Abstract
Background.
Enhanced influenza vaccines may improve protection for older adults, but comparative immunogenicity data are limited. Our objective was to examine immune responses to enhanced influenza vaccines, compared to standard-dose vaccines, in community-dwelling older adults.
Methods.
Community-dwelling older adults aged 65–82 years in Hong Kong were randomly allocated (October 2017–January 2018) to receive 2017–2018 Northern hemisphere formulations of a standard-dose quadrivalent vaccine, MF59-adjuvanted trivalent vaccine, high-dose trivalent vaccine, or recombinant-hemagglutinin (rHA) quadrivalent vaccine. Sera collected from 200 recipients of each vaccine before and at 30-days postvaccination were assessed for antibodies to egg-propagated vaccine strains by hemagglutination inhibition (HAI) and to cell-propagated A/Hong Kong/4801/2014(H3N2) virus by microneutralization (MN). Influenzaspecific CD4+ and CD8+ T cell responses were assessed in 20 participants per group.
Results.
Mean fold rises (MFR) in HAI titers to egg-propagated A(H1N1) and A(H3N2) and the MFR in MN to cell-propagated A(H3N2) were statistically significantly higher in the enhanced vaccine groups, compared to the standard-dose vaccine. The MFR in MN to cell-propagated A(H3N2) was highest among rHA recipients (4.7), followed by high-dose (3.4) and MF59-adjuvanted (2.9) recipients, compared to standard-dose recipients (2.3). Similarly, the ratio of postvaccination MN titers among rHA recipients to cell-propagated A(H3N2) recipients was 2.57-fold higher than the standard-dose vaccine, which was statistically higher than the high-dose (1.33-fold) and MF59-adjuvanted (1.43-fold) recipient ratios. Enhanced vaccines also resulted in the boosting of T-cell responses.
Conclusions.
In this head-to-head comparison, older adults receiving enhanced vaccines showed improved humoral and cell-mediated immune responses, compared to standard-dose vaccine recipients.
Clinical Trials Registration.
Keywords: influenza, vaccination, public health
Influenza vaccination is the cornerstone public health intervention to reduce the annual burden of influenza epidemics [1]. The most widely used influenza vaccines are inactivated influenza vaccines, administered intramuscularly [2]. Trivalent inactivated influenza vaccines contain a total of 45 μg of hemagglutinin (HA), with a “standard dose” (SD) of 15 μg each of influenza A(H1N1), A(H3N2), and B strains, while quadrivalent vaccines contain an additional 15 μg HA of a second B strain. While these are the most commonly used vaccines, their effectiveness is variable from year to year [3].
Enhanced influenza vaccines have the potential to overcome issues associated with an aging immune system and reduced vaccine effectiveness [4–7]. The MF59-adjuvanted vaccine (FluAD) includes MF59, a squalene-based emulsion, in addition to 45 μg of HA (trivalent formulation), and produces a stronger immune response than the SD vaccine [8, 9], including increased breadth, diversity, and avidity of HA antibodies [10]. However, there are no available trials comparing the clinical efficacy of the MF59-adjuvanted vaccine to the SD vaccine in older adults. The trivalent high-dose (HD) vaccine, FluZone, contains a total of 180 μg of HA (60 μg of each HA): that is, 4 times the antigen of a trivalent SD vaccine. Compared to an SD vaccine, the HD vaccine induced significantly higher antibody responses [11] and provided better efficacy than the SD vaccine against laboratory-confirmed influenza among older adults [12]. A recombinant HA-protein quadrivalent influenza vaccine, FluBlok, includes 180 μg of HA (45 μg of each HA), produced by Baculovirus expression in insect SF9 cell culture [13, 14]. This vaccine was more efficacious than an SD vaccine in preventing polymerase chain reaction–confirmed influenza among adults aged ≥50 years [15].
Although there is some evidence to support improved protection among older adults for these 3 enhanced influenza vaccines, multiple knowledge gaps remain. The immunogenicity of enhanced vaccines has not been directly compared against the same influenza vaccine antigens within the same population. This is especially important for the A(H3N2) vaccine component, given the lower clinical effectiveness against A(H3N2) illness among older adults [16] and the effects of egg adaptations observed in association with A(H3N2) vaccine components [17]. Therefore, we conducted a randomized, controlled trial to examine immune responses to 3 enhanced influenza vaccines, compared to SD vaccines, in community-dwelling older adults. Because antibody responses have limitations as predictors of vaccine efficacy, especially among older adults [18], we also compared T-cell responses to the vaccines [19]. Although large vaccine efficacy trials will ultimately be needed to clarify the relative preventive benefits of enhanced vaccines among older adults, over the course of this immunogenicity trial we aim to directly quantify and compare relative differences in immune responses to inform this substantial future investment.
METHODS
Recruitment and Follow-up of Participants
We enrolled community-dwelling older adults who were: (1) 65–82 years of age; (2) residing in Hong Kong; and (3) had not already received the Northern hemisphere 2017–2018 formulation of the influenza vaccine. Minor exclusion criteria and details on recruitment methods are described in the Supplementary Appendix.
Because we intend to examine alternative combinations of repeated vaccinations with SD and enhanced vaccines in future years of this trial, we randomized participants to 11 different groups (see the Supplementary Appendix). In Year 1, we randomized 3/11 (27%) participants to receive an SD quadrivalent vaccine (0.5 mL FluQuadri), 3/11 (27%) to receive a trivalent MF59-adjuvanted vaccine (0.5 mL FLUAD, Seqirus), 3/11 (27%) to receive a trivalent high-dose vaccine (0.5 mL Fluzone High-Dose, Sanofi Pasteur), and 2/11 (18%) to receive a quadrivalent recombinant-HA vaccine (0.5 mL Flublok, Sanofi Pasteur). All vaccines included the strains recommended for the Northern hemisphere 2017–2018 formulation: namely, the A/Michigan/45/2015(H1N1)-like virus (clade 6B.1), A/Hong Kong/4801/2014(H3N2)-like virus (clade 3C.2a), and B/Brisbane/60/2008-like virus (Victoria lineage; clade 1A), and the quadrivalent vaccines also included the B/Phuket/3073/2013-like virus (Yamagata lineage; clade 3).
We used R software to generate a block-randomized allocation sequence (see the Supplementary Appendix), which was concealed from the study staff. Study vaccines were repackaged into numbered boxes, based on the allocation sequence, by a research assistant who had no contact with participants. The study was blinded to participants, researchers, and laboratory staff, but was not blinded to the nurse who removed the vaccine from the numbered box and administered it. Randomization was done immediately prior to the administration of the vaccine for each participant.
We collected a 9 ml blood sample from each participant immediately prior to vaccination and 30 days (range, 26–35 days) after vaccination. A nonrandom, voluntary subset of 10% of participants (see the Supplementary Appendix) was invited to provide an additional 10 ml of heparinized whole-blood specimens at baseline, 7 days, and 30 days after vaccination for the extraction of peripheral blood mononuclear cells (PBMCs) and testing for cell-mediated immune responses to vaccination.
After vaccination, participants were observed for 15 minutes for acute reactions, and contacted at 1, 3, 7, and 14 days after vaccination to record any adverse events. During the 30-day postvaccination visit, we collected information on any serious adverse events since vaccination.
Ethics
Signed, informed consent was obtained from all participants. The study protocol was approved by the Institutional Review Board of the University of Hong Kong.
Laboratory Methods
A subset of 200 pairs of Day 0 and Day 30 sera from each of the 4 vaccine groups were selected, including all sera from the participants in the nonrandom 10% subset who provided PBMCs, plus a random sample of the other participants (see the Supplementary Appendix). Sera were tested by hemagglutination inhibition (HAI) assays against egg-propagated vaccine strains A/Singapore/GP1908/2015 (A/Michigan/45/2015[H1N1]-like virus), A/Hong Kong/4801/2014(H3N2), B/Brisbane/60/2008, and B/Phuket/3073/2013. In addition, we tested the sera by virus microneutralization (MN) against cell-propagated A/Hong Kong/4801/2014(H3N2), because the cell-propagated virus, which is more similar to the circulating strains, failed to agglutinate red blood cells (see the Supplementary Appendix).
In a subset of 20 participants in each of the 4 vaccine groups, influenza-specific CD4+ and CD8+ T-cell responses against influenza A(H1N1), A(H3N2), and B vaccine strains were assessed by flow cytometry for interferon (IFN)-γ+ production after in vitro stimulation (see the Supplementary Appendix) to determine CD8+ and CD4+ T-cell recall responses at Day 7 and memory responses at Day 30, compared to prevaccination (Day 0) [20].
Outcome Measures
Following the approach taken by a recent review of randomized trials comparing the immunogenicity of alternative influenza vaccine types among older adults [5], the primary outcome measures relate to the immune responses, measured by antibody titers against the vaccine strains in each enhanced vaccine group, compared to SD, including: (1) the proportion of participants who achieved a 4-fold or greater rise in postvaccination titers from Day 0 to Day 30 with a postvaccination titer ≥40; and (2) the geometric mean titer (GMT) at Day 30. In addition, we examined 3 protocol-specified secondary outcome measures: (1) the proportion of participants who achieved a postvaccination (Day 30) antibody titer ≥40 (and further elevated titers of ≥80 and ≥160); (2) the fold rise of vaccine-specific IFN-γ+ CD4+ and IFN-γ+ CD8+ T-cell responses at 7 (acute) and 30 (memory) days postvaccination, compared to prevaccination (Day 0); and (3) the rate of adverse events within 30 days after each vaccination.
Statistical Analysis
We estimated the proportion of participants with a ≥4-fold rise in antibody titers that led to a postvaccination titer ≥40, the GMTs at Day 0 and Day 30, and the mean fold rise (MFR) from Day 0 to Day 30, with 95% confidence intervals. We calculated the ratio of postvaccination GMTs in each enhanced vaccine group, compared to the postvaccination GMT in the SD group, with 95% confidence intervals. We estimated the proportions of participants with postvaccination titers ≥40, ≥80, and ≥160 in each vaccination group, and compared the proportions in the other 3 vaccination groups versus an SD quadrivalent influenza vaccine (QIV), using Chi-squared tests. Although P values <.05 were considered statistically significant in order to optimize sensitivity to differences between exposure groups, we considered as noteworthy any patterns of effects that were repeated across outcomes. As previously noted [21], with a P value threshold <.05, a statistically significant difference can be observed even when 95% confidence intervals for the estimate overlap. All statistical analyses were conducted using R version 3.4.3.
Sample Size Justification
For the present comparison of responses to 4 different vaccines in Year 1, we sought to detect small to moderate but meaningful relative differences in immunogenicity. A sample size of 200 participants per group would permit 80% power to detect increases of ≥14% in the proportion of enhanced vaccine recipients with ≥4-fold rises in titer, and GMT ratios of 1.4 or higher in the enhanced vaccine groups, compared to a SD vaccine. Overall, we aimed to enroll 2200 participants into our study, anticipating a 10% dropout rate per year and a final sample size of at least 146 participants in each group through to the fourth year of the planned trial (see the Supplementary Appendix).
RESULTS
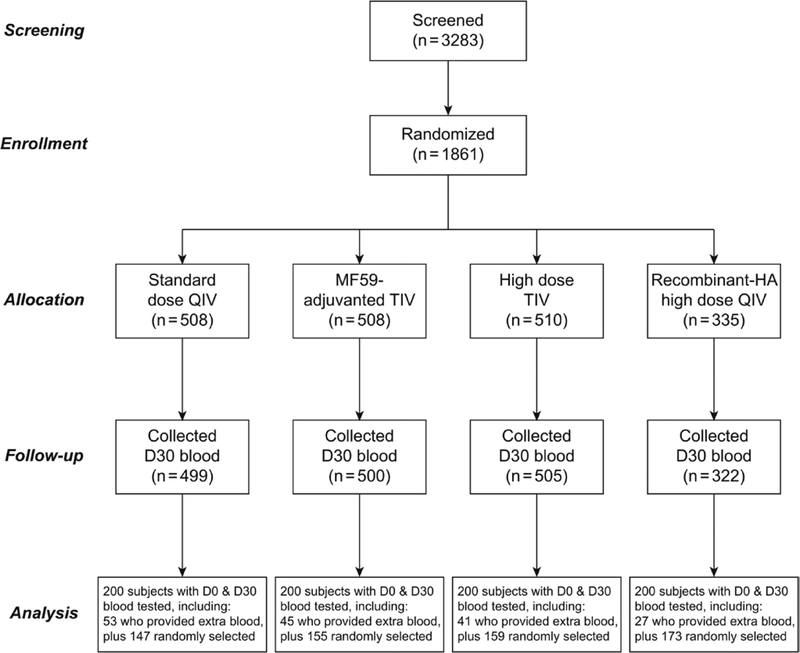
We screened 3283 older adults, of whom 2695 were eligible and 2039 agreed to participate. We obtained signed informed consent from 2008 persons and were able to randomize and vaccinate 1861 participants from 7 October 2017 through 12 January 2018, of whom 1826 provided Day 30 postvaccination serum samples (Figure 1). About 10% of participants were asked to give additional blood; 168 contributed PBMCs for analyses of cell-mediated immunity.
Figure 1.
Flow chart of participants through the study, and selection of 200 participants per group for serologic analysis. Abbreviations: D, day; HA, hemagglutinin; QIV, quadrivalent influenza vaccine; TIV, trivalent influenza vaccine.
Participants were balanced by age, sex, underlying medical conditions, and prior vaccination history between the 4 study groups, with no statistically significant differences in these key variables overall or among those contributing to the primary antibody findings reported here (Table 1). The most common underlying conditions were hypertension, osteoarthritis, and diabetes. Most participants (66%) had received an influenza vaccination in the preceding year (2016–2017), and only 22% had not received any influenza vaccination in the preceding 5 years. All prior influenza vaccinations were with an SD vaccine. We selected 200 participants from each group for a serologic analysis, and the characteristics of this subset were representative of the entire cohort (Table 2). Pre- and post-vaccination GMTs and corresponding MFRs are described in Table 3 for each vaccine group, and Figure 2 presents comparisons of the MFRs between the enhanced vaccine groups and the SD group. Table 4 shows the proportions of participants achieving specific titer thresholds after vaccination.
Table 1.
Baseline Characteristics of the 1861 Participants, by Vaccination Group
| Characteristics | All Participants | |||||||
|---|---|---|---|---|---|---|---|---|
| Standard-dose quadrivalent vaccine, n = 508 | MF59-adjuvanted trivalent vaccine, n = 508 | High-dose trivalent vaccine, n = 510 | Recombinant-HA quadrivalent vaccine, n = 335 | |||||
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Age, years | ||||||||
| 65–70 | 269 | (53%) | 248 | (49%) | 258 | (51%) | 171 | (51%) |
| 71–76 | 130 | (26%) | 149 | (29%) | 143 | (28%) | 82 | (24%) |
| 77–82 | 109 | (21%) | 111 | (22%) | 109 | (21%) | 82 | (24%) |
| Female sex | 301 | (59%) | 308 | (61%) | 327 | (64%) | 195 | (58%) |
| Underlying medical conditions | ||||||||
| Hypertension | 230 | (45%) | 262 | (52%) | 239 | (47%) | 161 | (48%) |
| Osteoarthritis | 110 | (22%) | 102 | (20%) | 109 | (21%) | 70 | (21%) |
| Diabetes | 98 | (19%) | 104 | (20%) | 88 | (17%) | 61 | (18%) |
| Heart diseases | 52 | (10%) | 47 | (9%) | 52 | (10%) | 29 | (9%) |
| Cancer | 46 | (9%) | 43 | (8%) | 40 | (8%) | 22 | (7%) |
| Othersa | 209 | (41%) | 221 | (44%) | 226 | (44%) | 141 | (42%) |
| Received influenza vaccination in 2016–2017 season | 328 | (65%) | 332 | (65%) | 351 | (69%) | 226 | (67%) |
| Number of times received influenza vaccination in the preceding 5 yearsb | ||||||||
| 0 | 126 | (25%) | 104 | (20%) | 111 | (22%) | 71 | (21%) |
| 1–2 | 113 | (22%) | 120 | (24%) | 112 | (22%) | 68 | (20%) |
| 3–4 | 54 | (11%) | 45 | (9%) | 61 | (12%) | 38 | (11%) |
| 5–6 | 195 | (38%) | 201 | (40%) | 212 | (42%) | 144 | (43%) |
Abbreviation: HA, hemagglutinin.
Other underlying conditions included: stroke, chronic lung disease, kidney disease, liver disease, depression or anxiety disorder, neurologic disorder, autoimmune disease, disease of the digestive system, hypothyroidism, dermatological disease, etc.
There were 6 possible influenza vaccines, including the 5 Northern hemisphere influenza vaccines from 2012–2013 through 2016–2017 plus the Southern hemisphere 2015 vaccine, which was available in Hong Kong for a special vaccination campaign [20]. The Southern hemisphere formulation is not usually available in Hong Kong.
Table 2.
Baseline Characteristics of the Subset of 800 Participants Selected for Immunogenicity Analyses
| Participants Selected for Immunogenicity Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Standard-dose quadrivalent vaccine, n = 200 | MF59-adjuvanted trivalent vaccine, n = 200 | High-dose trivalent vaccine, n = 200 | Recombinant-HA quadrivalent vaccine, n = 200 | |||||
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Age, years | ||||||||
| 65–70 | 105 | (52%) | 96 | (48%) | 106 | (53%) | 101 | (50%) |
| 71–76 | 44 | (22%) | 59 | (30%) | 52 | (26%) | 53 | (26%) |
| 77–82 | 51 | (26%) | 45 | (22%) | 42 | (21%) | 46 | (23%) |
| Female sex | 119 | (60%) | 119 | (60%) | 127 | (64%) | 120 | (60%) |
| Underlying medical conditions | ||||||||
| Hypertension | 95 | (48%) | 105 | (52%) | 92 | (46%) | 101 | (50%) |
| Osteoarthritis | 44 | (22%) | 36 | (18%) | 41 | (20%) | 49 | (24%) |
| Diabetes | 43 | (22%) | 41 | (20%) | 38 | (19%) | 37 | (18%) |
| Heart diseases | 22 | (11%) | 11 | (6%) | 20 | (10%) | 21 | (10%) |
| Cancer | 20 | (10%) | 14 | (7%) | 15 | (8%) | 15 | (8%) |
| Othersa | 83 | (42%) | 81 | (40%) | 82 | (41%) | 89 | (44%) |
| Received influenza vaccination in 2016–2017 season | 136 | (68%) | 130 | (65%) | 139 | (70%) | 137 | (69%) |
| Number of times received influenza vaccination in the preceding 5 yearsb | ||||||||
| 0 | 46 | (23%) | 45 | (22%) | 46 | (23%) | 47 | (24%) |
| 1–2 | 46 | (23%) | 43 | (22%) | 34 | (17%) | 41 | (20%) |
| 3–4 | 29 | (14%) | 21 | (10%) | 26 | (13%) | 19 | (10%) |
| 5–6 | 74 | (37%) | 78 | (39%) | 90 | (45%) | 87 | (44%) |
Abbreviation: HA, hemagglutinin.
Other underlying conditions included: stroke, chronic lung disease, kidney disease, liver disease, depression or anxiety disorder, neurologic disorder, autoimmune disease, disease of the digestive system, hypothyroidism, dermatological disease, etc.
There were 6 possible influenza vaccines, including the 5 Northern hemisphere influenza vaccines from 2012–2013 through 2016–2017 plus the Southern hemisphere 2015 vaccine, which was available in Hong Kong for a special vaccination campaign [20]. The Southern hemisphere formulation is not usually available in Hong Kong.
Table 3.
Summary of Pre- and Post-vaccination Antibody Titers and Fold Rises in Each Vaccination Group
| Assay | Strain | Vaccination Group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Standard-dose QIV, n = 200 | MF59-adjuvanted TIV, n = 200 | High-dose TIV, n = 200 | Recombinant-HA QIV, n = 200 | ||||||
| Estimate | (95% CI) | Estimate | (95% CI) | Estimate | (95% CI) | Estimate | (95% CI) | ||
| HAI | A/Michigan/45/2015 (H1N1) | ||||||||
| Day 0 GMT | 17 | (14–20) | 17 | (15–20) | 20 | (17–24) | 16 | (14–19) | |
| Day 30 GMT | 69 | (58–83) | 94* | (78–114) | 125* | (102–152) | 85 | (69–105) | |
| Mean fold rise from D0 to D30 | 4.1 | (3.5–4.9) | 5.5* | (4.6–6.6) | 6.1* | (5.1–7.3) | 5.3* | (4.4–6.3) | |
| % with ≥4-fold rise from D0 to D30b | 42% | (36–50%) | 60%* | (53–67%) | 59%* | (52–66%) | 60%* | (53–67%) | |
| HAI | A/Hong Kong/4801/2014 (H3N2) egg-like | ||||||||
| Day 0 GMT | 46 | (39–56) | 49 | (41–59) | 45 | (38–54) | 54 | (45–64) | |
| Day 30 GMT | 158 | (135–186) | 207* | (178–241) | 214* | (183–250) | 254* | (218–295) | |
| Mean fold rise from D0 to D30 | 3.4 | (2.8–4.1) | 4.2* | (3.5–5.1) | 4.7* | (3.9–5.7) | 4.7* | (3.9–5.7) | |
| % with ≥4-fold rise from D0 to D30a | 41% | (34–48%) | 48% | (40–55%) | 54%* | (46–61%) | 56%* | (48–63%) | |
| MN | A/Hong Kong/4801/2014 (H3N2) cell-like | ||||||||
| Day 0 GMT | 38 | (31–46) | 43 | (35–53) | 34 | (28–41) | 48 | (39–59) | |
| Day 30 GMT | 87 | (72–106) | 125* | (102–152) | 116* | (95–141) | 223* | (189–263) | |
| Mean fold rise from D0 to D30 | 2.3 | (2.0–2.6) | 2.9* | (2.5–3.4) | 3.4* | (2.9–4.0) | 4.7* | (3.9–5.6) | |
| % with ≥4-fold rise from D0 to D30a | 28% | (22–35%) | 39%* | (32–46%) | 47%* | (40–54%) | 57%* | (50–64%) | |
| HAI | B/Brisbane/60/2008 (Victoria lineage) | ||||||||
| Day 0 GMT | 24 | (20–28) | 31* | (26–38) | 31* | (26–37) | 29* | (25–35) | |
| Day 30 GMT | 89 | (75–105) | 95 | (81–112) | 132* | (112–157) | 90 | (76–107) | |
| Mean fold rise from D0 to D30 | 3.7 | (3.2–4.4) | 3.0 | (2.6–3.5) | 4.2 | (3.5–5.0) | 3.1 | (2.7–3.5) | |
| % with ≥4-fold rise from D0 to D30a | 48% | (41–56%) | 44% | (37–51%) | 52% | (45–60%) | 44% | (37–51%) | |
| HAI | B/Phuket/3073/2013b (Yamagata lineage) | ||||||||
| Day 0 GMT | 37 | (31–44) | 41 | (35–49) | 41 | (34–50) | 45 | (38–54) | |
| Day 30 GMT | 121 | (104–141) | 63* | (54–74) | 68* | (57–81) | 131 | (111–155) | |
| Mean fold rise from D0 to D30 | 3.3 | (2.8–3.8) | 1.5* | (1.4–1.7) | 1.6* | (1.5–1.8) | 2.9 | (2.5–3.3) | |
| % with ≥4-fold rise from D0 to D30a | 42% | (36–50%) | 12%* | (8–18%) | 15%* | (10–21%) | 42% | (36–50%) | |
Values that are significantly different from the corresponding value in the standard-dose group. Statistical significance was designated at P value <.05.
Abbreviations: CI, confidence interval; D, day; GMT, geometric mean titer; HA, hemagglutinin; HAI, hemagglutination inhibition assay; MN, microneutralization assay; QIV, quadrivalent influenza vaccine; TIV, trivalent influenza vaccine.
At least a 4-fold rise from D0 to D30 with D30 titer ≥40.
Note that a B/Yamagata lineage virus was not included in the MF59-adjuvanted TIV and the high-dose TIV.
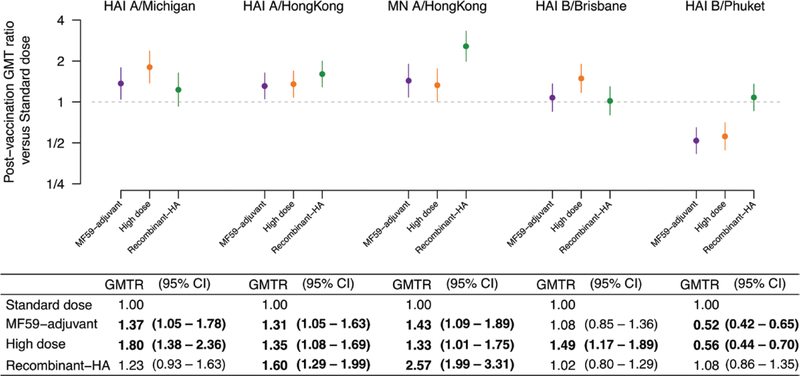
Figure 2.
Comparisons of postvaccination geometric mean antibody titers in each of the enhanced vaccine groups, compared to the postvaccination geometric mean titer in the standard-dose QIV group (n = 200 individuals per group). Values significantly different from 1.0 are highlighted in bold. Note that a B/Yamagata lineage virus was not included in the MF59-adjuvanted TIV and the high-dose TIV. Antigens used were A/Singapore/GP1908/2015 (A/Michigan/45/2015[H1N1]-like virus); A/Hong Kong/4801/2014(H3N2), with both egg-propagated and cell-propagated variants; B/Brisbane/60/2008; and B/Phuket/3073/2013. Abbreviations: CI, confidence interval; GMT, geometric mean titer; GMTR, geometric mean titer ratio comparing post-vaccination geometric mean titers between enhanced vaccine groups and the standard dose group; HA, hemagglutinin; HAI, hemagglutination inhibition assay; MN, microneutralization assay; QIV, quadrivalent influenza vaccine; TIV, trivalent influenza vaccine.
Table 4.
Proportions of Participants With Postvaccination Antibody Titers ≥40, ≥80, and ≥160 in Each Group
| Assay | Strain | Vaccination Group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Standard-dose QIV, n = 200 | MF59-adjuvantedTIV, n = 200 | High-doseTIV, n = 200 | Recombinant-HA QIV, n = 200 | ||||||
| Prop. | (95% CI) | Prop. | (95% CI) | Prop. | (95% CI) | Prop. | (95% CI) | ||
| Postvaccination (D30) titer ≥40 | |||||||||
| HAI | A/Michigan/45/2015 (H1N1) | 73% | (66–79%) | 82%* | (76–87%) | 83%* | (77–88%) | 76% | (69–81%) |
| HAI | A/Hong Kong/4801/2014 (H3N2) | 94% | (90–97%) | 96% | (92–98%) | 96% | (92–98%) | 96% | (92–98%) |
| MN | A/Hong Kong/4801/2014 (H3N2) | 78% | (72–84%) | 85% | (79–90%) | 82% | (77–87%) | 94%* | (90–97%) |
| HAI | B/Brisbane/60/2008 | 84% | (78–88%) | 88% | (83–93%) | 90% | (85–94%) | 88% | (82–92%) |
| HAI | B/Phuket/3073/2013a | 94% | (90–97%) | 77%* | (71–83%) | 78%* | (72–84%) | 90% | (86–94%) |
| Postvaccination (D30) titer ≥80 | |||||||||
| HAI | A/Michigan/45/2015 (H1N1) | 54% | (47–61%) | 64%* | (57–71%) | 69%* | (62–75%) | 62% | (54–68%) |
| HAI | A/Hong Kong/4801/2014 (H3N2) | 81% | (75–86%) | 88%* | (83–93%) | 88% | (82–92%) | 92%* | (8%–96%) |
| MN | A/Hong Kong/4801/2014 (H3N2) | 63% | (56–70%) | 72% | (65–78%) | 74%* | (67–79%) | 88%* | (83–92%) |
| HAI | B/Brisbane/60/2008 | 69% | (62–75%) | 70% | (63–76%) | 78% | (71–83%) | 64% | (57–71%) |
| HAI | B/Phuket/3073/2013a | 76% | (69–81%) | 58%* | (51–65%) | 58%* | (51–65%) | 77% | (71–83%) |
| Postvaccination (D30) titer ≥160 | |||||||||
| HAI | A/Michigan/45/2015 (H1N1) | 35% | (28–42%) | 45%* | (38–52%) | 55%* | (48–62%) | 46%* | (39–53%) |
| HAI | A/Hong Kong/4801/2014 (H3N2) | 64% | (56–70%) | 72% | (66–79%) | 75%* | (68–81%) | 81%* | (75–86%) |
| MN | A/Hong Kong/4801/2014 (H3N2) | 48% | (41–56%) | 54% | (47–61%) | 58% | (51–65%) | 74%* | (68–80%) |
| HAI | B/Brisbane/60/2008 | 40% | (34–48%) | 44% | (37–52%) | 54%* | (46–61%) | 39% | (32–46%) |
| HAI | B/Phuket/3073/2013a | 52% | (45–59%) | 32%* | (26–39%) | 33%* | (27–40%) | 56% | (49–63%) |
Significant differences in comparison to standard-dose QIV.
Abbreviagtions: D, day; HA, hemagglutinin; HAI, hemagglutination inhibition assay; MN, microneutralization assay; Prop., proportion; QIV, quadrivalent influenza vaccine; TIV, trivalent influenza vaccine.
Note that a B/Yamagata lineage virus was not included in the MF59-adjuvanted TIV and the high-dose TIV.
There were no statistically significant baseline differences between the groups in prevaccination GMTs for each of the antigens tested, except that the baseline GMTs to the B/Victoria antigen were statistically significantly lower in the SD group (Table 3). As shown in Table 3, among SD QIV recipients, the HAI GMT to A(H1N1) increased from 17 to 69. MF59-adjuvanted and HD recipients achieved a significantly higher postvaccination GMT to A(H1N1) than SD recipients; recombinant-HA recipients did not. Nonetheless, MFRs for A(H1N1) were statistically significantly higher for all 3 enhanced vaccines (range, 5.3–6.1) compared to the SD (4.1; Table 3; Figure 2). The proportions with ≥4-fold rises to titers ≥40 were statistically significantly higher for all 3 enhanced vaccines (range, 59–60%) compared to the SD QIV (42%). As shown in Table 4, the proportions achieving elevated titers of ≥40 were higher for the MF59-adjuvanted (82%) and HD (83%) groups, compared to the SD recipients (72%). Of note, the proportions achieving very high titers of ≥160 were significantly higher for all 3 enhanced vaccines (45–55%), compared to the SD vaccine (35%).
Recipients of all 3 enhanced vaccines achieved higher postvaccination GMTs and greater MFRs to both the egg-propagated A(H3N2) by HAI and the cell-propagated A(H3N2) by MN than recipients of SD vaccines (Table 3; Figure 2). The directions of the effects were similar using other indicators. Of note, only 28% of SD recipients achieved ≥4-fold rises in MN responses to cell-propagated A(H3N2), compared to 39% of MF59-adjuvant, 47% of HD QIV, and 57% of recombinant-HA QIV recipients (P < .01; Table 3).
On several indicators, antibody responses to cell-propagated A(H3N2) by MN were significantly higher among recombinant-HA QIV recipients, compared to SD vaccine, MF59-adjuvant, and HD recipients (Table 3). Specifically, recipients of recombinant-HA QIV achieved a postvaccination GMT to cell-propagated A(H3N2) that was 2.57-fold higher than that of SD recipients; the magnitude of this difference was significantly higher than the 1.43- and 1.33-fold differences of MF59-adjuvanted and HD recipients, respectively, compared to SD recipients (Figure 2). Recombinant-HA recipients were the only enhanced vaccine group with a significantly larger proportion (74%) of very high titers (≥160) against cell-propagated A(H3N2), compared to SD recipients (48%; Table 4).
Responses to the B/Victoria component of both the trivalent influenza vaccine (TIV) and QIV in the study year (B/Brisbane/60/2008) were similar across vaccines, with the exception of a significantly higher GMT of HD TIV, compared with SD QIV (Figure 2). Although the MFRs against B/Phuket/3073/2013 were statistically significantly >1 for both SD and enhanced vaccines (Table 3), the GMTs were significantly lower, compared with the SD QIV, for the MF-59-adjuvanted and HD TIVs, which did not include this B/Yamagata component (Figure 2). The response to recombinant-HA QIV that did include B/Yamagata was not significantly different to SD QIV on any indicator.
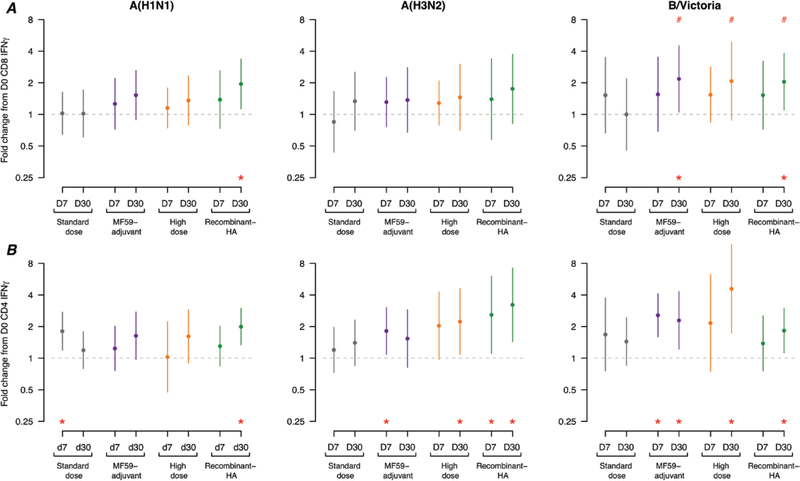
For T-cell responses at 7 and 30 days postvaccination, we noted a trend whereby enhanced influenza vaccines elicited increased peak acute IFN-γ+ T-cell responses and maintained higher average memory responses for selected time points and viruses (Figure 3; Supplementary Appendix Table 5). For influenza-specific IFN-γ+ CD8+ T-cell responses, statistically significant rises in T-cell responses from Day 0 to Day 30 were noted for the recombinant-HA vaccine against A(H1N1) and B/Victoria and for the MF59-adjuvanted vaccine against B/Victoria; no significant rises in the SD group were observed. IFN-γ+ CD8+ T cells to B/Victoria increased from Day 7 to Day 30 for all 3 enhanced vaccines; this trend was statistically significant when contrasted with a decline in T cells among SD vaccinees.
Figure 3.
The mean and standard deviations of the fold changes of Days 7 and 30 IFN-γ+ T cells, compared to Day 0 responses for A/Michigan/45/2015(H1N1), A/Switzerland/9715293/2013(H3N2), and B/Brisbane/60/2008 viruses after in vitro stimulation for (A) CD8+ and (B) CD4+ IFN-γ+ T cells (n = 20 individuals per group). Values marked with a # symbol indicate a statistically significant difference (P < .05), compared to the response in the standard-dose vaccine group at the same time point. Values marked with an * indicate a statistically significant difference (P < .05), compared to the baseline (Day 0) response within the same vaccine group. Abbreviations: D, day; HA, hemagglutinin; IFN, interferon.
For influenza-specific IFN-γ+ CD4+ T-cell responses, statistically significant rises in T-cell responses were noted at 7 and/or 30 days postvaccination for the enhanced vaccines, though none of these were statistically significantly higher than the trend for the SD vaccine. Overall, the recombinant-HA vaccine caused sustained memory IFN-γ+ CD4+ T-cell responses by significantly increased Day 30 mean fold rises for 3 of 3 viruses tested, whilst the HD vaccine increased CD4+ T-cell Day 30 memory fold rises for 2 of 3 viruses and the MF59-adjuvanted vaccine resulted in early Day 7 rises for 2 of 3 viruses and sustained memory rises for only 1 of 3 viruses. SD group had a significant rise in CD4+ T-cell response to 1 virus at day 7 and no significant rises for all 3 viruses tested at Day 30.
The rate of contraction of T-cell responses from acute Day 7 to memory Day 30 was determined by the fold change from Day 30, divided by Day 7 (Supplementary Appendix Table 5). We did not observe statistically significant contractions (or expansions) for virus-specific CD4+ T cells for any vaccines. For virus-specific CD8+ T cells, a significant difference for the fold change from acute Day 7 to memory Day 30 responses was noted for B/Victoria only. Among those receiving the SD vaccine, B/Victoria-specific CD8+ T cells significantly contracted by 0.65-fold from 7 to 30 days postvaccination. In contrast, among those receiving the enhanced vaccines, B/Victoria-specific CD8+ T cells continued to increase by 1.34- to 1.40-fold from Day 7 to Day 30 (P values all <.05, compared to the standard vaccine group); therefore, enhanced vaccines maintained and expanded a larger pool of circulating B/Victoria-specific CD8+ T cells than the SD vaccine.
The most frequently reported adverse events following vaccination included local reactions, such as tenderness (range across vaccine groups, 12–20%), pain (10–22%) and swelling (4–10%; Supplementary Appendix Figure 4). Compared to the SD group, recipients of the MF59-adjuvanted vaccine and HD vaccine experienced more tenderness and recipients of the HD vaccine experienced more swelling, mostly of a mild nature. Hospitalizations were not uncommon, but the rates of postvaccination hospitalizations were not statistically different between the 4 vaccine groups (Supplementary Appendix Table 3). We did not identify any potentially vaccine-related serious adverse events.
DISCUSSION
We found that all 3 of the 2017–2018 Northern hemisphere–enhanced vaccines had improved immunogenicity for influenza A(H1N1) and A(H3N2), compared to the SD vaccine, among older adults, while the recombinant-HA vaccine elicited a particularly high antibody response to the cell-like H3N2 strain, relative to the other enhanced vaccines and the SD vaccine (Figure 2). Improved CD4+ T-cell responses against H3N2 were also seen at Day 7 or Day 30 for each enhanced vaccine (Figure 3). Influenza A(H3N2) virus infection causes the greatest morbidity and mortality effects in older adults [22] and there have been particular concerns in recent years over poorer vaccine effectiveness against influenza A(H3N2) [16, 23], which may be at least partially due to egg adaptations in A(H3N2) vaccine strains and/or poorer immunogenicity in repeated vaccinees [17, 24–27].
Enhanced vaccines resulted in higher antibody responses on almost all indicators, compared to a SD vaccine, to the A/Michigan/45/2015(H1N1)-like virus, which was introduced in the 2017–2018 season. The ratios of postvaccination titers against the A(H1N1) component for the MF59-adjuvanted vaccine and the HD vaccine, compared to the SD vaccine (1.37-fold higher and 1.80-fold higher, respectively), were very similar to pooled estimates in a recent meta-analysis for this ratio across previous immunogenicity trials (1.28 and 1.72, respectively) [5]. The antibody response to A(H1N1) among recombinant-HA recipients in our study was better than the A(H1N1) response among older adults in a previous recombinant-HA trial [15]. Our findings for A(H3N2) were also within the range of postvaccination ratios for the HD vaccine and MF59-adjuvanted vaccine, compared to the SD vaccine, when observed across prior trials [5]. Our findings reinforce other studies suggesting that enhanced vaccines offer improved antibody responses over standard vaccines in older adults. However, our study was not designed to demonstrate efficacy against polymerase chain reaction–confirmed influenza, and comparisons of clinical efficacy and effectiveness are still needed.
However, the findings for influenza B viruses were less clear. Antibody responses to the influenza B/Victoria-lineage component, which was in the TIV and QIV formulations in 2017–2018 and the previous season, were high for all vaccines. There were also significant improvements over the SD vaccine in CD8+ T-cell responses to B/Victoria (Figure 3). Not surprisingly, the quadrivalent SD vaccine resulted in higher antibody responses to the influenza B/Yamagata-lineage strain, compared to the trivalent MF59-adjuvanted and HD vaccines, which did not include this component; nonetheless, both vaccines were associated with significant rises in B/Yamagata titers (Table 3). The QIV recombinant-HA vaccine did not result in a higher antibody response to the influenza B/Yamagata lineage, compared to the QIV SD vaccine, consistent with a previous trial [13].
Our study had a number of limitations. First, vaccine immunogenicity is not equivalent to vaccine efficacy or effectiveness, nor is there yet a way to directly extrapolate our findings into an epidemiologic measure of differences in actual vaccine protection. Second, we only tested sera from a subset of 800 participants, determining that this would be sufficient to compare vaccines in the first year and allowing us to save more of the sera for future cross-season analyses. Two-thirds of participants were vaccinated during the previous season; examining the association between vaccination histories and immune response will also require additional future testing. Finally, here we only examined the immediate response to vaccination; we did not examine the duration of protection or effects on subsequent vaccinations with enhanced or SD vaccines.
In conclusion, this randomized, controlled trial provides a unique comparison of the immune response to multiple enhanced influenza vaccine options for older adults. Although distinguishing the relative preventive value of alternative vaccine types among older adults will ultimately depend on future efficacy trials and/or well-controlled effectiveness studies, the findings from this ongoing immunogenicity trial can help to establish the conditions for those more definitive evaluations, including what differences in efficacy/effectiveness might plausibly be expected.
Supplementary Material
Acknowledgments.
The authors thank Ed Belongia for advice on the study design and implementation; the study staff for assistance in project implementation, including Tin-Kin Chau, Faith Ho, Jennifer Ho, Fiona Kee, Janisy Lai, Cecily Leung, Anita Li, Jessamine Luk, Loretta Mak, Yvonne Ng, Angel Wong, Miyuki Wong, Phoebe Wong, and Kitty Yu; Leo Luk, Emily Yau, Chi Tsang, and Kin Chan for laboratory support; Julie Au and Chi-Kin Lam for administrative support; and David Shay and Jerome Tokars for feedback on an earlier version of this manuscript.
Financial support. This study was supported by the Centers for Disease Control and Prevention (Cooperative Agreement Number IP001064-02).
Footnotes
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Publisher's Disclaimer: Disclaimer. The sponsor had no role in the data collection and analysis, or the decision to publish, but was involved in the study design and preparation of the manuscript. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Potential conflicts of interest. B. J. C. has received honoraria from Sanofi and Roche for advisory committees. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Barberis I, Martini M, Iavarone F, Orsi A. Available influenza vaccines: immunization strategies, history and new tools for fighting the disease. J Prev Med Hyg 2016; 57:E41–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Talbot HK, Nian H, Zhu Y, Chen Q, Williams JV, Griffin MR. Clinical effectiveness of split-virion versus subunit trivalent influenza vaccines in older adults. Clin Infect Dis 2015; 60:1170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belongia EA, Simpson MD, King JP, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016; 16:942–51. [DOI] [PubMed] [Google Scholar]
- 4.Xu C, Thompson MG, Cowling BJ. Influenza vaccination in tropical and subtropical areas. Lancet Respir Med 2017; 5:920–2. [DOI] [PubMed] [Google Scholar]
- 5.Ng TWY, Cowling BJ, Gao HZ, Thompson MG. Comparative immunogenicity of enhanced seasonal influenza vaccines in older adults: a systematic review and meta-analysis. J Infect Dis 2019; 219:1525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong SS, Webby RJ. Traditional and new influenza vaccines. Clin Microbiol Rev 2013; 26:476–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaffner W, van Buynder P, McNeil S, Osterhaus ADME. Seasonal influenza immunisation: Strategies for older adults. Int J Clin Pract 2018; 72:e13249. [DOI] [PubMed] [Google Scholar]
- 8.Gasparini R, Pozzi T, Montomoli E, et al. Increased immunogenicity of the MF59-adjuvanted influenza vaccine compared to a conventional subunit vaccine in elderly subjects. Eur J Epidemiol 2001; 17:135–40. [DOI] [PubMed] [Google Scholar]
- 9.Camilloni B, Basileo M, Valente S, Nunzi E, Iorio AM. Immunogenicity of intramuscular MF59-adjuvanted and intradermal administered influenza enhanced vaccines in subjects aged over 60: a literature review. Hum Vaccin Immunother 2015; 11:553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khurana S, Verma N, Yewdell JW, et al. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med 2011; 3:85ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couch RB, Winokur P, Brady R, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 2007; 25:7656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–45. [DOI] [PubMed] [Google Scholar]
- 13.Keitel WA, Treanor JJ, El Sahly HM, et al. Comparative immunogenicity of recombinant influenza hemagglutinin (rHA) and trivalent inactivated vaccine (TIV) among persons > or =65 years old. Vaccine 2009; 28:379–85. [DOI] [PubMed] [Google Scholar]
- 14.Treanor JJ, Schiff GM, Couch RB, et al. Dose-related safety and immunogenicity of a trivalent baculovirus-expressed influenza-virus hemagglutinin vaccine in elderly adults. J Infect Dis 2006; 193:1223–8. [DOI] [PubMed] [Google Scholar]
- 15.Dunkle LM, Izikson R, Patriarca P, et al. ; Protein Sciences Consortium 12 Study Team. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376:2427–36. [DOI] [PubMed] [Google Scholar]
- 16.Rondy M, El Omeiri N, Thompson MG, Levêque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75:381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skowronski DM, Janjua NZ, De Serres G, et al. Low 2012–13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLOS One 2014; 9:e92153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McElhaney JE, Xie D, Hager WD, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol 2006; 176:6333–9. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, McElhaney JE, Walrond L, et al. Cellular immune responses of older adults to four influenza vaccines: results of a randomized, controlled comparison. Hum Vaccin Immunother 2017; 13:2048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tam YH, Valkenburg SA, Perera RAPM, et al. Immune responses to twice-annual influenza vaccination in older adults in Hong Kong. Clin Infect Dis 2018; 66:904–12. [DOI] [PubMed] [Google Scholar]
- 21.Cumming G Inference by eye: reading the overlap of independent confidence intervals. Stat Med 2009; 28:205–20. [DOI] [PubMed] [Google Scholar]
- 22.Wu P, Presanis AM, Bond HS, Lau EHY, Fang VJ, Cowling BJ. A joint analysis of influenza-associated hospitalizations and mortality in Hong Kong, 1998–2013. Sci Rep 2017; 7:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell K, Chung JR, Monto AS, et al. Influenza vaccine effectiveness in older adults compared with younger adults over five seasons. Vaccine 2018; 36:1272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skowronski DM, Chambers C, Sabaiduc S, et al. A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin Infect Dis 2016; 63:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skowronski DM, Chambers C, De Serres G, et al. Early season co-circulation of influenza A(H3N2) and B(Yamagata): interim estimates of 2017/18 vaccine effectiveness, Canada, January 2018. Euro Surveill 2018; 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cobey S, Gouma S, Parkhouse K, et al. Poor immunogenicity, not vaccine strain egg adaptation, may explain the low H3N2 influenza vaccine effectiveness in 2012–2013. Clin Infect Dis 2018; 67:327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zost SJ, Parkhouse K, Gumina ME, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci USA 2017; 114:12578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.