Abstract
BACKGROUND
Lateral pelvic lymph node (LLN) metastasis (LLNM) occur in up to 28% of patients with low rectal tumours. While prophylactic lateral pelvic lymph node dissection (LLND) has been abandoned by most western institutions in the era of neoadjuvant chemoradiation therapy (CRT), the role of selective LLND in patients with enlarged LLN on pre-CRT imaging remains unclear. Some studies have shown improved survival and recurrence outcomes when LLNs show “response” to CRT. However, no management algorithm exists to differentiate treatment for “responders” vs “non-responders”.
AIM
To determine if selective LLND in patients with enlarged LLNs results in improved survival and recurrence outcomes.
METHODS
A systemic search of PubMed and Embase databases for studies reporting on patients with synchronous radiologically suspicious LLNM (s-LLNM) in rectal cancer receiving preoperative-CRT was performed.
RESULTS
Fifteen retrospective, single-centre studies were included. 793 patients with s-LLNM were evaluated: 456 underwent TME while 337 underwent TME with LLND post-CRT. In the TME group, local recurrence (LR) rates range from 12.5% to 36%. Five-year disease free survival (DFS) was 42% to 75%. In the TME with LLND group, LR rates were 0% to 6%. Five years DFS was 41.2% to 100%. Radiological response was seen in 58%. Pathologically positive LLN was found in up to 94% of non-responders vs 0% to 20% in responders. Young age, low tumour location and radiological non-response were associated with final positive LLNM and lowered DFS.
CONCLUSION
LLND is associated with local control in patients with s-LLNM. It can be performed in radiological non-responders given a large majority represent true LLNM. Its role in radiological responders should be considered in selected high risk patients.
Keywords: Lateral pelvic lymph node, Colorectal cancer, Lateral pelvic lymph node dissection
Core tip: The role of lateral pelvic lymph node (LLN) dissection in rectal cancer patients with synchronous radiologically suspicious LLN is unclear in the era of neoadjuvant chemoradiation. Our systemic review aims to define the role of LLN dissection for patients with synchronous LLNs.
INTRODUCTION
Lateral pelvic lymph node (LLN) involvement in advanced rectal cancer located below the peritoneal reflection is common, with incidence ranging from 15% to 28%[1-4]. As such, the Japanese Society for Cancer of the Colon and Rectum (JSCRR) proposes routine LLN dissection (LLND) in low T3 and 4 rectal tumours, citing the potential benefits of improved local control and survival[2]. On the contrary, western data suggest that additional LLND result in increased morbidity without conferring significant oncological benefits[5,6]. This East-West divergence may be partially attributed to the greater utilization of preoperative radiation therapy in the west. Given the reduced rates of local recurrence (LR) as reported by the Swedish and German group[7-9], neoadjuvant chemoradiation therapy (CRT) has since been adopted as mainstay in the management of locally advanced rectal tumours.
While the role of prophylactic LLND has been diminished with the advent of CRT, its role in patients with radiologically suspicious synchronous LLN metastasis (s-LLNM) has not been established. Though neoadjuvant CRT can potentially eradicate metastatic foci in the LLNs, long term recurrence outcomes remain unclear. To date, several studies have reported acceptable outcomes in patients who received only total meso-rectal excision (TME) with CRT in the presence of s-LLNM but this has not been directly compared with patients who underwent LLND[10-14]. Furthermore, the management of radiological “responders” vs “non-responders” to CRT has not clearly defined.
Given the lack of randomized trials, we aim to perform a systemic review of the current literature to evaluate the evidence for and against LLND for s-LLNM in rectal cancer post neoadjuvant CRT. We also hope to define a management strategy for “responders” vs “non-responders” to CRT to better select for patients who will benefit most from LLND.
MATERIALS AND METHODS
A literature search of PubMed, Ovid MEDLINE, and EMBASE databases was conducted for studies reporting on the management of LLNM in rectal cancer, published in English up to December 2018. Studies were included based on the predetermined selection criteria and additional relevant studies were identified from references cited in selected articles. This study was conducted in accordance to the PRISMA guidelines (Figure 1)[16].
Figure 1.
Flow diagram on selection of eligible studies.
Criteria for inclusion of study
Articles were included if they were: (1) Original articles published in English in peer-reviewed journals; (2) Involved rectal cancer patients who received neo-adjuvant CRT prior to surgery; (3) Included patients with s-LLNM as detected on imaging modalities such as computed topography, magnetic resonance imaging or positron emission tomography scans at diagnosis; and (4) Had clear documentation of patient survival, recurrence and morbidity outcomes.
Articles were excluded if they were: (1) Abstracts, letters, editorials or expert opinion; (2) Included patients with systemic metastases; (3) Included patients who did not received neo-adjuvant CRT; or (4) Patients in whom LLND was performed prophylactically i.e. without radiologically suspicious LLNM.
Definitions
Radiological response post neoadjuvant CRT was defined as any decrease in size in a previously radiologically suspicious s-LLNM. Pathological response was determined upon examination of tumour specimens after surgery, tumour regression grades were microscopically evaluated and a regression score of 4 was considered complete pathological response.
Data extraction and analysis
Two reviewers independently reviewed each article and discrepancies resolved by discussion and consensus. Data was then extracted using standardised forms, recording study methodology, patient demographics, surgery performed, post-operative morbidity and mortality, survival and recurrence outcomes. In particular, when available, radiological and pathological response of suspicious LLNM at diagnosis to neoadjuvant CRT was recorded. Subsequently, outcomes of patients who received TME only vs TME with LLND were compared.
All studies were assessed for their level of evidence using the Oxford Centre for Evidence-Based Medicine Levels of Evidence table[17].
Upon review of all available studies, the authors opted to perform a systemic review over a meta-analysis in view of heterogeneity of available studies as well as the absence of direct comparative data between patients with s-LLNM who had underwent TME vs TME with LLND. Furthermore, when there was potential for overlapping patient groups, studies were reviewed independently by the authors and a decision was made if they should be included in the final analysis based on the amount of additional information that was present in each study.
Outcome measures such as LR rates, DFS and OS were specifically evaluated. LR was defined as any recurrence of tumour within the pelvic cavity.
RESULTS
Fifteen articles published between 1998 and 2018 were included in our final analysis (Table 1).
Table 1.
Characteristics of included studies
| Ref. | Study period | Study centre | Study Type | Evidence | Intervention | Study sample | Description | Data on RR post CRT |
| Kim et al[10] | 2009-2011 | Goyang National Cancer Center, South Korea | Case control | 3b | TME | 157 | Compare outcomes of radio+ vs - LLNM post CRT | Yes |
| Inoue et al[11] | 2001-2013 | Mie University Hospital, Tsu, Japan | Case control | 3b | TME | 19 | Compare outcomes of radio+ vs - LLNM patients post CRT | Yes |
| Kim et al[12] | 2001-20091 | Goyang National Cancer Center and Seoul National university cancer hospital, South Korea | Case series | 4 | TME | 212 | Identify prognostic factors for LLN recurrence in locally advanced rectal ca post CRT | No |
| MERCURY Study group[18]2 | 2002-2003 | Participating centres in MECURY Group | Case control | 3b | TME | 38 | Compare outcomes of radio+ vs - LLNM patients post CRT | No |
| Dharnarajan et al[13] | 2000-2005 | Washington University School of Medicine, United States | Case control | 3b | TME | 30 | Compare outcomes of radio+ vs - LLNM patients post CRT | No |
| Kim et al[14] | 2001-20051 | Goyang National Cancer Center, South Korea | Case series | 4 | TME | 64 | Identify prognostic factors for LLN recurrence in locally advanced rectal ca post CRT | Yes |
| Ogura et al[21] | 2005-2014 | Cancer Institute Hospital, Tokyo, Japan | Case control | 3b | TME and LLND | 107 | Compare laparoscopic TME and LLND for patients with radio+ LLNM vs TME for radio- LLNM based on pre-CRT imaging3 | No |
| Ishihara et al[22] | 2003-2015 | University of Tokyo, Japan | Case control | 3b | TME and LLND | 31 | Compare TME and LLND for patients with radio+ LLNM and TME for radio- LLNM based on pre-CRT imaging3 | Yes |
| Toshiya et al[23] | 1985-20121 | Cancer Institute Hospital, Tokyo, Japan | Case control | 3b | TME and LLND | 30 | Evaluate outcomes preopCRT vs no CRT in patients undergoing open TME and LLND | No |
| Akiyoshi et al[24] | 2004-20131 | Cancer Institute Hospital, Tokyo, Japan | Case series | 3b | TME and LLND | 77 | Outcomes of TME and LLND for patients with radio+ LLNM based on pre-CRT imaging (MRI) | Yes |
| Otowa et al[25] | 2005 -2013 | Kobe University Graduate School of Medicine, Japan | Case series | 3b | TME and LLND | 10 | Outcomes of TME and LLND for patients with radio+ LLNM based on pre-CRT imaging (MRI) | No |
| Oh et al[26] | 2004-2011 | (1) Seoul National University Bundang Hospital; (2) Seoul National University Hospital; (3) National Cancer Center, South Korea | Cohort study | 2b | TME and LLND | 66 | Compare outcomes of patients with responsive vs non-responsive LLNM post-CRT who underwent TME and LLND based on pre-CRT imaging of radio+ LLNM | Yes |
| Akiyoshi et al[27] | 2004-20101 | Cancer Institute Hospital, Tokyo, Japan | Case control | 3b | TME and LLND | 38 | Compare TME and LLND for patients with radio+ LLNM vs TME for radio- LLNM based on pre-CRT imaging3 | No |
| Liang et al[28] | 20102 | National Taiwan University Hospital, Taiwan | Case series | 4 | TME and LLND | 34 | Outcomes of laparoscopic TME and LLND for patients with radio+ LLNM based on post-CRT imaging | No |
| Park et al[29] | 2003-2009 | Kyungpook National University hospital, South Korea | Case series | 4 | TME and LLND | 9 | Outcomes of laparoscopic/ robotic TME and LLND for patients with radio+ LLNM based on post-CRT imaging | No |
Study period not specified.
Potential overlapping data.
Additional LLND was performed or patients with radiologically +/enlarged LLN based on pre-CRT imaging. +: Positive; -: Negative; Radio: Radiologically; RR: Radiological response; CRT: Chemoradiation therapy; LLNM: Lateral pelvic lymph node metastases; CRT: Chemoradiation therapy; LLN: Lateral pelvic lymph node; LLND: Lateral pelvic lymph node dissection; TME: Total mesorectal excision; MRI: Magnetic resonance imaging.
Quality of evidence
“TME only” for s-LLNM: Six studies[10-14,18] reported on the outcomes of rectal cancer patients with radiologically suspicious LLN treated with pre-operative radiation therapy (RT) or CRT followed by conventional TME surgery[19,20].
Two studies were retrospective case series evaluating the relationship between the presence of s-LLNM with recurrence and survival outcomes[12,14]. Four were case control studies comparing the outcomes of patients with and without s-LLNM in the era of neoadjuvant CRT[10,11,13,18]. Radiological response of s-LLNM to CRT was evaluated in three studies and this was subsequently correlated with recurrence and survival outcomes[10,11,14].
Three of the six studies originated from Goyang National Cancer Centre, South Korea – two were likely to have non-overlapping patient groups from differing study periods of 2001 to 2009 and 2009 to 2011 respectively[10,12]. It was likely that studies by Kim et al[12,14] drew analysis from a common patient dataset, as such the former study was excluded from the total number of patients with s-LLNM. However as Kim et al[12] did report additional data regarding radiological response, this information was included in a descriptive manner.
“TME + LLND” for s-LLNM: Nine studies reported on the outcomes of rectal patients with radiologically suspicious LLN that were treated with pre-operative CRT followed by TME and LLND[21-29]. Laparoscopic LLND was adopted in two case series and one case-control study: These were feasibility studies aimed at establishing safety and oncological outcomes with the laparoscopic approach[21,28-29]. Conventional open TME and LLND was the approach in six retrospective studies of which there was one cohort study, two case series, and three case-control study[22-27].
Radiological response of s-LLNM to CRT was evaluated in three studies[22,24,26]. Two studies reported only on radiological non-responders, that is, the presence of persistently enlarged s-LLN post CRT while all other articles were based on pre-CRT imaging findings of suspicious s-LLN[28,29].
Four out of nine studies originated from Cancer Institute Hospital, Tokyo. While patient data from Toshiya and Akiyoshi et al[27] were excluded in the final calculation for s-LLNM in view of the likelihood of duplication; information from these studies was included in a descriptive manner in our report.
In total, our systemic review evaluated a total of 793 patients with s-LLNM for which 456 underwent TME only and 337 underwent TME with additional LLND.
Radiological response of s-LLNM to neoadjuvant CRT
In both “TME” and “TME+LLND” groups, preoperative CRT was administered 4 to 8 wk prior to surgery. When long course CRT was prescribed, a 5-fluorouracil (FU) based regime or S1 with a total dose of 45 to 50.4 Gy of radiation in 25 fractions was given to both the primary tumour and lateral pelvic area over 5 to 5.5 wk. With short course CRT, 20 to 25 Gy in 4 or 5 fractions was given with concurrent chemo-therapy[11,26].
At diagnosis, most authors considered LLN to be “positive” if it was more than 5 mm in short or long axis diameter[10,12-14,26,29,30]; or had morphological features suspicious of metastasis, such as mixed signal intensity or an irregular or spiculated border[18]. A 7 mm LLN size cut-off was adopted in some series[21-24,27]. Magnetic resonance imaging was the most common modality used for radiological evaluation, but if unavailable, computed topography or positron emission tomography scan was used.
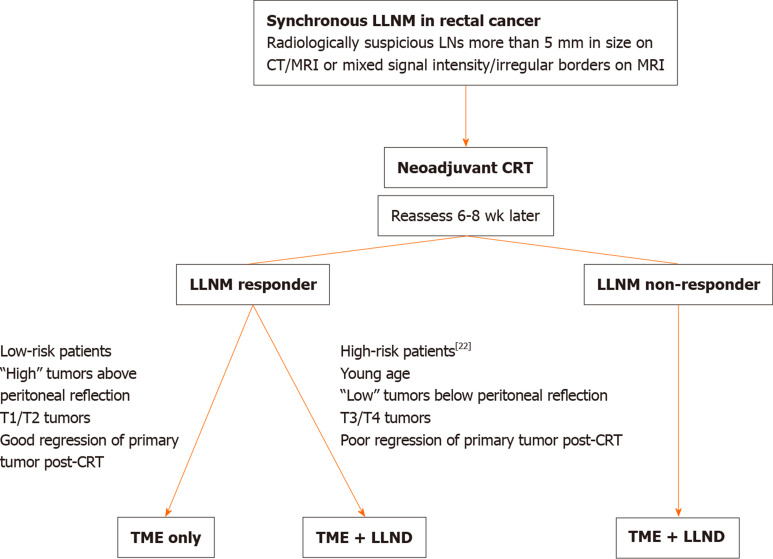
Radiological response of s-LLNM to CRT was evaluated in three studies in both “TME only”[10,11,14] and “TME + LLND” groups[22,24,26]. Mean diameter of s-LLN pre-CRT was 10.2 mm (range 5-45) and was 7 mm (range 0-45) post-CRT. “Responsive LLNs” were defined as having a short axis diameter > 5 mm pre-CRT and < 5 mm post-CRT; “non-responsive LLNs” were > 5 mm both pre and post CRT[10,24,26]. A 7 mm and 8 mm cut-off were adopted in Inoue’s and Ishihara’s groups respectively[11,22] (Figure 2).
Figure 2.
Flow diagram on the strategy of management for suspicious lateral pelvic lymph node metastases. LLNM: Lateral pelvic lymph node metastases; CRT: Chemoradiation therapy; TME: Total mesorectal excision; LLND: Lateral pelvic lymph node dissection.
Amongst 414 patients in whom radiological response was reported, there were 241 responders and 173 non-responders (Tables 2-3). Response rate to CRT was 58% (range 35-72).
Table 2.
Outcomes of total mesorectal excision only in suspicious lateral pelvic lymph node metastases
| Ref. | Primary surgery | T stage | No. of patients with s-LLNM | No. of “Responders” post CRT | Recurrence | Overall survival | Disease free survival |
| Kim et al[10] | Sphinc-sav1 83%; sphinc-sac3 17% | All T3/4 | 157 | 98 (62%) | LR 15%; SR+LR 6% | 5-yr2 85.7%; 5-yr4 74.9% | 5-yr2 76.6%; 5-yr4 56.9% |
| Inoue et al[11] | Sphinc-sav1 63%; sphinc-sac3 37% | All T3/4 | 19 | 7 (37%) | 3-yr LR 12.5% | 5-yr2 84.8%; 5-yr4 72.9% | 5-yr2 77.1%; 5-yr4 32.4% |
| Kim et al[12] | NA | All T2/3/4 | 212 | NA | 5-yr LR 36% | 5-yr 70.3%5 | 5-yr 51.4%5 |
| MECURY Study group[18] | NA | T1/2 18%; T3/4 82% | 38 | NA | NA | NA | 5-yr 42% |
| Dharnarajan et al[13] | Sphinc-sav1 77%; sphinc-sac3 23% | T1/216%; T3/4 83% | 30 | NA | 13% | 5-yr 54% | 5-yr 42% |
| Kim et al[14] | Either Sphinc-sav/sac | All T3/4 | 64 | 46 (72%) | 19.5%2; 44.4%4 | NA | NA |
Sphincter-saving.
Radiological responder post CRT.
Sphincter-sacrificing.
Radiological non-responder post CRT.
Average value taken. s-LLNM: Suspicious lateral pelvic lymph node metastases; CRT: Chemoradiation therapy; LR: Local recurrence; SR: Systemic recurrence; NA: Not available.
Table 3.
Outcomes of total mesorectal excision and lateral pelvic lymph node dissection in suspicious lateral pelvic lymph node metastases
| Ref. | Primary Surgery | T Stage | No. of patients with s-LLNM | No. of “responder” post CRT | No. of pathologic (+) LLN | Morbidity (%) | Recurrence | Overall survival | Disease free survival |
| Ogura et al[21] | Sphinc-sav1 65% | T2 2% | 107 | NA | 26 (24%) | 33.60% | 3-yr 3.2% | 3-yr 95.8% | 3-yr 84.7% |
| Sphinc-sac2 35% | T3/4 98% | ||||||||
| Ishihara et al[22] | NA | T1/2 42% | 31 | 11 (35%) | 1 (9%)3 | NA | 5-yr 0% | 5-yr 81.2% | 5-yr 100% |
| T3/4 58% | 15 (75%)4 | ||||||||
| Toshiya et al[23] | NA | All T3/4 | 30 | NA | NA | NA | 5-yr 3.5% | 5-yr 78.2% | 5-yr 72.1% |
| Akiyoshi et al[24] | Sphinc-sav1 61% | T2 1% | 77 | 49 (64%) | 10 (20%)3 | NA | NA | NA | 3-yr3 90% |
| Sphinc-sac2 39% | T3/4 99% | 21 (75%)4 | 3-yr4 78% | ||||||
| Otowa et al[25] | NA | All T3/4 | 10 | NA | 3 (30%) | NA | NA | NA | NA |
| Oh et al[26] | Sphinc-sav1 78% | T2 3% | 66 | 30 (45%) | 3 | 43.90% | LR 2%5 5%6 | 5-yr 58.7% | 5-yr 41.2% |
| Sphinc-sac2 22% | T3/4 97% | 224 (61%) | SR+LR 2%5 27%6 | 5-yr3 77.1% | 5-yr3 72.5% | ||||
| SR 16%5 32%6 | 5-yr4 44.6% | 5-yr4 33.7% | |||||||
| Akiyoshi et al[27] | Sphinc-sav1 55% | All T3/T4 | 38 | NA | 25 (66%) | 36.80% | LR 2.7% | NA | 3-yr 83.8% |
| Sphinc-sac2 45% | |||||||||
| Liang et al[28] | Sphinc-sav1 82% | All T3/T4 | 34 | NA | 324 (71%) | 20.60% | LR 3% | 2-yr4 97.1% | NA |
| Sphinc-sac2 18% | SR+LR 3% | ||||||||
| SR 21% | |||||||||
| Park et al[29] | Sphinc-sav1 88% | All T3 | 9 | NA | 64 (66%) | 18.70% | LR 6% | NA | NA |
| Sphinc-sac2 12% | SR 13% |
Sphincter-saving.
Sphincter-sacrificing.
Radiological responder post CRT.
Radiological non-responder post CRT.
Pathological responder.
Pathological non-responder. s-LLNM: Suspicious lateral pelvic lymph node metastases; CRT: Chemoradiation therapy; LLN: Lateral pelvic lymph node; LR: Local recurrence; SR: Systemic recurrence; NA: Not available.
Outcomes of “TME only” for s-LLNM
A total of 456 patients received neoadjuvant CRT followed by TME only despite the presence of s-LLNM at diagnosis (Table 1). Meticulous sharp dissection and complete removal of the mesorectum to a level that is below the distal margin of the tumour or to the pelvic floor as described by Heald et al[19] and MacFarlane et al[20] was adopted in all cases.
Rates of LR range from 12.5% to 36%. Five-year overall survival (OS) and disease- free survival (DFS) range from 54% to 83.9% and 42% to 75% respectively. When comparing between patients with and without s-LLNM receiving CRT, Inoue, Dharnarajan and the MECURY group did not find a significant difference in survival and recurrence outcomes[11,13,18].
Comparing “responders” vs “non-responders”: From Kim et al[10]’s study, LR was 8.2% in responders vs 25.4% in non-responders (P < 0.05). Five-year OS and DFS was 85.7% and 76.6% in responders vs 74.9% and 56.9% in non-responders respectively (P = 0.006). Inoue et al[11] reported similar findings concluding improved cancer specific survival in LLN responders.
Outcomes of “TME and LLND” for s-LLNM
Three hundred and thirty-seven patients with suspicious s-LLNM at diagnosis underwent TME with LLND after neoadjuvant CRT (Table 2). As defined by the JSCCR[2], lymph nodes along internal iliac, obturator, external iliac and common iliac basins are considered LLNs. The lateral, medial, cranial, caudal, and dorsal anatomical borders in a standard LLND are the external iliac artery, pelvic plexus, bifurcation of the common iliac artery, levator ani, and the sciatic nerve respectively. Autonomic nerve preservation was performed whenever possible.
Median number of LLNs harvested was 7.5 (range 3-19). Peri- operative complications were evaluated in five studies[21,25,26,28,29]. Mean operative time with additional LLND was 338.4 (range 42-890) min with a mean blood loss of 272 (range 0-1190) mL. Mean length of hospital stay was 11.7 (range 3-100) d. No surgery-related mortality was reported. Morbidity rates ranged from 18.7% to 43.9% and they included superficial skin infections (SSIs), intra-abdominal collections, anastomotic leak, bleeding, chest infections and ileus. Genitourinary dysfunction occurred in 20% to 40%. When comparing patients who received “TME” vs “TME with LLND”, Ogura et al[21] found a significantly longer operative time, greater blood loss, and more prolonged hospital stay in the latter group. Though overall complication rates were worse with additional LLND (33.6% vs 24.5%, P = 0.0839), there was no significant difference in Calvein-Dindo grade 3 and above complications[30]. Similarly, Akiyoshi et al[27] reported no difference in overall complications rates when comparing TME with TME and LLND (29.2% vs 36.8%, P = 0.4).
Rates of LR range from 0% to 6%. Five-year OS and DFS range from 58.7 to 81.2% and 41.2% to 100% respectively. When TME with LLND was performed for patients with s-LLNM based on pre-CRT imaging, Ogura, Ishihara and Akiyoshi et al[27] (2014) found survival outcomes to be comparable with patients without s-LLNM.
Comparing “responders” vs “non-responders”: True responders post-CRT have pathologically negative LLN after LLND. Of 379 patients in whom final pathology was reported, 57% were true responders.
Both radiological and pathological response was evaluated in 3 studies[22,24,26]. Radiological response was seen in 35% to 64% of patients. Amongst radiological responders, Oh et al[26] reported a true response rate of 100%. Ishihara and Akiyoshi et al[24] however found pathologically positive LLN in 9% and 20% of patients respectively despite radiological response. Amongst radiological non-responders, pathological positive LLN occurred in 61% to 94%.
From Oh’s study, LR was 20% in radiological responders vs 47.2% in non-responders (P = 0.012)[26]. Five-year OS and DFS was 77.1% and 72.5% in responders vs 44.6% and 33.7% in non-responders respectively (P = 0.034, 0.011). Akiyoshi et al[24] reported similar findings concluding improved cancer specific survival in radiologically responsive LLNs.
In true pathological responders, recurrence rates was 22.7% vs 59.1% in true non-responders (P = 0.001)[26]. Ishihara et al[22] found that 5-year OS was 100% in true responders vs 60% in patients with and without a pathologically positive LLNM (P = 0.05). Five-year LR rates was 0% in both groups. Factors associated with a final pathological positive LLNM were: Younger age, shorter distance from anal verge, larger tumour size, radiologically non-responsive LLNM, less frequent “T” down staging and histological regression.
DISCUSSION
Extended lymph node dissection in low rectal cancer was first described in the 1950s by Dr Stearns and Deddish[15] and was aimed at reducing LR. Results from the recent JCOG0212 trial from Japan comparing TME alone with TME and LLND lend support to prophylactic dissection by showing reduced rates of LR in the latter group[31]. However, as pre-operative CRT was not utilised, the applicability of this trial to institutions that adopt CRT is questionable. While high level evidence has shown superior recurrence-free survival in recipients of neoadjuvant CRT, improvements in OS have not been conclusively reported[7-9,32,33]. Akiyoshi et al[1], at an attempt to determine if LLNM in rectal cancer represent “local-regional” or “distant” disease, found LLNM patients to have survival outcomes that were comparable to American classified N1/2 disease and superior to those with stage IV disease. Hence, the role of CRT or LLND appears to be confined to providing improved local control.
While preoperative CRT with LLND might be an overkill in the absence of a clinically positive LLNM and may result in increased morbidity without corresponding improvements in local control[34], its role in patients with radiologically persistently suspicious LLNs remains less debateable. Proponents of CRT claim that the presence of suspicious nodes on pre-CRT imaging did not impact OS when TME alone was performed, but fail to show comparable results in local control[18]. In fact, our review found that when pre-CRT LLN size was > 5 mm, up to 43% had pathological metastases[22,24,26]. Even in the radiological responders, residual disease in the LLN is documented to be between 9%-20%, corresponding to a reported LR rate of 8%. Though OS was not significantly different between the “TME only” and “TME with LLND” groups; a difference was seen in DFS and LR rates.
LRs involving the LLN portend a dismal prognosis. Moore and Yamada et al[36] attempted to classify pelvic recurrences in rectal cancer and found patients with “lateral” invasive types of recurrence to have the lowest 5-yr survival rates[35]. Furthermore, genito-urinary complications were common occurring in up to 58% of patients post intervention for locally recurrent rectal cancer[37]. Quality of life was also substantially reduced with patients reporting chronic pain, gastrointestinal and genitourinary symptoms[38]. Opponents of LLND often cite increased urinary and sexual dysfunction, greater intraoperative blood loss and operative time as a significant morbidities[6]. Our review found a 20% to 40% rate of genitourinary and sexual dysfunction with no difference in major complication rates. Though genitourinary dysfunction post LLND remains a concern, it must be weighed against the increased risk of LR and its implicating consequence without extended surgery in the presence of radiologically suspicious LLNs.
Radiological response to CRT was seen in up to 64% of patients. While Oh et al[26] reported a 100% true response rate, others found pathological positive LLNM in up to 20% of patients despite radiological response. Potentially, 80% of radiological responders would have received unnecessary LLND given a final pathologically negative LLN. Ishihara et al[22] identified young age, low location and greater T stage of the primary tumour, and radiological non-response to CRT as pre-surgical factors that result in final positive LLNM. With this, we can infer that these high-risk features if present should indicate the need for LLND, and the inverse may predict low-risk patients that might have a final negative LLN and in whom additional LLND may be avoided despite the presence of an enlarged pre-CRT LLN.
Our review highlights that currently, all available studies on the management of s-LLNM are retrospective in nature and limited by small sample size. In addition, as LLND is more widely performed in the East and majority of studies conducted from Japanese or Korean centers, results tend to be bias toward these institutions. There is also heterogeneity in the studies included, each having differing independent control groups. Furthermore radiological cut-offs used to defined radiologically suspicious LLNs varied. The regime of pre-operative CRT use was not standardized with some adopting short course and others long course CRT.
In light of evidence showing potential improvements in LR and DFS in patients with s-LLNM undergoing LLND after CRT, randomized control trials are required to determine the optimal course of management for these patients and to better select for patients that will benefit most from LLND.
s-LLNM is common in locally advanced low rectal cancer. Despite preoperative CRT, LLNM may persist necessitating surgical removal. LLND is associated with lowered rates of LR and may improve DFS. In radiological responders, LLND may be considered in patients with “high” risk features to prevent LR.
In the absence of data from RCTs to guide our management strategy in patients with s-LLNM in rectal cancer, the authors propose the following algorithm base on the results of our systemic review.
ARTICLE HIGHLIGHTS
Research background
Up to 28% of patients with locally advanced low rectal cancer present with synchronous lateral pelvic lymph node metastasis (LLNM). While neoadjuvant chemoradiation therapy followed by surgery has become the mainstay of treatment, the role of lateral pelvic lymph node dissection (LLND) remains unclear. As such, our study aims to define its role in patients who present with synchronous LLNM.
Research motivation
An understanding on the optimal management for patients who present with s-LLNM is essential to prevent local recurrence rates. The examination of responders vs non-responders to neoadjuvant chemoradiation can also serve to guide future research to optimise response rates.
Research objectives
We aim to evaluate if there is a difference in recurrence and survival outcomes in patients with s-LLNM post neoadjuvant therapy that is treated with TME only vs TME + LLND. This can serve as a guide to surgeons on the management of such patients.
Research methods
A systemic review was performed for all relevant articles from 1958. To our knowledge, there has been no such review on s-LLNM patients post neoadjuvant chemoradiation therapy.
Research results
Fifteen studies were included. Local recurrence rates was found to be higher in s-LLNM patients who had underwent only TME when compared with those who had additional LLND. True pathological response after neoadjuvant therapy was mixed and an absence of radiological response reflected final pathological findings.
Research conclusions
LLND is associated with local control in patients with s-LLNM. It can be performed in radiological non-responders given that a large majority represent true LLNM. Its role in radiological responders should be considered in selected high risk patients.
Research perspectives
Future research should focus on how to predict pathological non-response after neoadjuvant therapy such that super selective LLND may be performed only in non-responders that are more likely to recur.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Manuscript source: Unsolicited manuscript
Peer-review started: December 7, 2019
First decision: December 17, 2019
Article in press: April 28, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liang JW, Rutegard J S-Editor: Ma YJ L-Editor: A E-Editor: Liu JH
Contributor Information
Jolene Si Min Wong, Department of Sarcoma, Peritoneal and Rare Tumours (SPRinT), Division of Surgery and Surgical Oncology, National Cancer Centre Singapore, Singapore 169610, Singapore.
Grace Hwei Ching Tan, Department of Sarcoma, Peritoneal and Rare Tumours (SPRinT), Division of Surgery and Surgical Oncology, National Cancer Centre Singapore, Singapore 169610, Singapore; Duke-NUS Medical School, 8 College Road, Singapore 169857, Singapore. gracethc@gmail.com.
Claramae Shulyn Chia, Department of Sarcoma, Peritoneal and Rare Tumours (SPRinT), Division of Surgery and Surgical Oncology, National Cancer Centre Singapore, Singapore 169610, Singapore; Duke-NUS Medical School, 8 College Road, Singapore 169857, Singapore.
Chin-Ann Johnny Ong, Department of Sarcoma, Peritoneal and Rare Tumours (SPRinT), Division of Surgery and Surgical Oncology, National Cancer Centre Singapore, Singapore 169610, Singapore; Duke-NUS Medical School, 8 College Road, Singapore 169857, Singapore; Laboratory of Applied Human Genetics, Division of Medical Sciences, National Cancer Centre Singapore, Singapore 169610, Singapore; Institute of Molecular and Cell Biology, A*STAR Research Entities, 61 Biopolis Drive, Singapore 138673, Singapore.
Melissa Ching Ching Teo, Department of Sarcoma, Peritoneal and Rare Tumours (SPRinT), Division of Surgery and Surgical Oncology, National Cancer Centre Singapore, Singapore 169610, Singapore; Duke-NUS Medical School, 8 College Road, Singapore 169857, Singapore.
References
- 1.Akiyoshi T, Watanabe T, Miyata S, Kotake K, Muto T, Sugihara K Japanese Society for Cancer of the Colon and Rectum. Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer: is it regional or distant disease? Ann Surg. 2012;255:1129–1134. doi: 10.1097/SLA.0b013e3182565d9d. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishihara S, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Boku N, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20:207–239. doi: 10.1007/s10147-015-0801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugihara K, Kobayashi H, Kato T, Mori T, Mochizuki H, Kameoka S, Shirouzu K, Muto T. Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum. 2006;49:1663–1672. doi: 10.1007/s10350-006-0714-z. [DOI] [PubMed] [Google Scholar]
- 4.Ueno M, Oya M, Azekura K, Yamaguchi T, Muto T. Incidence and prognostic significance of lateral lymph node metastasis in patients with advanced low rectal cancer. Br J Surg. 2005;92:756–763. doi: 10.1002/bjs.4975. [DOI] [PubMed] [Google Scholar]
- 5.Monson JR, Weiser MR, Buie WD, Chang GJ, Rafferty JF, Buie WD, Rafferty J Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of rectal cancer (revised) Dis Colon Rectum. 2013;56:535–550. doi: 10.1097/DCR.0b013e31828cb66c. [DOI] [PubMed] [Google Scholar]
- 6.Georgiou P, Tan E, Gouvas N, Antoniou A, Brown G, Nicholls RJ, Tekkis P. Extended lymphadenectomy versus conventional surgery for rectal cancer: a meta-analysis. Lancet Oncol. 2009;10:1053–1062. doi: 10.1016/S1470-2045(09)70224-4. [DOI] [PubMed] [Google Scholar]
- 7.Swedish Rectal Cancer Trial, Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, Wilking N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 8.Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644–5650. doi: 10.1200/JCO.2005.08.144. [DOI] [PubMed] [Google Scholar]
- 9.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 10.Kim MJ, Chan Park S, Kim TH, Kim DY, Kim SY, Baek JY, Chang HJ, Park JW, Oh JH. Is lateral pelvic node dissection necessary after preoperative chemoradiotherapy for rectal cancer patients with initially suspected lateral pelvic node? Surgery. 2016;160:366–376. doi: 10.1016/j.surg.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Inoue Y, Saigusa S, Hiro J, Toiyama Y, Araki T, Tanaka K, Mohri Y, Kusunoki M. Clinical significance of enlarged lateral pelvic lymph nodes before and after preoperative chemoradiotherapy for rectal cancer. Mol Clin Oncol. 2016;4:994–1002. doi: 10.3892/mco.2016.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MJ, Kim TH, Kim DY, Kim SY, Baek JY, Chang HJ, Park SC, Park JW, Oh JH. Can chemoradiation allow for omission of lateral pelvic node dissection for locally advanced rectal cancer? J Surg Oncol. 2015;111:459–464. doi: 10.1002/jso.23852. [DOI] [PubMed] [Google Scholar]
- 13.Dharmarajan S, Shuai D, Fajardo AD, Birnbaum EH, Hunt SR, Mutch MG, Fleshman JW, Lin AY. Clinically enlarged lateral pelvic lymph nodes do not influence prognosis after neoadjuvant therapy and TME in stage III rectal cancer. J Gastrointest Surg. 2011;15:1368–1374. doi: 10.1007/s11605-011-1533-7. [DOI] [PubMed] [Google Scholar]
- 14.Kim TH, Jeong SY, Choi DH, Kim DY, Jung KH, Moon SH, Chang HJ, Lim SB, Choi HS, Park JG. Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol. 2008;15:729–737. doi: 10.1245/s10434-007-9696-x. [DOI] [PubMed] [Google Scholar]
- 15.Stearns MW, Jr, Deddish MR. Five-year results of abdominopelvic lymph node dissection for carcinoma of the rectum. Dis Colon Rectum. 1959;2:169–172. doi: 10.1007/BF02616711. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howick J, Chalmers NI, Glasziou P. ‘‘The Oxford 2011 Levels of Evidence’’. Oxford Centre for Evidence-Based Medicine. [accessed 23 Jan 2012] Available from: http://www.cebm.net/ index.aspx?o=5653.
- 18.MERCURY Study Group, Shihab OC, Taylor F, Bees N, Blake H, Jeyadevan N, Bleehen R, Blomqvist L, Creagh M, George C, Guthrie A, Massouh H, Peppercorn D, Moran BJ, Heald RJ, Quirke P, Tekkis P, Brown G. Relevance of magnetic resonance imaging-detected pelvic sidewall lymph node involvement in rectal cancer. Br J Surg. 2011;98:1798–1804. doi: 10.1002/bjs.7662. [DOI] [PubMed] [Google Scholar]
- 19.Heald RJ. The 'Holy Plane' of rectal surgery. J R Soc Med. 1988;81:503–508. doi: 10.1177/014107688808100904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457–460. doi: 10.1016/0140-6736(93)90207-w. [DOI] [PubMed] [Google Scholar]
- 21.Ogura A, Akiyoshi T, Nagasaki T, Konishi T, Fujimoto Y, Nagayama S, Fukunaga Y, Ueno M, Kuroyanagi H. Feasibility of Laparoscopic Total Mesorectal Excision with Extended Lateral Pelvic Lymph Node Dissection for Advanced Lower Rectal Cancer after Preoperative Chemoradiotherapy. World J Surg. 2017;41:868–875. doi: 10.1007/s00268-016-3762-0. [DOI] [PubMed] [Google Scholar]
- 22.Ishihara S, Kawai K, Tanaka T, Kiyomatsu T, Hata K, Nozawa H, Morikawa T, Watanabe T. Oncological Outcomes of Lateral Pelvic Lymph Node Metastasis in Rectal Cancer Treated With Preoperative Chemoradiotherapy. Dis Colon Rectum. 2017;60:469–476. doi: 10.1097/DCR.0000000000000752. [DOI] [PubMed] [Google Scholar]
- 23.Nagasaki T, Akiyoshi T, Fujimoto Y, Konishi T, Nagayama S, Fukunaga Y, Ueno M. Preoperative Chemoradiotherapy Might Improve the Prognosis of Patients with Locally Advanced Low Rectal Cancer and Lateral Pelvic Lymph Node Metastases. World J Surg. 2017;41:876–883. doi: 10.1007/s00268-016-3748-y. [DOI] [PubMed] [Google Scholar]
- 24.Akiyoshi T, Matsueda K, Hiratsuka M, Unno T, Nagata J, Nagasaki T, Konishi T, Fujimoto Y, Nagayama S, Fukunaga Y, Ueno M. Indications for Lateral Pelvic Lymph Node Dissection Based on Magnetic Resonance Imaging Before and After Preoperative Chemoradiotherapy in Patients with Advanced Low-Rectal Cancer. Ann Surg Oncol. 2015;22 Suppl 3:S614–S620. doi: 10.1245/s10434-015-4565-5. [DOI] [PubMed] [Google Scholar]
- 25.Otowa Y, Yamashita K, Kanemitsu K, Sumi Y, Yamamoto M, Kanaji S, Imanishi T, Nakamura T, Suzuki S, Tanaka K, Kakeji Y. Treating patients with advanced rectal cancer and lateral pelvic lymph nodes with preoperative chemoradiotherapy based on pretreatment imaging. Onco Targets Ther. 2015;8:3169–3173. doi: 10.2147/OTT.S89752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh HK, Kang SB, Lee SM, Lee SY, Ihn MH, Kim DW, Park JH, Kim YH, Lee KH, Kim JS, Kim JW, Kim JH, Chang TY, Park SC, Sohn DK, Oh JH, Park JW, Ryoo SB, Jeong SY, Park KJ. Neoadjuvant chemoradiotherapy affects the indications for lateral pelvic node dissection in mid/low rectal cancer with clinically suspected lateral node involvement: a multicenter retrospective cohort study. Ann Surg Oncol. 2014;21:2280–2287. doi: 10.1245/s10434-014-3559-z. [DOI] [PubMed] [Google Scholar]
- 27.Akiyoshi T, Ueno M, Matsueda K, Konishi T, Fujimoto Y, Nagayama S, Fukunaga Y, Unno T, Kano A, Kuroyanagi H, Oya M, Yamaguchi T, Watanabe T, Muto T. Selective lateral pelvic lymph node dissection in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy based on pretreatment imaging. Ann Surg Oncol. 2014;21:189–196. doi: 10.1245/s10434-013-3216-y. [DOI] [PubMed] [Google Scholar]
- 28.Liang JT. Technical feasibility of laparoscopic lateral pelvic lymph node dissection for patients with low rectal cancer after concurrent chemoradiation therapy. Ann Surg Oncol. 2011;18:153–159. doi: 10.1245/s10434-010-1238-2. [DOI] [PubMed] [Google Scholar]
- 29.Park JS, Choi GS, Lim KH, Jang YS, Kim HJ, Park SY, Jun SH. Laparoscopic extended lateral pelvic node dissection following total mesorectal excision for advanced rectal cancer: initial clinical experience. Surg Endosc. 2011;25:3322–3329. doi: 10.1007/s00464-011-1719-9. [DOI] [PubMed] [Google Scholar]
- 30.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita S, Mizusawa J, Kanemitsu Y, Ito M, Kinugasa Y, Komori K, Ohue M, Ota M, Akazai Y, Shiozawa M, Yamaguchi T, Bandou H, Katsumata K, Murata K, Akagi Y, Takiguchi N, Saida Y, Nakamura K, Fukuda H, Akasu T, Moriya Y Colorectal Cancer Study Group of Japan Clinical Oncology Group. Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial. Ann Surg. 2017;266:201–207. doi: 10.1097/SLA.0000000000002212. [DOI] [PubMed] [Google Scholar]
- 32.Ma B, Gao P, Wang H, Xu Q, Song Y, Huang X, Sun J, Zhao J, Luo J, Sun Y, Wang Z. What has preoperative radio(chemo)therapy brought to localized rectal cancer patients in terms of perioperative and long-term outcomes over the past decades? A systematic review and meta-analysis based on 41,121 patients. Int J Cancer. 2017;141:1052–1065. doi: 10.1002/ijc.30805. [DOI] [PubMed] [Google Scholar]
- 33.Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS, Bessell E, Griffiths G, Thompson LC, Parmar M. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagawa H, Muto T, Sunouchi K, Higuchi Y, Tsurita G, Watanabe T, Sawada T. Randomized, controlled trial of lateral node dissection vs. nerve-preserving resection in patients with rectal cancer after preoperative radiotherapy. Dis Colon Rectum. 2001;44:1274–1280. doi: 10.1007/BF02234784. [DOI] [PubMed] [Google Scholar]
- 35.Moore HG, Shoup M, Riedel E, Minsky BD, Alektiar KM, Ercolani M, Paty PB, Wong WD, Guillem JG. Colorectal cancer pelvic recurrences: determinants of resectability. Dis Colon Rectum. 2004;47:1599–1606. doi: 10.1007/s10350-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 36.Yamada K, Ishizawa T, Niwa K, Chuman Y, Akiba S, Aikou T. Patterns of pelvic invasion are prognostic in the treatment of locally recurrent rectal cancer. Br J Surg. 2001;88:988–993. doi: 10.1046/j.0007-1323.2001.01811.x. [DOI] [PubMed] [Google Scholar]
- 37.Mannaerts GH, Schijven MP, Hendrikx A, Martijn H, Rutten HJ, Wiggers T. Urologic and sexual morbidity following multimodality treatment for locally advanced primary and locally recurrent rectal cancer. Eur J Surg Oncol. 2001;27:265–272. doi: 10.1053/ejso.2000.1099. [DOI] [PubMed] [Google Scholar]
- 38.Harji DP, Griffiths B, Velikova G, Sagar PM, Brown J. Systematic review of health-related quality of life issues in locally recurrent rectal cancer. J Surg Oncol. 2015;111:431–438. doi: 10.1002/jso.23832. [DOI] [PubMed] [Google Scholar]