Abstract
Purpose:
Urinary tract cancer can be pure urothelial carcinoma (PUC), pure non-UC, or variant UC (VUC, defined here as mixed UC). Little is known regarding outcomes for patients with VUC receiving immune checkpoint inhibitors (ICI). We hypothesized that VUC does not compromise ICI efficacy in patients with advanced (a)UC.
Materials and Methods:
We performed a retrospective cohort study across 18 institutions. Demographic, clinicopathologic, treatment and outcomes data were collected for patients with aUC who received ICI. Patients were divided into PUC vs. VUC; VUC further divided by type of variant (i.e. squamous, neuroendocrine, etc). We compared overall response rate (ORR) using univariate and multivariate logistic regression and progression free survival (PFS) and overall survival (OS) using Kaplan-Meier and univariate and multivariate Cox proportional hazards.
Results:
519 patients were identified; 395, 406 and 403 included in ORR, OS and PFS analyses, respectively. ORR to ICI between patients with PUC and VUC was comparable (28% vs. 29%, p=0.90) without significant differences for individual subtypes vs. PUC. Median OS for patients with PUC was 11.0 months vs. 10.1 months for VUC (p=0.60), but only 4.6 months for patients with neuroendocrine (NE) features (n=9; HR=2.75 [95% CI 1.40-5.40] vs. PUC; p=0.003). Median PFS was 4.1 months for PUC vs. 5.2 months for VUC (p=0.43) and 3.7 months for NE (HR=1.87 [95% CI 0.92-3.79] vs. PUC, p=0.09).
Conclusions:
ORR to ICI was comparable across histologic types. However, OS was worse for patients with tumors containing NE features. VUC should not exclude patients from receiving ICI.
Keywords: Bladder Cancer, urothelial carcinoma, variant urothelial carcinoma, transitional cell, immunotherapy, neuroendocrine bladder cancer
Introduction:
Bladder cancer is an aggressive malignancy, with approximately 165,000 worldwide deaths annually1. Most bladder tumors demonstrate pure urothelial carcinoma (PUC) histology, but there is an increasingly recognized fraction of “urothelial carcinoma (UC) with divergent differentiation”2 or variant urothelial carcinoma (VUC), which has been documented in 33% of cystectomy2 and 25% of TURBT3 specimens. The most common subtypes of VUC are UC with squamous cell features, ~20%, and UC with glandular features, 16-18%2. VUC can be more aggressive; VUC with squamous differentiation has been shown to present at higher stage4 with increased recurrence rate after radical cystectomy5-6; while rarer variants, such as plasmacytoid and sarcomatoid are thought to carry the poorest prognosis7.
Conflicting reports exist regarding the responsiveness of VUC to conventional therapies; one study of patients with advanced UC treated with systemic chemotherapy found significantly shorter OS and PFS for patients with VUC compared to PUC4. In addition, UC with squamous cell differentiation has had reduced response to radiotherapy8. However, larger studies have shown that UC with squamous and/or glandular features has comparable pathologic response to PUC after neoadjuvant cisplatin-based chemotherapy9, and that VUC treated with tri-modality bladder-sparing therapy (including chemotherapy/radiation) has similar response rates, overall survival (OS), and disease-specific survival to PUC10. Conversely, tumors with neuroendocrine (NE) features often present at more advanced stage with overall poor prognosis11.
Immune checkpoint inhibitor therapies (ICI) have changed UC treatment, with 13-29% of patients with locally advanced (unresectable) or metastatic UC (both defined as aUC in this study) experiencing durable responses to anti-PD-(L)1 agents12-14. While patients with minority components of variant histology were included in ICI clinical trials, there is no data regarding the overall response rate (ORR) of tumors with variant histology, especially when urothelial is not the dominant histologic type. A trial found that patients with mixed histology had higher OS after receiving pembrolizumab than patients with UC (HR 0.58 vs 0.80 vs. chemotherapy15. Limited case studies of ICI in VUC illustrate that striking responses are possible16-18. We investigated treatment outcomes of VUC to ICI in a multi-institution retrospective cohort of 519 patients with aUC treated with ICI. We hypothesized that outcomes after ICI initiation would not be significantly different in patients with tumors harboring individual histology variants compared to those with PUC.
Materials and Methods:
Patients and Data Collection:
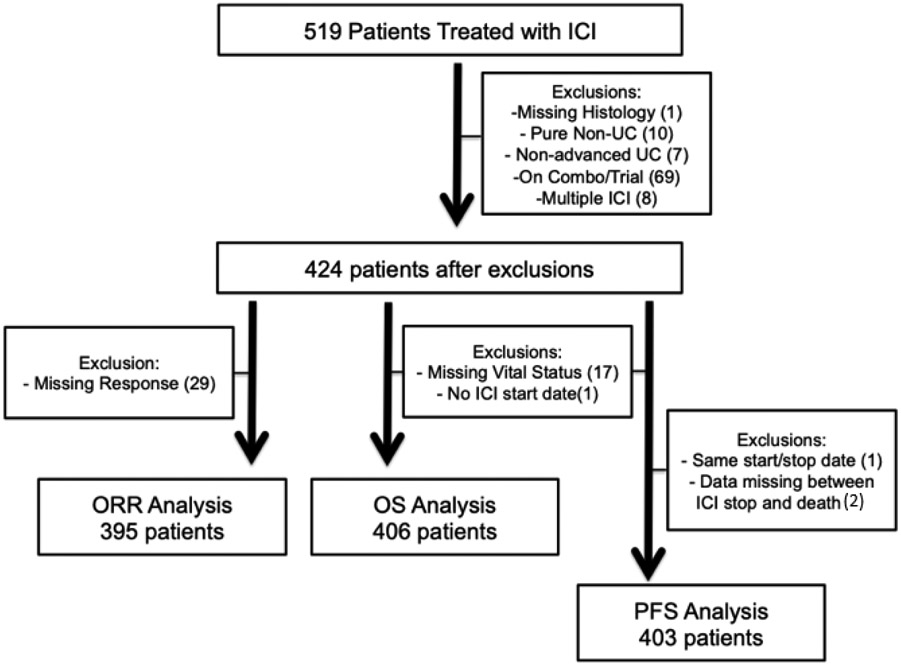
After IRB-approval, data was obtained from 18 institutions to build a database of patients who received anti-PD-1 or PD-L1 for aUC. Patients initiated treatment with ICI between May 2013 and May 2019. The number of patients derived from each institution for response and survival analyses can be found in Supplemental Table 1. Each institution identified patients using provider-driven and electronic health record search algorithms. Variables included demographics, clinicopathologic, laboratory, treatment response and clinical outcomes. Data was collected using secure, web-based, standardized REDCap electronic data capture tools hosted at the Institute of Translational Health Sciences19. Patients were excluded if ICI was given for alternate diagnosis, in (neo)adjuvant setting, in combination with chemotherapy, as part of a clinical trial, or if they received >1 anti-PD-(L)1 agent (Figure 1).
Figure 1:
Consort diagram of patients for multi-institution cohort
Patients underwent standard of care imaging as per treating provider. Evaluation of best response was determined by the data collector based on radiographic studies and clinic notes and did not include formal central radiology review. Progression was defined as clinical or radiologic progression based on investigator review of clinical notes and radiographic study reports. Overall response rate (ORR) was calculated as the sum of patients with complete or partial response among all patients in the analytical cohort. Overall survival (OS) was defined as the time from first ICI dose to death from any cause; alive patients were censored at date of last follow-up. Progression free survival (PFS) was defined as the time interval from first ICI dose to the date of progression or death, whichever occurred earlier. Patients who were alive and without disease progression or lost to follow-up were censored at the time of last follow-up (Figure 1).
Histology definitions
Investigators reported histology based on pathology report without central pathology review. Patients were classified as PUC or VUC (UC plus ≥1 variant histologic subtype; tumors with two or more variant histologies were a minority n= 13 in ORR analysis and n= 12 in survival analysis). For subsequent analysis, PUC was compared to each variant subtype individually (i.e., squamous). Variant histologic subtypes included squamous, micropapillary, sarcomatoid, adenocarcinoma, plasmacytoid, NE/small cell, nested and ‘other’. A subset of VUC excluding NE features was used for comparison to NE VUC.
Statistical Analysis
Baseline characteristics were summarized with descriptive statistics. Categorical and continuous variables were compared with chi-squared and two-sample t-tests, respectively. Associations between tumor histology subtype (PUC vs. VUC; PUC vs. individual variant) and ORR were assessed using univariate and multivariate logistic regression. OS and PFS were estimated using the Kaplan-Meier method. Between-group differences in OS and PFS were determined via univariate and multivariate Cox regression. Multivariate logistic regression and Cox regression was performed utilizing two models, one derived from covariates with p<0.1 on univariate analysis and a second based on Bellmunt risk factors20. Covariates tested included age, sex, race (white vs. other), smoking history, ICI treatment line (first line vs subsequent), history of extirpative surgery, and hemoglobin, albumin, liver metastases and ECOG performance status at ICI initiation (Table 1). Statistical analysis was performed with STATA 16.0 (College Station, Texas). Alpha level was set to 0.05 for all analyses.
Table 1.
Baseline characteristics of patients for overall survival analysis cohort.
| Histological Type | Pure UC (PUC) |
Variant UC (VUC) |
Neuro- endocrine (NE) |
p value (PUC vs. VUC) |
p value (PUC vs. NE) |
|---|---|---|---|---|---|
| Number of Patients | 286 | 120 | 9 | ||
| Age (mean ± SD) | 69 +/− 11 | 69 +/− 10 | 70 +/− 10 | 0.91 | 0.73 |
| Male | 76% | 71% | 67% | 0.33 | 0.54 |
| Ever Smoker | 65% | 69% | 78% | 0.45 | 0.44 |
| White race | 80% | 82% | 89% | 0.77 | 0.53 |
| Upper Tract Tumors | 14% | 16% | 14% (1 of 7) | 0.74 | 1.00 |
| Cystectomy or (Nephro)ureterectomy | 51% | 60% | 29% (2 of 7) | 0.12 | 0.24 |
| Hgb<10 | 24% | 28% | 22% | 0.45 | 0.88 |
| Liver Metastasis | 20% | 15% | 33% | 0.28 | 0.31 |
| Albumin < 4 g/dL | 66% | 66% | 67% | 0.88 | 0.95 |
| Bellmunt score | 0.42 | 0.03 | |||
| 0 | 15% | 21% | 11% | ||
| 1 | 53% | 45% | 67% | ||
| 2 | 28% | 30% | 0% | ||
| 3 | 4% | 4% | 22% | ||
| ECOG PS | 0.33 | 0.94 | |||
| 0 | 23% | 26% | 22% | ||
| 1 | 50% | 53% | 56% | ||
| 2 | 23% | 21% | 22% | ||
| 3 | 4% | 1% | 0% | ||
| Type of ICI (# patients) | 0.80 | 0.42 | |||
| Atezolizumab | 51% | 50% | 67% | ||
| Avelumab | 1% | 0% | 0% | ||
| Durvalumab | 3% | 5% | 11% | ||
| Nivolumab | 7% | 7% | 11% | ||
| Pembrolizumab | 38% | 38% | 11% |
VUC tumors contain both urothelial plus at least one variant histology. In this cohort, variants included squamous (n=51), micropapillary (n=27), sarcomatoid (n=16), plasmacytoid (n=11), adenocarcinoma (n=9), neuroendocrine (n=9), nested (n=2) and other (n=9).
Characteristics and laboratory data represented for date of ICI initiation. Bellmunt score 0-3 based on number of the following present at time of ICI: Hgb <10, presence of liver metastases, or ECOG PS >0.
Abbreviations: ICI, immune checkpoint inhibitor; Hgb, hemoglobin; ECOG, Eastern Cooperative Oncology Group; PS, performance status; NE, neuroendocrine; PUC, pure urothelial carcinoma; VUC, variant urothelial carcinoma
Results:
Data on 519 consecutive patients across 18 institutions was collected. After exclusions (Figure 1), 406 patients were included in OS analysis; 70% had PUC and 30% had VUC. Baseline characteristics of patients by histological subtype, including known prognostic features20-21, are in Table 1. There were no significant differences among characteristics between PUC vs. VUC. Patients with tumors containing NE features (n=9) had significantly different distribution of Bellmunt risk factors (p=0.03).
A total of 395 patients were included in the ORR analysis; 70% with PUC and 30% with VUC. ORR was not significantly different between PUC vs. VUC (28% vs. 29%, OR 1.03, 95% confidence interval [CI] 0.64-1.66, p=0.90) nor among patients with any individual histologic variant (Table 2A). Notably, ORR of patients whose tumors contained NE features was 25%, not significantly different than those with PUC (p=0.83). There was no significant difference between the ORR of PUC vs. VUC when patients were stratified by receiving ICI as first line (n=199, p=0.46) vs. subsequent therapy (n=196, p=0.36, Table 2B), nor when patients were stratified by primary tumor in upper (p=0.22) or lower tract (p=0.47, Table 2B). Two multivariate models were used, adjusting for 1) age, Hgb, and albumin, or 2) Bellmunt risk factors. There were no significant differences between ORR for PUC vs. VUC or between any individual histology type (including NE) compared to PUC with either model (Table 2C).
Table 2A.
Observed objective response rate (ORR) by tumor histology subtype
| N | ORR (%) | OR (95% CI) vs. PUC | p value | |
|---|---|---|---|---|
| PUC | 278 | 28% | ||
| VUC | 117 | 29% | 1.03 (0.64 - 1.66) | 0.90 |
| Squamous | 50 | 28% | 0.98 (0.50 - 1.91) | 0.95 |
| Micropapillary | 25 | 28% | 0.98 (0.39 - 2.44) | 0.97 |
| Sarcomatoid | 16 | 38% | 1.51 (0.53 – 4.30) | 0.44 |
| Plasmacytoid | 12 | 17% | 0.50 (0.11 - 2.35) | 0.38 |
| Adenocarcinoma | 10 | 20% | 0.63 (0.13 – 3.03) | 0.56 |
| NE | 8 | 25% | 0.84 (0.17 – 4.25) | 0.83 |
| OR (95% CI) vs. VUC w/o NE | ||||
| VUC without NE | 109 | 29% | ||
| NE | 8 | 25% | 0.80 (0.15 – 4.19) | 0.79 |
NE: neuroendocrine, PUC, pure urothelial carcinoma; VUC, variant urothelial carcinoma
Table 2B.
Observed ORR by tumor histology subtype, per treatment line or primary tumor site
| ORR (%) | PUC | VUC | OR (95% CI) vs. PUC | p value |
|---|---|---|---|---|
| First line (n= 199; 134 PUC, 65 VUC) | 33% | 28% | 0.78 (0.41 - 1.50) | 0.46 |
| Subsequent (n = 196; 144 PUC, 52 VUC) | 24% | 31% | 1.38 (0.69 - 2.79) | 0.36 |
| Upper tract (n= 51; 35 PUC, 16 VUC) | 29% | 13% | 0.36 (0.07 – 1.87) | 0.22 |
| Lower tract (n= 325; 229 PUC, 96 VUC) | 29% | 33% | 1.21 (0.73 – 2.02) | 0.47 |
PUC, pure urothelial carcinoma; VUC, variant urothelial carcinoma
Table 2C.
Multivariate analysis of observed ORR by tumor histology subtype compared to PUC, utilizing two separate models with significant covariates
| PUC vs. VUC | OR (95% CI) | p value |
|---|---|---|
| VUC | 1.08 (0.66 - 1.77) | 0.75 |
| Age | 1.03 (1.01 - 1.06) | 0.007 |
| Hgb | 1.07 (0.93 - 1.22) | 0.33 |
| Albumin | 1.47 (0.90 - 2.42) | 0.13 |
| VUC | 1.07 (0.66 – 1.74) | 0.77 |
| Bellmunt 0 | reference | reference |
| Bellmunt 1 | 1.36 (0.73 - 2.52) | 0.33 |
| Bellmunt 2 | 0.90 (0.45 - 1.79) | 0.75 |
| Bellmunt 3 | 0.60 (0.15 - 2.33) | 0.46 |
| PUC vs NE | OR (95% CI) | p value |
| NE | 0.75 (0.15 - 3.88) | 0.73 |
| Age | 1.04 (1.01 - 1.06) | 0.01 |
| Hgb | 1.10 (0.94 - 1.29) | 0.21 |
| Albumin | 1.37 (0.77 – 2.44) | 0.29 |
| NE | 0.89 (0.17 – 4.69) | 0.89 |
| Bellmunt 0 | reference | reference |
| Bellmunt 1 | 1.69 (0.77 - 3.68) | 0.19 |
| Bellmunt 2 | 1.09 (0.45 - 2.64) | 0.85 |
| Bellmunt 3 | 0.57 (0.11 – 3.04) | 0.51 |
Logistic regression odds ratio (OR) was calculated to compare ORR of patients with PUC versus histology subtype (VUC or NE).
Subsequent therapy = patients received ICI during 2nd and beyond line therapy.
Abbreviations: Hgb, hemoglobin; NE, neuroendocrine; PUC, pure urothelial carcinoma; VUC, variant urothelial carcinoma
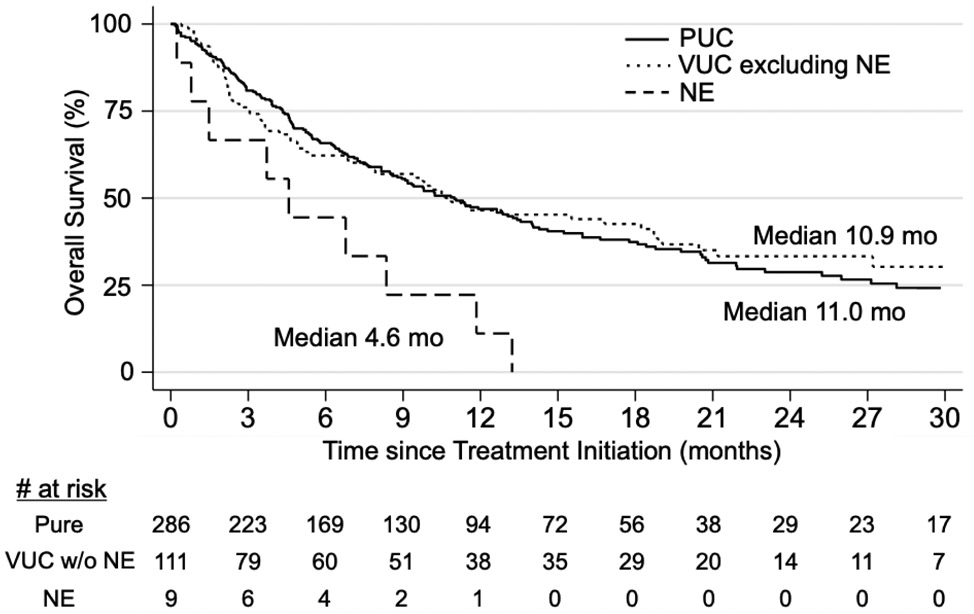
A total of 406 patients were included in the OS analysis with a median follow-up of 7.5 months. Patients with PUC had median OS of 11.0 months (95% CI 8.8-13.4); those with VUC had median OS of 10.1 months (95% CI 6.8-15.5). OS was not significantly different between PUC and VUC (HR 1.07, 95% CI 0.82-1.41, p=0.60, Table 3A). All individual variants had similar OS compared to PUC except for NE. Patients with NE features (n=9) had significantly shorter median OS of 4.6 months (95% CI 0.2-11.8) compared to PUC (HR 2.75, 95% CI 1.40-5.40, p=0.003) and compared to VUC without NE features (HR 2.65, 95% CI 1.30-5.37, p=0.007) on univariate analysis (Table 3A, Figure 2). Similar to ORR, there was no significant OS difference between PUC and VUC among patients receiving ICI as first line (n=211, p=0.30) vs. subsequent therapy (n=195, p=0.81, Table 3B). However, when stratifying by primary site, patients with upper tract VUC had poorer OS vs. PUC (p=0.03), while there was no difference in OS among lower tract tumors between histology subtypes (p=0.7, Table 3B). Two multivariate models were used, adjusting for 1) Hgb, albumin, ECOG PS 0-3 and presence of liver metastases, or 2) Bellmunt risk factors. There was no significant difference in OS between PUC and VUC with either model (p=0.62 and p=0.41, Table 3C). However, patients with NE features continued to exhibit shorter OS compared to PUC in both multivariate analyses (HR 2.11, 95% CI 1.06-4.19, p=0.03 in model 1; HR 3.76, 95% CI 1.36-5.59, p=0.005 in model 2, Table 3C).
Table 3A.
Overall survival (OS) by tumor histology subtype
| N | OS, months (95% CI) | HR (95% CI) vs. PUC | p value | |
|---|---|---|---|---|
| PUC | 286 | 11.0 (8.8-13.4) | ||
| VUC | 120 | 10.1 (6.8-15.5) | 1.08 (0.82 – 1.41) | 0.60 |
| Squamous | 51 | 10.5 (4.9-18.8) | 1.06 (0.73 – 1.53) | 0.78 |
| Micropapillary | 27 | 9.8 (3.0-NR) | 0.97 (0.57 – 1.65) | 0.91 |
| Sarcomatoid | 16 | 11.5 (2.0-21.2) | 1.27 (0.69-2.34) | 0.44 |
| Plasmacytoid | 11 | 10.1 (1.0-16.8) | 1.16 (0.55 – 2.48) | 0.70 |
| Adenocarcinoma | 10 | NR (1.5-NR) | 0.52 (0.19 – 1.41) | 0.20 |
| NE | 9 | 4.6 (0.2-11.8) | 2.75 (1.40 – 5.40) | 0.003 |
| OS, months (95% CI) | HR (95% CI) vs. VUC w/o NE | p value | ||
| VUC without NE | 111 | 10.9 (7.6 – 18.7) | ||
| NE | 9 | 4.6 (0.2-11.8) | 2.65 (1.31 – 5.37) | 0.007 |
NE: Neuroendocrine; NR: not reached; PUC, pure urothelial carcinoma; VUC, variant urothelial carcinoma
Figure 2.
Overall survival (OS) after initiating ICI in patients with tumors containing neuroendocrine features compared to patients with PUC or VUC.
Overall survival analysis of patients with aUC receiving ICI, with Kaplan-Meier curve depicting OS among patients with PUC (n= 286, solid line), VUC excluding NE (n= 111, dotted line) or tumors with NE features (n= 9, dashed line). Median OS was 11.0 months for patients with PUC vs. 4.6 months for patients with NE features (PUC vs. NE HR 2.75, 95%CI 1.40 – 5.40, p = 0.003). Median OS was 10.9 months for patients with VUC excluding NE (vs. NE HR 2.65, 95%CI 1.30 – 5.37, p = 0.007).
Table 3B.
Overall survival (OS) by tumor histology subtype, per treatment line or primary tumor site
| OS, months (95% CI) | PUC | Variant UC | HR (95% CI) vs. PUC | p value |
|---|---|---|---|---|
| First line (n = 211; 142 PUC, 69 VUC) | 13.3 (10.2-20.6) | 10.1 (6.8 -16.8) | 1.22 (0.84-1.77) | 0.30 |
| Subsequent (n = 195; 144 PUC, 51 VUC) | 9.2 (7.7-11.4) | 10.9 (4.6 -19.0) | 0.95 (0.64-1.42) | 0.81 |
| Upper tract (n= 57; 39 PUC, 18 VUC) | 14 (10-28) | 4 (2-19) | 2.18 (1.10-4.32) | 0.03 |
| Lower tract (n= 330; 233 PUC, 97 VUC) | 9 (7-13) | 11 (7-19) | 0.94 (0.69-1.28) | 0.07 |
PUC, pure urothelial carcinoma; VUC, variant urothelial carcinoma
Table 3C.
Multivariate analysis of observed OS by tumor histology subtype (VUC or NE) compared to PUC, utilizing two distinct models
| PUC vs. VUC | HR (95% CI) | p value |
|---|---|---|
| VUC | 1.07 (0.81 – 1.43) | 0.62 |
| Hgb | 0.96 (0.89 – 1.04) | 0.32 |
| Liver Metastasis | 1.67 (1.22 – 2.29) | 0.001 |
| Albumin | 0.45 (0.34 – 0.60) | <0.001 |
| ECOG PS 0 | reference | reference |
| ECOG PS 1 | 1.26 (0.89 – 1.77) | 0.20 |
| ECOG PS 2 | 1.36 (0.91 – 2.04) | 0.14 |
| ECOG PS 3 | 2.13 (0.94 – 4.80) | 0.07 |
| VUC | 1.12 (0.85 – 1.58) | 0.41 |
| Bellmunt 0 | reference | reference |
| Bellmunt 1 | 1.79 (1.19 – 2.69) | 0.005 |
| Bellmunt 2 | 2.90 (1.90 – 4.45) | <0.001 |
| Bellmunt 3 | 4.27 (2.25 – 8.11) | <0.001 |
| PUC vs. NE | HR (95% CI) | p value |
| NE | 2.11 (1.06 – 4.19) | 0.03 |
| Hgb | 1.01 (0.92 – 1.09) | 0.90 |
| Liver Metastasis | 1.80 (1.25 – 2.60) | 0.001 |
| Albumin | 0.41 (0.30 – 0.57) | <0.001 |
| ECOG PS 0 | Reference | reference |
| ECOG PS 1 | 1.01 (0.68 – 1.48) | 0.98 |
| ECOG PS 2 | 1.03 (0.65 – 1.64) | 0.90 |
| ECOG PS 3 | 1.79 (0.74 – 4.34) | 0.19 |
| NE | 3.76 (1.36 – 5.59) | 0.005 |
| Bellmunt 0 | reference | reference |
| Bellmunt 1 | 1.29 (0.82 – 2.05) | 0.27 |
| Bellmunt 2 | 2.32 (1.42 – 3.80) | 0.001 |
| Bellmunt 3 | 2.75 (1.35 – 5.58) | 0.005 |
Cox hazard ratio (HR) was calculated to compare OS of patients with pure urothelial cancer versus each histology subtype (VUC as defined previously, or each variant histology).
Subsequent therapy = patients received ICI during 2nd and beyond line therapy.
Abbreviations: Hgb, hemoglobin; ECOG, Eastern Cooperative Oncology Group; PS, performance status, NR, not reached; NE, neuroendocrine; PUC, pure urothelial carcinoma; VUC, variant urothelial carcinoma.
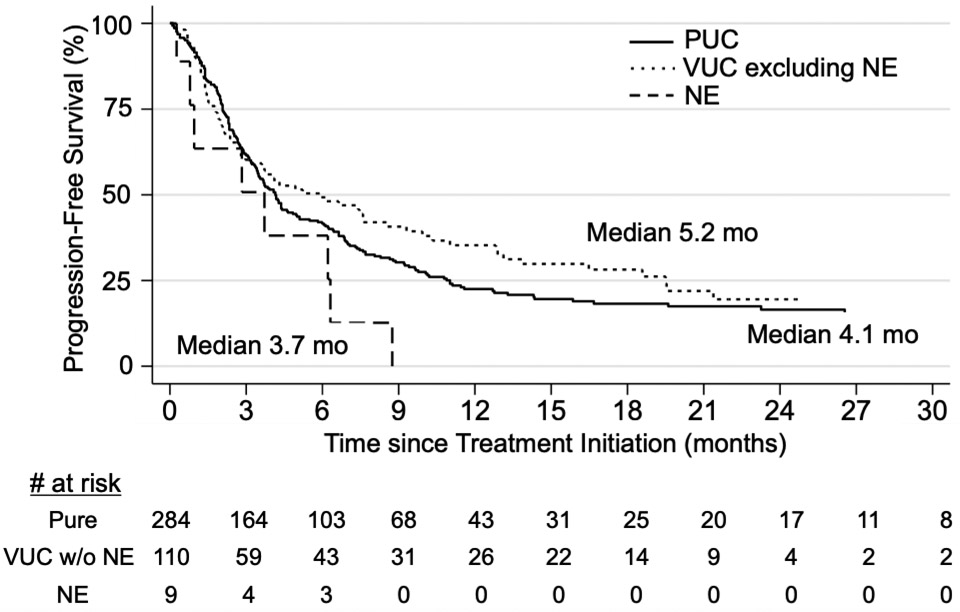
A total of 403 patients were included in the PFS analysis with a median follow-up of 3.5 months. PFS for PUC was not significantly different compared to VUC (HR 0.90, 95% CI 0.70-1.17, p=0.43). Conversely, PFS was significantly worse for NE vs. VUC without NE (HR 2.24, 95% CI 1.07-4.69, p=0.03, Table 4A, Figure 3). Of patients with tumors containing NE features who had clinical response (n=2/8), the median duration of response was 13 weeks. There was no significant difference in the PFS between PUC and VUC among patients receiving ICI as first line (n=209, p=0.62) vs. subsequent therapy (n=194, p=0.60, Table 4B), nor when patients were stratified by primary tumor in upper (p=0.38) or lower tract (p=0.23, Table 4B). Again, two multivariate models were used, adjusting for 1) age, hemoglobin, albumin, ECOG PS 0-3 and presence of liver metastases, or 2) Bellmunt risk factors. There was no significant difference in PFS between PUC vs. VUC (p=0.56 and p=0.50 for model 1 and 2, respectively, Table 4C) or between PUC and NE (p=0.24 and p=0.18 for model 1 and 2, respectively, Table 4C).
Table 4A.
Progression-free survival (PFS) by tumor histology subtype
| N | PFS, months (95% CI) | HR (95% CI) vs. PUC | p value | |
|---|---|---|---|---|
| PUC | 284 | 4.1 (3.5 – 5.0) | ||
| VUC | 119 | 5.2 (3.0 - 7.6) | 0.90 (0.70 -1.17) | 0.43 |
| Squamous | 51 | 4.1 (2.1 - 7.5) | 1.00 (0.70- 1.44) | 0.99 |
| Micropapillary | 26 | 7.6 (2.1 - 21.4) | 0.71 (0.43 - 1.19) | 0.20 |
| Sarcomatoid | 16 | 10.3 (1.6 - 18.6) | 0.92 (0.50 – 1.70) | 0.80 |
| Plasmacytoid | 11 | 5.9 (1.0 – NR) | 0.99 (0.49 - 2.01) | 0.98 |
| Adenocarcinoma | 10 | 7.6 (1.2 - NR) | 0.57 (0.25 - 1.27) | 0.17 |
| NE | 9 | 3.7 (0.3 - 6.3) | 1.87 (0.92 - 3.79) | 0.09 |
| PFS, months (95% CI) | HR (95% CI) vs. VUC w/o NE | p value | ||
| VUC without NE | 110 | 5.9 (3.0 - 8.5) | ||
| NE | 9 | 3.7 (0.3 - 6.3) | 2.24 (1.07 - 4.69) | 0.03 |
NE: Neuroendocrine; UC: urothelial carcinoma; PUC, pure urothelial carcinoma; VUC, variant urothelial carcinoma
Figure 3:
Progression free survival (PFS) after initiating ICI among patients with tumors containing neuroendocrine features compared to patients with PUC or VUC.
PFS analysis of patients with aUC receiving ICI, with Kaplan-Meier curve depicting PFS among patients with PUC (n= 284, solid line), VUC excluding NE (n= 110, dotted line) or tumors with NE features (n= 9, dashed line). Median PFS was 4.1 months for patients with PUC, 5.2 months for patients with VUC excluding NE, and 3.7 months for patients with NE features (HR 2.24 compared to VUC without NE, 95%CI 1.07 - 4.69, p = 0.03).
Table 4B.
Progression-free survival (PFS) by tumor histology subtype, per treatment line or primary tumor site
| PFS, months (95% CI) | PUC | VUC | HR (95% CI) vs. PUC | p value |
|---|---|---|---|---|
| First line (n= 209) | 4.2 (3.1 – 6.2) | 5.9 (2.7 – 9.3) | 0.92 (0.65-1.30) | 0.62 |
| Subsequent (n = 194) | 4.1 (3.5 - 5.5) | 5.2 (2.8 – 8.5) | 0.90 (0.61-1.33) | 0.60 |
| Upper tract (n= 57; 39 PUC, 18 VUC) | 4 (2-7) | 2 (1-5) | 1.33 (0.70-2.50) | 0.38 |
| Lower tract (n= 327; 231 PUC, 96 VUC) | 4 (3-6) | 6 (4-10) | 0.84 (0.63-1.12) | 0.23 |
PUC, pure urothelial carcinoma; VUC, variant urothelial carcinoma
Table 4C.
Multivariate analysis of PFS by tumor histology subtype compared to PUC, with Bellmunt covariates
| PUC vs. VUC | HR (95% CI) | p value |
|---|---|---|
| Variant UC | 0.92 (0.70 – 1.21) | 0.56 |
| Age | 0.98 (0.97-1.00) | 0.01 |
| Hgb | 1.01 (0.94 – 1.08) | 0.81 |
| Liver Metastasis | 1.85 (1.36 – 2.50) | <0.001 |
| Albumin | 0.58 (0.44 – 0.77) | <0.001 |
| ECOG PS 0 | Reference | reference |
| ECOG PS 1 | 1.19 (0.87 – 1.62) | 0.29 |
| ECOG PS 2 | 1.26 (0.86 – 1.84) | 0.24 |
| ECOG PS 3 | 2.02 (0.98 – 4.15) | 0.06 |
| Variant UC | 0.91 (0.70 – 1.19) | 0.50 |
| Bellmunt 0 | reference | reference |
| Bellmunt 1 | 1.37 (0.97 – 1.95) | 0.08 |
| Bellmunt 2 | 2.02 (1.40 – 2.93) | <0.001 |
| Bellmunt 3 | 3.09 (1.70 – 5.60) | <0.001 |
| Pure UC vs. NE | HR (95% CI) | p value |
| Neuroendocrine | 1.57 (0.75 – 3.29) | 0.24 |
| Age | 0.98 (0.97 – 0.99) | 0.003 |
| Hgb | 1.05 (0.97 – 1.13) | 0.24 |
| Liver Metastasis | 2.04 (1.44 – 2.89) | <0.001 |
| Albumin | 0.55 (0.40 – 0.76) | <0.001 |
| ECOG PS 0 | reference | reference |
| ECOG PS 1 | 1.00 (0.69 – 1.42) | 0.97 |
| ECOG PS 2 | 1.11 (0.72 – 1.70) | 0.65 |
| ECOG PS 3 | 1.88 (0.90 – 3.93) | 0.09 |
| Neuroendocrine | 1.67 (0.78 – 3.55) | 0.18 |
| Bellmunt 0 | reference | reference |
| Bellmunt 1 | 1.14 (0.76 - 1.71) | 0.52 |
| Bellmunt 2 | 1.86 (1.20 – 2.87) | 0.01 |
| Bellmunt 3 | 2.30 (1.17 – 4.54) | 0.02 |
Cox hazard ratio (HR) was calculated to compare OS of patients with pure urothelial cancer versus each histology subtype (VUC as defined previously, or each variant histology).
Abbreviations: Hgb, hemoglobin; ECOG, Eastern Cooperative Oncology Group; NE, neuroendocrine; PS, performance status; PUC, pure urothelial carcinoma; VUC, variant urothelial carcinoma
Discussion:
In our retrospective analysis of 519 patients with aUC who received ICI, histologic subtype did not impact ORR, PFS, or OS, except for NE features that portended shorter PFS and OS. Among patients with upper tract tumors, VUC has significantly shorter OS compared to PUC (p= 0.03). There is conflicting data about the response of VUC to first-line or neoadjuvant chemotherapy and/or radiotherapy4,8-9. No robust data exists from clinical trials regarding the impact of histology on ICI outcomes, with histology reported as a baseline characteristic without response data in one phase Ib study of anti-PD(L)122. Considering the prevalence of VUC, our findings contribute to the ongoing discussion about optimal use of ICI in aUC.
Putative biomarkers that might predict ICI response in aUC patients include tumor tissue PD-L1 expression, molecular subtype by gene expression signature, tumor mutational burden (TMB), T cell receptor clonality/diversity, among others12-15. Among VUC, PD-L1 expression by IHC has been found to be comparable between PUC and VUC and highest among VUC with squamous differentiation23. In addition, compared to UC, squamous cell bladder cancer24 and UC with NE features25 were associated with higher TMB, while bladder adenocarcinoma discordantly had lower TMB and reduced PD-L1 gene amplification24. These studies illuminate significant variation among subtypes of VUC and provide a molecular-based rationale for why VUC might respond as well as PUC to ICI, further supporting its use in these patients.
In our study, patients with tumors containing NE features had a shorter OS which remained significant in multivariate analysis. The identification of VUC by pathologic review remains a significant challenge given intra- and inter-tumor heterogeneity. Recently, genomic studies have defined subsets of bladder cancer, often discordant with histologic classification. For example, a subset of tumors termed ‘neuronal’ based on NE gene expression pattern from TCGA, notably without NE features on histology, had the shortest five-year survival26. A separate study identified NE-like tumors without concordant histology, which demonstrated poor OS compared to UC when treated with neoadjuvant chemotherapy and/or radical cystectomy27. Surprisingly, the TCGA ‘neuronal’ group demonstrated the highest ORR and OS of all subsets treated with atezolizumab in the IMvigor 210 phase II trial, hypothesized secondary to high TMB and/or low TGF-β expression28. It is unclear why there is a different impact of NE features on OS in our study with similar sample size (n=11). We hypothesize that the different methods of classification of “neuronal” (molecular vs histologic) might have played a role. NE tumors in our study were classified by histology rather than assessment for ‘neuronal’ gene expression pattern. Further, potential selection and confounding factors may exist, including enrollment of healthier patients in other studies compared to our “real world” study. Overall, data suggests that NE tumors warrant further evaluation for new therapies in clinical trials and that gene expression may be used in the future, in addition to histology, for the identification of such cases. Given that overall only 28% of patients with aUC in our study responded to ICI, with a short median PFS, combination therapies may be clinically relevant. Gene expression analysis may identify more aggressive tumors and guide treatment selection. For instance, a small cell (NE-like) subset was found that is associated with poorer prognosis, yet it can overexpress the surface Delta Like Canonical Notch Ligand 3 (DLL3) that can be targeted by DLL3-targeted antibody-drug conjugates in clinical trials29.Another study demonstrated that paired urothelial and squamous components from the same tumor have significant transcriptional differences despite having similar driver mutations30, and thus may be susceptible to distinct targeted molecular therapies.
The strengths of our study include utilization of “real-world” data, multi-institution representation and a reasonably large cohort size overall. There are several limitations inherent to the retrospective nature of our study, such as lack of randomized comparisons or external validation, heterogeneity in clinical practice and data collection across institutions, selection and confounding biases and missing data. Patients received different ICI across various lines of therapy and were not stratified by ICI; however, most received pembrolizumab or atezolizumab, which have randomized phase III trial data in platinum-refractory aUC. Patients with NE features had a small sample size. Median follow-up was relatively short. Data was derived from academic centers with UC pathology experts; however, there was no central radiology or pathology review, which is a major limitation. Fraction of VUC in each tumor was not reported and may have varied significantly. Rare tumors with ≥1 VUC subtype were included in ≥1 individual subtype analysis. Lastly, we note the ORRs observed in this retrospective analysis were higher than in most clinical trials, which we attribute possibly to differences in methodology. In our study, ORR was assessed by data collected based on reviewing clinician notes and radiographic studies, which might not correspond strictly to RECIST v.1.1 criteria in all cases. Despite these limitations, our study generated relevant hypotheses to be tested in larger cohorts and prospective studies.
Conclusions:
In summary, there was no significant association between histologic variant subtypes and ORR among patients treated with ICI in this retrospective study. PFS and OS were comparable between PUC and VUC, but shorter in patients with tumors harboring NE features. Our data suggests that histology subtype may not be a biomarker of response to ICI, though patients with NE tumors seem to have poor long-term outcomes. In the absence of high-level definitive evidence, retrospective studies can provide “real world” information relevant to clinical practice. This study supports both routine use and further clinical trial investigation of ICI, including novel combinations, across histologic subtypes in aUC.
Supplementary Material
Acknowledgments:
ARK was supported by the National Cancer Institute under training grant, award #T32CA009515. REDCap at ITHS is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR002319. EY and PG acknowledge the support from Seattle Translational Tumor Research Program at the Fred Hutchinson Cancer Research Center. DJP acknowledges support from the Imperial Experimental Cancer Medicine Centre, Cancer Research UK Imperial Centre and grant funding from the Wellcome Trust Strategic Fund (PS3416).
References:
- 1.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A & Bray F Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017;71:96–108. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey PA, Moch H, Cubilla AL, Ulbright TM & Reuter VE The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol 2016;70:106–119. [DOI] [PubMed] [Google Scholar]
- 3.Wasco MJ, Daignault S, Zhang Y, Kunju LP, Kinnaman M, Braun T, et al. Urothelial carcinoma with divergent histologic differentiation (mixed histologic features) predicts the presence of locally advanced bladder cancer when detected at transurethral resection. Urology 2007;70:69–74. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh MC, Sung MT, Chiang PH, Huang CH, Tang Y & Su YL The Prognostic Impact of Histopathological Variants in Patients with Advanced Urothelial Carcinoma. PLoS One 2015;10:e0129268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antunes AA, Nesrallah LJ, Dall’Oglio MF, Maluf CE, Camara C, Leite KR, et al. The role of squamous differentiation in patients with transitional cell carcinoma of the bladder treated with radical cystectomy. Int Braz J Urol 2007;33:339–345; discussion 346. [DOI] [PubMed] [Google Scholar]
- 6.Honma I, Masumori N, Sato E, Takayanagi A, Takahashi A, Itoh N, et al. Local recurrence after radical cystectomy for invasive bladder cancer: an analysis of predictive factors. Urology 2004;64:744–748. [DOI] [PubMed] [Google Scholar]
- 7.Chalasani V, Chin JL & Izawa JI Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer. Can Urol Assoc J 2009;3:S193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin JE, Jenkins BJ, Zuk RJ, Blandy JP & Baithun SI Clinical importance of squamous metaplasia in invasive transitional cell carcinoma of the bladder. J Clin Pathol 1989;42:250–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scosyrev E, Ely BW, Messing EM, Speights VO, Grossman HB, Wood DP, et al. Do mixed histological features affect survival benefit from neoadjuvant platinum-based combination chemotherapy in patients with locally advanced bladder cancer? A secondary analysis of Southwest Oncology Group-Directed Intergroup Study (S8710). BJU Int 2011;108:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krasnow RE, Drumm M, Roberts HJ, Niemierko A, Wu CL, Wu S, et al. Clinical Outcomes of Patients with Histologic Variants of Urothelial Cancer Treated with Trimodality Bladder-sparing Therapy. Eur Urol 2017;72:54–60. [DOI] [PubMed] [Google Scholar]
- 11.Wang G, Xiao L, Zhang M, Kamat AM, Siefker-Radtke A, Dinney CP, et al. Small cell carcinoma of the urinary bladder: a clinicopathological and immunohistochemical analysis of 81 cases. Hum Pathol 2018;79:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fradet Y, Bellmunt J, Vaughn DJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol 2019;30:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powles T, Duran I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391:748–757. [DOI] [PubMed] [Google Scholar]
- 14.Sharma P, Siefker-Radtke A, de Braud F, et al. Nivolumab Alone and With Ipilimumab in Previously Treated Metastatic Urothelial Carcinoma: CheckMate 032 Nivolumab 1 mg/kg Plus Ipilimumab 3 mg/kg Expansion Cohort Results. J Clin Oncol 2019;37:1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilde L, Ali SM, Solomides CC, Ross JS, Trabulsi E & Hoffman-Censits J Response to Pembrolizumab in a Patient With Chemotherapy Refractory Bladder Cancer With Small Cell Variant Histology: A Case Report and Review of the Literature. Clin Genitourin Cancer 2017;15:e521–e524. [DOI] [PubMed] [Google Scholar]
- 17.Hunter L, Moser J, Sturge C, Barraza G & Colonna S First-line pembrolizumab therapy in a cisplatin-ineligible patient with plasmacytoid urothelial carcinoma: A case report. J Oncol Pharm Pract 2019:1078155219835006. [DOI] [PubMed] [Google Scholar]
- 18.Nadal RM, Mortazavi A, Stein M, Pal SK, Davarpanah NN, Parnes HL, et al. Results of phase I plus expansion cohorts of cabozantinib (Cabo) plus nivolumab (Nivo) and CaboNivo plus ipilimumab (Ipi) in patients (pts) with with metastatic urothelial carcinoma (mUC) and other genitourinary (GU) malignancies. Journal of Clinical Oncology 2018;36:515–515.29267131 [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N & Conde JG Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellmunt J, Choueiri TK, Fougeray R, Schutz FA, Salhi Y, Winquist E, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol 2010;28:1850–1855. [DOI] [PubMed] [Google Scholar]
- 21.Bajorin DF, Dodd PM, Mazumdar M, Fazzari M, McCaffrey JA, Scher HI, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999;17:3173–3181. [DOI] [PubMed] [Google Scholar]
- 22.Plimack ER, Bellmunt J, Gupta S, Berger R, Chow LQ, Juco J, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol 2017;18:212–220. [DOI] [PubMed] [Google Scholar]
- 23.Reis H, Serrette R, Posada J, Lu V, Chen YB, Gopalan A, et al. PD-L1 Expression in Urothelial Carcinoma With Predominant or Pure Variant Histology: Concordance Among 3 Commonly Used and Commercially Available Antibodies. Am J Surg Pathol 2019;43:920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacob J, Bratslavsky G, Shapiro O, Liu N, Ferry EK, Basnet A, et al. Adenocarcinoma (ACB), urothelial carcinoma (UCB) and squamous cell carcinoma (SCCB) of the bladder: A Comprehensive Genomic Profiling (CGP) Study [abstract]. In: 2019 ASCO Annual Meeting; Chicago, IL: J Clin Oncol 37, 2019 (suppl; abstr 4533) [Google Scholar]
- 25.Chang MT, Penson A, Desai NB, Socci ND, Shen R, Seshan VE, et al. Small-Cell Carcinomas of the Bladder and Lung Are Characterized by a Convergent but Distinct Pathogenesis. Clin Cancer Res 2018;24:1965–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017;171:540–556.e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batista da Costa J, Gibb EA, Bivalacqua TJ, Liu Y, Oo HZ, Miyamoto DT, et al. Molecular Characterization of Neuroendocrine-like Bladder Cancer. Clin Cancer Res 2019;25:3908–3920. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Kwiatkowski D, McConkey DJ, Meeks JJ, Freeman SS, Bellmunt J, et al. The Cancer Genome Atlas Expression Subtypes Stratify Response to Checkpoint Inhibition in Advanced Urothelial Cancer and Identify a Subset of Patients with High Survival Probability. Eur Urol 2019;75:961–964 [DOI] [PubMed] [Google Scholar]
- 29.Koshkin VS, Garcia JA, Reynolds J, Elson P, Magi-Galluzzi C, McKenney JK, et al. Transcriptomic and Protein Analysis of Small-cell Bladder Cancer (SCBC) Identifies Prognostic Biomarkers and DLL3 as a Relevant Therapeutic Target. Clin Cancer Res 2019;25:210–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hovelson DH, Udager AM, McDaniel AS, Grivas P, Palmbos P, Tamura S, et al. Targeted DNA and RNA Sequencing of Paired Urothelial and Squamous Bladder Cancers Reveals Discordant Genomic and Transcriptomic Events and Unique Therapeutic Implications. Eur Urol 2018;74:741–753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.