Abstract
Synovial sarcoma, an aggressive soft tissue sarcoma with a predilection for the extremities of young adults, harbors the pathognomonic t(X;18)(p11;q11) translocation, resulting in SS18-SSX rearrangements. Synovial sarcoma includes monophasic, biphasic, and poorly differentiated variants, which show considerable histologic overlap with a range of other tumor types, making the diagnosis challenging on limited biopsies. Immunohistochemistry (IHC) is routinely used in the differential diagnosis; however, presently available markers lack specificity. Thus, cytogenetic or molecular genetic techniques are often employed to confirm the diagnosis. Here, we report the development and characterization of two novel antibodies: an SS18-SSX fusion-specific antibody (E9X9V, designed to the breakpoint) as well as an SSX-specific antibody (E5A2C, designed to the SSX C-terminus). We validated the selectivity and specificity of the antibodies using immunoblotting, immunoprecipitation, and chromatin immunoprecipitation followed by next-generation sequencing (ChIP-seq) in SS cell lines and demonstrated that both antibodies capture SS18-SSX on chromatin at established target sites (e.g., TLE1 and BCL2) genome-wide. Using IHC in whole sections from 400 tumors including 100 genetically-confirmed cases of SS and 300 histologic mimics, the SS18-SSX fusion-specific antibody revealed strong diffuse nuclear staining in 95 of 100 (95%) synovial sarcoma cases, whereas none of the 300 control tumors showed any staining. The SSX antibody showed strong diffuse nuclear staining in all 100 (100%) synovial sarcoma cases; 13 (4%) of the 300 other tumors were also positive, five of which displayed greater than 50% nuclear staining. In summary, a novel SS18-SSX fusion-specific antibody is highly sensitive (95%) and specific (100%) for synovial sarcoma, and an antibody to the SSX C-terminus is also highly sensitive (100%), but slightly less specific (96%). IHC using the SS18-SSX antibody could replace molecular genetic or cytogenetic testing in most cases, and these reagents together will also provide the research community with valuable tools for further biochemical and genomic interrogation of the SS18-SSX fusion protein.
Keywords: synovial sarcoma, SS18-SSX, antibody, immunohistochemistry, gene fusion, fusion oncoprotein
INTRODUCTION
Synovial sarcoma (SS) is a malignant mesenchymal neoplasm with variable epithelial differentiation that accounts for up to 10% of soft tissue sarcomas overall and is the second most common pediatric sarcoma.1 While SS predominantly affects adolescents and young adults, with a peak incidence in the third decade of life and a predilection for the extremities, SS affects all ages and is anatomically ubiquitous.1,2 SS is an aggressive sarcoma with 5- and 10-year survival rates of ~60% and ~50%, respectively, in adults and ~80% and ~75% in children and adolescents.2 Late recurrences or metastases are not infrequent; large tumor size, high disease stage, and high-grade histology at diagnosis are associated with a worse prognosis.1
SS is defined by the hallmark translocation t(X;18)(p11;q11), which has not been found in other neoplasms and occurs as the sole genetic aberration in up to a third of cases.3 This recurrent translocation results in fusion of the SS18 gene (formerly SYT) on chromosome 18 with one of three highly homologous SSX genes on the X chromosome (SSX1 in two thirds of SS, SSX2 in one third, and SSX4 rarely), creating a chimeric gene fusion identified in >95% of cases.3,4 Although most SS18-SSX gene fusions involve the same intronic breakpoints, rare variant chimeric transcripts and cryptic rearrangements have been described.3,5 While SS18 is ubiquitously expressed in normal human tissue, expression of SSX is reported only in spermatogonia, at low levels in the thyroid, and in a variety of non-sarcoma tumor types (including melanoma, lymphomas, and various carcinomas).6,7 SS18 encodes a protein member of the mSWI/SNF chromatin remodeling complex, whereas SSX genes encode histone-binding proteins, both of which localize to the nucleus.1 Several recent studies have elucidated the molecular mechanism underpinning the oncogenic activity of the SS18-SSX chimeric fusion protein, namely, that SS18-SSX binds mSWI/SNF (BAF) chromatin remodeling complexes, altering their subunit composition (specifically, replacing wild-type SS18 and displacing the BAF47 (INI1; SMARCB1) subunit) and hijacking their gene activating functions to aberrant locations genome wide.8–10 The altered BAF complexes target to and activate genes found in polycomb-repressed domains, driving aberrant activation of genes such as SOX2, PAX3, PAX7, and MYC.8,9
Histologically, SS is heterogeneous in appearances, exhibiting three dominant subtypes – monophasic, biphasic, and poorly differentiated (PD) – each of which shows considerable morphologic overlap with a range of other tumor types. Monophasic SS is composed of uniform spindle cells with overlapping hyperchromatic ovoid-to-elongated nuclei arranged in dense fascicles within a variably collagenous matrix containing wiry collagen fibers and sometimes chunky calcifications. Myxoid stroma may be a focal or dominant feature, and some cases contain prominent staghorn vasculature reminiscent of that seen in solitary fibrous tumor (SFT). Biphasic SS contains a morphologically similar spindle cell component along with a variably prominent epithelial component of glands, nests, or cords. PDSS is composed of round or spindle cells with a high mitotic rate, mimicking round cell sarcomas (such as Ewing, CIC or BCOR-rearranged sarcomas) or high-grade malignant peripheral nerve sheath tumor (MPNST), among other aggressive neoplasms. The varied histology of SS and overlapping features with other tumor types render the diagnosis challenging on small biopsies or with unusual presentations.
While immunohistochemistry (IHC) is routinely used for the diagnosis of SS, no currently available antibody is sufficiently specific or sensitive. Historically, EMA has been used with high sensitivity (keratins with less sensitivity) to highlight epithelial differentiation, usually at least focally staining spindle cells in SS and consistently staining epithelial cells in biphasic SS.11,12 The vast majority of SS also stain for CD99, sometimes with the membranous pattern seen in Ewing sarcoma, potentially adding to diagnostic uncertainty.11,12 Up to 40% of SS show immunoreactivity for S100 protein.11 More recently, gene expression profiling studies revealed overexpression of the transcriptional corepressor TLE1 in SS, with subsequent studies finding moderate-to-strong TLE1 nuclear staining by IHC in over 90% of SS.13,14 Importantly, several histologic mimics of SS may express TLE1, including benign and malignant nerve sheath tumors, SFT, mesothelioma, and poorly differentiated carcinomas.13–17 By IHC, INI1 shows decreased (but not absent) staining in most (85–90%) SS,18,19 correlating with the recently discovered relationship between SS18-SSX and BAF47 (INI1) in the mSWI/SNF complex, which results in BAF47 degradation.8
Given these diagnostic challenges, detection of t(X;18) by conventional cytogenetics, SS18 rearrangements by break-apart FISH, or SS18-SSX fusion transcripts by RT-PCR or next-generation sequencing has become the gold standard for diagnosis of SS. However, RT-PCR for SS18-SSX1 and SS18-SSX2 is not widely available, will not detect rare SS18-SSX4 fusion transcripts, and may miss variant chimeric transcripts.20 FISH also lacks complete sensitivity, can present interpretative challenges, and may not identify cryptic rearrangements.20 The purpose of this study was to evaluate a novel fusion-specific SS18-SSX antibody and an SSX C-terminal antibody on a set of genetically well-characterized SS cases and a large cohort of histologic mimics.
MATERIALS AND METHODS
Antibody Development
A rabbit monoclonal antibody (clone E9X9V) was produced by immunization of animals with a synthetic peptide containing amino acid residues surrounding the SS18-SSX fusion site (corresponding to the breakpoints in approximately 95% of SS cases): Gln379 to Ile111 of human SSX. A second rabbit monoclonal antibody (clone E5A2C) was produced by immunizing animals with a synthetic peptide corresponding to conserved residues surrounding Gln173 of human SSX proteins.
Cell Lines and Cell Culture
The synovial sarcoma cell lines Aska and SYO1 were generously provided as gifts from Kazuyuki Itoh, Norifumi Naka, and Satoshi Takenaka (Osaka University, Japan) and Akira Kawai (National Cancer Center Hospital, Japan). The synovial sarcoma cell line HSSY2 was purchased from RIKEN. The human fibroblast cell line CRL7250 was acquired from Drs. Berkeley Gryder and Javed Khan (National Cancer Institute, Bethesda, MD). Each cell line was cultured using standard protocols in DMEM medium (Gibco) supplemented with 10–20% fetal bovine serum, 1% Glutamax (Gibco), 1% Sodium Pyruvate (Gibco) and 1% Penicillin-Streptomycin (Gibco) and grown in a humidified incubator at 37°C with 5% CO2.
Cell Lysate Collection
Whole cell extractions (WCE) were obtained by washing harvested cell pellets with PBS pH 7.4, resuspending in whole cell lysis buffer (PBS pH 7.4 and 1% SDS) and then heating for 3 minutes at 95°C. Nuclear extractions were obtained by suspending the harvested cells in Buffer 0 (50 mM Tris pH 7.5, 0.1% NP-40, 1 mM EDTA, 1 mM MgCl2 with protease inhibitor, 1 mM DTT and 1 mM phenylmethylsulfonyl fluoride [PMSF]), centrifuging at 5,000 rpm for 5 minutes at 4°C, and discarding the supernatant. The pellet (nuclei) was resuspended in Buffer 300 (50 mM Tris pH 7.5, 0.1% NP-40, 1 mM EDTA, 1 mM MgCl2, 300 mM NaCl with protease inhibitor, 1 mM DTT and 1 mM PMSF), vortexed, incubated on ice, centrifuged at 15,000 rpm for 10 minutes at 4°C, and supernatant containing the nuclear extract was collected.
Immunoprecipitation
Nuclear extracts were quantified by Bradford assay and 500 μg of protein was incubated with 1.5 μg of antibody in Buffer 150 (50 mM Tris pH 7.5, 0.1% NP-40, 1 mM EDTA, 1 mM MgCl2, 150 mM NaCl with protease inhibitor, 1 mM DTT and 1 mM PMSF) overnight at 4°C. Each sample was then incubated with Protein G Dynabeads (Thermo Scientific) for 3 hours. Beads were washed three times with Buffer 300 followed by elution with 20 μL of elution buffer (NuPage LDS buffer (2X), containing 100 mM DTT and water; Life Technologies).
Western Blot Analysis
Detection of proteins by immunoblot (IB) analysis was achieved using standard protocols with primary antibodies. Following incubation with the primary antibody, membranes were incubated with IRDye (LI-COR Biosciences) secondary antibodies and then visualized by the LI-COR Odyssey Imaging System (LI-COR Biosciences).
Chromatin Immunoprecipitation with Sequencing (ChIP-seq)
For chromatin immunoprecipitation (ChIP) experiments, prepared cells were harvested, and capture of chromatin bound proteins was performed using standard protocols (Millipore, Billerica, MA). Briefly, cells were cross-linked with 1% formaldehyde for 10 minutes at 37°C, reaction was quenched by addition of 125 mM glycine for 5 min and then 5 million fixed cells were used per experiment. Chromatin was fragmented by sonication with a Covaris E220, and the solubilized chromatin was incubated with 1.5 μg of primary antibody overnight at 4°C to form antibody-chromatin complexes. These complexes were incubated with Protein G-Dynabeads (Thermo Scientific) for 3 hours at 4°C; beads were washed 3X and eluted. The samples then underwent crosslink reversal, treatment with RNase A (Roche), and treatment with proteinase K (Thermo Scientific) followed by DNA capture with AMP Pure beads (Beckman Coulter).
The DNA from these ChIP-seq samples was then prepared for sequencing using the NEBNext® Ultra™ II (New England BioLabs) to amplify and barcode each sample. The fragment sizes were determined using a D1000 ScreenTape system (Agilent), and the DNA was quantified by Kapa Library Quantification Kit Illumina Platforms (Kapa Biosystems). The samples were then diluted and loaded on a buffer cartridge for 75bp single end sequencing on the NextSeq 500 system (Illumina).
Alignment of ChIP-seq data was done using Bowtie2, version 2.1.0, and reads were mapped to the hg19 human reference genome, using the parameter –k 1. To process the aligned data, peaks were called using MACS2 version 2.1.0 against an input sample with a q = 0.001 cutoff; broad peaks were called for each antibody in each cell line and condition. Those peaks that mapped to unmappable chromosomes (any that were not chr1–22, X or Y) or were located in blacklisted regions of ENCODE were excluded. For downstream analysis of data, bam files were generated with duplicates removed using the samtools rmdup command and the –b option. All ChIP-seq tracks were obtained from the bedGraphToBigWig script (UCSC) using bedgraph files generated with MACS2 using the –B –SPMR options. ChIP-seq tracks were visualized using IGV version 2.4.16 (Broad Institute).
Immunohistochemistry
Cases were retrieved from the surgical pathology and consultation files of Brigham and Women’s Hospital, Boston, MA, the surgical pathology files of the University of Iowa, Iowa City, IA, and the consultation files of two of the authors (C.D.M.F. and J.L.H.). In total, 400 tumors were evaluated: 100 SS (41 monophasic, 18 biphasic, and 41 poorly differentiated) and 300 histologic mimics of SS (20 each MPNST, SFT, dedifferentiated liposarcoma, leiomyosarcoma, fibrosarcomatous variant of dermatofibrosarcoma protuberans (DFSP), Ewing sarcoma, CIC sarcoma, alveolar rhabdomyosarcoma, embryonal rhabdomyosarcoma, spindle cell rhabdomyosarcoma, mesenchymal chondrosarcoma, desmoplastic small round cell tumor, and clear cell sarcoma; 10 each biphenotypic sinonasal sarcoma, BCOR-rearranged sarcoma, sarcomatoid mesothelioma, and biphasic mesothelioma). SS cases were genetically confirmed at the time of original diagnosis by SS18 break-apart FISH, conventional cytogenetics, SS18-SSX RT-PCR, or next-generation sequencing. Dedifferentiated liposarcoma, Ewing sarcoma, CIC sarcoma and clear cell sarcoma cases were also all genetically confirmed at the time of diagnosis. Representative hematoxylin and eosin-stained slides were reviewed by two authors (E.B. and J.L.H.) to confirm the diagnoses. IHC was performed by manual methods on 4-μm thick formalin-fixed paraffin-embedded (FFPE) whole tissue sections following pressure cooker antigen retrieval (Target Retrieval Solution, pH 6.1; Dako, Carpinteria, CA) using an SS18-SSX fusion-specific rabbit monoclonal antibody (clone E9X9V, Cell Signaling Technology, Danvers, MA) at 1:7500 dilution (40 minute incubation) and an SSX C-terminus rabbit monoclonal antibody (clone E5A2C, Cell Signaling Technology) at 1:7500 dilution (40 minute incubation) and the EnVision+ System-HRP (Dako). The staining intensity in each case for both antibodies was characterized as weak, moderate, or strong, and the extent of immunoreactivity was scored according to the percentage of tumor cells with nuclear staining: 0 (0%), 1+ (<5%), 2+ (5–25%), 3+ (26–50%), 4+ (51–75%), and 5+ (76–100%). The presence of nuclear staining in greater than 5% of tumor cells (i.e., score of 2+ or greater) was considered positive.
Fluorescence in situ Hybridization (FISH) for SS18 rearrangement
Break-apart FISH was performed on 4-μm FFPE whole tissue sections of 5 cases (2 MPNST, 1 dedifferentiated liposarcoma, 1 alveolar rhabdomyosarcoma, and 1 sarcomatoid mesothelioma), which were baked at 60°C for at least two hours then de-paraffinized and digested using methods described previously.21 The Vysis LSI SS18 (Cen, SpectrumGreen), Vysis LSI SS18 (Tel, SpectrumOrange), and CEP18 (D18Z1, SpectrumAqua) probes were co-hybridized to tissue sections following manufacturer’s directions (Abbott Molecular/Vysis, Inc.). Tissues and probes were co-denatured at 80°C, hybridized at least 16 hours at 37°C in a darkened humid chamber, washed in 2X SSC at 71°C for two minutes, rinsed in room temperature 2X SSC, and counterstained with DAPI (4’,6-diamidino-2-phenylindole, Abbott Molecular/Vysis, Inc.). Slides were imaged using a Zeiss AX10 fluorescence microscope. Individual images were captured using an Applied Imaging system running CytoVision Genus version 7.5.
Next Generation Sequencing (NGS)
Targeted sequencing of all exonic and selected intronic regions of 447 cancer-associated genes (OncoPanel POPv3) was performed on one case (MPNST) using hybrid capture with a custom probe set (Agilent SureSelect, Agilent Technologies, Santa Clara, CA) and massively parallel sequencing (HiSeq 2500, Illumina, San Diego, CA), as previously described.22 Data processing and variant classification were performed as previously described.23
RESULTS
Development and assessment of an antibody specific for the SS18-SSX oncogenic fusion
A synthetic peptide was designed to contain amino acid residues surrounding the SS18-SSX fusion site (identical to fusion breakpoints in ~95% of SS cases). The amino acid sequence encompassed the fusion junction where the 78 N-terminal amino acids of SSX (SSX1, SSX2 or SSX4) replace the 8 C-terminal amino acids of SS18 (Figure 1A). Rabbit monoclonal antibodies were then raised against this synthetic fusion peptide, followed by collection and purification (clone E9X9V). IB of this antibody against whole cell extracts from human fibroblast cells CRL7250 (expressing wild-type SS18) and synovial sarcoma cell lines (Aska, HSSY2, SYO1) revealed that E9X9V detects the SS18-SSX fusion protein without non-specific protein detection or cross-reactivity with wild-type SS18 protein (Figure 1B). Further, expression of an shRNA hairpin targeting the 3’UTR of SS18-SSX transcripts (shSSX) reduced detected protein levels, further confirming that E9X9V selectively detects the fusion protein (Figure 1B–1C). Finally, the antibody was shown to detect both SS18-SSX1 (in HSSY2 and Aska cell lines) and SS18-SSX2 (in SYO1 cell line) fusion forms. These findings demonstrate that the developed antibody (E9X9V) successfully detects the SS18-SSX oncogenic fusion protein in a highly specific manner. An additional monoclonal antibody (clone E5A2C) was developed against the SSX C-terminus and analyzed by IB to reveal detection of the SS18-SSX fusion protein in synovial sarcoma cell lines (without non-specific protein detection) but not human fibroblast cells, which do not express wild-type SSX protein (Figure 1B–1C). Similarly, E5A2C detected both SS18-SSX1 and SS18-SSX2 fusion forms, demonstrating that E5A2C also successfully detects the SS18-SSX oncogenic fusion protein in a specific manner.
Figure 1.

Diagram of the SS18-SSX fusion sequence targeted by the fusion-specific antibody (E9X9V) (A). Immunoblotting of E9X9V and E5A2C antibodies in whole cell extracts from human fibroblast cells (CRL7250) and SS cell lines (HSSY2, Aska, SYO1, with and without shRNA-mediated knockdown of SS18-SSX expression) showing specific detection of SS18-SSX fusion protein (B) without cross reactivity with wild-type SS18 (C).
The SS18-SSX-specific antibody purifies mSWI/SNF (BAF) complexes and captures chromatin-bound fusion protein
The SS18-SSX fusion protein has been previously shown to incorporate into mSWI/SNF complexes;8 we therefore tested the ability of E9X9V and E5A2C to capture these intact complexes endogenously from human cell lines. To explore this, nuclear extracts from synovial sarcoma cell lines were collected and co-immunoprecipitations were performed with both antibodies (Figure 2A). In all three synovial sarcoma cell lines tested (Aska, SYO1 and HSSY2), E9X9V and E5A2C successfully captured the SS18-SSX bait as well as core subunit members of the mSWI/SNF complex (i.e., BRG1 and BAF155). Furthermore, ChIP-seq with both antibodies was carried out in the Aska, SYO1, and HSSY2 synovial sarcoma cell lines, revealing that these antibodies successfully capture SS18-SSX fusion-bound BAF complexes on chromatin at previously established SS18-SSX target sites.9 This genomic targeting of the fusion oncoprotein captured by both antibodies is exemplified at TLE1 and BCL2, two genes known to be overexpressed in synovial sarcoma (Figure 2B). Overall, these results establish that E9X9V and E5A2C both capture SS18-SSX-containing BAF complexes on chromatin.
Figure 2.

Co-immunoprecipitations with E9X9V, E5A2C, and an antibody to BRG1 (a core subunit of the mSWI/SNF complex) from SS cell line nuclear extracts show that E9X9V and E5A2C both capture SS18-SSX-containing mSWI/SNF complexes (A). ChIP-seq with E9X9V and E5A3C in SS cell lines reveals that these antibodies capture SS18-SSX on chromatin at previously established genomic sites, such as TLE1 and BCL2 (B).
Immunohistochemical analysis with the SS18-SSX fusion-specific and SSX C-terminus antibodies
Whole tissue sections of 100 genetically confirmed SS cases and 300 histologic mimics were examined by IHC. The results are summarized in Table 1. The SS18-SSX fusion-specific antibody (E9X9V) was positive in 95 of 100 (95%) SS cases and 0 of 300 (0%) histologic mimics. While antibody positivity was defined as the presence of nuclear staining in greater than 5% of total tumor nuclei, all positive SS cases showed strong crisp nuclear staining in over 75% of tumor cell nuclei (5+), with most cases approaching 100% nuclear positivity (Figures 3, 4 and 5). Five SS cases (two monophasic and three poorly differentiated SS) revealed no reactivity with E9X9V (Figure 5); other routine antibodies showed expected staining of internal controls. No cases showed predominantly weak or moderate staining, though the central areas in large sections (presumably less well fixed) showed somewhat diminished staining intensity. Small biopsies and recent cases showed uniform, strong staining. All histologic mimics revealed no antibody reactivity whatsoever.
TABLE 1.
Summary of immunohistochemical staining with SS18-SSX fusion-specific and SSX C-terminus antibodies.
| Tumor type | Total Cases | SS18-SSX Positive (%) | SSX C-terminus Positive (%) |
|---|---|---|---|
| Synovial sarcoma | 100 | 95 (95) | 100 (100) |
| Monophasic synovial sarcoma | 41 | 39 (95) | 41 (100) |
| Biphasic synovial sarcoma | 18 | 18 (100) | 18 (100) |
| Poorly differentiated synovial sarcoma | 41 | 38 (93) | 41 (100) |
| Non-synovial sarcoma tumors | 300 | 0 (0) | 13 (4) |
| Malignant peripheral nerve sheath tumor | 20 | 0 (0) | 2 (10) |
| Solitary fibrous tumor | 20 | 0 (0) | 0 (0) |
| Dedifferentiated liposarcoma | 20 | 0 (0) | 2 (10) |
| Leiomyosarcoma | 20 | 0 (0) | 0 (0) |
| Fibrosarcomatous variant of DFSP | 20 | 0 (0) | 0 (0) |
| Ewing sarcoma | 20 | 0 (0) | 0 (0) |
| CIC sarcoma | 20 | 0 (0) | 0 (0) |
| Spindle cell rhabdomyosarcoma | 20 | 0 (0) | 0 (0) |
| Alveolar rhabdomyosarcoma | 20 | 0 (0) | 1 (5) |
| Embryonal rhabdomyosarcoma | 20 | 0 (0) | 1 (5) |
| Mesenchymal chondrosarcoma | 20 | 0 (0) | 2 (10) |
| Desmoplastic small round cell tumor | 20 | 0 (0) | 2 (10) |
| Clear cell sarcoma | 20 | 0 (0) | 0 (0) |
| Biphenotypic sinonasal sarcoma | 10 | 0 (0) | 1 (10) |
| BCOR-rearranged sarcoma | 10 | 0 (0) | 0 (0) |
| Sarcomatoid mesothelioma | 10 | 0 (0) | 2 (20) |
| Biphasic mesothelioma | 10 | 0 (0) | 0 (0) |
DFSP, dermatofibrosarcoma protuberans.
Figure 3.
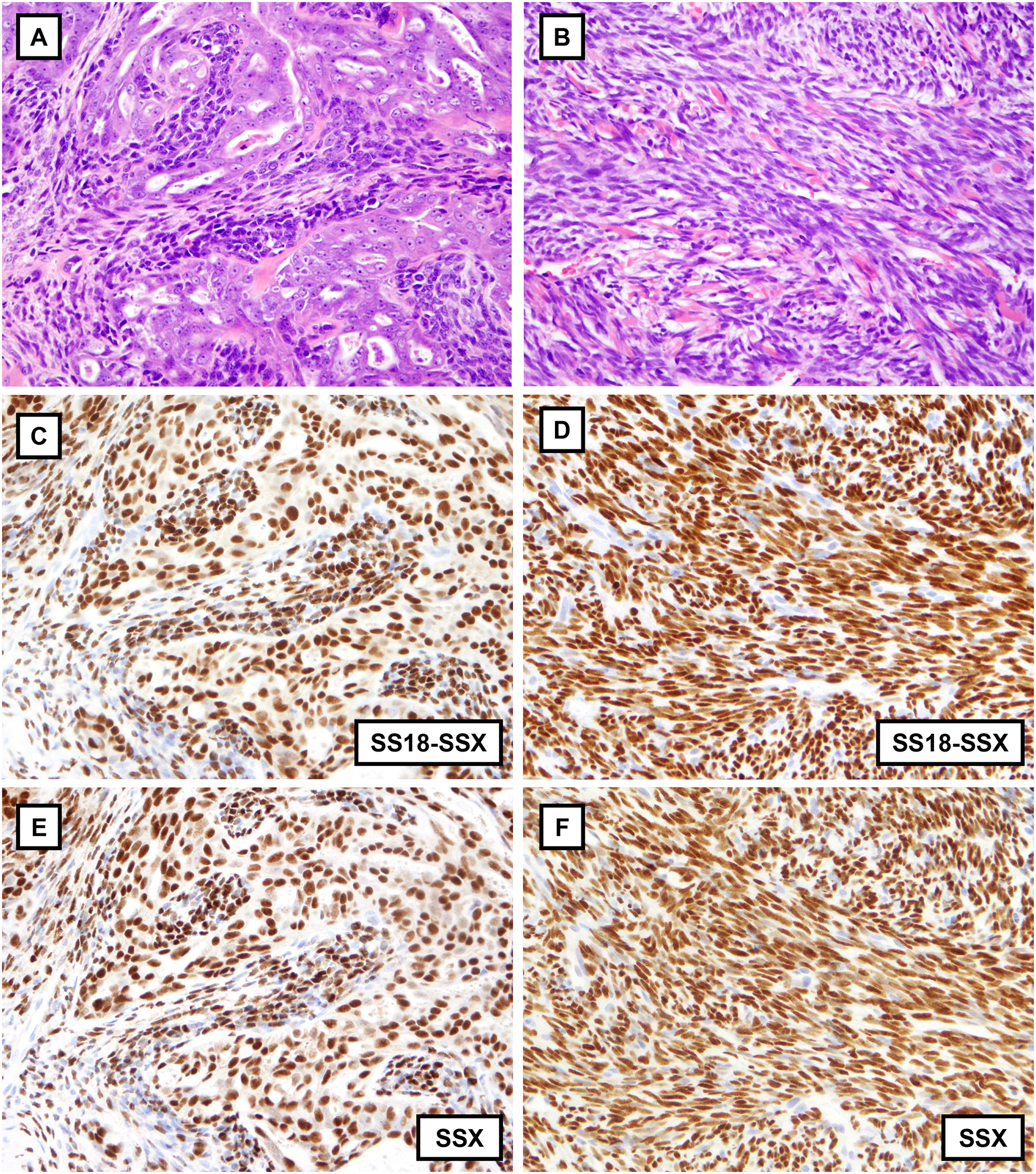
Biphasic synovial sarcoma with intermixed glandular and spindle cell components (A) and monophasic synovial sarcoma consisting of spindle cells arranged in fascicles with prominent wiry collagen (B). The SS18-SSX fusion-specific antibody (E9X9V) reveals strong diffuse nuclear positivity in both cases (C and D), including in the glandular component of biphasic synovial sarcoma. The SSX C-terminus antibody (E5A2C) is likewise strongly and diffusely positive in both cases (E and F).
Figure 4.

Hypocellular monophasic synovial sarcoma with abundant collagenous stroma, mimicking neurofibroma (A); a predominantly myxoid monophasic synovial sarcoma with prominent vasculature (C), raising the possibility of a myxoid solitary fibrous tumor among other tumor types; and monophasic synovial sarcoma within the lung showing entrapped alveolar epithelium mimicking a glandular component (E). All three cases of synovial sarcoma, including the two rare histologic variants, display strong diffuse positivity with the SS18-SSX fusion-specific antibody (B, D, F) and the SSX C-terminus antibody (not shown). The SS18-SSX antibody highlights negative benign entrapped pulmonary epithelium within a background of positively staining tumor cells (F).
Figure 5.
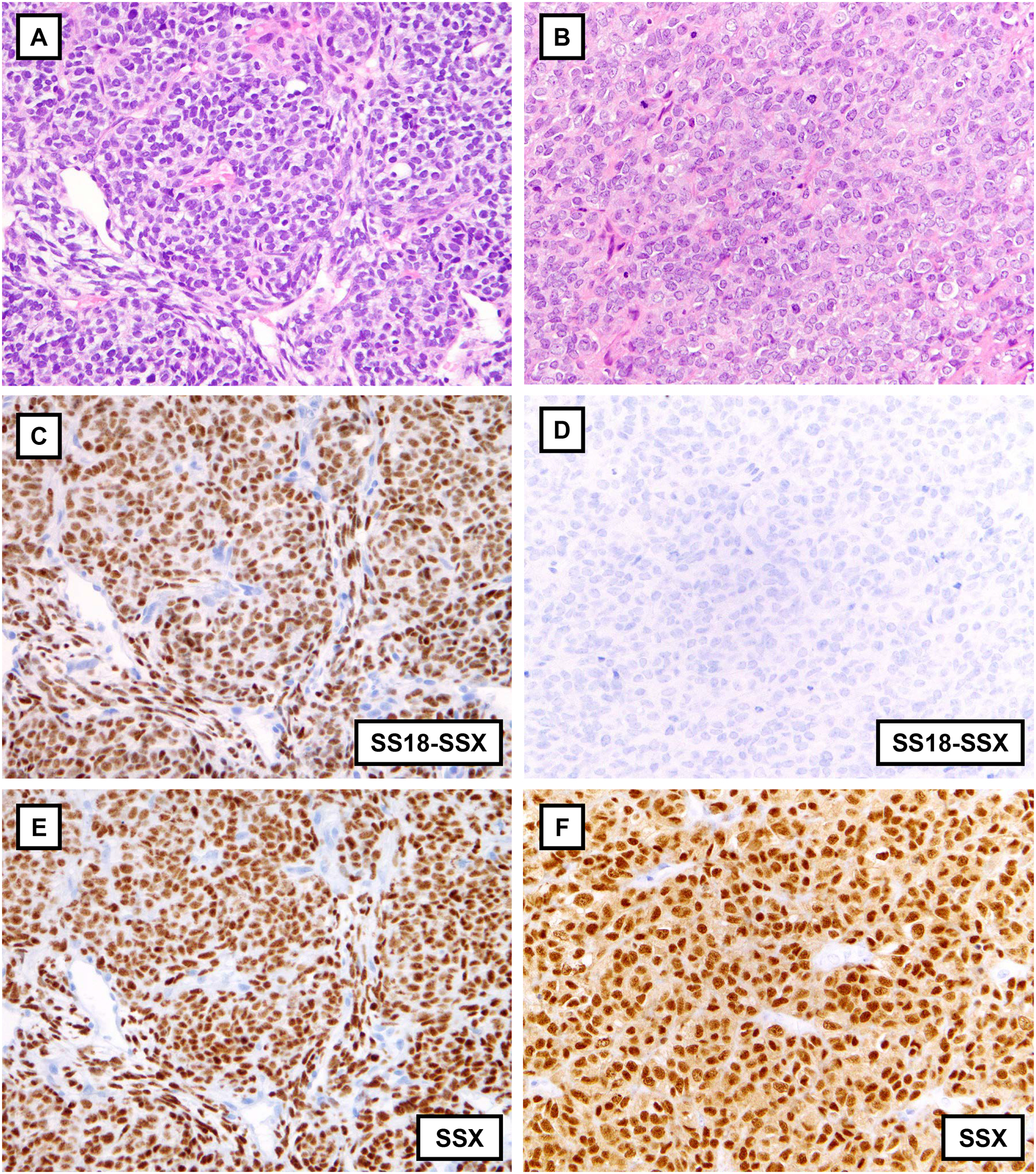
Two poorly differentiated synovial sarcomas displaying sheets of monomorphic primitive round cells reminiscent of Ewing sarcoma or other round cell sarcomas (A, B). The first case is strongly and diffusely positive for the SS18-SSX fusion-specific (C) and the SSX C-terminus (E) antibodies. The second case is negative for the SS18-SSX fusion-specific antibody (D), though strongly and diffusely positive for the SSX C-terminus antibody (F), one of five SS cases with this immunophenotype.
The SSX C-terminus antibody (E5A2C) was immunoreactive in all 100 (100%) SS cases and 13 of 300 (4%) histologic mimics. Of these 13 positive non-SS cases, 5 displayed nuclear staining in greater than 50% of tumor cell nuclei (3 cases with 5+, 2 cases with 4+; see Figure 6), while the remaining 8 cases revealed 2+ staining. All 13 non-SS cases positive for the SSX C-terminus antibody (E5A2C) were negative for the SS18-SSX fusion-specific antibody (E9X9V). Of all the cases negative for the SSX C-terminus antibody, 263 (88%) revealed no staining (score 0), whereas 24 (8%) revealed 1+ staining, though most of these latter cases showed nuclear staining in only rare cells (<1% of total tumor cells). As with the SS18-SSX fusion antibody, all positive SS cases showed strong crisp 5+ nuclear staining for the SSX C-terminus antibody, with most cases approaching 100% nuclear staining (Figures 3, 4, and 5). The staining intensity in all but two of the positive non-SS cases was strong, including in 24 cases with 1+ nuclear staining. Each of these two cases (one embryonal rhabdomyosarcoma and one mesenchymal chondrosarcoma) showed moderate intensity staining with 2+ nuclear positivity. As with the other antibody, none of the cases showed weak staining. In summary, these results reveal 95% sensitivity and 100% specificity for SS with the SS18-SSX fusion-specific antibody (E9X9V) and 100% sensitivity and 96% specificity with the SSX C-terminus antibody (E5A2C).
Figure 6.

Malignant peripheral nerve sheath tumor (MPNST) composed of fascicles of markedly atypical spindle cells (A). The tumor cells revealed loss of H3K27me3 (not shown). The tumor cells are negative with the SS18-SSX fusion-specific antibody (C) and strongly and diffusely positive with the SSX C-terminus antibody. A monophasic synovial sarcoma with alternating cellular and myxoid areas, reminiscent of MPNST (B), found to have strong diffuse staining with the SS18-SSX fusion-specific (D) and SSX C-terminus antibodies (F). This case was found to harbor an SS18-SSX4 rearrangement by next-generation sequencing.
Fluorescence in situ hybridization (FISH) and next generation sequencing (NGS)
The five non-SS cases revealing 4+ or 5+ staining with the SSX C-terminus antibody (2 MPNST, 1 dedifferentiated liposarcoma, 1 alveolar rhabdomyosarcoma, and 1 sarcomatoid mesothelioma, all negative with the SS18-SSX antibody) each underwent analysis by SS18 break-apart FISH. Of the five, one case (an MPNST with heterologous rhabdomyoblastic differentiation revealing H3K27me3 loss by IHC) showed a complex pattern in 26/50 nuclei that initially suggested SS18 rearrangement. However, this finding could not be confirmed by targeted sequencing analyses and, therefore, against the background of a complex genome, the FISH result was re-interpreted as negative for SS18 rearrangement. Notably, the same targeted NGS assay revealed SUZ12, NF1 and TP53 inactivating mutations (findings characteristic of MPNST), as well as numerous copy number alterations, which support the original diagnosis.
DISCUSSION
In this study, we evaluated two novel monoclonal antibodies for the diagnosis of synovial sarcoma: (1) an antibody directed against the SS18-SSX fusion sequence found in ~95% of SS; and (2) a robust antibody against the SSX C-terminus. We first tested these antibodies against control and SS cell lines, showing that both antibodies successfully detect SS18-SSX1 and SS18-SSX2 fusion proteins by immunoblotting without cross-reactivity with wild-type SS18 (a protein ubiquitously expressed across human cell types). Immunoprecipitation with both antibodies successfully captured SS18-SSX fusion proteins as well as core subunits of the mSWI/SNF complex, as expected. ChIP-seq with each antibody demonstrated capture of the SS18-SSX fusion protein on chromatin at known upregulated target sites.9 These experimental data confirm the specificity of each antibody for the SS18-SSX fusion oncoprotein and highlight their utility in enabling further research efforts on the role of SS18-SSX in SS.
We then tested these two antibodies by IHC in a large series of genetically confirmed SS and known histologic mimics (400 cases total) as potential surrogates for molecular genetic testing. The SS18-SSX fusion-specific antibody (E9X9V) was found to be 95% sensitive and 100% specific for SS, surpassing the specificity of all IHC markers currently in clinical practice and likely obviating confirmatory molecular genetic or cytogenetic testing in positive cases. The sensitivity of this antibody is similar to that of RT-PCR and greater than that of SS18 break-apart FISH.20 The SSX C-terminus antibody (E5A2C) was found to be 100% sensitive and 96% specific for SS, which should also prove valuable for the diagnosis of SS, particularly given its high sensitivity. Together, these two antibodies could replace the ‘gold standard’ of molecular genetic or cytogenetic testing in the majority of SS, allowing pathologists to make a definitive diagnosis without the need for more time consuming and less widely available assays.
While the diagnosis of SS can be rendered based on histologic features by expert soft tissue pathologists in many cases, and with the assistance of currently available IHC in most cases, there remains a subset of cases that requires ancillary testing to confirm the diagnosis. In fact, many practices routinely test all possible SS cases by SS18 break-apart FISH or RT-PCR in order to avoid misdiagnosis. TLE1, likely the best IHC marker for SS to date, shows up to 90% sensitivity but only moderate specificity,14,15 as this marker is positive in 15–30% of MPNST and around 10% of SFT (tumor types that closely mimic SS), as well as nearly all mesotheliomas16 and around 10% of poorly differentiated carcinomas.17
The generation of a diagnostically useful antibody specific to the amino acid fusion sequence of a chimeric oncoprotein in a translocation-associated malignant neoplasm is in itself novel. While other antibodies have been successfully generated for the diagnosis of translocation-associated neoplasms by IHC, these antibodies have been developed either against the C-terminus of a fusion partner (e.g., STAT6 in SFT with NAB2-STAT6 fusions; CAMTA1 in epithelioid hemangioendothelioma with WWTR1-CAMTA1 fusions; FOSB in pseudomyogenic hemangioendothelioma with SERPINE1-FOSB fusions),24–26 or against proteins upregulated by the fusion oncoprotein (e.g., TLE1 in SS, MUC4 in low-grade fibromyxoid sarcoma with FUS-CREB3L2 or FUS-CREB3L1 fusions, and NKX2.2 in Ewing sarcoma).14,27,28 While these antibodies successfully aid in the diagnosis of translocation-associated neoplasms and most are extremely sensitive, none are entirely specific. Thus, the 100% specificity of this SS18-SSX fusion-specific antibody is thus far unique and suggests the possibility of generating additional fusion-specific antibodies in other translocation-associated neoplasms in which particular breakpoints predominate. Moreover, the specificity of this antibody should replace the need for ancillary genetic testing in cases of SS in which IHC is positive.
Qualitatively, the SS18-SSX fusion-specific antibody displayed diffusely, strongly positive or completely negative IHC results, rendering interpretation straightforward; all positive cases displayed over 90% nuclear positivity. The 5% of SS cases in this study negative by IHC with the fusion-specific antibody (E9X9V) likely represent cases with uncommon fusion variants, in keeping with prior data showing alternate fusion breakpoints in around 8% of SS, including breakpoints resulting in extended SSX C-terminal amino acid tails.9 Extension of the SSX tail and other breakpoint variants would change the identity of the fusion sequence and thus prevent recognition by the fusion-specific antibody. These cases, however, would be expected to be recognized by the SSX C-terminus antibody (E5A2C), as seen in the five E9X9V negative cases in this study (Figure 5). The SSX C-terminus antibody had similar qualitative features to the fusion-specific antibody; positive cases (all SS cases and the few positive non-SS cases) revealed strong and diffuse staining, with only two non-SS cases showing moderate staining, again making interpretation with this antibody straightforward. Most non-SS tumors (88%) were entirely negative with the SSX C-terminus antibody, though 8% showed 1+ staining (usually only rare cells) and 3% showed 2+ staining.
Of the five non-SS cases (2%) showing 4+ or 5+ staining with the SSX C-terminus antibody, one case showed a potential SS18 rearrangement that was reclassified as negative when orthogonal testing did not confirm the finding. This tumor was an MPNST with heterologous rhabdomyoblastic differentiation, showing complete loss of staining for H3K27me3. Targeted NGS of this tumor not only failed to confirm SS18 fusion, but also revealed inactivating mutations characteristic of MPNST (SUZ12, NF1, TP53). This example highlights inherent challenges in FISH interpretation while highlighting the importance of ancillary molecular testing in rare cases positive for the SSX C-terminus antibody, but negative for the SS18-SSX fusion-specific antibody.
Following completion of this study, one of the authors (J.L.H.) encountered an interesting consult case, emphasizing the utility of these antibodies in the diagnosis of SS. The H&E of a posterior mediastinal mass in a 40-year-old woman revealed a high-grade spindle cell sarcoma with alternating myxoid and cellular, fascicular areas (see Figure 6B). The case had been previously reviewed at another institution and found to be negative for SS18 rearrangement by FISH, as well as negative for SS18-SSX1 and SS18-SSX2 by RT-PCR. Although IHC for S100 protein and SOX10 were both negative, and H3K27me3 was retained, given the anatomic site, histologic appearances, and apparent lack of an SS18 rearrangement (evaluated by multiple modalities), MPNST was favored. The case later underwent NGS and was found to have an SS18-SSX4 gene fusion, leading to revision of the diagnosis to monophasic SS. The tumor was subsequently evaluated by IHC with the SS18-SSX fusion-specific and SSX C-terminus antibodies; both were strongly and diffusely positive (see Figure 6D, F). This case highlights the value of these antibodies in detecting SS18-SSX fusions that are missed by RT-PCR and/or FISH.
In summary, a novel SS18-SSX fusion-specific antibody is highly sensitive and specific for SS. IHC using this antibody could replace molecular genetic or cytogenetic testing in most cases. An antibody to the SSX C-terminus is also highly sensitive but slightly less specific. In addition to their clinical diagnostic application, these reagents will provide the research community with valuable tools for further biochemical and genomic interrogation of the oncogenic activity of the SS18-SSX fusion protein.
ACKNOWLEDGMENTS
We thank Curtis Desilets, Katrina Costa, Michelle Ferrante, and Christopher Grange (Cell Signaling Technology) for providing the antibodies; Mei Zheng and the immunohistochemistry laboratory (Department of Pathology, Brigham and Women’s Hospital); and the Center for Advanced Molecular Diagnostics (Department of Pathology, Brigham and Women’s Hospital).
Footnotes
Conflicts of Interest:
C.K. is the Scientific Founder, fiduciary Board of Directors Member, Scientific Advisory Board Member, Shareholder, and Consultant for Foghorn Therapeutics, Inc. (Cambridge, MA).
J.L.H. is a consultant to Eli-Lilly and Epizyme.
For the remaining authors, no conflicts were declared.
This paper was presented in part at the 109th Annual Meeting of the United States and Canadian Academy of Pathology in Los Angeles, CA, February 29 – March 5, 2020.
REFERENCES
- 1.Thway K, Fisher C. Synovial sarcoma: defining features and diagnostic evolution. Ann Diagn Pathol. 2014;18:369–380. [DOI] [PubMed] [Google Scholar]
- 2.Sultan I, Rodriguez-Galindo C, Saab R, et al. Comparing children and adults with synovial sarcoma in the Surveillance, Epidemiology, and End Results program, 1983 to 2005: an analysis of 1268 patients. Cancer. 2009;115:3537–3547. [DOI] [PubMed] [Google Scholar]
- 3.dos Santos NR, de Bruijn DR, van Kessel AG. Molecular mechanisms underlying human synovial sarcoma development. Genes Chromosomes Cancer. 2001;30:1–14. [DOI] [PubMed] [Google Scholar]
- 4.Ladanyi M, Antonescu CR, Leung DH, et al. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multi-institutional retrospective study of 243 patients. Cancer Res. 2002;62:135–140. [PubMed] [Google Scholar]
- 5.Dimitriadis E, Rontogianni D, Kyriazoglou A, et al. Novel SYT-SSX fusion transcript variants in synovial sarcoma. Cancer Genet Cytogenet. 2009;195:54–58. [DOI] [PubMed] [Google Scholar]
- 6.Türeci O, Chen YT, Sahin U, et al. Expression of SSX genes in human tumors. Int J Cancer. 1998;77:19–23. [DOI] [PubMed] [Google Scholar]
- 7.dos Santos NR, Torensma R, de Vries TJ, et al. Heterogeneous expression of the SSX cancer/testis antigens in human melanoma lesions and cell lines. Cancer Res. 2000;60:1654–1662. [PubMed] [Google Scholar]
- 8.Kadoch C, Crabtree GR. Reversible disruption of mSWI/SNF (BAF) complexes by the SS18-SSX oncogenic fusion in synovial sarcoma. Cell. 2013;153:71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McBride MJ, Pulice JL, Beird HC, et al. The SS18-SSX Fusion Oncoprotein Hijacks BAF Complex Targeting and Function to Drive Synovial Sarcoma. Cancer Cell. 2018;33:1128–1141.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McBride MJ, Kadoch C. Disruption of mammalian SWI/SNF and polycomb complexes in human sarcomas: mechanisms and therapeutic opportunities. J Pathol. 2018;244:638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelmus M, Guillou L, Hostein I, et al. Monophasic fibrous and poorly differentiated synovial sarcoma: immunohistochemical reassessment of 60 t(X;18)(SYT-SSX)-positive cases. Am J Surg Pathol. 2002;26:1434–1440. [DOI] [PubMed] [Google Scholar]
- 12.Olsen SH, Thomas DG, Lucas DR. Cluster analysis of immunohistochemical profiles in synovial sarcoma, malignant peripheral nerve sheath tumor, and Ewing sarcoma. Mod Pathol. 2006;19:659–668. [DOI] [PubMed] [Google Scholar]
- 13.Terry J, Saito T, Subramanian S, et al. TLE1 as a diagnostic immunohistochemical marker for synovial sarcoma emerging from gene expression profiling studies. Am J Surg Pathol. 2007;31:240–246. [DOI] [PubMed] [Google Scholar]
- 14.Foo WC, Cruise MW, Wick MR, et al. Immunohistochemical staining for TLE1 distinguishes synovial sarcoma from histologic mimics. Am J Clin Pathol. 2011;135:839–844. [DOI] [PubMed] [Google Scholar]
- 15.Kosemehmetoglu K, Vrana JA, Folpe AL. TLE1 expression is not specific for synovial sarcoma: a whole section study of 163 soft tissue and bone neoplasms. Mod Pathol. 2009;22:872–878. [DOI] [PubMed] [Google Scholar]
- 16.Matsuyama A, Hisaoka M, Iwasaki M, et al. TLE1 expression in malignant mesothelioma. Virchows Arch. 2010;457:577–583. [DOI] [PubMed] [Google Scholar]
- 17.Zaccarini DJ, Deng X, Tull J, et al. Expression of TLE-1 and CD99 in Carcinoma: Pitfalls in Diagnosis of Synovial Sarcoma. Appl Immunohistochem Mol Morphol. 2018;26:368–373. [DOI] [PubMed] [Google Scholar]
- 18.Kohashi K, Oda Y, Yamamoto H, et al. Reduced expression of SMARCB1/INI1 protein in synovial sarcoma. Mod Pathol. 2010;23:981–990. [DOI] [PubMed] [Google Scholar]
- 19.Arnold MA, Arnold CA, Li G, et al. A unique pattern of INI1 immunohistochemistry distinguishes synovial sarcoma from its histologic mimics. Hum Pathol. 2013;44:881–887. [DOI] [PubMed] [Google Scholar]
- 20.Amary MFC, Berisha F, Bernardi FDC, et al. Detection of SS18-SSX fusion transcripts in formalin-fixed paraffin-embedded neoplasms: analysis of conventional RT-PCR, qRT-PCR and dual color FISH as diagnostic tools for synovial sarcoma. Mod Pathol. 2007;20:482–496. [DOI] [PubMed] [Google Scholar]
- 21.Firestein R, Bass AJ, Kim SY, et al. CDK8 is a colorectal cancer oncogene that regulates beta-catenin activity. Nature. 2008;455:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sholl LM, Do K, Shivdasani P, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight. 2016;1:e87062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shanmugam V, Griffin GK, Jacobsen ED, et al. Identification of diverse activating mutations of the RAS-MAPK pathway in histiocytic sarcoma. Mod Pathol. 2019;32:830–843. [DOI] [PubMed] [Google Scholar]
- 24.Doyle LA, Vivero M, Fletcher CD, et al. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014;27:390–395. [DOI] [PubMed] [Google Scholar]
- 25.Doyle LA, Fletcher CDM, Hornick JL. Nuclear Expression of CAMTA1 Distinguishes Epithelioid Hemangioendothelioma From Histologic Mimics. Am J Surg Pathol. 2016;40:94–102. [DOI] [PubMed] [Google Scholar]
- 26.Hung YP, Fletcher CDM, Hornick JL. FOSB is a Useful Diagnostic Marker for Pseudomyogenic Hemangioendothelioma. Am J Surg Pathol. 2017;41:596–606. [DOI] [PubMed] [Google Scholar]
- 27.Doyle LA, Möller E, Dal Cin P, et al. MUC4 is a highly sensitive and specific marker for low-grade fibromyxoid sarcoma. Am J Surg Pathol. 2011;35:733–741. [DOI] [PubMed] [Google Scholar]
- 28.Hung YP, Fletcher CDM, Hornick JL. Evaluation of NKX2–2 expression in round cell sarcomas and other tumors with EWSR1 rearrangement: imperfect specificity for Ewing sarcoma. Mod Pathol. 2016;29:370–380. [DOI] [PubMed] [Google Scholar]


