Abstract
The opioid epidemic has become a severe public health problem, with approximately 130 opioid-induced deaths occurring each day in the United States. Prescription opioids are responsible for approximately 40% of these deaths. Oxycodone is one of the most commonly abused prescription opioids, but despite its prevalent misuse, the number of preclinical studies investigating oxycodone-seeking behaviors are relatively limited. Furthermore, preclinical oxycodone studies that include female subjects are even more scarce, and it is critical that future work includes both sexes. Additionally, the oral route of administration is one of the most common routes for recreational users, especially in the early stages of drug experimentation. However, currently only two studies have been published investigating operant oral oxycodone self-administration in rodents. Therefore, the primary goal of the present study was to establish an oral oxycodone operant self-administration model in adult male and female rats, as well as to examine a potential mechanism of stress-primed reinstatement. We found that females consumed significantly more oral oxycodone than males in operant self-administration sessions. We also found that active oxycodone self-administration was reduced by mu opioid receptor antagonism and by substitution of water for oxycodone solution. Lastly, we induced stress-primed reinstatement and found that this behavior was significantly attenuated by antagonism of the neurokinin-1 receptor, consistent with our prior work examining stress-induced reinstatement of alcohol- and cocaine-seeking.
Introduction
Prescription opioid abuse is a severe public health issue in the United States, with approximately 17,000 prescription opioid-related deaths annually (CDC), and an estimated economic burden of $78.5 billion (Florence et al., 2016). The current overdose rate is five times higher than in 1999 (CDC), highlighting the acceleration of the opioid addiction crisis over the last two decades. In addition to prescription opioid abuse being an issue in and of itself, epidemiological data suggest that it also increases the risk of later heroin abuse by approximately 40-fold (NIDA , Jones 2013, Jones, Logan et al. 2015). As of 2013, approximately 75% of people seeking treatment for heroin addiction first began with nonmedical use of prescription opioids (Cicero et al., 2014).
Oxycodone is one of the most frequently prescribed opioid analgesics, with approximately 59 million oxycodone containing prescriptions filled in 2013. This drug is available in several formulations, including under the trade name Roxicodone, as a high dose, slow release pill (OxyContin), and in combination with NSAIDs (Percocet) (DEA 2014), each of which act as a potent mu opioid receptor (MOR) agonist (Kalso, 2005). While MOR agonism is responsible for the strong analgesic effects of oxycodone, this property is also responsible for its off-target reinforcing effects, giving this drug high abuse potential (Olkkola et al., 2013). Due to these properties, and as the availability of oxycodone has continued to increase over time, misuse, dependence, and incidents of overdose have increased in parallel, culminating in our current epidemic (Olkkola et al., 2013; Paulozzi et al., 2006; Paulozzi et al., 2011; Warner et al., 2009).
Despite the prevalence of this issue, preclinical studies investigating oxycodone are underrepresented in the current addiction literature. A PubMed search for “rat oxycodone self-administration” yields only 27 results, compared to 583 results obtained for “rat morphine self-administration.” The vast majority of these oxycodone studies assessed intravenous (i.v.) self-administration (see, for example (Blackwood et al., 2018; Mavrikaki et al., 2017; Neelakantan et al., 2017; Nguyen et al., 2018; Pravetoni et al., 2014a; You et al., 2018)). While these studies and others have provided significant findings regarding i.v. intake, epidemiological data suggest that one of the preferred routes of administration (ROA) for prescription opioid abuse is oral, in that 72% of chronic abusers and 97% of recreational abusers report a preference for this method of use (Kirsh et al., 2012). While many opioid abusers also report a high preference for snorting prescription opioids, the pharmacokinetic profile of which is more similar to an i.v. ROA (Hines, Lynskey et al. 2017), it should be noted that the majority of this population initiated their abuse using the oral ROA (Hays 2004). However, only two operant self-administration studies to date have investigated oxycodone-seeking using the oral ROA in rodents (Enga et al., 2016; Jimenez et al., 2017). Therefore, an increase in preclinical studies assessing oral oxycodone administration is crucial.
In addition to the need for more studies assessing oral oxycodone intake, inclusion of female subjects is essential to elucidate the role of sex in the progression of oxycodone use disorders. Currently, only two rodent oxycodone self-administration studies using both male and female subjects have been published (Jimenez et al., 2017; Mavrikaki et al., 2017). While a marked increase in prescription opioid-induced overdoses has been observed since 1999, this effect is even more pronounced in females, in that a seven-fold increase occurred in females compared to a four-fold increase in males (NIDA). Some data also suggest that women transition from a state of regular opioid abuse to dependence more quickly than men, often referred to as the telescoping effect (Hernandez-Avila et al., 2004). Lastly, women with opioid use disorder report significantly higher subjective craving for opioids than men (Back et al., 2011), a factor that is known to contribute significantly to incidence of relapse (Northrup et al., 2015).
Several therapeutic targets such as the MOR and dopamine D3 receptor have been identified for their potential to prevent certain oxycodone-seeking behaviors (Bossert et al., 2018; Neelakantan et al., 2017; You et al., 2018). However, these studies have been conducted using solely male subjects and the i.v. ROA. The neurokinin 1 receptor (NK1R) is associated with stress, anxiety, and drug-seeking behaviors (Schank, 2014), and represents a valuable target for influencing opiate-related behaviors. Specifically, work from our group has demonstrated that NK1R antagonism significantly attenuates stress-primed reinstatement for various classes of drugs (Schank et al., 2014; Schank et al., 2015; Schank et al., 2011). Additionally, NK1R antagonism has been shown to attenuate the reinforcing properties of opioids (Barbier et al., 2013; Gadd et al., 2003; Ripley et al., 2002; Sandweiss et al., 2018), making the NK1R system an especially promising target for development of therapeutics for those suffering from opioid use disorders. Therefore, a secondary objective of our study was to examine the effect of NK1R antagonism on stress-primed reinstatement of oxycodone seeking.
Materials and Methods
Animals
Adult male and female Wistar rats (Charles River, Wilmington, MA) aged 10 weeks at the start of experimentation were used for the oxycodone concentration response curve experiment. Adult male and female Long Evans rats (Charles River, Wilmington, MA) aged 10 weeks at the start of experimentation were used for the remaining studies. After 1 week of acclimation to the animal facility, animals were handled daily for 3 days before beginning self-administration training. Animals were pair housed on a reverse 12:12 light/dark cycle, and all experiments were conducted during the dark phase. Food and water were provided ad libitum, except where stated. All procedures were approved by the University of Georgia Institutional Animal Care and Use Committee and were in accordance with NIH guidelines.
Oral self-administration
Med Associates (Fairfax, VT) self-administration chambers were used for all experiments. Upon being placed in the chambers, levers were extended indicating the start of the 1-hour self-administration session. Subjects had access to an inactive lever and an active lever, in which inactive lever presses were recorded but had no consequence, and active lever presses resulted in the delivery of a 100 μl reinforcer into a trough. Following each session, a paper towel was placed in the trough to check for excess, non-consumed liquid to ensure that each subject was consuming all of the delivered liquid reinforcer. If a subject did not consume all of the delivered reinforcers, this animal was dropped from the study.
Concentration response curve
Subjects were water deprived for 22 hours/day for the first 3 self-administration sessions to encourage lever pressing. Rats were trained on a fixed-ratio 1 (FR1) schedule with 0.2 % (w/v) saccharin as the reinforcer. Following the first 3 days of water deprivation, animals self-administered saccharin for 2 additional sessions. Oxycodone (NIDA Drug Supply Program, Research Triangle Park, NC) solution was then introduced as the reinforcer at a concentration of 0.03 mg/ml (dissolved in tap water). Upon the introduction of oxycodone, sessions were conducted using an FR1 schedule, with the addition of a cue light and 20-second timeout period following an active lever press. Rats self-administered the 0.03 mg/ml oxycodone concentration for 5 consecutive sessions before being exposed to increasing concentrations of oxycodone (0.1, 0.3, and 1.0 mg/ml). Each concentration was presented as the reinforcer for 5 consecutive sessions of FR1 self-administration. Following the 1.0 mg/ml concentration, the 0.1 mg/ml concentration was re-introduced to investigate whether lever presses would increase in efforts to titrate the dose of oxycodone consumed. For subsequent experiments, only 0.3 mg/ml oxycodone (no saccharin) was used in initial self-administration training, and the timeout period following active lever press was reduced from 20 to 5 seconds (see below).
Baseline self-administration
As in the previously described experiment, subjects were water deprived for 22 hours/day for the first 3 self-administration sessions. Subjects self-administered oxycodone solution in 1 hour sessions on a fixed-ratio 1 (FR1) schedule so that for every active lever press, 100 μl of 0.3 mg/ml oxycodone solution (dissolved in tap water) was delivered. The 0.3 mg/ml concentration was presented for 3–5 additional days following the end of water deprivation before being reduced to 0.1 mg/ml. Oxycodone concentrations and schedule used were determined based on preliminary experiments presented in Figures 1 and S1. When the oxycodone concentration was reduced to 0.1 mg/ml, animals continued self-administering on an FR1 schedule, and a 5 sec timeout with cue light illumination was introduced. Sessions were 1 hour in duration and were performed once daily for 5 days/week.
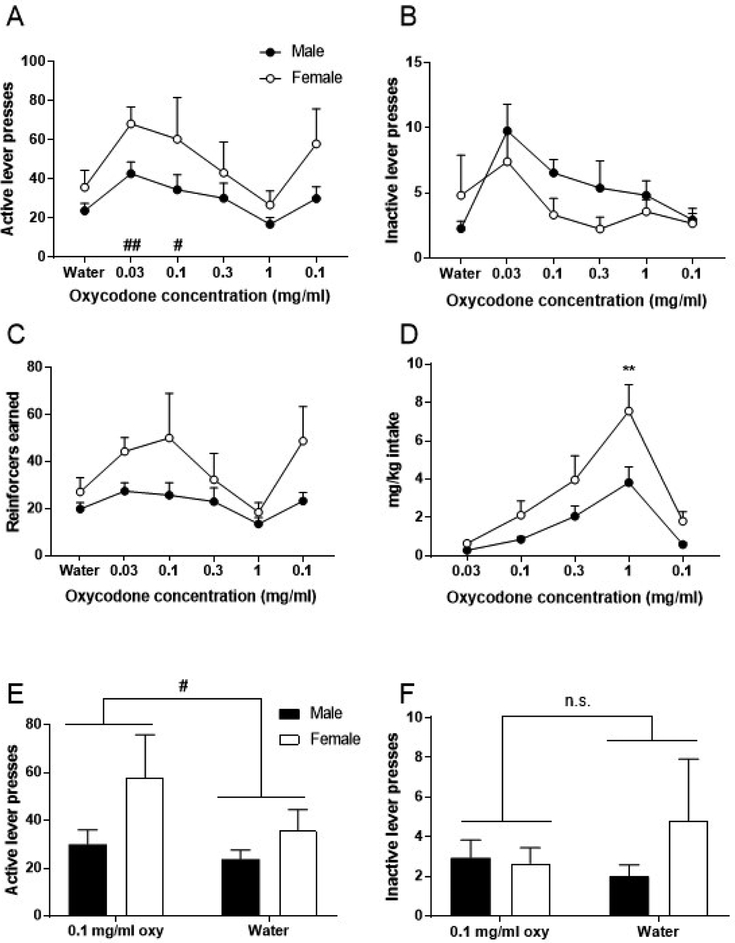
Figure 1. Oral oxycodone self-administration: concentration-response curve.
Data for each concentration represent the average of the last 3 days of self-administration. (A) Average active lever presses. (B) Average inactive lever presses. (C) Average reinforcers earned. (D) Average intake in mg/kg. (E) Average active lever presses for the last 3 days of re-introduced 0.1 mg/ml oxycodone compared to average of last 3 days of water. (F) Average inactive lever presses for the last 3 days of re-introduced 0.1 mg/ml oxycodone compared to average of last 3 days of water. Data expressed as mean values ± SEM. ##p<0.01, #p<0.05, compared to water. **p<0.01, compared to males. n=4–6/sex.
Estrous cycle monitoring
The estrous cycle in rodents consists of four phases, each phase lasting approximately 24 hours (McLean et al., 2012). Proestrus is characterized by peak estradiol and relatively high progesterone levels. This phase is followed by estrus, the phase in which estradiol levels begin to decrease and progesterone levels are relatively low. Estrus is then followed by the metestrus phase, which is characterized by low levels of both hormones. Metestrus is followed by diestrus, in which estradiol levels are low, and progesterone levels peak (McLean et al., 2012). After self-administration rates stabilized (less than 20% variation in active lever presses over 3 consecutive days, following a minimum of 15 sessions), estrous cycle was monitored daily via vaginal lavage for four consecutive days (n=6). Immediately following self-administration sessions, animals were lightly restrained, and 100 ul 0.9% sterile saline was pipetted into the vaginal opening. The solution was aspirated and expelled several times, and the sample was pipetted directly onto a clean microscope slide. Slides were allowed to dry at room temperature and were stained with Toluidine Blue O for visualization using a Zeiss light microscope at 20x. For representative images of vaginal cytology for each estrous cycle phase, see Figure S2. Estrous cycle was also monitored in drug-naïve animals using the same methodology (see Supplementary Material).
Progressive ratio
After responding stabilized (as defined above) on the 0.1 mg/ml oxycodone concentration, a progressive ratio (PR) session was conducted. Under this schedule, the number of active lever presses required to result in the delivery of a reinforcer increases over the course of the session using the following schedule: 1, 2, 3, 4, 6, 8, 10, 12, 16, and continuing to increase by 4. The session terminated if 30 minutes passed without the completion of the response requirement. We have used this specific PR schedule in studies examining oral alcohol self-administration (Schank et al., 2013). The largest response requirement that the subject completed for one reinforcer is referred to as the breakpoint.
Naloxone pretreatment
Animals (n=4–6/sex) self-administered 0.1 mg/ml oxycodone before being treated with 0, 1, 3, or 10 mg/kg naloxone (Sigma-Aldrich; 1 ml/kg injection volume, 0.9% saline vehicle, i.p.) (Pert et al., 1973) 15 minutes before beginning the self-administration session. Animals had undergone a total of 26 self-administration sessions before their first challenge with naloxone (8 sessions with the 0.3 mg/ml concentration and 18 sessions with the 0.1 mg/ml concentration). Doses and time of injection were selected based on previous literature (Alderson et al., 2000; Karami and Zarrindast, 2008; Koob et al., 1984; Ozawa et al., 2001). Doses of naloxone were administered in a counterbalanced design, with 2 days of self-administration occurring between each treatment day.
Because we observed no effect of single dose of naloxone on oxycodone self-administration, we conducted a follow-up experiment in which repeated 10 mg/kg naloxone treatments were administered. This second set of naloxone treatments occurred 7 sessions after final naloxone pretreatment described above. Subjects were injected with vehicle or 10 mg/kg naloxone 15 minutes before self-administration sessions for 3 consecutive days. Subjects underwent 3 days of normal self-administration before receiving the opposite pretreatment in a counterbalanced design.
Extinction and Reinstatement
Animals (n=10/sex) were allowed to self-administer 0.1 mg/ml oxycodone for 23 days before beginning extinction. Extinction sessions were run 3 times/day. Between each 1-hour extinction session, rats were placed in the home cage with food and water access for 10 minutes. During extinction sessions, oxycodone solution was replaced with water. This specific approach (water delivery as opposed to no lever press consequence) helped to confirm that rats were actively pressing for oxycodone containing solutions during previous baseline self-administration. The cue light continued to be activated following presses on the previously active lever. Animals underwent 3 extinction sessions/day until 75% of subjects met extinction criterion (active lever presses less than half of baseline), which took a total of 10 days, or 30 sessions. The animals that had not extinguished by this time point underwent 3 additional days of 3/day extinction sessions, and if criterion was not met by this point, self-administration data were excluded for these subjects. The data from 2 female subjects and 1 male subject were excluded based on this criterion. In efforts to establish most conservative comparison between extinction and reinstatement responding, extinction response rate was quantified as the number of active lever presses exhibited during the first of the 3 extinction sessions that occurred on the last day before reinstatement testing.
For the first stress-primed reinstatement session, rats were placed in self-administration chambers, with levers withdrawn, and exposed to 15 minutes of unpredictable footshocks (0.8 mA intensity, 0.5 sec duration, 45 sec average intershock interval). Immediately following the end of footshock, levers were extended, and animals underwent a 1-hour reinstatement session, in which only water was delivered. Because males and females appeared to differ slightly in their degree of reinstatement following 0.8 mA footshock, we conducted two additional reinstatement sessions at different shock intensities (first 0.4 and then 1.0 mA) to investigate whether shock intensity would affect reinstatement responding differently in males and females. Each stress-primed reinstatement session was separated by one day (3 sessions) of extinction. The reinstatement data from one male subject were excluded due identification as a statistically-significant outlier using Grubb’s test. After the reinstatement sessions detailed above, animals were given an additional day (3 sessions) of extinction. Next, subjects received i.p. injections of vehicle (2-hydroxypropyl-β-cyclodextrin; Sigma-Aldrich, 45% w/v) or the NK1R antagonist L822429 (15 mg/kg, 1 ml/kg injection volume; synthesized by K. Cheng and K. Rice at NIDA/NIAAA) 45 minutes prior to footshock exposure (0.8 mA, as above). Injection time and dose were based on previous studies (Schank et al., 2014). Following the 15-minute footshock, levers were extended, and animals underwent a 1-hour reinstatement session as described above. As with the previous stress-primed reinstatement experiments, each test day was separated by 1 day (3 sessions) of extinction. L822429 and vehicle pretreatments were administered using a counterbalanced design.
Serum oxycodone concentration analysis
Oxycodone serum concentration was analyzed as previously described (Laudenbach et al., 2018; Mavrikaki et al., 2017; Pravetoni et al., 2012a; Pravetoni et al., 2012b; Pravetoni et al., 2014b). Rats were sacrificed via live decapitation and trunk blood was collected 15 minutes following intragastric gavage of 0.87 mg/kg (males) or 1.49 mg/kg oxycodone (females) at 1 ml/kg volume, dissolved in tap water. Doses administered were determined based on the average mg/kg oxycodone consumed for each sex during baseline oxycodone self-administration. Samples were collected in 1.5 ml tubes and centrifuged at room temperature at 7500 rpm. Serum was then stored at −20° until analysis by gas chromatography-mass spectrometry.
Statistics
GraphPad Prism software was used for graphing all results and Statistica software was used for data analyses. Data were analyzed using mixed model two-way ANOVA, repeated measures one-way ANOVA, or unpaired student’s t-test as indicated in the Results section. When appropriate, post-hoc analyses were conducted using Bonferroni correction (Figures 1 and 4–5). Planned Comparisons analyses were conducted for data represented in Figures 2A, 2E, and 2G.
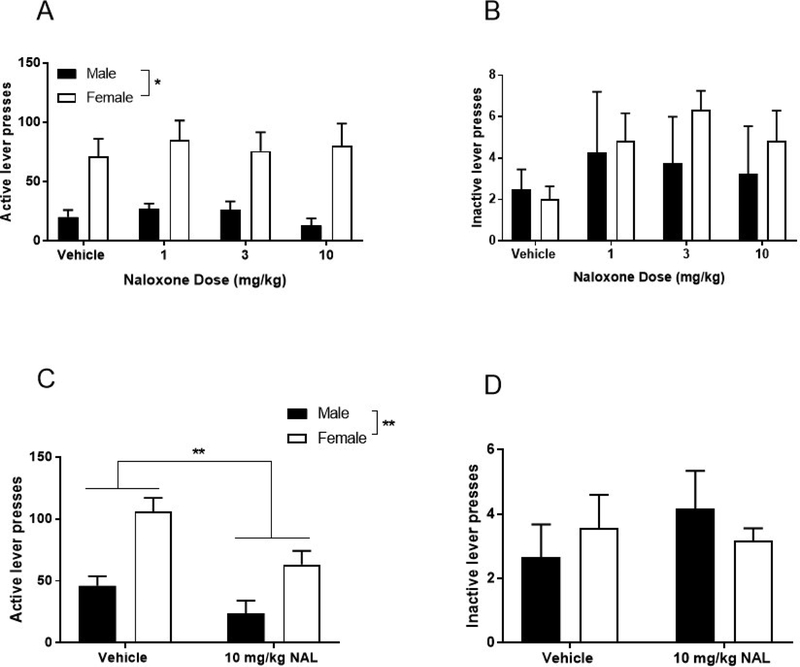
Figure 4. Effect of naloxone pretreatment on oral oxycodone self-administration.
(A) Active lever presses following single naloxone pretreatment at 1, 3, and 10 mg/kg doses. (B) Inactive lever presses following single naloxone pretreatment at 1, 3, and 10 mg/kg doses. (C) Average active lever presses after 3 consecutive days of 10 mg/kg naloxone pretreatment. (D) Average inactive lever presses after 3 consecutive days of 10 mg/kg naloxone pretreatment. Data expressed as mean values ± SEM. **p<0.01, compared to vehicle. n=4–6/sex.
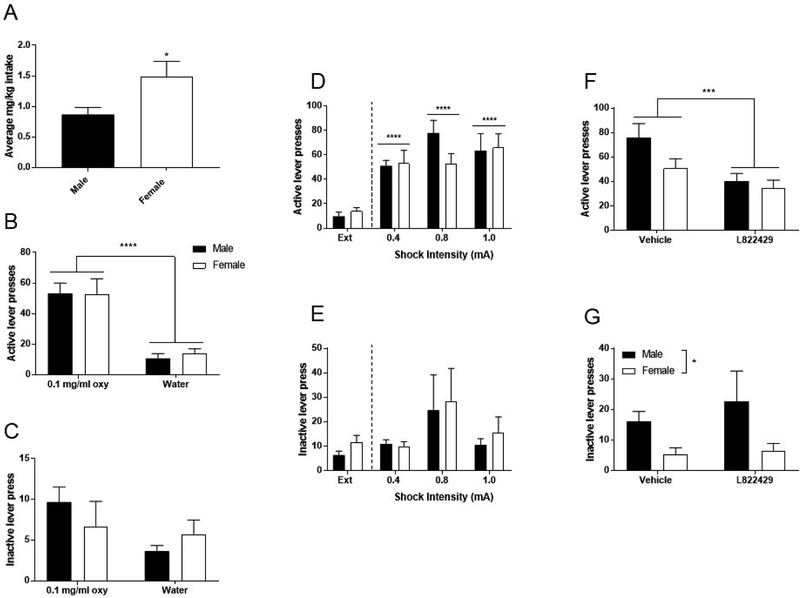
Figure 5. Extinction and stress-primed reinstatement.
(A) Average mg/kg intake over last three oxycodone self-administration sessions. (B) Average active lever presses over last three oxycodone self-administration sessions compared to the average active lever presses over the last three extinction (water only) sessions. (C) Average inactive lever presses over the last three oxycodone self-administration sessions compared to average active lever presses over the last three extinction (water only) sessions. (D) Effect of shock intensity on stress-primed reinstatement, active lever presses. (E) Effect of shock intensity on stress-primed reinstatement, inactive lever presses. (F) NK1R antagonism pretreatment and stress-primed reinstatement, active lever presses. (G) NK1R antagonism pretreatment and stress-primed reinstatement. inactive lever presses. Data expressed as mean values ± SEM. *p<0.05, compared to extinction, ***p<0.001, compared to vehicle, ****p<0.0001 compared to extinction. n=9–10/sex.
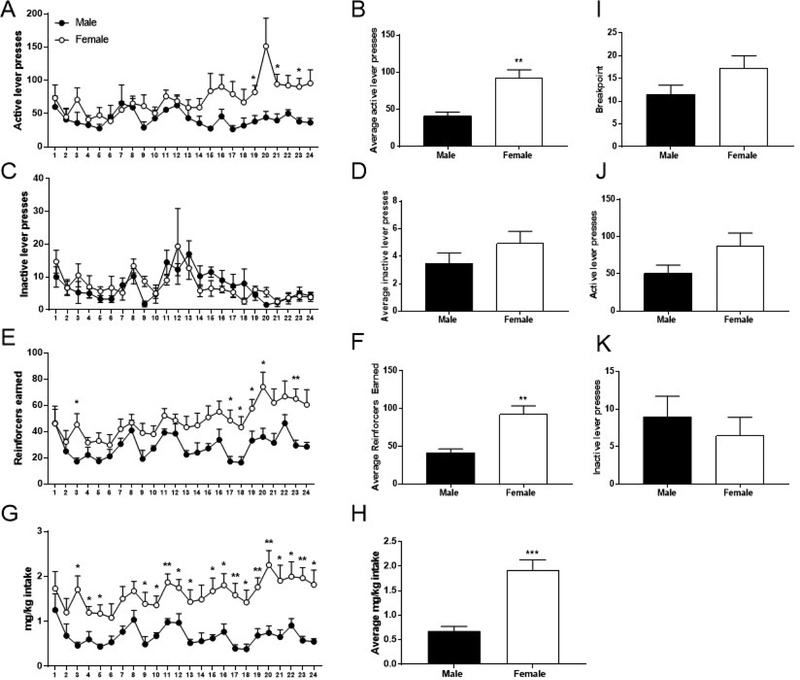
Figure 2. Baseline oral oxycodone self-administration and progressive ratio.
(A) Total active lever presses. (B) Average active lever presses over last three days of baseline self-administration. (C) Total inactive lever presses. (D) Average inactive lever presses over last three days of baseline self-administration. (E) Total reinforcers earned. (F) Average reinforcers earned over the last three days of baseline self-administration. (G) Total mg/kg consumed (H) Average mg/kg intake over the last three days of baseline self-administration. (I) Breakpoints obtained in progressive ratio (PR) session. (J) Total active lever presses during PR session. (K) Total inactive lever presses during PR session. Data expressed as mean values ± SEM. **p<0.01, *p<0.05, compared to males. n=4–6/sex.
Results
Self-administration concentration response-curve
Subjects were exposed to increasing concentrations of oxycodone solution and allowed to self-administer each solution on an FR1 schedule for 5 consecutive days. Data points represent the average of the last 3 days of self-administration for each oxycodone concentration. Repeated measures two-way ANOVA for active lever presses (Figure 1A) revealed a main effect of oxycodone concentration (F5,40=9.69, p<0.0001), no effect of sex (F1,8=2.74, p=0.14), and no interaction between these factors (F5,40=1.09, p=0.38). Post-hoc analysis across the factor of oxycodone concentration using Bonferroni correction revealed that active lever presses for the 0.03 and 0.1 mg/ml oxycodone concentrations were significantly different than active presses for water (p<0.001, p=0.024, respectively). For inactive lever presses (Figure 1B), repeated measures two-way ANOVA revealed a main effect of oxycodone concentration (F5,40=3.87, p<0.01), no effect of sex (F1,8=1.18, p=0.31), and no interaction between these factors (F5,40=1.10, p=0.37). Post-hoc analysis across the factor of oxycodone concentration using Bonferroni correction revealed that inactive lever presses for the 0.03 mg/ml oxycodone concentration were significantly different than inactive presses for water (p<0.01). However, no differences were observed for inactive presses for all other oxycodone concentrations when compared to water (p>0.9999 for all concentrations). Analysis of reinforcers earned (Figure 1C) using repeated measures two-way ANOVA revealed a main effect of oxycodone concentration (F5,40=7.42, p<0.0001), no effect of sex (F1,8=2.91, p=0.13), and no interaction between these factors (F5,40=1.93, p=0.11). Lastly, analysis of oxycodone intake (measured in mg/kg, Figure 1D) revealed that females consumed significantly more oxycodone than males. Repeated measures two-way ANOVA revealed a main effect of oxycodone concentration (F4,32=40.26, p<0.0001), a main effect of sex (F1,8=5.74, p=0.044), and a significant interaction between these factors (F4,32=3.76, p=0.013). Intake was influenced by concentration of the solution available, which higher levels of intake at higher solution concentration. Also, females consumed significantly more oxycodone at the 1 mg/ml concentration when compared to males (p=0.011).
To assess responding for 0.1 mg/ml oxycodone versus water, the average active lever presses for the last 3 days of re-introduced 0.1 mg/ml oxycodone were compared to average of last 3 days of water (Figure 1E). Repeated measures two-way ANOVA revealed a main effect of oxycodone (F1,8=5.33, p<0.049), no effect of sex (F1,8=3.21, p=0.11), and no interaction between these factors (F1,8=1.68, p=0.23), providing evidence that 0.1 mg/ml oxycodone was distinguishable from water. Average inactive lever presses (Figure 1F) for the last 3 days of re-introduced 0.1 mg/ml oxycodone were compared to average of last 3 days of water. Repeated measures two-way ANOVA revealed no effect of oxycodone (F1,8=0.18, p=0.68), no effect of sex (F1,8=0.84, p=0.39), and no interaction between these factors (F1,8=1.15, p=0.32).
Baseline self-administration
Baseline self-administration data (n=4–6/sex) demonstrates that females consumed significantly more oxycodone than males, as measured by active lever presses, reinforcers earned, and mg/kg intake. Repeated measures two-way ANOVA of active presses (Figure 2A) revealed a main effect of session (F23,184=2.06, p<0.01), and a significant sex x session interaction (F23,184=1.98, p<0.01), but no main effect of sex (F1,8=4.53, p=0.07). Planned comparisons analysis demonstrated that females pressed significantly more than males on sessions 19 (p=0.01), 21 (p=0.03), and 23 (p=0.01). Unpaired t-test comparing the average active presses over last three days of self-administration revealed a significant sex difference (Figure 2B, t8=3.63, p<0.01). While repeated measures two-way ANOVA of inactive lever presses (Figure 2C) revealed a main effect of session (F23,184=3.16, p<0.0001), males and females did not differ in the number of inactive lever presses, indicated by no significant effect of sex (F1,8=0.03, p=0.86), and no interaction (F23,184=0.87, p=0.64). Similarly, unpaired t-test comparing the average inactive presses earned over the last three days of self-administration revealed no significant difference between males and females (Figure 2D; t8=1.17, p=0.28). Additionally, to ensure that active lever presses were consistently higher than inactive presses for both sexes, a repeated measures three-way ANOVA was conducted with the factors of sex, session, and inactive/active lever. This analysis revealed a main effect of lever, in that active lever presses were significantly higher than inactive lever presses (F1,16=49.20, p<0.0001) over the course of oxycodone self-administration. Repeated measures two-way ANOVA of reinforcers (Figure 2E) revealed a main effect of sex (F1,8=6.01, p=0.040), a main effect of session (F23,184=4.64, p<0.0001), but no interaction between these two factors (F23,184=1.45, p=0.09). Planned comparisons analysis demonstrated that females received significantly more reinforcers than males on sessions 3 (p=0.03), 17–20 (p=0.02, p=0.04, p=0.047, p=0.03, respectively), and 23 (p<0.01). Unpaired t-test comparing the average reinforcers earned over the last three days of baseline oral self-administration (Figure 2F) also revealed a significant sex difference (t8=3.63, p<0.01). Repeated measures two-way ANOVA of mg/kg intake (Figure 2G) revealed a main effect of sex (F1,8=14.28, p<0.01), a main effect of session (F23,184=2.61, p<0.001), but no interaction between these two factors (F23,184=1.13, p=0.32). Planned comparisons analysis demonstrated that females consumed significantly more oxycodone than males on sessions 3–5 (p=0.01, p=0.03, p=0.02) and sessions 9–24 (p=0.03 for sessions 9, 10, 12, 13, and 22; p=0.02 for sessions 15, 16, 18, 21, and 24; p<0.01 for sessions 11, 17, 19, 20, and 23). Unpaired t-test revealed a significant sex difference in mg/kg intake (Figure 2H, t8=4.69, p<0.01). We have observed results consistent with this effect of sex in a second strain of rats (Wistar, Figure S1).
Progressive ratio
After responding stabilized on the FR1 self-administration schedule, subjects underwent a single PR session, a measure of motivation for drug delivery. While the average breakpoint of females was higher than that of male subjects (mean ± SEM: females: 17.3 ± 2.7, males: 11.5 ± 2.1), unpaired t-test failed to detect a significant difference (Figure 2I, t8=1.57, p=0.15). Similarly, the average number of active lever presses during PR was higher in females (mean ± SEM: females: 87.5 ± 17.4, males: 51 ± 10.9), but unpaired t-test did not reveal a significant difference between sexes (Figure 2J, t8=1.56, p=0.16). Lastly, inactive lever presses did not differ between males and females for this session (Figure 2K, t8=0.67, p=0.52).
Estrous cycle monitoring
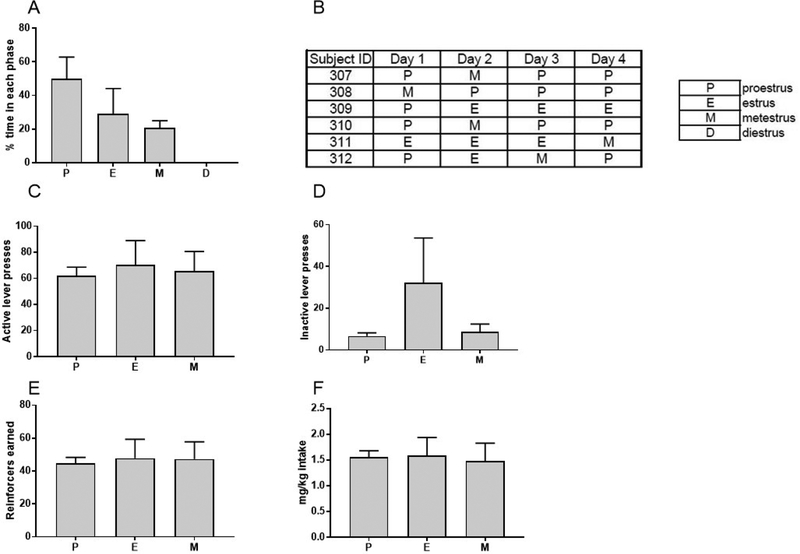
Estrous cycle was monitored every 24 hours via vaginal lavage immediately following oxycodone self-administration sessions for 4 consecutive days. Results of estrous cycle monitoring of Long Evans rats (n=6) suggested a dysregulation of the estrous cycle, in that the order in which phases were observed and the percentage of time spent in each phase seemed to be disrupted (Figure 3A–B). More specifically, the average percent time spent in each phase was 50.0 ± 12.9 SEM for proestrus, 29.2 ± 15.0 SEM for estrus, 20.8 ± 4.2 SEM for metestrus, and 0 for diestrus (Figure 3A). When estrous cycle was monitored for 4 consecutive days in age-matched, drug-naïve controls (n=6), we also found evidence of estrous cycle dysregulation, in that 4/6 rats were not observed in diestrus, and the percent time spent in each phase was irregular (see Figure S3). While our results assessing the effect of oxycodone on the estrous cycle remain inconclusive due to irregular cycling in rats that were not self-administering oxycodone, we found strong evidence that estrous cycle phase did not affect oxycodone self-administration (Figure 3C–F). One-way ANOVA revealed no effect of estrous cycle phase on active lever presses (Figure 3C, F2,10=0.09, p=0.92), inactive lever presses (Figure 3D, F2,10=2.25, p=0.16), reinforcers earned (Figure 3E, F2,10=0.03, p=0.97), or intake in mg/kg (Figure 3F, F2,12=0.04, p=0.96). Overall, these data demonstrate that oral oxycodone self-administration is not affected by estrous cycle phase. However, the effect of oral oxycodone self-administration in Long Evans rats remains unclear, in that estrous cycle phase also appeared to be somewhat abnormal in drug-naïve controls. Of note, we found no effect of estrous cycle phase on oxycodone self-administration or of oxycodone self-administration on estrous cycle phase in previous experiments conducted using the Wistar strain (Figure S4).
Figure 3. Estrous cycle monitoring.
(A) Percent time spent in each estrous cycle phase. (B) Estrous cycle phases for individual subjects over four days of estrous cycle monitoring. (C) Active lever presses for each estrous phase. (D) Inactive lever presses for each estrous phase. (E) Reinforcers earned for each estrous phase. (F) Intake in mg/kg for each estrous phase. Data expressed as mean values ± SEM. n=6.
Naloxone treatment
Pretreatments with single doses of naloxone (0, 1, 3, 10 mg/kg) had no effect on oral oxycodone self-administration response rates (Figure 4A). Repeated measures two-way ANOVA revealed a main effect of sex (F1,8=10.55, p=0.012), but no effect of naloxone treatment (F3,24=0.45, p=0.72), nor an interaction between these two factors (F3,24=0.33, p=0.81). Repeated measures two-way ANOVA also revealed that naloxone treatment had no effect on inactive lever presses (Figure 4B, F3,24=1.68, p=0.20). Additionally, there was no effect of sex on inactive lever presses (F1,8=0.46, p=0.52) and no interaction between sex and naloxone treatment (F3,24=0.50, p=0.69).
We hypothesized that the absence of a naloxone effect on active lever pressing may be the result of a delay in experiencing oxycodone’s reinforcing properties following its oral consumption. Thus, it may take multiple treatment days for the animal to learn that the reinforcing value of the oxycodone solution has been reduced because the animal may not fully sense this reduction in value until after the completion of the session. Therefore, we administered naloxone over 3 consecutive days, which effectively attenuated oxycodone self-administration rates in males and females (Figure 4C). Repeated measures two-way ANOVA revealed a main effect of sex (F1,8=14.15, p<0.01), and a main effect of naloxone treatment (F1,8=14.98, p<0.01), but no interaction between these two factors (F1,8=1.47, p=0.26). This result was specific for active lever presses, in that repeated naloxone treatment had no effect on inactive lever presses (Figure 4D, F1,8=0.61, p=0.46). Consistent with previous data, there was no effect of sex on inactive lever presses (F1,8=0.003, p=0.96) and no interaction between sex and naloxone treatment (F1,8=1.75, p=0.22).
Extinction and Reinstatement
A separate cohort of male and female Long Evans rats (n=10/sex) was trained to orally self-administer 0.1 mg/ml oxycodone until stable response rates were achieved (28 total sessions). Consistently with previous cohorts, female rats self-administered significantly more oxycodone when compared to males (Figure 5A, unpaired t-test of the average mg/kg consumed over the last 3 sessions of self-administration revealed a significant sex difference: t18=65.2, p<0.0001). When water was substituted for oxycodone solution, lever pressing extinguished over time (Figure 5B). Specifically, repeated measures two-way ANOVA of the average lever presses at baseline compared to extinction revealed a main effect of extinction (F1,18=65.2, p<0.0001), indicating that lever responding decreased when water was substituted for oxycodone. However, there was no effect of sex (F1,18=0.05, p=0.82), nor an interaction between these two factors (F1,18=0.13, p=0.73). This effect was specific for active lever presses, in that repeated measures two-way ANOVA of average inactive lever presses at baseline compared to extinction revealed no effect of extinction (Figure 5C, F1,18=2.68, p=0.12), no effect of sex (F1,18=0.05, p=0.82), and no interaction between these factors (F1,18=1.40, p=0.25). Overall, these data support the hypothesis that oral oxycodone functions as an effective reinforcer and that its self-administration is extinguished when substituted with water.
After extinction, subjects underwent 15-minute footshock exposure (0.4, 0.8, or 1.0 mA) before the 1-hour reinstatement session (Figure 5D). Repeated measures two-way ANOVA of active lever presses revealed a main effect of shock (F3,51=27.36, p<0.0001), no effect of sex (F1,17=0.38, p=0.55), nor an interaction between these two factors (F3,51=1.54, p=0.22). Post-hoc comparisons using Bonferroni correction across the factor of shock intensity indicated a significant increase in responding relative to extinction at all shock intensities used (p<0.0001 for all comparisons). This effect was specific to active lever presses, in that repeated measures two-way ANOVA of inactive lever presses (Figure 5E) revealed no effect of shock (F3,51=2.56, p=0.07), no effect of sex (F1,17=0.30, p=0.59), and no interaction between these factors (F3,51=0.09, p=0.97).
Collectively, these data indicate that males and females reinstate strongly following footshock stress exposure, and that this behavior is not sensitive to variations in shock intensity within the range used. While both males and females reinstated following each shock intensity, males exhibited peak responding at the 0.8 mA intensity, whereas females exhibited peak responding at the 1.0 mA. Additionally, responding at the 0.8 mA appeared to be slightly greater in males than females. These data suggest that males may be more sensitive to some aspects of footshock-induced reinstatement, but further experiments with larger group sizes will be necessary to detect these subtle differences.
Systemic NK1R antagonism
Subjects were administered vehicle or 15 mg/kg L822429 (i.p.) 45 minutes before a 15-minute footshock session (0.8 mA) and 1-hour reinstatement session (Figure 5F–G). Dose and time of injection were based on previous experiments demonstrating efficacy for attenuation of stress-induced reinstatement of cocaine or alcohol seeking, and minimization of non-specific effects in the Long Evans strain (Schank et al., 2014). Repeated measures two-way ANOVA of active lever presses revealed a main effect of NK1R antagonism (F1,17=10.02, p<0.001), but no effect of sex (F1,17=2.29, p=0.14), and no interaction between these two factors (F1,17=2.74, p=0.12). This indicates that antagonist pretreatment reduced reinstatement responding in both male and female rats. Repeated measures two-way ANOVA of inactive lever presses revealed a main effect of sex (F1,17=6.21, p=0.015), no effect of NK1R antagonism (F1,17=0.58, p=0.45), and no interaction between these factors (F1,17=0.28, p=0.60). These data demonstrate that systemic NK1R antagonism significantly attenuates stress-primed reinstatement in Long-Evans rats. Consistent with the results shown in figure 5D, reinstatement responding in males appeared to be slightly higher than female rats under vehicle pretreatment conditions, but the effect of sex did not reach statistical significance.
Oxycodone serum concentration
After completing stress-primed reinstatement sessions, subjects (n=11–12/sex) were intragastrically gavaged with 0.87 mg/kg (males) or 1.49 mg/kg oxycodone (females). Doses administered for oral gavage were determined based on the average mg/kg oxycodone consumed for each sex during baseline self-administration. The average serum concentrations were 2.36 ± 0.4 ng/ml and 15.42 ± 3.9 ng/ml for males and females respectively (data represented as mean ± SEM). These concentrations are similar to those obtained by humans following oral consumption of oxycodone.
Discussion
In addition to establishing a protocol for operant oral oxycodone self-administration in rats, these experiments revealed a significant sex difference in oxycodone intake. Females consumed significantly more than males, a phenomenon that has been observed under certain conditions in several preclinical studies of drugs of abuse (Cicero et al., 2003; Lynch and Carroll, 1999; Lynch and Taylor, 2004; Nieto and Kosten, 2017). Mavrikaki and colleagues specifically demonstrated that females self-administer significantly more i.v. oxycodone than males (Mavrikaki et al., 2017). These data, in conjunction with our current findings, provide strong evidence of a sex difference in oxycodone self-administration. Evidence that rats in our study self-administered oral oxycodone solution for its reinforcing properties include the inverted U-shaped concentration-response curve generated across increasing concentrations of oxycodone solution (Figure 1), extinguished lever pressing when water was substituted for oxycodone in multiple cohorts (Figures 1E, and 5B), and disruption of lever pressing following MOR antagonist treatment (Figure 4F). Additionally, we were able to induce a reinstatement of extinguished oxycodone seeking by exposing the rats to footshock stress, and this response was attenuated by NK1R antagonism (Figure 5D and 5F).
Some studies have shown that fluctuations in systemic hormone levels during the estrous cycle affects various drug-seeking behaviors. Generally, higher systemic estradiol levels tend to facilitate, and progesterone to inhibit, drug-related behaviors (Becker and Cha, 1989; Calipari et al., 2017; Carroll et al., 2016; Evans et al., 2002; Sofuoglu et al., 2002). In contrast, a study assessing the role of estrous cycle phase in i.v. oxycodone self-administration found no effect of estrous cycle on drug-seeking (Mavrikaki et al., 2017). Consistent with these data, we found no effect of estrous cycle phase on oral oxycodone self-administration. Though estrous cycle did not affect drug-seeking behavior (Figures 3, and S4), our data suggest that chronic oral oxycodone self-administration may lead to dysregulation of the estrous cycle (Figure 3A–B). However, because the cycle also appeared to be irregular in drug-naïve controls, the role of oxycodone self-administration on estrous cycle regularity in Long Evans rats remains ambiguous. Interestingly, we did not observe estrous cycle dysregulation in a different strain of rats (Wistar) exposed to chronic oral oxycodone self-administration (Figures S4). Of note, a paper by Goldman et al (Goldman, Murr et al. 2007) revealed that in addition to the presence of predominantly leukocytes, the diestrus phase can also be characterized by the presence of a combination of leukocytes and epithelial cells in the Long Evans strain. Thus, determination of estrous cycle phase in Long Evans rats may be particularly challenging.
As oxycodone is a potent MOR agonist (Gallego et al., 2007), we wanted to determine whether administration of an opioid receptor antagonist could disrupt oral self-administration of this drug. We found that single pretreatments of naloxone had no effect on oxycodone self-administration. While initially a surprising finding, it is important to consider the role of oxycodone ROA in the response to systemic naloxone treatment. Because subjects were self-administering oxycodone orally, we speculate that they experienced a longer delay between operant response and sensation of drug effects than would occur during iv self-administration. Thus, it is possible that the animals were not able to learn the effect of naloxone pretreatment on oxycodone reinforcement until later in the session, or even after the session had concluded. Therefore, we conducted repeated naloxone pretreatments for 3 consecutive days, and found that oxycodone-seeking was significantly attenuated. Thus, naloxone was capable of disrupting operant responding for oxycodone, but this response took multiple exposures to learn. The fact that lever pressing was decreased, as opposed to increased, is consistent with our dose-response data suggesting that 0.1 mg/ml oxycodone is near the peak, or on the ascending limb, of the dose-response function. We do not think that this attenuation in oxycodone self-administration is due to off target or general sedative effects because 10 mg/kg naloxone had no effect on responding when given acutely. Additionally, no somatic withdrawal symptoms were observed following any of the acute naloxone treatments or the repeated naloxone treatments, suggesting that animals were not physically dependent on oxycodone during the baseline self-administration phase, nor was acute withdrawal precipitated following naloxone treatments. However, it is important to note that the first day of single dose naloxone treatment occurred following 26 sessions, and the first day of the repeated naloxone treatments occurred 14 sessions after that, which may have also contributed to the increased efficacy of naloxone treatment.
The NK1R is known to have a role in stress and anxiety responses, as well as various drug-seeking behaviors (Schank, 2014). This system is particularly relevant in studying opioid abuse, in that antagonism of this receptor has been repeatedly shown to alter opioid-seeking behaviors. The majority of studies have found that NK1R antagonism attenuates the reinforcing properties of opiate drugs (Barbier et al., 2013; Gadd et al., 2003; Ripley et al., 2002; Sandweiss et al., 2018). In contrast, Walsh et al. have demonstrated that NK1R antagonism with aprepitant potentiated oxycodone’s reinforcing effects when consumed orally or intranasally in human recreational opioid users (Walsh, Heilig et al. 2013). This unexpected result could be the result of complex factors including the oxycodone experience of experimental subjects, where the dose of oxycodone falls on the dose-response function, and the potency of NK1R antagonism of the specific compound and dose used. However, the NK1R system has been consistently shown to mediate stress-primed reinstatement for both cocaine and alcohol (Schank et al., 2014; Schank et al., 2015; Schank et al., 2011). To our knowledge, we are the first to assess the role of the NK1R in reinstatement of drug seeking for any opiate drug. We found that systemic delivery of a NK1R antagonist significantly attenuated reinstatement of oxycodone-seeking following stress in both male and female subjects. This finding provides further support for targeting the NK1R system in the development of therapeutics for those suffering from substance use disorders, and suggests that its ability to suppress stress-elicited drug seeking extends to all classes of drugs examined thus far. In both the initial shock titration experiment, and in the antagonist experiment, it appeared that males exhibited greater lever pressing behavior following exposure to 0.8 mA shock intensity. While this was not a statistically significant effect, it suggests that males may be slightly more sensitive to stress-induced reinstatement of oxycodone seeking than females. It should also be noted that estrous cycle phase was not monitored during the reinstatement test days, and reinstatement behavior could be more intense on specific days of the estrous cycle. Future studies will assess the role of ovarian hormones in reinstatement to oxycodone-seeking and will follow up on this subtle effect of sex on stress-induced oxycodone seeking.
Lastly, we found that intragastric gavage of the same amount of oxycodone that was consumed during baseline self-administration produced blood levels that were comparable to those reached following oral oxycodone intake in human subjects (Kalso, 2005), suggesting that both males and females voluntarily self-administer pharmacologically relevant doses.
Collectively, these data demonstrate that operant self-administration can be used to study oral oxycodone intake in rats. Based on typical drug taking behaviors in humans, this preclinical model has high translational value and can be used as a platform for early development of pharmacotherapy for prescription opiate dependence as well as studying the transition from oral to intravenous routes of opioid administration that frequently occurs in human opioid abusers. Additionally, we observed a significant sex difference in oxycodone consumption that will be important to consider in the interpretation of future studies. Mechanistically, we identified a role of the NK1R system in stress-primed reinstatement, consistent with the effect of this receptor on stress-induced seeking of other drug classes, as well as its role in reward/reinforcement for other opiate drugs.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Gina Kim, DVM (University of Georgia Lab Animal Resident) for her training on methods in estrous cycle monitoring. We thank the NIDA Drug Supply Program for providing oxycodone.
This work was funded by the University of Georgia Research Foundation and the University of Georgia Office for the Vice President of Research. A portion of this work was supported by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse Intramural Research Programs.
References
- Alderson HL, Robbins TW, Everitt BJ, 2000. Heroin self-administration under a second-order schedule of reinforcement: acquisition and maintenance of heroin-seeking behaviour in rats. Psychopharmacology (Berl) 153, 120–133. [DOI] [PubMed] [Google Scholar]
- Back SE, Payne RL, Wahlquist AH, Carter RE, Stroud Z, Haynes L, Hillhouse M, Brady KT, Ling W, 2011. Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. Am J Drug Alcohol Abuse 37, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier E, Vendruscolo LF, Schlosburg JE, Edwards S, Juergens N, Park PE, Misra KK, Cheng K, Rice KC, Schank J, Schulteis G, Koob GF, Heilig M, 2013. The NK1 receptor antagonist L822429 reduces heroin reinforcement. Neuropsychopharmacology 38, 976–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Cha JH, 1989. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res 35, 117–125. [DOI] [PubMed] [Google Scholar]
- Blackwood CA, Hoerle R, Leary M, Schroeder J, Job MO, McCoy MT, Ladenheim B, Jayanthi S, Cadet JL, 2018. Molecular Adaptations in the Rat Dorsal Striatum and Hippocampus Following Abstinence-Induced Incubation of Drug Seeking After Escalated Oxycodone Self-Administration. Mol Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Hoots JK, Fredriksson I, Adhikary S, Zhang M, Venniro M, Shaham Y, 2018. Role of mu, but not delta or kappa, opioid receptors in context-induced reinstatement of oxycodone seeking. The European journal of neuroscience. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, Roman-Ortiz C, Ramakrishnan C, Deisseroth K, Han MH, Nestler EJ, 2017. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun 8, 13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Collins M, Kohl EA, Johnson S, Dougen B, 2016. Sex and menstrual cycle effects on chronic oral cocaine self-administration in rhesus monkeys: Effects of a nondrug alternative reward. Psychopharmacology (Berl) 233, 2973–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, Meyer ER, 2003. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav 74, 541–549. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, Kurtz SP, 2014. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry 71, 821–826. [DOI] [PubMed] [Google Scholar]
- Emanuele NV, LaPaglia N, Steiner J, Kirsteins L, Emanuele MA, 2001. Effect of chronic ethanol exposure on female rat reproductive cyclicity and hormone secretion. Alcohol Clin Exp Res 25, 1025–1029. [PubMed] [Google Scholar]
- Enga RM, Jackson A, Damaj MI, Beardsley PM, 2016. Oxycodone physical dependence and its oral self-administration in C57BL/6J mice. European journal of pharmacology 789, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW, 2002. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 159, 397–406. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE, 2007. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend 89, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence CS, Zhou C, Luo F, Xu L, 2016. The Economic Burden of Prescription Opioid Overdose, Abuse, and Dependence in the United States, 2013. Med Care 54, 901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadd CA, Murtra P, De Felipe C, Hunt SP, 2003. Neurokinin-1 receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouse. The Journal of neuroscience: the official journal of the Society for Neuroscience 23, 8271–8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego AO, Baron MG, Arranz EE, 2007. Oxycodone: a pharmacological and clinical review. Clinical & Translational Oncology 9, 298–307. [DOI] [PubMed] [Google Scholar]
- CDC “Prescription Opioid Data.” Opioid Overdose. [Google Scholar]
- DEA (2014). “Oxycodone (Trade Names: Tylox, Percodan, OxyContin).” Office of Diversion Control: Drug and Chemical Evaluation Section. [Google Scholar]
- Goldman JM, Murr AS and Cooper RL (2007). “The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies.” Birth Defects Res B Dev Reprod Toxicol 80(2): 84–97. [DOI] [PubMed] [Google Scholar]
- Hays LR (2004). “A profile of OxyContin addiction.” J Addict Dis 23(4): 1–9. [DOI] [PubMed] [Google Scholar]
- Hines LA, Lynskey M, Morley KI, Griffiths P, Gossop M, Powis B and Strang J (2017). “The relationship between initial route of heroin administration and speed of transition to daily heroin use.” Drug Alcohol Rev 36(5): 633–638. [DOI] [PubMed] [Google Scholar]
- Jones CM (2013). “Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002–2004 and 2008–2010.” Drug and Alcohol Dependence 132(1–2): 95–100. [DOI] [PubMed] [Google Scholar]
- Jones CM, Logan J, Gladden RM and Bohm MK (2015). “Vital Signs: Demographic and Substance Use Trends Among Heroin Users - United States, 2002–2013.” MMWR Morb Mortal Wkly Rep 64(26): 719–725. [PMC free article] [PubMed] [Google Scholar]
- NIDA “Sex and Gender Differences in Substance Use.” Substance Use in Women. [Google Scholar]
- NIDA “A subset of people who abuse prescription opioids may progress to heroin use.” Prescription Opioids and Heroin. [Google Scholar]
- Walsh SL, Heilig M, Nuzzo PA, Henderson P and Lofwall MR (2013). “Effects of the NK1 antagonist, aprepitant, on response to oral and intranasal oxycodone in prescription opioid abusers.” Addict Biol 18(2): 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR, 2004. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug and alcohol dependence 74, 265–272. [DOI] [PubMed] [Google Scholar]
- Jimenez SM, Healy AF, Coelho MA, Brown CN, Kippin TE, Szumlinski KK, 2017. Variability in prescription opioid intake and reinforcement amongst 129 substrains. Genes, brain, and behavior 16, 709–724. [DOI] [PubMed] [Google Scholar]
- Jones CM, 2013. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002–2004 and 2008–2010. Drug Alcohol Depen 132, 95–100. [DOI] [PubMed] [Google Scholar]
- Jones CM, Logan J, Gladden M, Bohm MK, 2015. Vital Signs: Demographic and Substance Use Trends Among Heroin Users - United States, 2002–2013. Mmwr-Morbid Mortal W 64, 719–725. [PMC free article] [PubMed] [Google Scholar]
- Kalso E, 2005. Oxycodone. J Pain Symptom Manage 29, S47–56. [DOI] [PubMed] [Google Scholar]
- Karami M, Zarrindast MR, 2008. Morphine sex-dependently induced place conditioning in adult Wistar rats. Eur J Pharmacol 582, 78–87. [DOI] [PubMed] [Google Scholar]
- King TS, Canez MS, Gaskill S, Javors MA, Schenken RS, 1993. Chronic cocaine disruption of estrous cyclicity in the rat: dose-dependent effects. J Pharmacol Exp Ther 264, 29–34. [PubMed] [Google Scholar]
- Kirsh K, Peppin J, Coleman J, 2012. Characterization of prescription opioid abuse in the United States: focus on route of administration. J Pain Palliat Care Pharmacother 26, 348–361. [DOI] [PubMed] [Google Scholar]
- Koob GF, Pettit HO, Ettenberg A, Bloom FE, 1984. Effects of opiate antagonists and their quaternary derivatives on heroin self-administration in the rat. J Pharmacol Exp Ther 229, 481–486. [PubMed] [Google Scholar]
- Laudenbach M, Baruffaldi F, Robinson C, Carter P, Seelig D, Baehr C, Pravetoni M, 2018. Blocking interleukin-4 enhances efficacy of vaccines for treatment of opioid abuse and prevention of opioid overdose. Sci Rep 8, 5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME, 1999. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 144, 77–82. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR, 2004. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology 29, 943–951. [DOI] [PubMed] [Google Scholar]
- Mavrikaki M, Pravetoni M, Page S, Potter D, Chartoff E, 2017. Oxycodone self-administration in male and female rats. Psychopharmacology 234, 977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SA, 2012. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp, e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan H, Holliday ED, Fox RG, Stutz SJ, Comer SD, Haney M, Anastasio NC, Moeller FG, Cunningham KA, 2017. Lorcaserin Suppresses Oxycodone Self-Administration and Relapse Vulnerability in Rats. ACS Chem Neurosci 8, 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Hwang CS, Grant Y, Janda KD, Taffe MA, 2018. Prophylactic vaccination protects against the development of oxycodone self-administration. Neuropharmacology 138, 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto SJ, Kosten TA, 2017. Female Sprague-Dawley rats display greater appetitive and consummatory responses to alcohol. Behav Brain Res 327, 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrup TF, Stotts AL, Green C, Potter JS, Marino EN, Walker R, Weiss RD, Trivedi M, 2015. Opioid withdrawal, craving, and use during and after outpatient buprenorphine stabilization and taper: a discrete survival and growth mixture model. Addict Behav 41, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkkola KT, Kontinen VK, Saari TI, Kalso EA, 2013. Does the pharmacology of oxycodone justify its increasing use as an analgesic? Trends Pharmacol Sci 34, 206–214. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Nakagawa T, Shige K, Minami M, Satoh M, 2001. Changes in the expression of glial glutamate transporters in the rat brain accompanied with morphine dependence and naloxone-precipitated withdrawal. Brain Res 905, 254–258. [DOI] [PubMed] [Google Scholar]
- Paulozzi LJ, Budnitz DS, Xi YL, 2006. Increasing deaths from opioid analgesics in the United States. Pharmacoepidem Dr S 15, 618–627. [DOI] [PubMed] [Google Scholar]
- Paulozzi LJ, Jones CM, Mack KA, Rudd RA, 2011. Vital Signs: Overdoses of Prescription Opioid Pain Relievers-United States, 1999–2008 (Reprinted from MMWR, vol 60, pg 1487–1492, 2011). Jama-J Am Med Assoc 306, 2444–2446. [Google Scholar]
- Pert CB, Pasternak G, Snyder SH, 1973. Opiate agonists and antagonists discriminated by receptor binding in brain. Science 182, 1359–1361. [DOI] [PubMed] [Google Scholar]
- Pravetoni M, Le Naour M, Harmon TM, Tucker AM, Portoghese PS, Pentel PR, 2012a. An oxycodone conjugate vaccine elicits drug-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. J Pharmacol Exp Ther 341, 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Pentel PR, Potter DN, Chartoff EH, Tally L, LeSage MG, 2014a. Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PloS one 9, e101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Raleigh MD, Le Naour M, Tucker AM, Harmon TM, Jones JM, Birnbaum AK, Portoghese PS, Pentel PR, 2012b. Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats. Vaccine 30, 4617–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Vervacke JS, Distefano MD, Tucker AM, Laudenbach M, Pentel PR, 2014b. Effect of currently approved carriers and adjuvants on the pre-clinical efficacy of a conjugate vaccine against oxycodone in mice and rats. PLoS One 9, e96547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley TL, Gadd CA, De Felipe C, Hunt SP, Stephens DN, 2002. Lack of self-administration and behavioural sensitisation to morphine, but not cocaine, in mice lacking NK1 receptors. Neuropharmacology 43, 1258–1268. [DOI] [PubMed] [Google Scholar]
- Sandweiss AJ, McIntosh MI, Moutal A, Davidson-Knapp R, Hu J, Giri AK, Yamamoto T, Hruby VJ, Khanna R, Largent-Milnes TM, Vanderah TW, 2018. Genetic and pharmacological antagonism of NK1 receptor prevents opiate abuse potential. Mol Psychiatry 23, 1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, 2014. The neurokinin-1 receptor in addictive processes. The Journal of pharmacology and experimental therapeutics 351, 2–8. [DOI] [PubMed] [Google Scholar]
- Schank JR, King CE, Sun H, Cheng K, Rice KC, Heilig M, Weinshenker D, Schroeder JP, 2014. The role of the neurokinin-1 receptor in stress-induced reinstatement of alcohol and cocaine seeking. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 39, 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Nelson BS, Damadzic R, Tapocik JD, Yao M, King CE, Rowe KE, Cheng K, Rice KC, Heilig M, 2015. Neurokinin-1 receptor antagonism attenuates neuronal activity triggered by stress-induced reinstatement of alcohol seeking. Neuropharmacology 99, 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Pickens CL, Rowe KE, Cheng K, Thorsell A, Rice KC, Shaham Y, Heilig M, 2011. Stress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. Psychopharmacology 218, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Tapocik JD, Barbier E, Damadzic R, Eskay RL, Sun H, Rowe KE, King CE, Yao M, Flanigan ME, Solomon MG, Karlsson C, Cheng K, Rice KC, Heilig M, 2013. Tacr1 gene variation and neurokinin 1 receptor expression is associated with antagonist efficacy in genetically selected alcohol-preferring rats. Biological psychiatry 73, 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK, 2002. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav 72, 431–435. [DOI] [PubMed] [Google Scholar]
- Warner M, Chen LH, Makuc DM, 2009. Increase in fatal poisonings involving opioid analgesics in the United States, 1999–2006. NCHS Data Brief, 1–8. [PubMed] [Google Scholar]
- You ZB, Bi GH, Galaj E, Kumar V, Cao J, Gadiano A, Rais R, Slusher BS, Gardner EL, Xi ZX, Newman AH, 2018. Dopamine D3R antagonist VK4–116 attenuates oxycodone self-administration and reinstatement without compromising its antinociceptive effects. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.