-2) in patients planned for surgery-continuing the service and ‘suppressing’ the pandemic. Br J Oral Maxillofac Surg. 2020 doi: 10.1016/j.bjoms.2020.04.014. published online April 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tysome JR, Bhutta MF. COVID-19: protecting our ENT workforce. Clin Otolaryngol. 2020;45:311–312. doi: 10.1111/coa.13542. [DOI] [PubMed] [Google Scholar]
- 18.Paleri V, Hardman J, Tikka T, Bradley P, Pracy P, Kerawala C. Rapid implementation of an evidence-based remote triaging system for assessment of suspected referrals and patients with head and neck cancer on follow-up after treatment during the COVID-19 pandemic: model for international collaboration. Head Neck. 2020 doi: 10.1002/hed.26219. published online May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382:1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 20.Wu V, Noel CW, Forner D, et al. Considerations for head and neck oncology practices during the coronavirus disease 2019 (COVID-19) pandemic: the Wuhan and Toronto experience. Head Neck. 2020 doi: 10.1002/hed.26205. published online April 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The American Cancer Society Cancer Action Network Survey: COVID-19 affecting patients' access to cancer care. April 16, 2020. https://www.fightcancer.org/releases/survey-covid-19-affecting-patients'-access-cancer-care-7
- 22.Ku PK, Holsinger C, Chan JY, et al. Management of dysphagia in the patient with head and neck cancer during COVID-19 pandemic: practical strategy. Head Neck. 2020 doi: 10.1002/hed.26224. published online April 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JS, El-Sayed IH, Young VN, Pletcher SD. Development of clinical care guidelines for faculty and residents in the era of COVID-19. Head Neck. 2020 doi: 10.1002/hed.26225. published online April 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MD Anderson Head and Neck Surgery Treatment Guidelines Consortium Head and neck surgical oncology in the time of a pandemic: subsite-specific triage guidelines during the COVID-19 pandemic. Head Neck. 2020 doi: 10.1002/hed.26206. published online April 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crosby DL, Sharma A. Evidence-based guidelines for management of head and neck mucosal malignancies during the COVID-19 pandemic. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820923623. published online April 28. [DOI] [PubMed] [Google Scholar]
- 26.Kiong KL, Guo T, Yao CM, et al. Changing practice patterns in head and neck oncologic surgery in the early COVID-19 era. Head Neck. 2020 doi: 10.1002/hed.26202. published online April 27. [DOI] [PubMed] [Google Scholar]
- 27.Jozaghi Y, Zafereo ME, Perrier ND, et al. Endocrine surgery in the Coronavirus Disease 2019 pandemic. Head Neck. 2020 doi: 10.1002/hed.26169. published online April 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalkey NC. RAND Corporation; Santa Monica, CA: 1969. The Delphi method: an experimental study of group opinion. [Google Scholar]
- 29.Hogikyan ND, Shuman AG. Otolaryngologists and the doctor-patient relationship during a pandemic. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820922990. published online April 28. [DOI] [PubMed] [Google Scholar]
- 30.Williamson V, Murphy D, Greenberg N. COVID-19 and experiences of moral injury in front-line key workers. Occup Med (Lond) 2020 doi: 10.1093/occmed/kqaa052. published online April 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.