Abstract
Background.
Tens of millions of children are exposed to Mycobacterium tuberculosis globally every year; however, there are no contemporary estimates of the risk of developing tuberculosis in exposed children. The effectiveness of contact investigations and preventive therapy remains poorly understood.
Methods.
We conducted an individual participant data meta-analysis of cohort studies in which children (<19 years of age) with close tuberculosis exposure were investigated for tuberculosis and followed for incident disease. We estimated the odds of prevalent tuberculosis with mixed-effects logistic models, and estimated adjusted hazard ratios (AHR) for incident tuberculosis with mixed-effects Poisson regression models. The effectiveness of preventive therapy against incident tuberculosis was estimated through propensity score matching.
Findings.
We pooled participant-level data from 46 cohort studies in 34 countries. We included 137,647 exposed children followed for 429,538 child-years, during which 1,299 prevalent and 999 incident cases were diagnosed. The two-year risk of developing tuberculosis among infected children not receiving preventive therapy was 19.0% from 0 to 5 years of age. The effectiveness of preventive therapy was 63% (AHR, 0.37, 95% confidence intervals [CI], 0.30–0.47) among all exposed children, and 85% (AHR, 0.15, 95% CI, 0.11–0.20) among those with a positive test of infection. Among all children <5 years of age who developed tuberculosis, 83% were diagnosed within 90 days of the baseline visit.
Interpretation.
The risk of developing tuberculosis among exposed infants and young children is very high. The majority of cases occurred within weeks of contact investigation initiation and may not be preventable through prophylaxis. This suggests that alternative strategies for prevention, such as earlier initiation of preventive therapy through earlier diagnosis of adult cases or community-wide screening approaches, are needed.
Keywords: tuberculosis, children, prevention, pediatrics
INTRODUCTION
Tens of millions of children are exposed to Mycobacterium tuberculosis every year,1,2 and tuberculosis remains a leading infectious cause of global childhood morbidity and mortality.3–5 Historically, pediatric tuberculosis has been largely understudied, and its natural history in children remains poorly understood. Due to this, there is considerable uncertainty regarding the effectiveness of public health strategies for detection and prevention of tuberculosis among exposed children.
The majority of evidence concerning the natural history of tuberculosis in children relies upon studies performed prior to 1950.6–11 Many changes have occurred in the control of tuberculosis and in the health of populations more broadly, including the introduction of tuberculosis drug chemotherapy, widespread administration of the BCG vaccination, substantial decline of the prevalence of undernutrition in children, and the HIV-epidemic.12–16 A re-assessment of age-specific risks of tuberculosis and identifying risk factors for disease in exposed children is necessary to inform clinical and policy decision-making. Public health interventions targeting exposed children are urgently needed but remain poorly measured; the population-impact of pediatric case-finding and preventive interventions is currently unknown.
To address these knowledge gaps, we pooled data from longitudinal cohort studies conducted over the past 20 years. We estimated the risk of developing tuberculosis in children after close exposure, stratified by age and individual-level determinants of risk. We also examined how disease risk was impacted by preventive therapy, BCG vaccination, and time since tuberculosis exposure to better understand the role of various public health interventions.
METHODS
Search Strategy and Study Selection
We conducted a systematic review investigating development of tuberculosis in children closely exposed to a tuberculosis case. We registered a protocol with PROSPERO (CRD42018087022) that includes a prespecified analytical plan; this article follows PRISMA for Individual-Patient Data reporting guidelines (Supplementary Appendix).17
Our search entailed several steps which are detailed in the appendix. Briefly, we searched for cohort studies from January 1, 1998 to April 6, 2018 in MEDLINE, Web of Science, BIOSIS, and Embase electronic databases. Since incident tuberculosis was our primary study outcome, we restricted our search to cohort studies – case-control studies and outbreak reports were excluded. Search terms included “mycobacterium tuberculosis”, “TB”, “tuberculosis”, and “contact” (full search can be found in the appendix), and articles were unrestricted by language. The 20-year time-frame was chosen based on expected availability of individual-participant data. We additionally reviewed reference lists of other systematic reviews and selected primary or narrative review articles of contact investigations.18–21 We included data that was unpublished, deposited on data storage repositories, conference abstracts, and dissertations if eligible.
Due to the broad nature of our search terms, we developed a list of exclusionary words (Supplementary Appendix) that ruled out articles if present in manuscript titles. In order to evaluate the accuracy of this process, we implemented the algorithm on a random list of 100 titles and manually screened them for eligibility in the study. Our exclusionary algorithm eliminated all articles that were screened out by manual screening with 100% specificity. Two reviewers (LM and OC) independently reviewed articles in two stages: evaluation of titles and abstracts followed by full-text review. At each stage, the two reviewers discussed discrepancies and re-evaluated articles until consensus was reached.
Individual-participant data and a pre-specified list of variables was requested from authors of all eligible studies. These included characteristics of the exposed child, the index case, and environmental characteristics (Supplementary Appendix). To be eligible for inclusion in the final analysis, a dataset needed to include: (1) individuals below 19 years of age; (2) follow-up for tuberculosis for a minimum of six months; (3) individuals with household or close exposure to an individual with tuberculosis; (4) information on the age and sex of the child; (4) provide start and end follow-up dates. Studies assessing incident tuberculosis but without dates or time of follow-up were excluded. All data was appropriately de-identified prior to sharing and, due to this, the project was deemed exempt from further review by Stanford University’s institutional review board. Two reviewers (LM, OC) independently assessed quality of each study using a modified rubric of the Newcastle-Ottawa scale.21 Each study was judged based on a 9-point scale using three broad criteria: selection of participants (4 points), comparability of studies (2 points), and ascertainment of outcome of interest (3 points). High study quality was defined as a score of 6 or greater, moderate quality as 3 to 6 points, and low quality as <3 points. Discrepancies between the two reviewers were resolved by re-evaluating the study for consensus. To assess potential selection bias, we compared characteristics of studies that contributed participant-level data to studies that did not.
Study Definitions
Tuberculosis-exposed children were defined as participants <19 years of age with reported ‘close’ contact, either living in the same household or with substantial interaction outside the household, to a microbiologically or radiologically diagnosed tuberculosis case. Exposure and index case diagnoses were defined by the investigators leading each cohort, and we used study definitions among included studies (Supplementary Appendix).
Tuberculosis infection was defined as a positive QuantiFERON-TB Gold In-Tube (QFT) (interferon-γ-nil ≥0.35 IU/mL), T-SPOT.TB (>8 spot forming cells per well), or tuberculin skin test (TST) (≥10-millimeter induration) was used to indicate tuberculosis infection. Preventive therapy was assigned to participants according each study’s protocol or local guidelines and practices. We included any reported preventive therapy regimen in our analysis. A preventive therapy regimen was defined as initiation of any preventive drug regimen given and started to children. Treatment adherence was not assessed in most studies. These regimens included isoniazid for six months, isoniazid for nine months, rifampin for three months, and rifapentine for three months, among others.
Prevalent and incident tuberculosis were defined based on the time from the baseline enrollment of the participant in the contact investigation. Prevalent tuberculosis was defined as any diagnosis of tuberculosis at the initial visit or within 90 days of baseline evaluation based on a conventional definition19 (further discussion in the Supplementary Appendix). Incident tuberculosis was defined as a new tuberculosis case diagnosed >90 days after the initial evaluation. We utilized each study’s classification of tuberculosis case. Definitions for tuberculosis diagnosis, diagnostic tests, and algorithms used for diagnosis at baseline and follow-up in each study are listed in the Supplementary Appendix.
Statistical Analyses
We pooled individual participant-level data from all included cohorts. Our primary study outcomes were prevalent and incident tuberculosis. We calculated follow-up time from the first baseline visit to development of tuberculosis, loss to follow-up, death, or study completion. Heterogeneity was assessed using the I2 statistic.
Our analysis had two primary aims: (i) estimating the risk of developing tuberculosis by time-period of follow-up, demographic (age, region) and clinical attributes (HIV, tuberculosis infection status, prior tuberculosis); and (ii) estimating the effectiveness of preventive therapy and BCG vaccination on the risk of developing tuberculosis.
To estimate the 2-year cumulative incidence of tuberculosis, we included only prospective studies to avoid potential biases associated with case ascertainment from retrospective studies. Only children not given preventive therapy are included in this analysis. The cumulative incidence included both prevalent and incident tuberculosis in the first two years of follow-up in these studies. We stratified these results by age and baseline results of tuberculin skin test or interferon gamma release assay.
The analysis of tuberculosis risk factors was performed using separate outcomes measures: prevalent tuberculosis, incident tuberculosis, and cumulative incidence outcome (ie, including both prevalence and incidence together). For the prevalent and cumulative incidence outcomes, we used mixed-effects logistic regression analyses. For the incident tuberculosis outcome, we used mixed-effects Poisson and parametric survival-time models. In incident regression models, variables were modelled with time fixed effects. For this analysis prospective and retrospective cohort studies were used, both separately and pooled (stratified analysis in the Supplementary Appendix). Each statistical model accounted for clustering at the study-level and was adjusted for the variable of interest, baseline child age and sex, and whether data was collected prospectively or retrospectively.
We estimated tuberculosis prevalence using a mixed-effects logistic regression and tuberculosis incidence through mixed-effects Poisson regression models, with study-level random effects for all analyses. Tuberculosis incidence was stratified by days following study enrollment: 91–365, 366–730, and >730 days. To assess the influence of demographic and clinical factors on tuberculosis risk, we used mixed-effects Poisson and parametric survival-time models with a Weibull distribution. The likelihood ratio test was used to derive P values. Because of the large sample size of one study relative to the other included cohort studies, we re-analyzed our risk factor analysis without this study to assess the influence of this study on our results.
When evaluating the protective impact of preventive therapy, we performed a propensity score analysis, matching based on individual-level covariates of age, sex, study design (see the Supplementary Appendix). We then matched children who began preventive therapy with children who did not start using a nearest neighbor matching algorithm. In this matched cohort, we repeated our parametric survival-time models to estimate covariate-adjusted risk of prevalent and incident tuberculosis between groups when examining the protective effectiveness of preventive therapy. We repeated this analysis for children with and without tuberculosis infection. We evaluated several alternative propensity scores using additional variables. The Supplementary Appendix provides additional details about the analytical methodologies used.
We conducted several sensitivity analyses of different thresholds for prevalent and incident tuberculosis. We compared prevalence using the primary analysis cutoff of 90 days from the baseline investigation to other cutoffs including 0, 30, and 60 days.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Description of study population
From our multi-database search, we found 14,927 original titles and reviewed 7,924 abstracts and titles published after January 1, 1998 (Supplementary Figure 1). After title, abstract, and full-text review, 80 study groups were contacted for individual-participant data. In all, study groups from 53 cohorts in 46 studies – 29 (63%) prospective studies and 17 (37%) retrospective – agreed to share their data and were included in the final analysis (Table 1; references listed in Supplementary Appendix). Studies were from geographically diverse settings in 34 countries, and the majority rated as high or moderate quality (Table 1). Microbiological testing was used to diagnose tuberculosis in child contacts in 32 studies (70%). Among studies with household clustering data, we found that the median number of children per household included in the study was 2 (Interquartile Range, 1–4). Characteristics of studies that contributed participant-level data were generally similar to those that were not included (Supplementary Appendix).
Table 1.
Demographic Descriptions of Included Cohort Studies.
| Characteristic | Number of Studies (N=46) | Percentage |
|---|---|---|
| Prospective Study Design | 28 | 61 |
| World Health Organization High-burden† | 18 | 39 |
| Tuberculosis Incidence Burden of Country, per 100 thousand persons‡ | ||
| <50 | 16 | 36 |
| 50–100 | 9 | 19 |
| >100–200 | 9 | 19 |
| >200 | 12 | 23 |
| World Health Organization Region | ||
| African | 9 | 20 |
| Americas | 16 | 33 |
| Eastern Mediterranean | 1 | 2 |
| European | 7 | 15 |
| Southeast Asia | 4 | 9 |
| Western Pacific | 9 | 20 |
| Income Group§ | ||
| High | 14 | 30 |
| Upper-middle | 18 | 39 |
| Lower-middle | 8 | 17 |
| Low | 6 | 13 |
| HIV Status of Child Reported | 23 | 49 |
| Study Quality Assessment | ||
| High | 33 | 72 |
| Moderate | 11 | 24 |
| Low | 2 | 4 |
| Mean Duration of Study Follow-up | ||
| <2 years | 24 | 56 |
| 2–4 years | 13 | 28 |
| 5–7 years | 3 | 11 |
| >7 years | 3 | 7 |
| Cohort size | ||
| <1000 | 20 | 43 |
| 1000–5000 | 14 | 30 |
| >5000 | 12 | 26 |
| Exposed to Drug Resistant Index Cases | ||
| Only Drug-Resistant Index Cases | 3 | 6 |
| Both Drug-Resistant and Susceptible Index Cases | 12 | 26 |
| Only Drug-Susceptible Index Cases | 2 | 4 |
| Preventive Therapy included* | 32 | 70 |
| QuantiFERON or Tuberculin Skin Testing | 38 | 78 |
| Total | ||
| Persons-years | 429,538 | … |
| Total Individuals Evaluated for Prevalence | 137,647 | … |
| Total Individuals Evaluated for Incidence | 130,512 | … |
| Median age (IQR) | 10.5 (5.7, 15.2) | … |
| Mean age (SD) | 10.3 (5.4) | … |
Abbreviations: HIV, human immunodeficiency virus. BCG, bacillus Calmette–Guérin.
Percentages may not total 100% because within-column percentages were rounded to the nearest integer.
Studies were designated as being located in a “high-burden” country as classified by the World Health Organization.
Country-level tuberculosis incidence data was collected from World Health Organization databases for each study.
Studies were grouped into World Health Organization global regions and World Bank country-level economies (high-income, upper-middle-income, lower-middle-income, and low-income) as of October 2018.
This refers to preventive therapy being given to some participants and includes any type of preventive therapy regimen.
Tuberculosis prevalence and incidence among exposed children
Of 137,647 children evaluated at baseline, 1,299 (1%) were diagnosed with prevalent tuberculosis. For the cohort analysis, 130,512 children were followed for 429.538 child-years, including 395,531 years after the 90 day initial evaluation window, leading to 999 incident tuberculosis cases. Baseline TST or interferon gamma release assay (IGRA) results were available for 117,712 children, among whom 34,692 (random-effects prevalence estimate: 34.7%, 95% confidence intervals [CI], 29.6%–40.1%) had positive tests, with prevalence increasing with age (Supplemental Figure 2).
We calculated the risk of prevalent tuberculosis (cases diagnosed within 90 days of enrollment) and incident tuberculosis, among individuals not receiving preventive therapy, over two years of follow up (Figure 1). The risk of tuberculosis over follow-up was highest within 90 days of enrollment (2.9%, 95% CI: 1.7–4.9%). Prevalence was much higher among children with a baseline positive TST/IGRA (6.5% versus 0.8% among children with a negative TST/IGRA at baseline). Incident tuberculosis consistently decreased over time (2.1, 0.7, and 0.3 cases per 100 person-years during follow-up days 91–365, 365–730, and >730, respectively). Among children with a baseline positive TST/IGRA, incidence per 100 person-years was 3.9 at 91–365 days, 1.2 at 366–730 days, and 1.1 at >730 days from baseline. Among children with a baseline negative TST/IGRA, incidence over these same intervals was 1.1, 0.5, and <0.1 cases per 100 person-years (Figure 2).
Figure 1.
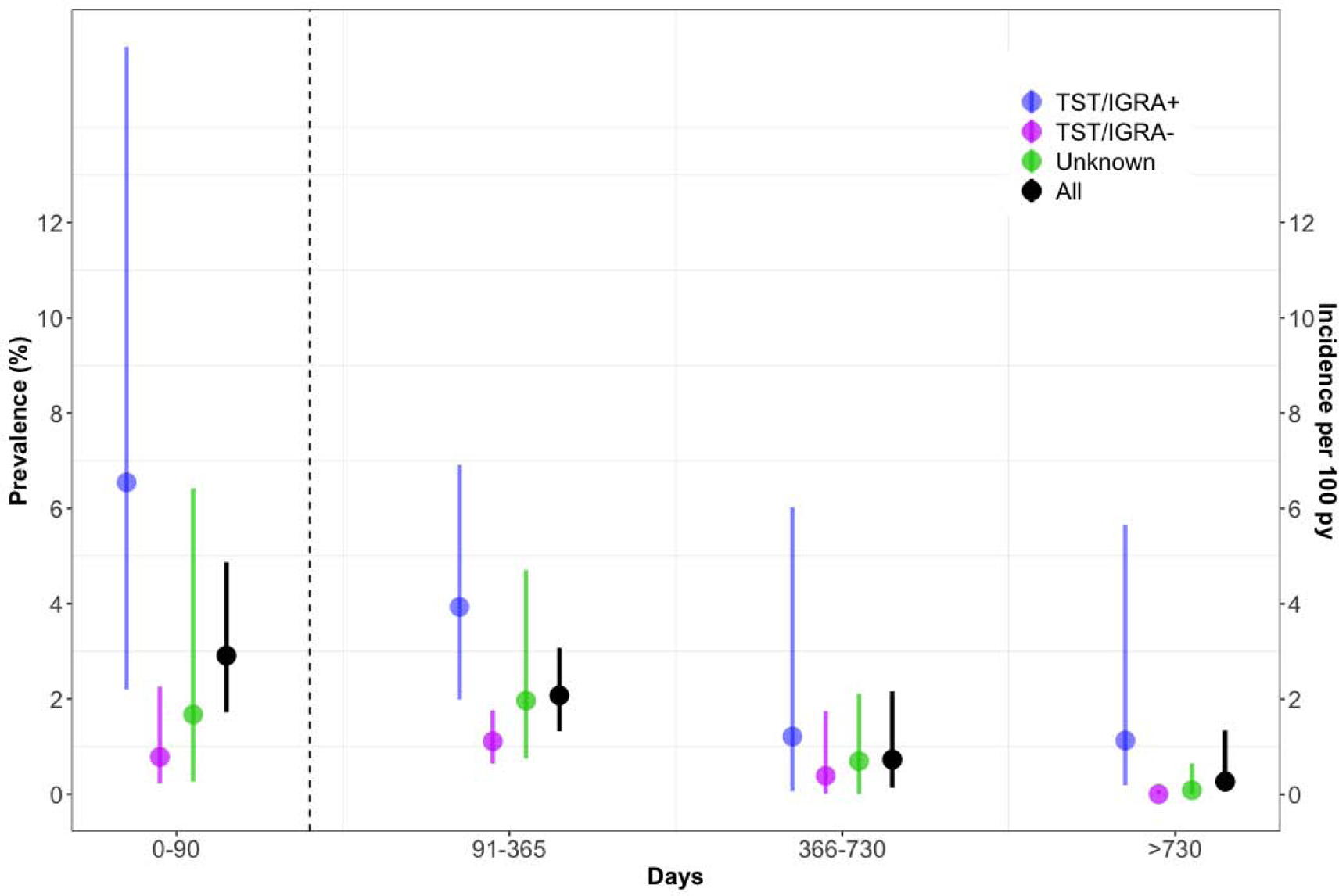
Risk of Developing Tuberculosis Over Time Among Exposed Children Not Receiving Preventive Therapy.
Abbreviations. py, person-years. TST, Tuberculin Skin Test. IGRA, Interferon Gamma Release Assay.
Only prospective studies are included in this analysis. Only children who did not receive preventive chemotherapy were included. The dotted vertical line represents 90 days. Circles represent mean estimates and bars represent 95% confidence intervals for each estimate. Bars may not be visible for some estimates at ‘>730 days’ because the confidence intervals are narrow. Tuberculosis prevalence and incidence are measured on distinct left and right y-axes on the left and right of the Figure. Shown are tuberculosis prevalence within 90 days of enrollment (left y-axis) and subsequent tuberculosis incidence over various intervals (right y-axis), stratified by baseline tuberculin skin test (TST) or interferon gamma release assay (IGRA) status. A positive tuberculin skin test was defined as an induration ≥10 mm, and a positive IGRA result was defined as a positive QuantiFERON-TB Gold In-Tube (QFT) (interferon-γ - nil ≥0.35 IU/mL), or TB-Spot (>8 spot forming cells per well).
Figure 2.
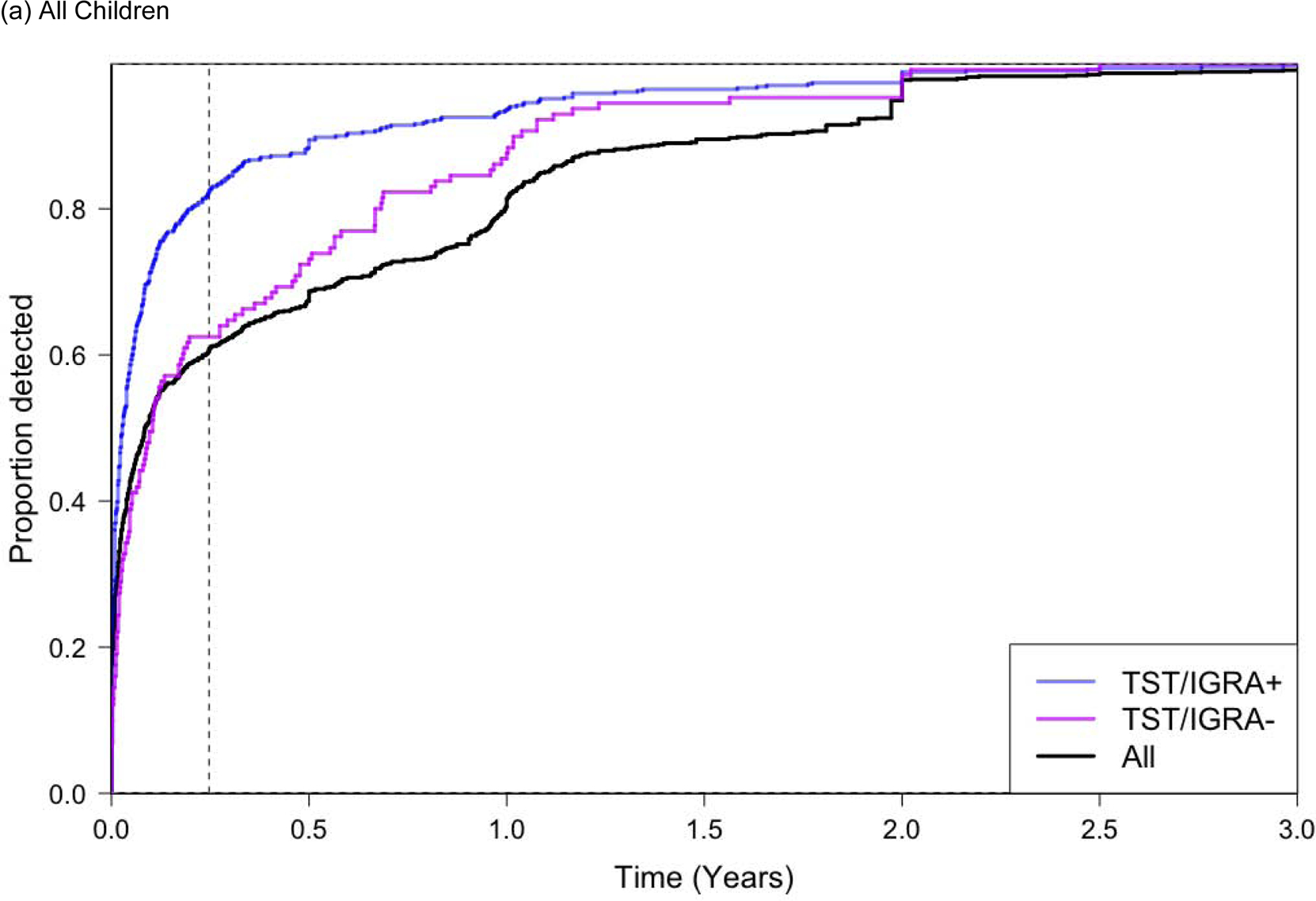
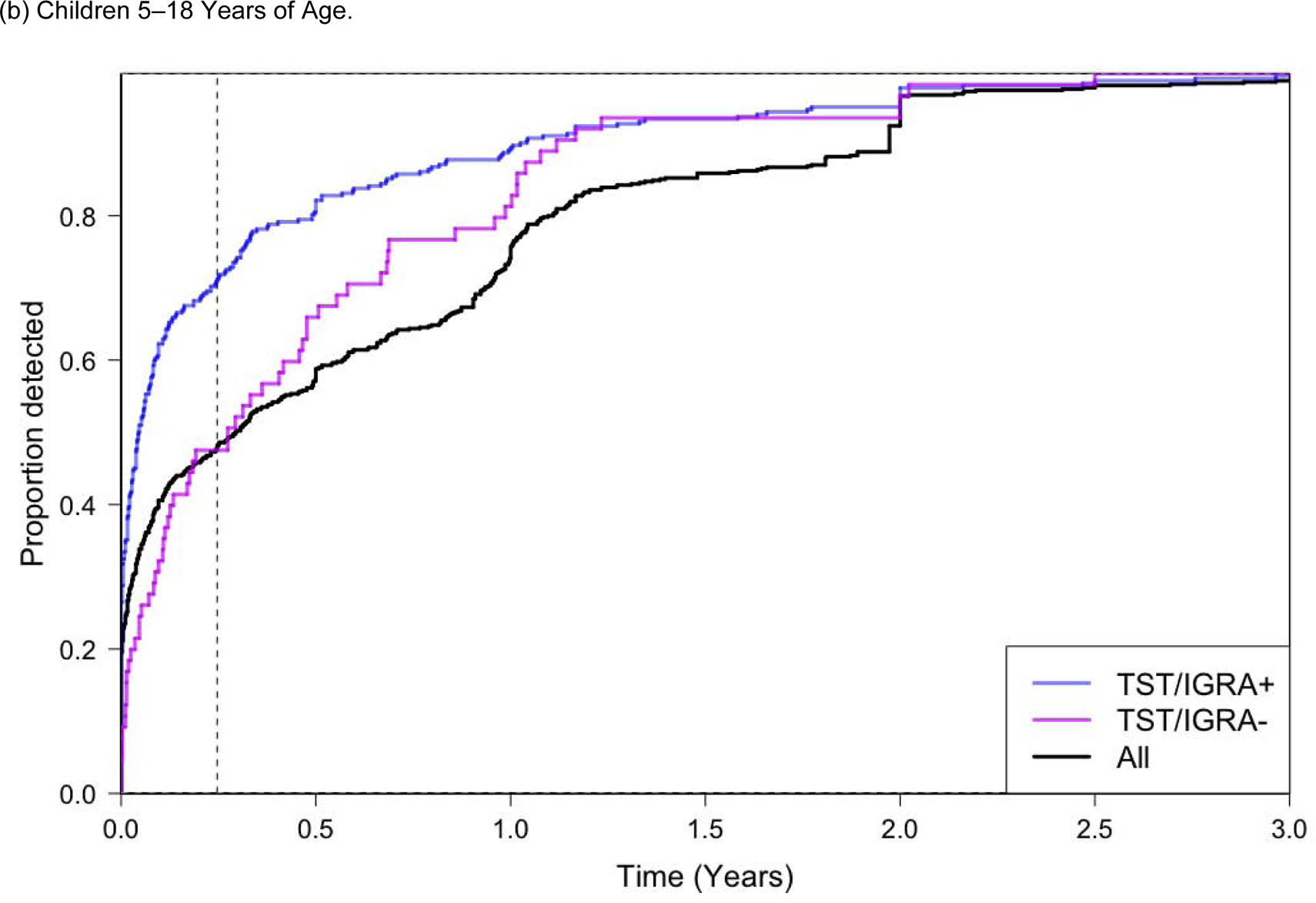
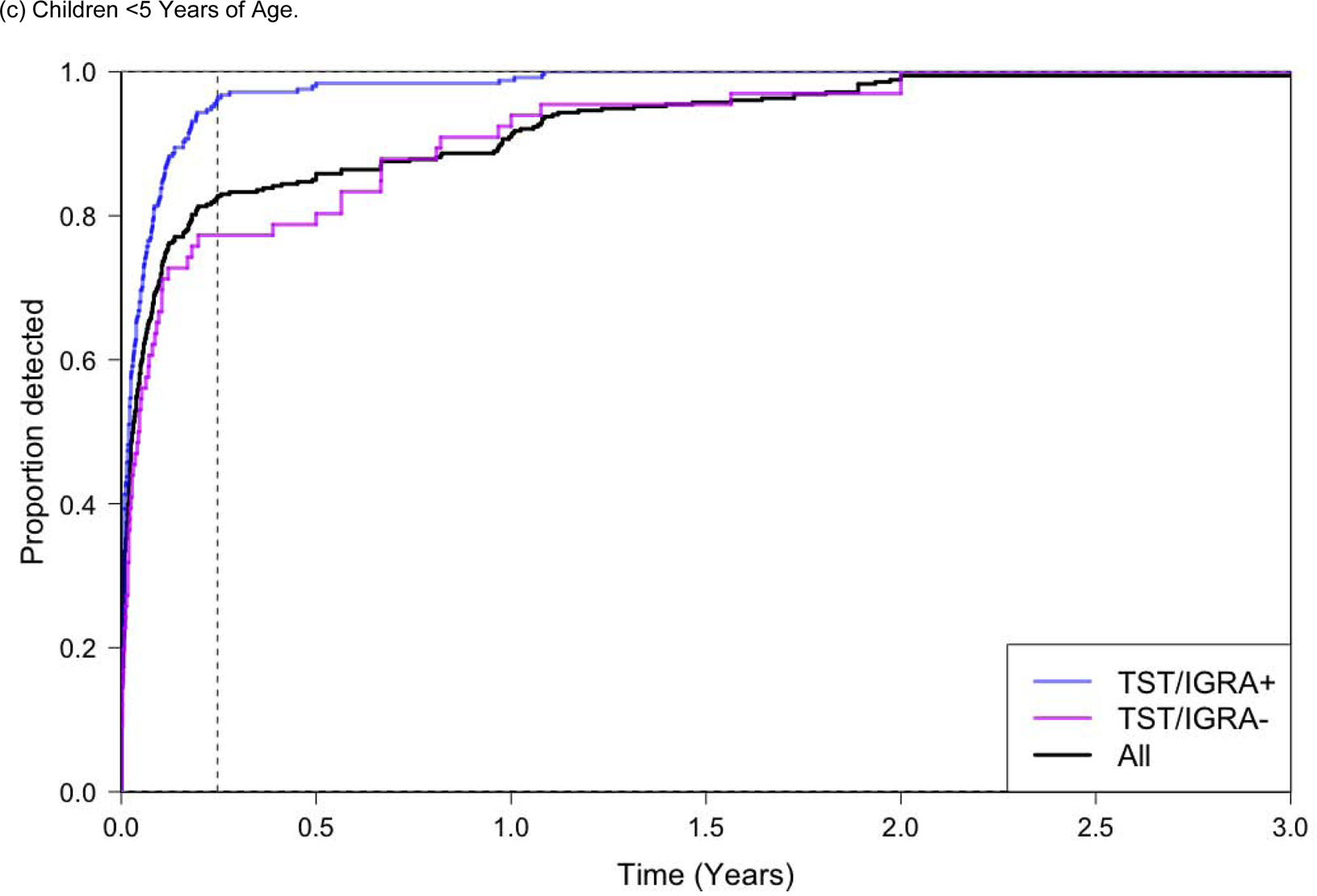
Proportion of All Tuberculosis Cases Diagnosed Over Follow-up Time.
Abbreviations. py, person-years. TST, Tuberculin Skin Test. IGRA, Interferon Gamma Release Assay.
Only prospective studies are included in this analysis. Only children who did not receive preventive chemotherapy were included. The ‘All’ group represents all participants regardless of TST and/or IGRA testing, which is a much larger group of children than those with TST/IGRA+ or TST/IGRA−; the detection proportion for ‘all children’ therefore does not appear as a weighted average between those two groups.
A positive tuberculin skin test was defined as an induration ≥10 mm, and a positive IGRA result was defined as a positive QuantiFERON-TB Gold In-Tube (QFT) (interferon-γ - nil ≥0.35 IU/mL), or TB-Spot (>8 spot forming cells per well). Dotted vertical line represents 90 days in both Figure 2a, 2b, and 2c.
Among all children who developed tuberculosis, 61% were diagnosed in the first 90 days of screening (Figure 2a). This number increased to 82% among children with a baseline positive TST/IGRA. Among children <5 years of age that developed tuberculosis, 83% were diagnosed within 90 days; among these young children with a positive TST/IGRA, 96% were diagnosed within 90 days (Figure 2b). The proportion of children that developed tuberculosis in the first 90 days of screening was much higher for children <5 years of age compared to children 5–18 years of age (Figure 2b and 2c).
The two-year cumulative risk of developing tuberculosis among children not receiving preventive therapy varied substantially by age and infection status. Among all children not on preventive therapy, the 2-year cumulative risk was U-shaped by age (Figure 3c), ranging from 7.6% in children under 5 years of age, decreasing to 5.2% in children 5–9 (P=0.0027 compared to <5 year old children), 5.6% in children 10–14 years old (P=0.0145 compared to <5 year old children), followed by a subsequent increase in risk to 6.7% among children >15 years old P=0.3491 compared to <5 year old children). Children with a negative baseline TST/IGRA had a similar U-shaped curve, but slightly lower rates (Figure 3b). Children with positive baseline TST/IGRAs had significantly higher 2-year cumulative tuberculosis incidence (Figure 3a), greatest among children <5 years of age (19.0%; 95% CI, 8.4–37.4%) (Supplementary Table 2). The cumulative risk among children <5 years old with positive baseline TST/IGRAs was statistically higher when compared to 5–9 year old TST/IGRA positive children (P<0.0001), 10–14 year old TST/IGRA positive children (P<0.0001), and 15–18 year old TST/IGRA positive children (P=0.0006). Among children <5 years of age with a positive baseline TST/IGRA, the 2-year cumulative tuberculosis incidence was relatively consistent in one-year age bins ranging from 16% to 22%.
Figure 3.
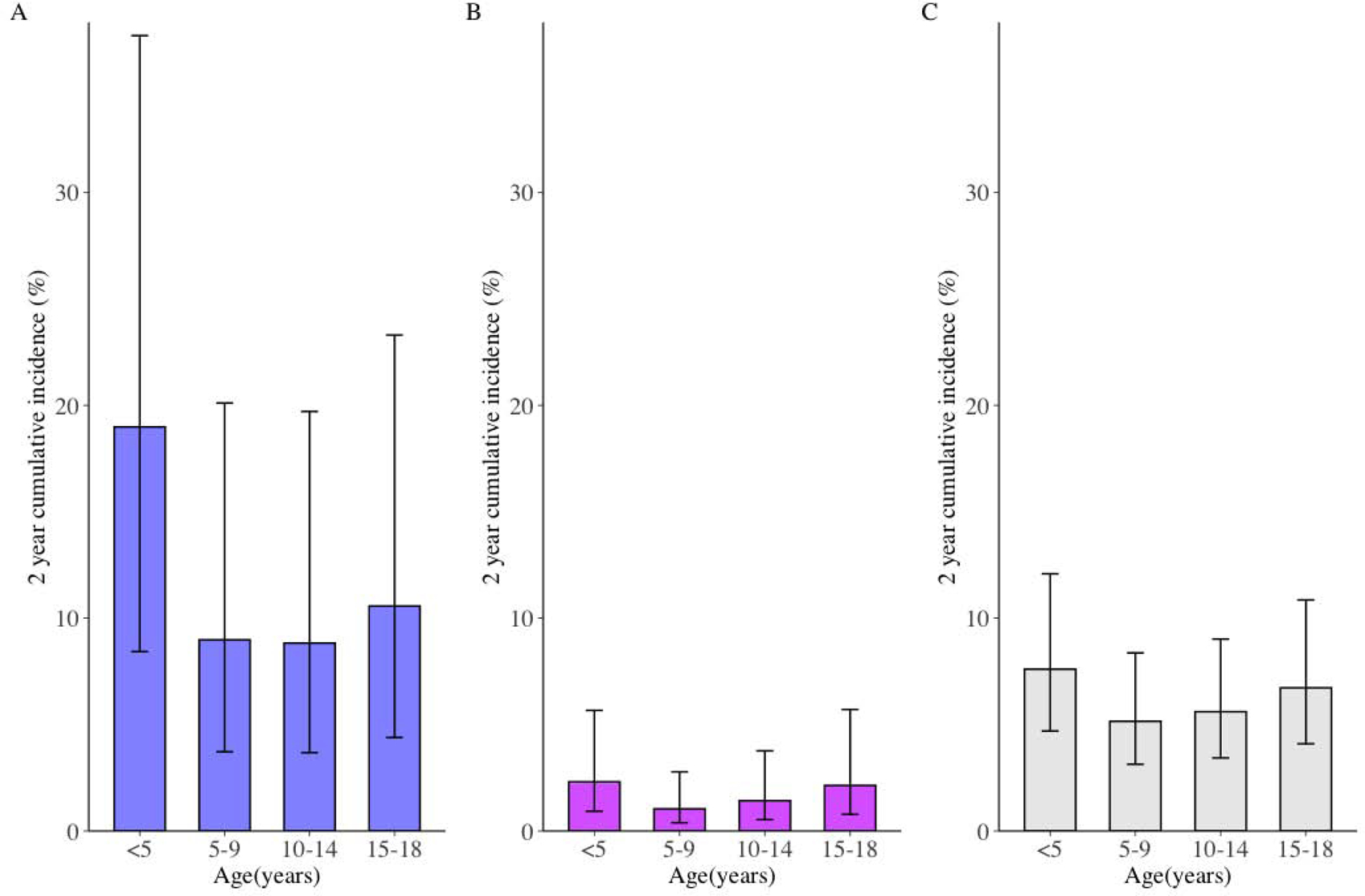
Two-year Cumulative Incidence of Tuberculosis Development in Children Not on Preventive Therapy, Stratified by Age and Infected (left), Uninfected (middle), and All (right) Children.
Abbreviations. py, person-years. TST, Tuberculin Skin Test. IGRA, Interferon Gamma Release Assay.
The two-year cumulative incidence of tuberculosis includes prevalent and incident tuberculosis in the first two years of follow-up from prospective cohort studies, stratified by age and baseline results of tuberculin skin test or interferon gamma release assay.
Only children not given preventive therapy are included in this analysis. Panel A includes only children with tuberculosis infection. Panel B includes only children without tuberculosis infection. Panel C includes all children, including those not tested for tuberculosis infection. A positive infection was determined by one of the following criteria: a tuberculin skin test induration ≥10 mm, a QuantiFERON-TB Gold In-Tube (QFT) (interferon-γ - nil ≥0.35 IU/mL), or a positive TB-Spot (>8 spot forming cells per well). Bars represent mean estimates and lines represent 95% confidence intervals. The two-year cumulative incidence of tuberculosis for children with tuberculosis infection was consistent within each age group bin. For example, the two-year cumulative incidence of tuberculosis was 19% for infected children <5 years of age and ranged from 17% to 21%. Risk of tuberculosis for one-age year bins can be seen in the Supplementary Appendix. In Panel A, the cumulative risk among children <5 years old with positive baseline TST/IGRAs was statistically higher when compared to 5–9 year old TST/IGRA positive children (P<0.0001), 10–14 year old TST/IGRA positive children (P<0.0001), and 15–18 year old TST/IGRA positive children (P=0.0006). In Panel B, the cumulative risk among children <5 years old with negative baseline TST/IGRAs was statistically higher when compared to 5–9 year old TST/IGRA negative children (P=0.0189), but not compared to 10–14 year old TST/IGRA negative children (P=0.1576) or 15–18 year old TST/IGRA positive children (P=0.8335). In Panel C, the cumulative risk among all children <5 years old with positive baseline TST/IGRAs was statistically higher when compared to 5–9 year old TST/IGRA positive children (P=0.0027) and 10–14 year old TST/IGRA positive children (P=0.0145), but not compared to 15–18 year old TST/IGRA positive children (P=0.3491).
Children living with HIV had higher risk of prevalent (Adjusted Odds Ratio [AOR], 2.80, 95% CI, 1.62–4.85) and incident (Adjusted Hazard Ratio [AHR], 5.31, 95% CI, 2.39–11.81) disease (Table 2). Children with a previous tuberculosis episode were more likely to be diagnosed with tuberculosis at baseline (AOR, 6.58, 95% CI, 4.40–9.84) and during follow up (AHR, 3.20, 95% CI, 2.22–4.51). There was substantial between-study heterogeneity in prevalent and incident tuberculosis, with differences by study design and region (Figure 4).
Table 2.
Risk Factors for Tuberculosis Amongst Children Less than 19 Years of Age.
| Characteristic | Coprevalent Tuberculosis, Adjusted Odds Ratio (95% CI) | Incident Tuberculosis, Adjusted Hazard Ratio (95% CI) | All Tuberculosis, Adjusted Odds Ratio (95% CI) |
|---|---|---|---|
| All Studies (N = 137,647) | |||
| Male Sex | 1.05 (0.96, 1.13) | 0.99 (0.88, 1.13) | 1.03 (0.94, 1.12) |
| Tuberculosis Infection‡ | |||
| Tuberculin Skin Test Induration ≥10 mm | 18.30 (14.87, 22.52) | 3.34 (2.86, 3.89) | 7.05 (6.27, 7.94) |
| QuantiFERON Gold In-Tube Test, ≥0.35 IU/mL | 21.90 (8.41, 57.06) | 6.47 (2.21, 18.90) | 14.26 (6.94, 29.28) |
| ELISPOT, >8 spot-forming cells* | 7.77 (1.69, 35.63) | 1.91 (0.64, 5.70) | 3.06 (6.94, 29.28) |
| HIV infection | 2.80 (1.62, 4.85) | 5.31 (2.39, 11.81) | 3.55 (2.20, 5.74) |
| Prior Tuberculosis Event | 6.58 (4.40, 9.84) | 3.20 (2.22, 4.51) | 5.30 (3.99, 7.06) |
| Preventive Drug Therapy Regimen† | |||
| All children | … | 0.37 (0.30, 0.47) | … |
| TST+ or IGRA+ | … | 0.15 (0.11, 0.20) | … |
| TST+ or IGRA+, Propensity-Score Matched | … | 0.09 (0.05, 0.15) | … |
| TST- or IGRA- | … | 0.65 (0.40, 1.06) | … |
| TST- or IGRA-, Propensity-Score Matched | … | 0.66 (0.40, 1.10) | … |
| BCG vaccination | |||
| 5–18 years of age | 0.96 (0.70, 1.31) | 0.91 (0.70, 1.18) | 0.90 (0.73, 1.10) |
| <5 years of age | 0.62 (0.45, 0.85) | 0.71 (0.46, 1.08) | 0.64 (0.50, 0.84) |
| Prospective (versus Retrospective) Data Collection | 3.00 (1.45, 6.21) | 3.42 (1.83, 6.42) | 2.38 (1.38, 4.13) |
Abbreviations: TST, Tuberculin Skin Test. IGRA, Interferon Gamma Release Assay. CI, confidence interval. HIV, human immunodeficiency virus. BCG, bacillus Calmette–Guérin.
Both prospective and retrospective studies are included in this analysis. This analysis was repeated with stratification of the prospective/retrospective nature of the data collection; this stratified analysis can be seen in the Supplementary Appendix. Each row represents a distinct statistical model. Each statistical model is adjusted for the variable of interest, baseline child age and sex, whether data was collected prospectively or retrospectively, and the study. The referent group for each row is the opposing value of the listed characteristic. For example, for HIV infection the reference group is children living without HIV. This includes sub-characteristics of variables. For example, the referent group for the sub-characteristic ‘Tuberculin Skin Test Induration ≥10 mm’ under the variable ‘Tuberculosis Infection’ is participants with a ‘Tuberculin Skin Test Induration <10 mm’. Measures of association are reported with 95% confidence intervals for all outcomes. Odds ratios are reported for “All Tuberculosis” which includes both prevalent and incident tuberculosis as one outcome. Prevalent tuberculosis was defined as any diagnosed disease before 90 days from the baseline evaluation. Incident tuberculosis was defined as diagnosed tuberculosis at or after 90 days from the initial contact investigation visit. In this case, contacts with prevalent tuberculosis are not given or protected by preventive therapy.
All tests for tuberculosis infection (tuberculin skin test, QuantiFERON Gold In-Tube test, and ELISpot tests) were administered at baseline. TST/IGRAs may be used in the case definition for tuberculosis, potentially leading to diagnostic bias. Odds Ratios for tests of tuberculosis infection may be understood as “Diagnostic Odds Ratios”.
Administration of preventive therapy, including any type of preventive therapy regimen.
Propensity score matching is based on the age and sex of the contact and whether the study design is prospective or retrospective.
A preventive drug therapy regimen was defined as iniitation of any regimen given and started to children at the baseline visit. These included isoniazid for six months, isoniazid for nine months, rifampin for three months, and rifapentine for three months. Preventive therapy was administered to children at the discretion of each study site and we accepted each study’s decision to administer preventive therapy. Completion of preventive therapy was not reported for almost all studies.
Figure 4.
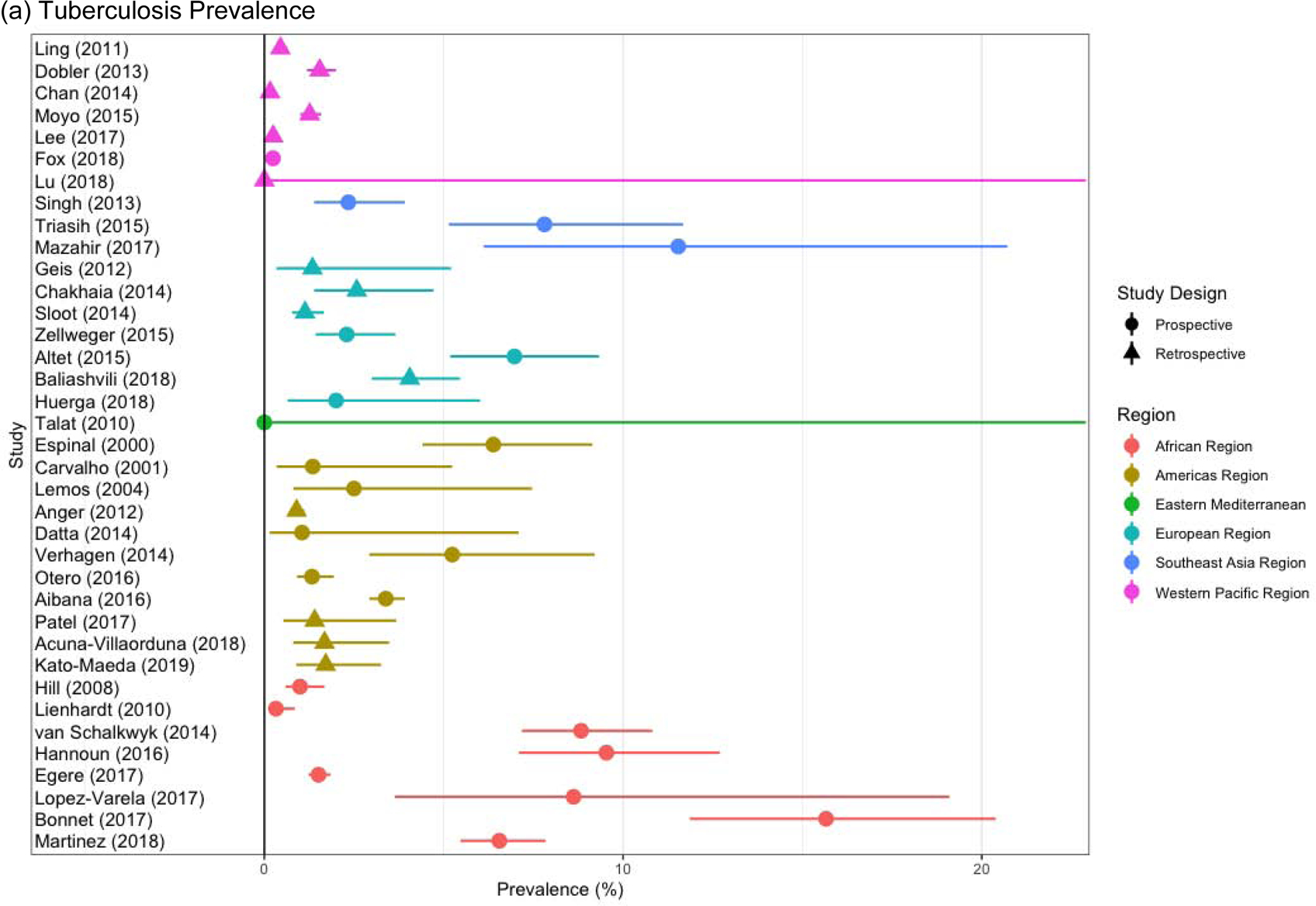
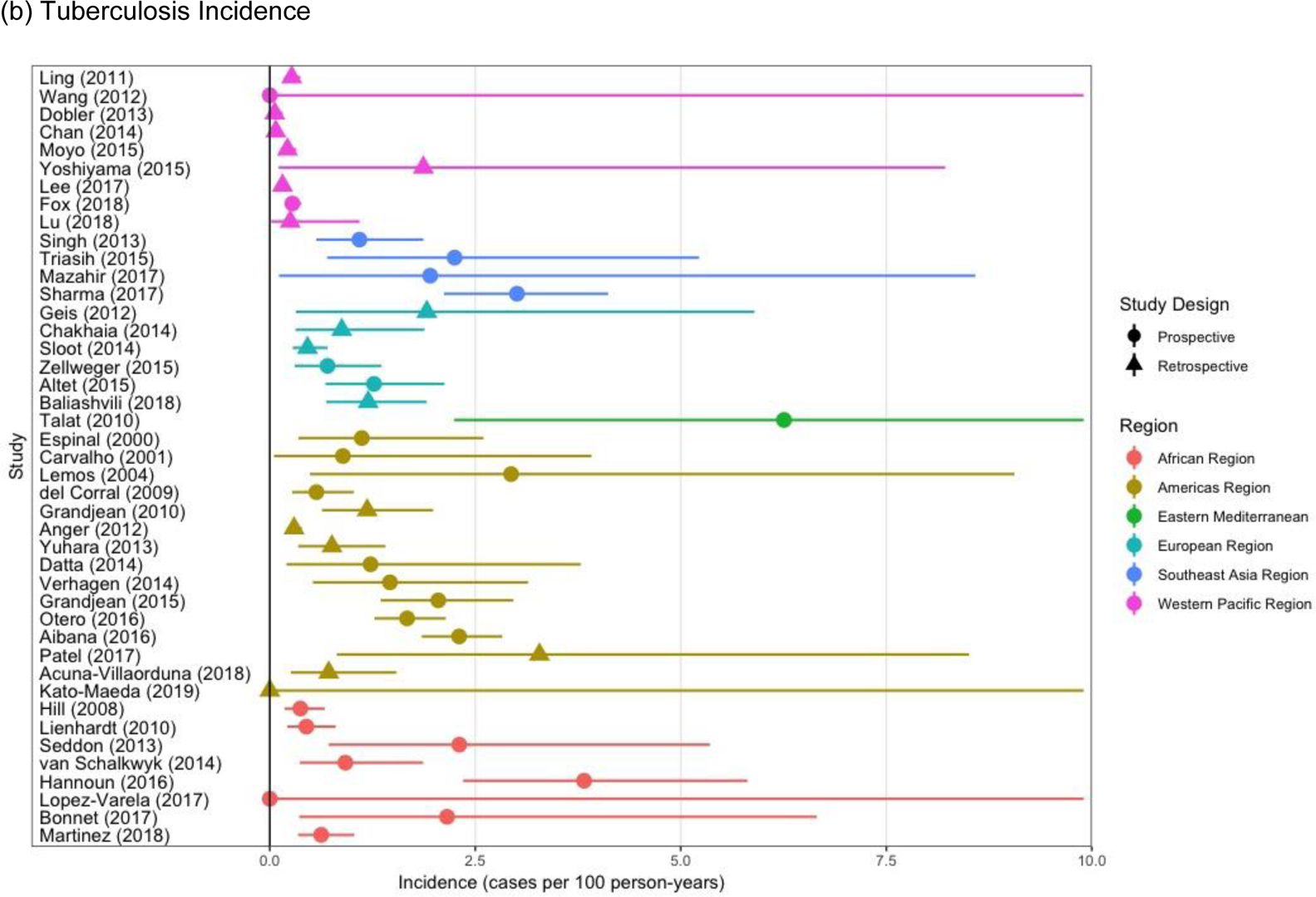
Study-specific Prevalent (a) and Incident (b) Tuberculosis in Children, Stratified by the Study Design and Region.
All children were included in Figure 4a and 4b
Prevalent and incident tuberculosis rates changed substantially based on the cutoff threshold used (Supplementary Appendix). Among all children, for cutoff thresholds from baseline of 0, 30, and 60 days from baseline, prevalence rates were 0.4% (95% CI, 0.2–1.2%), 1.2% (95% CI, 0.4–3.5%), and 1.7% (95% CI, 0.7–4.3) (Supplementary Table S5). Among children with a positive TST/IGRA, for cutoff thresholds from baseline of 0, 30, and 60 days from baseline, prevalence rates were 0.9% (95% CI, 0.2–3.7%), 3.8% (95% CI, 1.6–9.1%), and 4.6% (95% CI, 1.8–10.8) (Supplementary Table S5).
Protective Effectiveness of Preventive Therapy and BCG Vaccination
Children given preventive therapy were at substantially lower risk of developing tuberculosis compared to those who were not, and this effect was modified by infection status. The effectiveness of preventive therapy was 63% (AHR, 0.37, 95% CI, 0.30–0.47) among all exposed children. The effectiveness was greater in children with baseline infection (AHR, 0.09, 95% CI, 0.05–0.15), and a strong but nonsignificant relation in children without baseline infection (AHR, 0.66, 95% CI, 0.40–1.10). This analysis was reasonably robust to alternative statistical models without use of propensity score matching and alternative propensity scores (Supplementary Appendix). Additionally, the effect of preventive therapy in drug for incident tuberculosis was present in contacts of drug-susceptible (AHR, 0.33, 95% CI, 0.20–0.54) and drug-resistant (AHR, 0.44, 95% CI, 0.21–0.93) tuberculosis index cases (Pinteraction=0.454).
In children <5 years old, BCG vaccination was protective against all forms of tuberculosis (AOR, 0.64, 95% CI, 0.50, 0.84). However, among children five years and above, those receiving a BCG vaccination had similar risk of tuberculosis compared to those that did not (Table 2).
Study Heterogeneity
There was between-study heterogeneity in prevalent and incident tuberculosis. Prevalent tuberculosis ranged from 0–15% (Figure 4a). The rate of incident tuberculosis per 100 person-years ranged from 0–3.3% (Figure 4b). Much of the heterogeneity for both prevalent and incident tuberculosis was due to the global region of the study and the prospective/retrospective nature of data collection (Figure 4a and Figure 4b).
Compared to studies in the African region, studies demonstrated substantially lower rates of prevalent tuberculosis in the Americas Region (AOR, 0.48, 95% CI, 0.21–1.12) and the Western Pacific Region (AOR, O.10, 95% CI, 0.04–0.23). Incident tuberculosis was also lower in the Western Pacific Region versus the African Region (AHR, 0.16, 95% CI, 0.07–0.35). Prospective studies identified more prevalent (AOR 3.26, 95% CI, 1.49–7.12) and incident tuberculosis (AHR 3.12, 95% CI, 1.65–5.90) (Table 2).
The region and design of studies were correlated; all studies from the African Region were prospective and all but one study in the Western Pacific Region22 were retrospective. Therefore, we were unable to evaluate whether between-study heterogeneity was due to regional epidemiological differences, prospective or retrospective study design, or a combination of both.
DISCUSSION
Using individual-level data from 137,647 exposed children followed for 429,538 child-years, we found that the two-year cumulative risk of tuberculosis in children is very high, approaching 20% in tuberculosis-infected children under the age of 5. Preventive therapy was 63% effective among all children, and 91% effective among those with a positive TST/IGRA. However, we also found that nearly two-thirds of all pediatric tuberculosis cases, and >80% of cases among young children, were diagnosed within 90 days of contact investigation initiation, suggesting a large proportion of cases may not be avoided by preventive therapy. As over 15 million children are exposed to tuberculosis globally every year,1–2 these estimates indicate that many exposed children, especially those with recent infection, are at substantial risk of developing tuberculosis and must be prioritized by development of new prevention and early case finding strategies.
These results provide the first contemporary estimates of tuberculosis risk in children after close exposure. Historical studies on children performed prior to 1950 were recently synthesized.6,7 These historical studies suggested that the risk of tuberculosis after recent infection was between 30–50% in early infancy.8–11 We found that exposed, TST/IGRA positive children <1 year of age who did not receive preventive therapy had 18% risk of developing disease within two years of enrollment. In contrast to previous estimates suggesting risk falls to 5% in 2–5-year-olds,6,7 we found that this age group had 19% two-year cumulative tuberculosis risk. Additionally, although our results indicate that young children have the highest risk of developing tuberculosis, adolescents also face an increasing risk following childhood.23,24
We believe these findings have several important clinical and public health implications. First, we found marked protection of preventive therapy against incident tuberculosis. Protection was greatest among children with a positive TST/IGRA (91%), but there was a relationship among all children. Among children with a negative TST/IGRA, there was a 44% protective effect however this association was not statistically significant (95% CI, −10–60%). A meta-analysis of seven trials including 10,320 children (8,537 recruited prior to 1975) found that efficacy was 59% among children over 4 months of age, comparable to our overall estimate of 63%, but lacked analyses stratified by infection status.25 Second, we found that 61% of all tuberculosis cases in children were diagnosed within 90 days of initial screening, and thus are not targetable by preventive therapy. This number increased to 82% and 83% in children with tuberculosis infection and below 5 years of age, suggesting the importance of early case-finding. While preventive therapy and contact tracing are effective and have value in averting disease among children,3 most children are reached too late to prevent disease. Although cost-effectiveness analyses and implementation barriers should be assessed, earlier diagnosis of adult cases or community-wide screening approaches in children may be needed to improve prevention of tuberculosis in children.26 Third, we provide robust estimates of tuberculosis risk in children living with HIV infection or with a prior tuberculosis diagnosis. These children should be prioritized for prevention interventions and monitoring for development of disease. Fourth, there has been concern that IGRAs may perform poorly in young children; however, recent studies have found good performance in infants <2 years of age.27,28 Our study confirms these results in all children, finding that a child <19 years of age with a positive IGRA test has 6–7 times higher risk of incident tuberculosis than a child with a negative IGRA test.
The results of our analyses should be understood within the context of the limitations of the observational data from the multiple cohorts included in this study. First, there was heterogeneity in the definition of close exposure and tuberculosis diagnosis across studies. Diagnosis of tuberculosis in children is inherently challenging,3,27,29 as available diagnostics lack sensitivity, particularly among young children. As a result, experts typically recommend using composite definitions for diagnosis.29 Most studies included in this analysis used composite definitions that included microbiological testing as part of the diagnostic criteria. Due to poor ascertainment of pediatric tuberculosis during passive case finding, we limited our analysis of the tuberculosis incidence to prospective cohort studies. When assessing the effectiveness of preventive therapy, confounding by indication may occur if therapy was given to the children at higher or lower tuberculosis risk. We used propensity score matching to account for covariates predicting receipt of preventive therapy. However, residual confounding is possible and could bias these efficacy estimates in either direction. We also did not have dates of preventive therapy initiation. Additionally, TST/IGRAs may be used in the case definition for tuberculosis, potentially leading to diagnostic bias. These factors may partially explain the high proportion of tuberculosis cases diagnosed within 90 days. We defined prevalent tuberculosis as cases diagnosed within 90 days of enrollment, to account for diagnostic delays inherent in establishing a tuberculosis diagnosis in children; we examined multiple other thresholds (0, 30, 60 days) in sensitivity analyses and found an increased prevalence between 0 and 90 days of age which may reflect rapid development of incident cases.
In summary, this study represents a combined analysis of data from 46 cohort studies in 34 countries, representing diverse sociodemographic and epidemiological settings. These results identify key age and risk-factor specific groups of children that can be prioritized by tuberculosis control programs and find that while preventive therapy is highly effective for the individual child, this strategy can only be targeted to a minority of children and must be used as a supplementary intervention with intensified case-finding efforts to address the global burden of pediatric tuberculosis.
Supplementary Material
Research in context.
Evidence before this study
No contemporary studies have attempted to quantify the risk of developing paediatric tuberculosis after close exposure to a tuberculosis case or recently acquired tuberculosis infection. One narrative review of seven historical studies conducted prior to 1940 exists. This study synthesized results from these historical studies and found that approximately 50% of children <1 year of age with recent infection developed tuberculosis. This risk in children dropped to 10–15% in children 1–2 years of age, 5–6% in children 2–5 years of age, 2% in children 5–10 years of age, and rises to 10% among children >10 years old.
We searched MEDLINE and Google Scholar for articles published prior October 1, 2019. We used the search terms “child”, “tuberculosis”, “transmission”, “household”, “pediatric”, “paediatric”, “contact”, “close”, among others. We also reviewed reference lists, bibliographies, and other narrative reviews on incident tuberculosis for additional relevant articles. We found several contemporary household contact exposure studies that included children but none that focused on children or that included a large sample size. We did not identify estimates of longitudinal risk of tuberculosis in infants and young children with close exposure or recent infection. Due to this knowledge gap, the effectiveness of contact investigations and preventive therapy remains poorly understood.
Added value of this study
Using individual-level data from 46 cohort studies including 137,647 exposed children followed for 429,538 child-years, these results provide the first contemporary estimates of tuberculosis risk in children after close exposure. We found that exposed, TST/IGRA positive children <1 year of age who did not receive preventive therapy had 18% risk of developing disease within two years of enrollment. In contrast to previous estimates suggesting risk falls to 5% in 2–5-year-olds, we found that this age group had 19% two-year cumulative tuberculosis risk. In addition, the effectiveness of preventive therapy to prevent incident tuberculosis was high, 85% among children with tuberculosis infection. Despite this, the majority of children developed tuberculosis within weeks of the initial baseline contact investigation visit.
Implications of all the available evidence
Results from this multi-cohort collaboration indicate that greater focus should be placed on the first five years of life as a period of high risk of progression from tuberculosis infection to disease. The risk of developing tuberculosis among exposed infants and young children was very high, approaching 20% two years after exposure. Despite the effectiveness of preventive therapy, the majority of cases occurred within weeks of contact investigation initiation. While contact tracing is a high yield means for early case detection, many children are reached too late to prevent disease. Earlier diagnosis of adult cases or community-wide screening approaches in children may be needed to improve prevention of tuberculosis in children.
Acknowledgements.
We acknowledge and thank the participants and investigators in these studies. We would also like to acknowledge Catherine Paulsen and Mary Lou Egedahl for their contributions.
Funding Sources: LM was supported by an NIH T32 AI 052073 grant award.
Funding. National Institutes of Health.
Footnotes
Conflicts of Interest:None
Contributor Information
C. Robert Horsburgh, Department of Medicine, Boston University School of Medicine, Boston, USA; Department of Epidemiology, Biostatistics and Global Health, Boston University School of Public Health, Boston, United States..
Jason R. Andrews, Stanford University, School of Medicine, Division of Infectious Diseases and Geographic Medicine, Stanford, California, United States.
References.
- 1.Dodd PJ, Gardiner E, Coghlan R and Seddon JA, 2014. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. The lancet global health, 2(8), pp. e453–e459. [DOI] [PubMed] [Google Scholar]
- 2.Yuen CM, Jenkins HE, Chang R, Mpunga J and Becerra MC, 2016. Two methods for setting child-focused tuberculosis care targets. Public health action, 6(2), pp.83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez L, le Roux DM, Barnett W, Stadler A, Nicol MP and Zar HJ, 2018. Tuberculin skin test conversion and primary progressive tuberculosis disease in the first 5 years of life: a birth cohort study from Cape Town, South Africa. The lancet child & adolescent health, 2(1), pp.46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenkins HE, Tolman AW, Yuen CM, Parr JB, Keshavjee S, Pérez-Vélez CM, Pagano M, Becerra MC and Cohen T, 2014. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. The Lancet, 383(9928), pp.1572–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodd PJ, Sismanidis C and Seddon JA, 2016. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. The Lancet infectious diseases, 16(10), pp.1193–1201. [DOI] [PubMed] [Google Scholar]
- 6.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, Enarson DA, Donald PR and Beyers N, 2004. The natural history of childhood intra-thoracic tuberculosis: A critical review of literature from the pre-chemotherapy era [state of the art]. The International Journal of Tuberculosis and Lung Disease, 8(4), pp.392–402. [PubMed] [Google Scholar]
- 7.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Nelson LJ, Enarson DA, Donald PR and Beyers N, 2004. The clinical epidemiology of childhood pulmonary tuberculosis: A critical review of literature from the pre-chemotherapy era [state of the art]. The International Journal of Tuberculosis and Lung Disease, 8(3), pp.278–285. [PubMed] [Google Scholar]
- 8.Brailey M A study of tuberculous infection and mortality in the children of tuberculous households. Am J Hygiene 1940; 31: Sec.A1–43. [Google Scholar]
- 9.Bentley FJ, Grzybowski S, Benjamin B. Tuberculosis in childhood and adolescence The National Association for the Prevention of Tuberculosis. London, England: Waterlow and Sons Ltd, 1954: 1–213 and 238–253. [Google Scholar]
- 10.Lincoln EM, Sewell EM. Tuberculosis in children. New York, NY: McGraw-Hill Book Company Inc, 1963: 1–315. [Google Scholar]
- 11.Miller FJW, Seal RME, Taylor MD. Tuberculosis in children. London, UK: J and A Churchill Ltd, 1963: 163–275 and 466–587. [Google Scholar]
- 12.Comstock GW, Livesay VT and Woolpert SF, 1974. The prognosis of a positive tuberculin reaction in childhood and adolescence. American Journal of Epidemiology, 99(2), pp.131–138. [DOI] [PubMed] [Google Scholar]
- 13.Curry FJ, 1967. Prophylactic effect of isoniazid in young tuberculin reactors. New England Journal of Medicine, 277(11), pp. 562–567. [DOI] [PubMed] [Google Scholar]
- 14.Zar HJ, Cotton MF, Strauss S, Karpakis J, Hussey G, Schaaf HS, Rabie H and Lombard CJ, 2007. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomised controlled trial. BMJ, 334(7585), p.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowenthal ED, Bakeera-Kitaka S, Marukutira T, Chapman J, Goldrath K and Ferrand RA, 2014. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: a review of emerging challenges. The Lancet Infectious Diseases, 14(7), pp.627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trunz BB, Fine PEM and Dye C, 2006. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. The Lancet, 367(9517), pp.1173–1180. [DOI] [PubMed] [Google Scholar]
- 17.Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G and Tierney JF, 2015. Preferred reporting items for a systematic review and meta-analysis of individual participant data: the PRISMA-IPD statement. JAMA, 313(16), pp.1657–1665 [DOI] [PubMed] [Google Scholar]
- 18.Martinez L, Shen Y, Mupere E, Kizza A, Hill PC and Whalen CC, 2017. Transmission of Mycobacterium tuberculosis in households and the community: a systematic review and meta-analysis. American Journal of Epidemiology, 185(12), pp.1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox GJ, Barry SE, Britton WJ and Marks GB, 2013. Contact investigation for tuberculosis: a systematic review and meta-analysis. European Respiratory Journal, 41(1), pp.140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah NS, Yuen CM, Heo M, Tolman AW and Becerra MC, 2013. Yield of contact investigations in households of patients with drug-resistant tuberculosis: systematic review and meta-analysis. Clinical infectious diseases, 58(3), pp.381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison J, Pai M and Hopewell PC, 2008. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. The Lancet infectious diseases, 8(6), pp.359–368. [DOI] [PubMed] [Google Scholar]
- 22.Wells GA, Shea B, O’Connell D, Petersen J, Welch V, Losos M, & Tugwell P (2012). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses Department of Epidemiology and Community Medicine, University of Ottawa, Canada: University of Ottawa, Canada: Available at: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 23.Snow KJ, Sismanidis C, Denholm J, Sawyer SM and Graham SM, 2018. The incidence of tuberculosis among adolescents and young adults: a global estimate. European Respiratory Journal, 51(2), p.1702352. [DOI] [PubMed] [Google Scholar]
- 24.García-Basteiro Alberto L., Schaaf H. Simon, Diel Roland, and Battista Migliori Giovanni. “Adolescents and young adults: a neglected population group for tuberculosis surveillance.” (2018): 1800176. [DOI] [PubMed] [Google Scholar]
- 25.Ayieko J, Abuogi L, Simchowitz B, Bukusi EA, Smith AH and Reingold A, 2014. Efficacy of isoniazid prophylactic therapy in prevention of tuberculosis in children: a meta–analysis. BMC Infectious Diseases, 14(1), p.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez L, Lo NC, Cords O, Hill PC, Khan P, Hatherill M, Mandalakas A, Kay A, Croda J, Horsburgh CR and Zar HJ, 2019. Paediatric tuberculosis transmission outside the household: challenging historical paradigms to inform future public health strategies. The Lancet Respiratory Medicine, 7(6), pp.544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrews JR, Nemes E, Tameris M, Landry BS, Mahomed H, McClain JB, Fletcher HA, Hanekom WA, Wood R, McShane H and Scriba TJ, 2017. Serial QuantiFERON testing and tuberculosis disease risk among young children: an observational cohort study. The Lancet Respiratory Medicine, 5(4), pp.282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandalakas AM, Kirchner HL, Walzl G, Gie RP, Schaaf HS, Cotton MF, Grewal HM and Hesseling AC, 2015. Optimizing the detection of recent tuberculosis infection in children in a high tuberculosis–HIV burden setting. American Journal of Respiratory and Critical Care Medicine, 191(7), pp.820–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham SM, Cuevas LE, Jean-Philippe P, Browning R, Casenghi M, Detjen AK, Gnanashanmugam D, Hesseling AC, Kampmann B, Mandalakas A and Marais BJ, 2015. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clinical Infectious Diseases, 61(suppl_3), pp.S179–S187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


