Abstract
Previous research has shown that African Americans (AA) report higher pain intensity and pain interference than other racial/ethnic groups as well as greater levels of other risk factors related to worse pain outcomes, including PTSD symptoms, pain catastrophizing, and sleep disturbance. Within a Conservation of Resources theory framework, we tested the hypothesis that socioeconomic status (SES) factors (i.e., income, education, employment, perception of income meeting basic needs) largely account for these racial/ethnic differences. Participants were 435 women [AA, 59.1%; Hispanic/Latina (HL), 25.3%; Non-Hispanic/White (NHW), 15.6%] who presented to an Emergency Department (ED) with an acute pain-related complaint. Data were extracted from psychosocial questionnaires completed at the participants’ baseline interview. Structural Equation Modeling was used to examine whether racial/ethnic differences in pain intensity and pain interference were mediated by PTSD symptoms, pain catastrophizing, sleep quality, and sleep duration, and whether these mediation pathways were, in turn, accounted for by SES factors. Results indicated that SES factors accounted for the mediation relationships linking AA race to pain intensity via PTSD symptoms and the mediation relationships linking AA race to pain interference via PTSD symptoms, pain catastrophizing, and sleep quality. Results suggested that observed racial/ethnic differences in AA women’s pain intensity, pain interference, and common risk factors for elevated pain may be largely due to racial/ethnic differences in SES. These findings highlight the role of social inequality in persistent health disparities facing inner-city, AA women.
Keywords: acute pain, SES, race, ethnicity, health disparities
INTRODUCTION
Pain conditions affect approximately 69.6 million Americans in a given year (Dahlhamer et al., 2018) and have been shown to differ considerably based on social and demographic factors, including race/ethnicity and socioeconomic status (SES; Riskowski, 2014). African Americans (AAs), in particular, report greater acute pain intensity and pain-related interference than other racial/ethnic groups (Booker, 1989; Meints, Miller & Hirsh, 2016; Portenoy et al., 2004; Kim et al., 2017; Rahim‐Williams et al., 2012; Ostom et al., 2017). In addition, low socioeconomic status (SES; as measured by factors including income, employment status, education level, and neighborhood poverty level) has also been consistently associated with worse pain outcomes, including higher pain severity and more pain-related interference (Davies et al, 2009; Portenoy et al., 2004; Brekke, Hiortdahl, Kvien, 2002).
Importantly, national data consistently show that inequalities in SES are strongly patterned by race (Williams, Mohammed, Leavell, & Collins, 2010). Likely due to longstanding a history of systemic disadvantage, AAs, in particular, have persistent levels of overall poverty that are higher than Whites, and despite increases in education across all racial/ethnic groups since the 1960s (i.e., high school graduation, college graduation), AAs continue to have lower rates of educational attainment when compared to Whites (U.S. Census Bureau, 2017). Further, these increases in educational attainment among AAs have not led to commensurate increases in their earning potential; AAs earn approximately 59 cents for every dollar of income that Whites receive (Ryan & Siebens, 2012). Collectively, these persistent and pervasive racial inequalities in SES require that exploration of race-related differences in pain outcomes address concurrent race-related associations with SES.
Previous work that has sought to disentangle the effects of race from SES in pain outcomes has generated mixed results. Some studies have found that low SES explains adverse pain outcomes in AAs (Urwin et al., 1998; Elliot et al., 1999; Portenoy et al., 2004; Schneider et al., 2005; Fuentes, Hart-Johnson, & Green, 2007) while other studies have found that AAs have significantly worse pain outcomes even after controlling for SES (Plesh, Crawford, & Gansky, 2002; Day & Thorn, 2010; Green & Hart-Johnson, 2012). These discrepancies could be due to a number of methodological factors, including variations in the definition and assessment of SES and race (Edwards, Fillingim, & Keefe, 2001; Poleshuck & Green, 2008; Chan, Hamamura, & Janschewitz, 2013; Baldassari et al., 2016; Kim et al., 2017). For example, some studies have measured SES at the individual level, with only one indicator [e.g., individual income; Day & Thorn, 2010] while others have measured SES with multiple indicators, at the neighborhood level [e.g., percent of homes living below poverty line, percent of homes with high school education or higher, percent of homes with employed labor force; Green & Hart-Johnson, 2012]. Other studies have been limited methodologically by failing to distinguish acute and chronic pain processes (Riskowski, 2014), which is problematic, given that chronic pain has been defined as an acute pain complaint lasting longer than 3–6 months (Steingrímsdóttir et al., 2017). Thus, studies that conflate acute and chronic pain complaints lack specificity as to which risk factors are associated with the development of chronic pain from those pain complaints that may go away on their own. Still other studies have lacked assessment of other potential explanatory variables contributing to worse pain outcomes among AAs. Specifically, well-known pain-related risk factors, including symptoms of Posttraumatic Stress Disorder (PTSD), pain catastrophizing, and indicators of poor or insufficient sleep, have also been shown to differ by race (Burns et al, 2003; Smith, 2004; Beck & Clapp, 2011).
As many as 80% of individuals with PTSD report co-morbid pain (Otis, Keane, Kerns, 2003; Beck & Clapp, 2011; Brennstuhl, Tarquinio & Montel, 2015) and AAs have consistently been shown to have a higher lifetime prevalence of PTSD than other racial/ethnic groups, despite not necessarily experiencing more traumatic events than other racial/ethnic groups (Alim, Charney, & Mellman, 2006; Roberts, Gilman, Breslau, Breslau & Koenen, 2011; McLaughlin et al., 2018). Existing theories that have sought to explain the overlap among PTSD and pain-related complaints point to common underlying physiological and psychological factors [i.e., hyperarousal, attentional biases, and anxiety sensitivity; (Holley, Wilson, Noel, & Palermo, 2016; Beck & Clapp, 2011; Sharp & Harvey, 2001)]. These factors, in turn, have been highlighted as potential mechanisms underlying the persistent associations observed between higher PTSD symptom severity and both increased pain intensity and pain-related interference (Geisser, et al., 1996; Jenewein et al, 2009; Sherman et al., 2000; Phifer et al., 2011).
In addition, AAs have been found to be more likely than other racial/ethnic groups to report engaging in pain catastrophizing, a maladaptive cognitive style characterized by inflexibility, rumination, and increased vigilance to painful stimuli (Aldrich, Eccleston & Crombez, 2000; Carty, O-Donnell, Evans, Kazantzis, & Creamer, 2011). Pain catastrophizing has consistently been linked with worse pain outcomes, including increased pain interference and greater pain intensity (Forsythe et al., 2011; Meints, Miller & Hirsh, 2016). Furthermore, reflecting shared attentional biases and vigilance to potentially threatening stimuli, previous research has also highlighted increased vulnerability towards the use of pain catastrophizing among individuals with comorbid PTSD and pain (Alschuller & Otis, 2012).
Finally, AAs are nearly twice as likely as other racial/ethnic groups to report short sleep durations and low-quality sleep (Stamatakis, Kaplan, & Roberts, 2007; Krueger & Friedman, 2009; Hale & Do., 2007; Grandner et al., 2010). Previous research has linked shortened sleep times (< 6.5 hours per night) and reports of low-quality sleep with more severe pain intensity and pain-related interference (Affleck et al., 1996; Raymond et al. 2001; Finan, Goodin, Smith, 2013; Koffel et al., 2016). In addition, PTSD symptoms and sleeping difficulties are often comorbid, with difficulty falling and/or staying asleep and nightmares representing two diagnostic criteria of PTSD (American Psychological Association, 2013). Recent studies have also implicated disrupted sleep as a potential explanatory mechanism linking symptoms of PTSD to increased pain-related complaints (Bigatti et al, 2008; Lillis et al., 2018a; Aaron et al, 2019).
Collectively, these findings may indicate that differences in pain intensity and pain interference among AAs could be explained, in part, by underlying differences in PTSD symptoms, pain catastrophizing, and sleep. That is, AAs may have worse pain than other racial/ethnic groups because they have worse PTSD, pain catastrophizing, and sleep. However, such findings would still leave the question as to why AAs differ from other racial/ethnic groups on pain intensity and pain interference, and on PTSD, pain catastrophizing and sleep.
Accordingly, from the perspective of the Conservation of Resource Theory (COR Theory; Hobfoll, 1989), we sought to test the hypothesis that socioeconomic disadvantage (i.e., being low SES) largely accounts for both observed AA-race-related differences in self-reported pain outcomes (i.e., pain intensity and pain interference) and the impact of known risk factors associated with poor self-reported pain outcomes (i.e., PTSD symptoms, pain catastrophizing, poor sleep quality, and short sleep duration). We tested this hypothesis in a sample of racially diverse, inner-city women presenting to the Emergency Department with an acute pain complaint.
COR theory was chosen as our conceptual framework for this study as it has been applied in previous research exploring health outcomes following stress (Schumm, Hobfoll, & Keogh, 2004; Hou et al., 2010; Cook, Aten, Moore, Hook, & Davis, 2013; Lillis et al., 2018b) and provides a theoretical bridge across the PTSD, pain, and health disparities literature to implicate (both historical and current) socioeconomic disadvantage as a potential mechanism by which AAs would be disproportionately affected by pain and pain-related risk factors. Specifically, COR theory’s central tenant is that individuals are motivated to protect their resource (personal, social, material, financial, etc.) and that psychological distress arises when actual or threatened resource loss occurs. COR theory further asserts that those who have the fewest resources are the most vulnerable to further loss and distress because they lack sufficient resources to apply towards management of acute stressors.
Thus, viewed through the lens of COR theory, AAs who are also low SES would have fewer resources to recover from previous stressors, which may make them more vulnerable to developing PTSD symptoms. PTSD symptoms, in turn, could increase vulnerability to worse pain intensity and pain interference directly (Geisser, et al., 1996; Jenewein et al, 2009; Sherman et al., 2000; Phifer et al., 2011; Lillis et al., 2018b) as well as indirectly through increasing the use of maladaptive cognitive coping strategies, like pain catastrophizing (Alschuller & Otis, 2012), and through disrupting sleep (Lillis et al., 2018a, Aaron et al., 2019, Bigatti et al., 2008).
Importantly, COR theory could also potentially explain worse pain outcomes among economically disadvantaged AAs independent of PTSD symptoms. Specifically, AAs who are low SES may be more likely to be vigilant to threats of further resource loss and thus engage in more pain catastrophizing (leading to higher pain intensity and pain-interference). In addition, AAs who are low SES may also lack the resources needed to provide a sufficient opportunity for sleep. For example, previous research has shown that occupational instability, whether from working multiple part-time jobs or jobs with long or unpredictable hours, may restrict the amount of time available to obtain a sufficient amount of sleep (Basner et al., 2007; Luckhaupt, Tak, & Calvert, 2010). Further, living in low-income neighborhoods has also been shown to have a negative impact on sleep through increased exposure to nighttime noise and light (Hill et al., 2009; Hale et al., 2013; Johnson, Brown, Morgenstern, Meurer, & Lisabeth, 2015). These SES-related intrusions on sleep duration and sleep quality, in turn, could lead to higher pain intensity and pain-interference.
Study Hypotheses
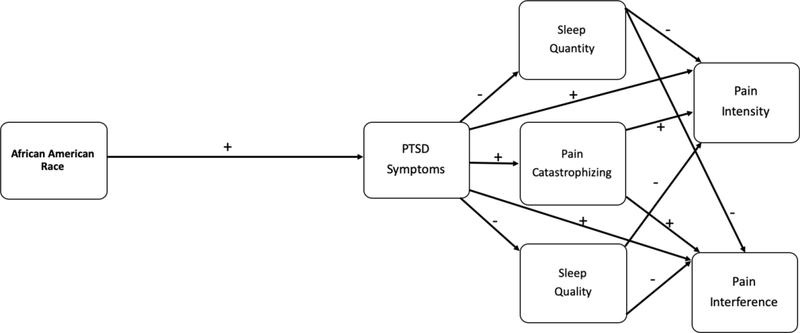
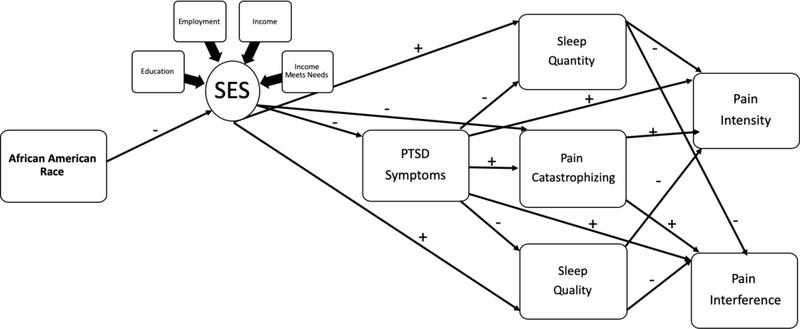
Following Figure 1 (Model #1), generalizing from the PTSD and pain literature, we first hypothesized that AA race would be significantly related to pain intensity and pain interference through a direct positive relationship with PTSD, an indirect positive relationship with pain catastrophizing (via PTSD), and indirect negative relationships with sleep duration and sleep quality (via PTSD). Second, following Figure 2 (Model #2), based on U.S. Census data (2017) and previous research, we expected a negative direct relationship between AA race and SES. Third, as informed by COR theory, we hypothesized that SES would be linked indirectly with pain intensity and pain interference through a negative direct relationship with PTSD, a negative direct relationship with pain catastrophizing, and positive direct relationships with sleep duration and sleep quality. Finally, in the models reflected in both figures, we expected positive direct relationships from pain catastrophizing to pain intensity and pain interference, and negative direct relationships from sleep duration and sleep quality to pain intensity and interference, respectively.
Figure 1.
Conceptual Model #1 linking African American race to pain intensity and pain interference via direct relationship with PTSD Symptoms and indirect relationships with Pain Catastrophizing, Sleep Quality, and Sleep Quantity.
Figure 2.
Conceptual Model #2 linking African American race to pain intensity and pain interference via direct relationship with SES factors and indirect relationships with PTSD symptoms, Pain Catastrophizing, Sleep Quality, and Sleep Quantity.
METHODS
Participants
The data from the current study are part of an ongoing longitudinal study exploring the relationship between trauma and pain among women who presented to our institution’s inner-city Chicago ED with an acute pain complaint. At the time of the current analysis, 1,921 women had been approached for recruitment. Of those 1,921 women, 1,702 (88.6%) expressed interest in participating. Of the 1702 interested 663 (39.1%) were screened eligible to participate. Of those 663 eligible women, 477 (72%) completed a baseline interview.
In the current study, only data collected at the baseline interview were used for analyses (which excluded 25 cases who listed their race as “other” or who identified as more than one race as these totals were too small to analyze separately). This baseline sample (n=435) was approximately 28 years-old (SD = 6.19) and predominantly AA (59.1%), followed by Hispanic/Latina (HL; 25.3%), and Non-Hispanic/White (NHW; 15.6%). Racial/ethnic composition of the sample of women who completed baseline interviews was not significantly different from the sample of women who did not complete baseline interviews (i.e., those who refused to participate, were ineligible, or were lost to follow up). Demographic characteristics are presented in Table 1 by race/ethnicity and for the total sample.
Table 1.
Characteristics of Total Sample and by Race.
| African American n = 257 | Hispanic/Latina n = 110 | Non-Hispanic/White n = 68 | Total Sample N = 435 | Significance Tests | ||||||
| Variables | M | SD | M | SD | M | SD | M | SD | F | p |
| Age (18–40) | 28.49 | 6.31 | 28.46 | 6.27 | 28.78 | 5.70 | 28.53 | 6.19 | .071 | n.s. |
| n | % | n | % | n | % | n | % | χ2 | p | |
| Education | 91.52 | .00 | ||||||||
| High School or Less | 108a | 42.0 | 44a | 40.0 | 10b | 14.7 | 162 | 37.2 | ||
| Some college | 115a | 44.7 | 49a | 44.5 | 13b | 19.1 | 177 | 40.7 | ||
| College degree or Higher | 34a | 13.2 | 17a | 15.5 | 45b | 66.2 | 96 | 22.1 | ||
| Employment | 18.59 | .01 | ||||||||
| Unemployed | 76a | 29.6 | 28a | 25.5 | 8b | 11.8 | 112 | 25.7 | ||
| Part-Time/Multiple Jobs | 66a,b | 25.7 | 39a | 35.5 | 14b | 20.6 | 119 | 27.4 | ||
| Full-Time | 115a | 44.7 | 43a | 39.1 | 46b | 67.6 | 204 | 46.9 | ||
| Annual Incomec | 72.32 | .00 | ||||||||
| < $10,000 | 110a | 43.8 | 23b | 21.3 | 9b | 13.2 | 142 | 33.3 | ||
| $10,000–$39,999 | 95a | 37.8 | 56b | 51.9 | 15c | 22.1 | 166 | 38.9 | ||
| > $40,000 | 46a | 18.3 | 29a | 26.9 | 44b | 64.7 | 119 | 27.9 | ||
| Income meets basic needs? | 57.07 | .00 | ||||||||
| Not enough | 160a | 62.3 | 53b | 48.2 | 14c | 20.6 | 227 | 52.2 | ||
| Enough | 89a | 34.6 | 48a,b | 43.6 | 37b | 54.4 | 174 | 40.0 | ||
| More than enough | 8a | 3.1 | 9b | 8.2 | 17c | 25.0 | 34 | 7.8 | ||
| M | SD | M | SD | M | SD | M | SD | F | p | |
| PTSD Symptoms | 7.69 | .00 | ||||||||
| PCL-5 Total Scored | 39.48a | 17.50 | 37.71a | 15.06 | 30.93b | 10.52 | 37.69 | 16.24 | ||
| n | % | n | % | n | % | n | % | χ2 | p | |
| 8.72 | .01 | |||||||||
| PCL-5 Total Score >33e | 144a | 56.0 | 58a,b | 52.7 | 24b | 35.8 | 226 | 52.1 | ||
| M | SD | M | SD | M | SD | M | SD | F | p | |
| Pain Catastrophizing | 9.99 | .00 | ||||||||
| PCS Total Scoref | 29.95a | 12.58 | 29.39a | 11.60 | 22.65b | 11.18 | 28.66 | 12.39 | ||
| Sleep | ||||||||||
| PROMIS Sleep Duration | 6.12 | 1.71 | 6.35 | 1.34 | 6.58 | 1.29 | 6.25 | 1.57 | 2.68 | n.s. |
| PROMIS Sleep Quality | 1.95a | 1.05 | 2.05a | .83 | 2.28b | .88 | 2.03 | .99 | 3.04 | .04 |
| Pain | ||||||||||
| Pain Intensity rating | 2.60a | 2.62 | 2.46a | 2.45 | 1.31b | 1.47 | 2.36 | 2.47 | 7.69 | .00 |
| PROMIS Pain Interference | 16.62a | 9.17 | 15.94a | 8.14 | 12.47b | 5.87 | 15.80 | 8.59 | 6.45 | .00 |
= each unique subscript letter denotes a race/ethnicity category whose column proportions differ significantly from each other at the p =.05 level.
= Income data missing for n = 7.
= (α=.93).
= a score greater than 33 is the clinical cutoff for a total score consistent with a PTSD diagnosis on the PCL-5.
= (α=.94).
= (α=.94).
Procedure
The following study procedures were approved by our institution’s Institutional Review Board. Study staff approached women at the ED with information about participating in a study about trauma and pain. For women who expressed interest in participating, study staff collected contact information and then conducted a brief telephone-screening interview within 72-hours of the ED visit to determine eligibility to participate in the study. Inclusion criteria were as follows: (1) female, (2) 18–40 years old, (3) premenopausal, (4) able to read and write English sufficiently to provide informed consent, and (5) present to our institution’s ED with an acute pain complaint of the chest, abdomen/pelvis, neck/shoulder, or back (i.e., not extremity or head pain). Exclusion criteria were as follows: (1) pain intensity or any injury or illness great enough to impair concentration or capacity to understand study instructions or the nature of being in the study, (2) current chronic illness that involved constant or frequent pain, (3) history of chronic pain on presentation in ED or documented in the Electronic Medical Record (EMR), (4) appearing intoxicated or under the influence of drugs at the ED visit, (5) self-reported or EMR-documented daily opiate use over the prior 3 months, or (6) the presenting ED pain complaint was due to a traumatic circumstance (e.g., a motor vehicle accident (MVA), physical assault, sexual assault, etc.). This latter exclusionary criterion was established in order to avoid the confounding effects of the presenting pain complaint and any reported PTSD symptoms being from the same event [e.g., a MVA survivor may have both pain and PTSD from the MVA, not because pain and PTSD impact each other].
Following completion of the telephone screening interview, eligible participants were scheduled for a baseline interview where they completed the informed consent. The baseline interview collected information on participants’ demographic characteristics, psychosocial functioning, pain intensity, pain-related interference, pain catastrophizing, current/past PTSD symptoms, and self-reported sleep characteristics. Participants were compensated for their time in the form of gift cards from a local retail store and could earn $150 for completion of all baseline measures.
Measures
Socioeconomic Status (SES)
Participant SES was measured via four demographic variables: highest education level, current employment status, current annual household income, and perception of current annual household income’s ability to meet basic needs. Highest education level was assessed with the question, “What is the highest grade of school you have completed?” Composition for highest education level was recoded for subsequent analyses as follows: (1) high school diploma/G.E.D. or less; (2) some college or technical school; and (3) college degree or higher. Current employment status was assessed with the question, “Which of these categories best describes your employment status: Full-time; Part-time; Working multiple jobs; Disability/SSI; Unemployed?” Composition for current employment status was recoded for subsequent analyses as follows: (1) unemployed or on disability/SSI; (2) part-time employment/multiple jobs; and (3) full-time employment. Current annual household income was assessed with the question, “Which category best represents your total household income before taxes: less than $10,000; $10,000-$29,999; $30,000-$39,999; $40,000-$49,999; $50,000-$59,999; $60,000-$69,999; $70,000-$79,999; greater than $80,000.” Composition for current employment status was recoded for subsequent analyses as follows: (1) <$10,000; (2) $10,000-$39,999; and (3) >$40,000. Composition of perception of income’s ability to meet basic needs was assessed with the following question “Do you consider your household income to be…:” (1) not enough to meet basic needs; (2) enough to meet basic needs; and (3) more than enough to meet basic needs. This latter measure was included as several past studies have found this to be a key indicator of SES (Shi & Stevens, 2005; Sachs-Ericsson et al., 2006; Nobles, Weintraub, & Adler, 2013; Präg, Mills, & Wittek, 2016).
Sleep Duration & Sleep Quality
Self-reported sleep duration and sleep quality were measured with two items from the PROMIS Sleep Disturbance Short-Form (Yu et al., 2011). Participants were asked to report the average number of hours they typically sleep per night and rate their average nightly sleep quality over the previous seven days on a scale from 1 (very poor) to 5 (very good). These items were developed using rigorous psychometric testing methods (i.e., Classical Test Theory, Item Response Theory), comprehensive literature reviews, qualitative item reviews, and focus groups and were found to have good face and construct validity as well as internal consistency (α = .95; Buysse et al., 2010).
Pain Catastrophizing
The total score from the 13-item Pain Catastrophizing Scale [(PCS); Sullivan, Bishop, & Pivik, 1995] was used to measure symptoms of pain catastrophizing. Respondents were asked to rate, from 0 (not at all) to 4 (all the time), the degree to which they had thoughts and feelings related to rumination, magnification, or helplessness when experiencing pain. Total scores can range from 0–52, with higher scores indicating greater catastrophic thinking. The measure has adequate reliability and validity [(α = .94); Osman et al., 2000].
PTSD Symptoms
The total score from the 20-item PTSD Checklist for DSM-5 [(PCL-5); Weather et al., 2013] was used to measure PTSD symptoms. Prior to administration of the PCL-5, participants were asked to reflect on their worst or most distressing trauma (i.e., their index trauma) and were then asked to rate the degree to which over the previous month, on a 1 (not at all) to 5 (extremely) scale, they had experienced PTSD symptoms related to re-experiencing, avoidance, hyperarousal, and negative alterations in cognition and mood. Total scores range from 20–100 with higher scores indicating more severe symptoms of PTSD. Scores above 33 are suggestive of a potential PTSD diagnosis. The measure has adequate reliability and validity [(α = .93); Blevins et al., 2015].
Pain Intensity
Pain intensity was measured on 11-point numeric rating scale (NRS-11; Farrar et al., 2001) of how much pain they were experiencing at that moment (0 = none at all – 10 = in extreme pain) in the same body area as presented to the ED. A systematic review of different clinical rating scales of pain intensity (Hjermstad et al., 2008) highlighted the superior reliability and face, convergent, divergent, and criterion-related validity of the NRS-11 when compared with visual analog and verbal pain rating scales.
Pain Interference
Pain interference was measured with the PROMIS 8-item short form scale (Amtmann et al., 2010) that asks respondents to rate, on a scale from 1 (not at all) to 5 (very much), how much their pain that initially brought them to the ED had interfered with their engagement in and enjoyment of daily work, home and social-related activities at the time of the interview. Total raw scores were converted into T-scores, with higher scores indicating higher pain-related interference. The measure has adequate reliability and validity [(α = .94); Broderick et al., 2013].
Data Analytic Strategy
Descriptive statistics and bivariate correlations were first examined to characterize our primary study variables. Structural equation modeling (SEM) was conducted with Mplus v.8.0 software (Muthen & Muthen, 2017) to examine the hypothesized direct and indirect relationships among our primary study variables (see Figures 1 and 2). In addition, SEM techniques afforded the opportunity to create and analyze a latent SES variable, which represents the common variance underlying our four observed SES variables: highest education level, current employment status, current annual household income, and perception of current annual household income’s ability to meet needs. Race/ethnicity was entered as a dichotomous variable in the SEM, where 1 = AA (n = 257, 59.1%) and 0 = Non-AA (n = 178, 40.9%). All other continuous variables in the model were measured at the indicator level as described above in the Measures section.
Bootstrapping techniques and maximum likelihood estimation were used in the estimation of our SEMs. Bootstrapping techniques provided 95% confidence intervals to determine the significance of indirect effects. Maximum likelihood estimation allowed all cases in the dataset to be analyzed, even those with missing data (of which < .03% were missing). Model fit was determined via several fit indices, including the root mean square error of approximation (RMSEA), the comparative fit index (CFI), and the standardized root mean square residual (SRMR). Adequate model fit was determined based on published recommendations (Marsh, Hau, & Wen, 2004) with RMSEA values < .06, CFI values > .95 and SRMR values < .09. In addition, prior to inclusion in the structural model, we evaluated the fit of our latent SES variable in a measurement model, which demonstrated good fit [(X2 (2) = 2.08, p = .35, CFI = .99, RMSEA = .01, 90% C.I. [.00, .09], SRMR = .01] and was retained in subsequent analyses.
RESULTS
Sample Characteristics
Table 1 provides descriptive statistics for demographic and primary study variables by race/ethnicity and for the total sample. Less than half (46.9%) of the total sample reported full-time employment outside the home and 37.2% reported obtaining a high school education or less. Just over a third (37.2%) reported an annual household income of less than $10,000 and over half (52.2%) described their annual income as not enough to meet their basic needs. Relative to AA and HL participants, NHW participants had significantly higher annual incomes, higher levels of education, higher levels of employment, and were more likely to report that their income was enough to meet their basic needs. NHW participants also had significantly lower- PCL-5 scores, PCS scores, pain intensity ratings, and pain interference ratings, and significantly higher sleep quality ratings than AA and HL participants. Just over half (52.1%) of the sample scored above the clinical cutoff that would be consistent with a PTSD diagnosis and significantly smaller proportion of NHW’s scored above the clinical cutoff for a suspect PTSD diagnosis as compared to AA participants (all p’s < .05). Table 2 provides bivariate correlations for the primary study variables. AA race was significantly correlated with all primary study variables.
Table 2.
Bivariate correlations among primary study variables.
| Study Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. African American | 1 | |||||||||||
| 2. White/Non-Hispanic | −.53** | 1 | ||||||||||
| 3. Hispanic/Latina | −.69** | −.24** | 1 | |||||||||
| 4. Education | −.25** | .39** | −.04 | 1 | ||||||||
| 5. Employment | −.12* | .17** | −.01 | .34** | 1 | |||||||
| 6. Annual Income | −.35** | .37** | .09 | .51** | .47** | 1 | ||||||
| 7. Income Meets Basic Needs | −.29** | .35** | .04 | .28** | .20** | .42** | 1 | |||||
| 8. PTSD | .13* | −.19** | .01 | −.22** | −.07 | −.12* | −.20** | 1 | ||||
| 9. Pain Catastrophizing | .12* | −.21** | .04 | −.18** | −.11* | −.15** | −.17** | .38** | 1 | |||
| 10. Sleep Quantity | −.15* | .13* | .07 | .03 | −.03 | −.06 | .09 | −.21** | −.13* | 1 | ||
| 11. Sleep Quality | −.13* | .14* | .04 | .06 | .01 | .01 | .11* | −.34** | −.19** | .61** | 1 | |
| 12. Pain Intensity | .13* | −.21** | .02 | −.12* | −.08 | −.14** | −.17** | .30** | .20** | −.16** | −.21** | 1 |
| 13. Pain Interference | .13* | −.21** | .02 | −.13* | −.08 | −.14** | −.14** | .33** | .31** | −.16** | −.26** | .70 |
Note. Significant bivariate correlations are bolded and denoted *p<.05 and **p < .01
Hypothesized Model #1 Fit and Results
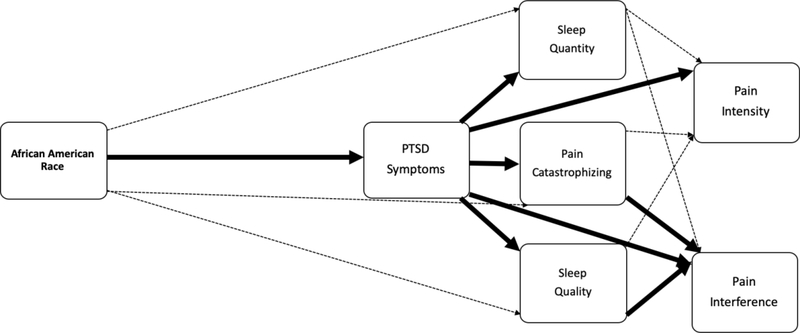
Model #1, in which SES variables were not included, demonstrated good fit [(X2 (7) = 8.67, p = .27, CFI = .99, RMSEA = .02, 90% C.I. [.00, .06], SRMR = .03; See Table 3 and Figure 3]. As hypothesized, there was a positive direct relationship between AA race and PCL-5 scores, the indicator of PTSD symptoms (β = .132). In addition, PCL-5 scores accounted for the direct relationships between: AA race and PCS scores (the indicator of Pain Catastrophizing), AA race and Sleep Quality, and AA race and Sleep Duration, respectively. Significant direct effects also indicated that PCL-5 scores were positively related to PCS scores (β = .399), and negatively related to Sleep Quality (β = −.297) and Sleep Duration ((β = −.181). Pain Interference scores, in turn, were positively related to PCL-5 scores (β = .187), PCS scores (β = .180), and negatively related to Sleep Quality (β = −.141). The only significant direct association with Pain Intensity was a positive relationship with PCL-5 scores (β = .196).
Table 3.
Standardized path coefficients of direct and indirect effects from Conceptual Model #1.
| Standardized Beta (SE) | Two-Tailed p-value | |
| Direct Effects | ||
| Effects on PCL-5 Scores | ||
| AA Race | .132 (.04) | .00 |
| Effects on Pain Catastrophizing | ||
| PTSD symptoms | .399 (.04) | .00 |
| AA Race | .073 (.05) | .12 |
| Effects on Sleep Quality ratings | ||
| PTSD symptoms | −.297 (.05) | .00 |
| AA Race | −.053 (.05) | .24 |
| Effects on Sleep Quantity | ||
| PTSD symptoms | −.181 (.05) | .00 |
| AA Race | −.078 (.05) | .10 |
| Effects on Pain Intensity | ||
| PTSD symptoms | .196 (.06) | .00 |
| Pain Catastrophizing | .081 (.05) | .09 |
| Sleep Quality | −.080 (.06) | .21 |
| Sleep Quantity | −.014 (.05) | .44 |
| Effects on Pain Interference | ||
| PTSD symptoms | .187 (.06) | .00 |
| Pain Catastrophizing | .180 (.05) | .00 |
| Sleep Quality | −.141 (.06) | .01 |
| Sleep Quantity | .014 (.05) | .79 |
| Correlation of Sleep Quality with Sleep Quantity | .559 (.04) | .00 |
| Correlation of Pain Intensity with Pain Interference | .661 (.03) | .00 |
| Standardized Beta (SE) | 95% Confidence Interval | |
| Indirect Effects | ||
| AA Race to Pain Intensity | ||
| via PTSD Symptoms | .026 (.01) | [.010, .048] |
| via PTSD Symptoms and Pain Catastrophizing | .004 (.01) | [.000, .010] |
| via PTSD Symptoms and Sleep Quality | .003 (.01) | [.000, .010] |
| via PTSD Symptoms and Sleep Quantity | .001 (.0) | [−.001, .005] |
| Total Indirect Effect | .034 (.01) | [.014, .059] |
| AA Race to Pain Interference | ||
| via PTSD Symptoms | .025 (.01) | [.009, .048] |
| via PTSD Symptoms and Pain Catastrophizing | .010 (.03) | [.004, .018] |
| via PTSD Symptoms and Sleep Quality | .006 (.01) | [.001, .013] |
| via PTSD Symptoms and Sleep Quantity | .001 (.01) | [−.003, .002] |
| Total Indirect Effect | .039 (.02) | [.016, .067] |
Note. Significant direct effects bolded when p <.05 and significant indirect effects bolded with 95%.C.I.
Figure 3.
Visual depiction of results from evaluation of Conceptual Model #1. Solid dark lines represent significant direct effects (p < .05) and dashed light lines represent non-significant direct paths (p >.05). Please refer to Table 3 for corresponding standardized path coefficients of direct and indirect effects.
Significant indirect effects were also observed, such that the indirect association of AA race on Pain Intensity ratings through PCL-5 scores was significant (β = .026), as was the indirect effect on Pain Interference scores through PCL-5 scores (β = .025). In addition, the indirect association of AA race on Pain Interference scores through PCL-5 scores and PCS scores was significant (β = .010) as was the indirect association through PCL-5 scores and Sleep Quality (β = .006). Collectively, these results suggested that race-related differences in pain intensity were explained by PCL-5 scores and differences in pain interference scores were explained by PCL-5 scores, PCS scores, and Sleep Quality. No significant direct or indirect effects through Sleep Duration on either pain outcome were observed.
Hypothesized Model #2 Fit and Results
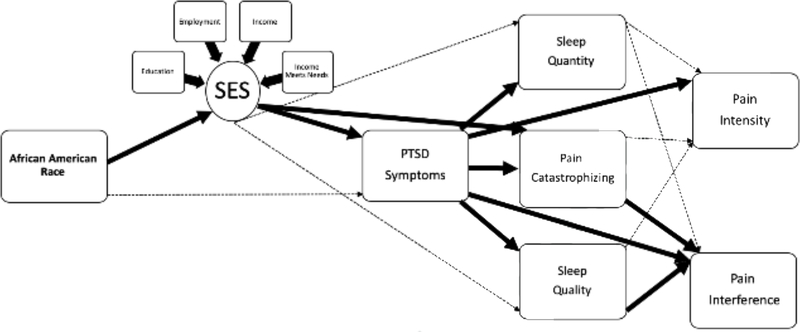
The second hypothesized model, in which the latent SES variable was included, demonstrated good fit [(X2 (30) = 57.13, p = .01, CFI = .98, RMSEA = .04, 90% C.I. [.02, .06], SRMR = .04; See Table 4 and Figure 4]. As expected, there were significant negative direct relationships between AA race and SES factors (β = −.368) and SES factors and PCL-5 scores (β = −.212). Consistent with our hypothesis, SES factors accounted for the direct relationship between AA race and PCL-5 scores. In addition, SES factors were negatively related to PCS scores (β = −.153), but not were not significantly related to Sleep Quality or Sleep Duration. Similar to Model #1, PCL-5 scores were positively related to PCS scores (β = .366), and negatively related to Sleep Quality (β = −.298) and Sleep Duration (β = −.194). Pain Interference scores, in turn, were positively related to PCL-5 scores (β = .187) and PCS scores (β = .180) and negatively related to Sleep Quality (β = −.141). The only significant direct association with Pain Intensity was a positive relationship with PCL-5 scores (β = .196).
Table 4.
Standardized path coefficients of direct and indirect effects from Conceptual Model #2.
| Standardized Beta (SE) | Two-Tailed p-value | |
| Latent SES Variable | ||
| Highest Education Level | .607 (.04) | .00 |
| Annual Household Income | .829 (.04) | .00 |
| Employment Status | .528 (.04) | .00 |
| Perception of Income Meeting Basic Needs | .511 (.05) | .00 |
| Direct Effects | ||
| Effects on SES Factors | ||
| AA Race | −.368 (.05) | .00 |
| Effects on PTSD Symptoms | ||
| SES Factors | −.212 (.05) | .00 |
| AA Race | .067 (.05) | .17 |
| Effects on Pain Catastrophizing | ||
| SES Factors | −.153 (.05) | .01 |
| PTSD symptoms | .366 (.04) | .00 |
| Effects on Sleep Quality | ||
| SES Factors | −.004 (.04) | .94 |
| PTSD symptoms | −.298 (.05) | .00 |
| Effects on Sleep Quantity | ||
| SES Factors | −.063 (.06) | .27 |
| PTSD Symptoms | −.194 (.05) | .00 |
| Effects on Pain Intensity | ||
| PTSD symptoms | .196 (.05) | .00 |
| Pain Catastrophizing | .081 (.05) | .08 |
| Sleep Quality | −.080 (.06) | .20 |
| Sleep Quantity | −.047 (.06) | .44 |
| Effects on Pain Interference | ||
| PTSD symptoms | .187 (.05) | .00 |
| Pain Catastrophizing | .180 (.05) | .01 |
| Sleep Quality | −.141 (.06) | .01 |
| Sleep Quantity | .014 (.06) | .79 |
| Correlation of Sleep Quality with Sleep Quantity | .550 (.04) | .00 |
| Correlation of Pain Intensity with Pain Interference | .661 (.03) | .00 |
| Standardized Beta (SE) | 95% Confidence Interval | |
| Indirect Effects | ||
| AA Race to Pain Intensity | ||
| via SES Factors and PTSD Symptoms | .015 (.01) | [.006, .029] |
| via SES Factors and Pain Catastrophizing | .005 (.01) | [.000, .012] |
| via SES Factors, PTSD Symptoms, and Pain Catastrophizing | .002 (.01) | [.000, .006] |
| via SES Factors and Sleep Quality | .000 (.01) | [−.004, .003] |
| via SES Factors, PTSD Symptoms, and Sleep Quality | .002 (.01) | [.000, .005] |
| via SES Factors and Sleep Quantity | −.001 (.01) | [−.006, .001] |
| via SES Factors, PTSD Symptoms, and Sleep Quantity | .001 (.01) | [−.001, .003] |
| Total Indirect Effect | .023 (.01) | [.010, .041] |
| AA Race to Pain Interference | ||
| via SES Factors and PTSD Symptoms | .015 (.01) | [.006, .028] |
| via SES Factors and Pain Catastrophizing | .010 (.01) | [.003, .021] |
| via SES Factors, PTSD Symptoms, and Pain Catastrophizing | .005 (.01) | [.002, .010] |
| via SES Factors and Sleep Quality | .000 (.01) | [−.005, .005] |
| via SES Factors, PTSD Symptoms, and Sleep Quality | .003 (.01) | [.001, .007] |
| via SES Factors and Sleep Quantity | .000 (.01) | [−.001, .005] |
| via SES Factors, PTSD Symptoms, and Sleep Quantity | .000 (.01) | [−.002, .001] |
| Total Indirect Effect | .033 (.01) | [.016, .054] |
Note. Significant direct effects bolded when p <.05 and significant indirect effects bolded with 95%.C.I.
Figure 4.
Visual depiction of results from evalution of Conceptual Model #2. Solid dark lines represent significant direct effects (p < .05) and dashed light lines represent non-significant direct paths (p >.05). Please refer to Table 4 for corresponding standardized path coefficients of direct and indirect effects.
Significant indirect effects were found, such that the indirect association of AA race on Pain Intensity through SES factors and PCL-5 scores was significant (β = .015). In addition, a number of significant indirect effects of AA race on Pain Interference scores were observed: 1) through SES factors and PCL-5 scores (β = .015); 2) through SES factors and PCS scores (β = .010); 3) through SES factors, PCL-5 scores, and PCS scores (β = .005); and 4) through SES factors, PCL-5 scores, and Sleep Quality (β = .0003). Collectively, these results suggest that SES factors accounted for race-related differences in Pain Intensity ratings through PCL-5 scores and race-related differences in Pain Interference scores through PCL-5 scores, PCS scores, and Sleep Quality. As with Model #1, no significant direct or indirect effects through Sleep Duration on either pain outcome were observed.
DISCUSSION
Previous research has shown that AAs report worse pain intensity and pain-related interference than other racial/ethnic groups as well as greater levels of risk factors commonly associated with poor pain outcomes, including higher PTSD symptoms and pain catastrophizing, shorter sleep duration, and lower sleep quality. From the perspective of COR theory (Hobfoll, 1989), we sought to explore whether SES factors explained AA-race-related differences in self-reported pain outcomes (i.e., pain intensity and pain interference) and known risk factors associated with worse self-reported pain outcomes (i.e., greater PTSD symptoms and pain catastrophizing, short sleep duration, and low sleep quality).
Consistent with past findings (Rahim‐Williams et al., 2012; Booker, 2016; Ostom et al., 2017), we found AA women to report higher pain intensity and pain-related interference than non-AA women. As expected, these differences were mediated by AA women’s higher PTSD symptoms and pain catastrophizing symptoms and lower sleep quality. In partial contrast to our original hypotheses, differences in AA women’s pain intensity and pain interference were not accounted for by differences in their sleep duration. In examination of our model with the inclusion of SES factors, we found that SES factors accounted for the mediation relationship linking AA race to pain intensity via PTSD symptoms and the mediation relationship linking AA race to pain interference via PTSD symptoms, pain catastrophizing, and sleep quality.
Our findings indicate that SES factors may play a role in explaining racial differences in risk factors associated with worse pain outcomes. To our knowledge, our study is the first to show that SES factors largely account for the relationship between AA race and PTSD symptoms. This is important given that previous research often highlights the increased severity in reported PTSD symptoms among AAs when compared to other racial/ethnic groups, but does not further contextualize these findings in terms socioeconomic differences among racial/ethnic minorities. As such, viewed through the lens of COR theory (Hobfoll, 1989), this finding may indicate that socioeconomically disadvantaged AAs may lack the resources needed to effectively recover from an acute stressor and, thus, are more vulnerable to developing PTSD.
Our results also indicated that SES factors explained the relationship linking AA race to higher pain intensity and pain inference, in part, via their association with higher PTSD symptoms. Given that low SES neighborhoods in Chicago have a high incidence of violent crime, it is possible that our participants of low SES were likely to experience greater exposure and threatened exposure to traumatic events, which may result in more severe symptoms of PTSD. The increased vigilance and attentional biases towards further threats of violent crime may, in turn, amplify pain intensity and efforts to cope effectively with pain (i.e., increase pain-related impairment; Geisser, et al., 1996; Jenewein et al, 2009; Sherman et al., 2000; Phifer et al., 2011). Importantly, although we did not measure neighborhood-level SES characteristics in the current study, the ED from which we recruited participants primarily serves the historically racially-segregated south and west-side neighborhoods of Chicago, where the annual rate of violent crime is nearly three times higher than the national average (Bureau of Justice Statistics, 2015; Berman, 2017) and the median household income is below $10,000 (Census Reporter, 2016).
Finally, previous studies have found that racial disparities in self-reported sleep quality and sleep duration are at least partly accounted for by SES factors (Stamatakis, Kaplan, & Roberts, 2007; Hale & Do., 2007; Grandner et al., 2010). For the most part, our sleep-related hypotheses were unsupported. SES factors were only found to partially account for the relationship between AA race and pain interference through a small indirect association with higher sleep quality (via PTSD). This finding may reveal more about the influence of PTSD symptoms on the relationship between sleep quality and pain interference than influences specific to socioeconomic factors. Indeed, our initial model (without SES factors) showed a slightly larger indirect effect of sleep quality on the relationship between AA race and pain interference (via PTSD).
In addition, we observed no significant direct or indirect effects through sleep quality on pain outcomes nor did we observe and significant direct or indirect links between SES factors and sleep duration. Although our study is not the first to demonstrate a lack of relationship between SES factors and self-reported sleep duration (Okun, Tolge, & Hall, 2014), our descriptive data paint a picture of relatively short self-reported sleep durations across racial/ethnic groups (Range Average hrs/night = 6.12 – 6.58). Consistent with the number of non-significant bivariate correlations between sleep duration and our primary study variables, there may not be enough variability in our sample’s self-reported sleep duration to pick up on differences in pain outcomes or unique associations with SES variables.
At the same time, it is also possible that the indicators of SES selected for this study (i.e., income, education, employment, perception of income meeting basic needs) were inadequate to explain these specific differences in self-reported sleep duration and sleep quality. It may be that factors related to lower SES in the urban context, like indices of household crowding (Rona et al., 1998) and nighttime exposure to light and neighborhood noise (Hill, Burdette, & Hale, 2009; Casey et al., 2017) better account for the relationship between race and sleep characteristics reported here.
A major strength of this study is the contextualization of race-related difference in pain risk-factors and pain outcomes with concurrent SES associations. Previous studies have reported independent effects of race on pain even after controlling for SES factors (Plesh, Crawford, & Gansky, 2002; Day & Thorn, 2010; Green & Hart-Johnson, 2012) while others have found mediational effects of SES similar to the ones presented here (Urwin et al., 1998; Elliot et al., 1999; Portenoy et al., 2004; Schneider et al., 2005; Fuentes, Hart-Johnson, & Green, 2007). Although there is no agreed upon “gold standard” measurement of SES in the literature (Shavers, 2007), a major strength of the current study is the use of multiple indicators of SES measured at the individual level, including perception of income meeting basic needs. Previous studies (Shi & Stevens, 2005; Sachs-Ericsson et al., 2006; Nobles, Weintraub, & Adler, 2013; Präg, Mills, & Wittek, 2016) have highlighted the value of measuring individual perception of SES and resource deficits alongside more traditional indicators of SES (i.e., annual income, education history) in predicting a variety of health and functional disability outcomes. At the same time, given the numerous ways in which SES can and has been measured, it is possible that our results could be different had we used different or additional indicators of SES.
A number of important limitations in the current study should be noted. Methodologically, the cross-sectional and self-reported nature of our data precludes drawing causal inferences from our findings. In addition, mediation, as described in the current study, was in the statistical sense only; replication with prospective measurement of the mediators and outcomes would be needed in order to validate these models. Along these lines, the conclusions of our study can only speak to associations of our primary study variables with acute pain processes, as we are not yet able to investigate whether and how our variables of interest relate to the transition from acute to chronic pain. From a study sample perspective, although a major strength of our study is its representation of low-income, inner-city women, this sample representation may also limit the generalizability of our findings to men, in general, women who reside outside low-income, inner-city environments, and individuals with acute pain complaints who did not present to the ED. Similarly, as our sample was restricted to pre-menopausal-age women (18–40), our results could differ with the inclusion of post-menopausal women, given known age-related changes in the frequency and intensity of pain-related complaints (Braden et al., 2012; El Tumi et al, 2017), age-related changes in sleep quality and quantity (Krystal et al., 1998), and age-related differences in financial stability and/or educational attainment.
Finally, a key limitation of our study is that we could not address the systemic effects of institutional racism that likely drive the relationships observed between low SES and poor pain outcomes among AAs in our sample. For example, we did not include a measurement of participants’ neighborhood-level health resources (i.e., distance from medical facilities, availability of pharmacies, access to fresh food, pollution index, etc.), which would be influenced by institutional policies and practices like residential segregation. Given Chicago’s long history of residential segregation that largely persists today (i.e., 13th most racially segregated city in the United States; Chicago Tribune, 2019), our participants’ health outcomes may have differed as a function of their disproportionate neighborhood access to health-related resources. Along these lines, an additional byproduct of institutional racism is the well-documented non-equivalence of SES indicators among racial/ethnic minorities, which we did not adjust for in our analyses. Specifically, AAs have been shown to receive less income at the same education levels as Whites and accumulate less wealth at equivalent income levels to Whites (Williams, Mohammed, et al., 2010). In this way, although we found low SES to explain some of the associations between AA race and worse pain-related risk factors and pain outcomes, we would not necessarily expect incremental gains in SES to result in commensurate improvements in AA’s risk-factor profiles or pain outcomes.
This study has important clinical implications. Foremost among them is the finding that SES factors largely explained associations among AA race, higher PTSD symptoms, higher pain catastrophizing, and worse subjective pain outcomes. These findings underscore the role of economic inequality in persistent health disparities facing racial/ethnic minorities and emphasize the need for population-level interventions improves access to health-related resources for racial/ethnic minorities and low-income populations. Importantly, many interventions aimed at improving population health actually widen the disparity between advantaged and disadvantaged groups because advantaged groups start at a higher level of health than disadvantaged groups and, thus, stand to reap greater benefits from these interventions (Mechanic, 2002). Accordingly, any population-level intervention that proposes to improve access to health-related resources for marginalized groups should be aimed specifically at marginalized groups and accelerate improvement in health outcomes more rapidly than for the rest of the population (Williams, Priest, & Anderson, 2016).
In addition, our findings also extend the literature on the impact of PTSD symptoms, pain catastrophizing, and poor sleep on subjective pain complaints by demonstrating these associations in a historically understudied population of racially diverse, low-income women. In addition to improving equity in access to medical resources and treatment among economically disadvantaged groups and racial/ethnic minorities, these findings may also indicate the potential utility of prophylactic, cognitive-behavioral interventions for PTSD symptoms, pain catastrophizing and poor sleep (e.g., Pigeon et al., 2012). Such an approach may reduce the likelihood of pain problems persisting, which could obviate treatment escalation to consideration of pharmacological pain management strategies with known risk profiles (i.e., opioid-based medications).
In summary, our findings highlight the critical role of social inequality in explaining persistent racial disparities in subjective pain complaints. These data provide an important perspective on the health-related burden affecting economically disadvantaged AA women and underscore the importance of assessing concurrent socioeconomic factors in race/ethnicity-related health research.
Acknowledgments
This study was funded by the National Institute of Drug Abuse (NIDA; R01DA039522).
References
- Aaron R, Noel M, Dudney J, Wilson A, Holley A, & Palermo T (2019). The role of sleep quality on the relationship between posstraumatic stress symptoms and pain in women. Journal of Behavioral Medicine, 10.1007/s10865-019-00016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affleck G, Urrows S, Tennen H, Higgins P, & Abeles M (1996). Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain, 68(2–3), 363–368. [DOI] [PubMed] [Google Scholar]
- Aldrich S, Eccleston C, Crombez G. Worrying about chronic pain: vigilance to threat and misdirected problem solving. Behaviour research and therapy. 2000. May 1;38(5):457–70. [DOI] [PubMed] [Google Scholar]
- Alim TN, Charney DS, Mellman TA. An overview of posttraumatic stress disorder in African Americans. J Clin Psychol. 2006;62(7):801–813. [DOI] [PubMed] [Google Scholar]
- Alschuler KN, & Otis JD (2012). Coping strategies and beliefs about pain in veterans with comorbid chronic pain and significant levels of posttraumatic stress disorder symptoms. European Journal of Pain, 16(2), 312–319. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Amtmann D, Cook KF, Jensen MP, Chen WH, Choi S, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L, Lai JS. Development of a PROMIS item bank to measure pain interference. Pain. 2010. July 31;150(1):173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassari AR, Cleveland RJ, Luong ML, Jonas BL, Conn DL, Moreland LW, Bridges SL, Callahan LF. Socioeconomic factors and self-reported health outcomes in African Americans with rheumatoid arthritis from the Southeastern United States: the contribution of childhood socioeconomic status. BMC musculoskeletal disorders. 2016. January 12;17(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basner MB, Fomberstein KM, Razavi FM, et al. (2007). American Time Use Survey: Sleep time and its relationship to waking activities. Sleep, 30:1085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JG, Clapp JD. A different kind of co-morbidity: understanding posttraumatic stress disorder and chronic pain. Psychol Trauma. 2011;3(2):101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M “With a push from Chicago, violent crime in U.S. rose in 2016 for second straight year.” Washington Post; Retrieved 10 April 2018 http://www.chicagotribune.com/news/local/breaking/ct-violent-crime-fbi-statistics-20170925-story.html [Google Scholar]
- Bigatti SM, Hernandez AM, Cronan TA, & Rand KL (2008). Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Care & Research: Official Journal of the American College of Rheumatology, 59(7), 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The posttraumatic stress disorder checklist for DSM‐5 (PCL‐5): Development and initial psychometric evaluation. Journal of Traumatic Stress. 2015. December 1;28(6):489–98. [DOI] [PubMed] [Google Scholar]
- Booker SQ. African Americans’ perceptions of pain and pain management: A systematic review. Journal of Transcultural Nursing. 2016. January;27(1):73–80. [DOI] [PubMed] [Google Scholar]
- Braden JB, Young A, Sullivan MD, Walitt B, LaCroix AZ, & Martin L (2012). Predictors of change in pain and physical functioning among post-menopausal women with recurrent pain conditions in the women’s health initiative observational cohort. The Journal of Pain, 13(1), 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brekke M, Hjortdahl P, Kvien TK. Severity of musculoskeletal pain: relations to socioeconomic inequality. Social science & medicine. 2002. January 31;54(2):221–8. [DOI] [PubMed] [Google Scholar]
- Brennstuhl MJ, Tarquinio C, Montel S. Chronic pain and PTSD: evolving views on their comorbidity. Perspectives in psychiatric care. 2015. October 1;51(4):295–304. [DOI] [PubMed] [Google Scholar]
- Broderick JE, Schneider S, Junghaenel DU, Schwartz JE, Stone AA. Validity and Reliability of Patient‐Reported Outcomes Measurement Information System Instruments in Osteoarthritis. Arthritis care & research. 2013. October 1;65(10):1625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Justice Statistics, Offenses Known to Law Enforcement by City, 2015. Retrieved 10 April 2018 https://ucr.fbi.gov/crime-in-the-u.s/2015/crime-in-the-u.s.-2015/tables/table-8/table-8-state-pieces/table_8_offenses_known_to_law_enforcement_illinois_by_city_2015.xls
- Burns JW, Kubilus A, Bruehl S, Harden RN, Lofland K. Do changes in cognitive factors influence outcome following multidisciplinary treatment for chronic pain? a cross-lagged panel analysis. J Consult Clin Psych. 2003;71(1):81–91. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Yu L, Moul DE, Germain A, Stover A, Dodds NE and Pilkonis PA 2010. development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairment. Sleep, 33: 781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Mayo A, Ventimiglia M. Coping, pain severity, interference, and disability: the potential mediating and moderating roles of race and education. The Journal of Pain. 2006. July 31;7(7):459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty J, O’Donnell M, Evans L, Kazantzis N, Creamer M. Predicting posttraumatic stress disorder symptoms and pain intensity following severe injury: The role of catastrophizing. European Journal of Psychotraumatology. 2011. January 1;2(1):5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JA, Morello-Frosch R, Mennitt DJ, Fristrup K, Ogburn EL, James P. Race/Ethnicity, Socioeconomic Status, Residential Segregation, and Spatial Variation in Noise Exposure in the Contiguous United States. Environmental Health Perspectives. 2017. July 25;77017:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Census Reporter, Median Household Income, Census Tract 3504, Cook, IL, 2016. Retrieved April 10th, 2018 https://censusreporter.org/profiles/14000US17031350400-census-tract-3504-cook-il/
- Chan MY, Hamamura T, Janschewitz K. Ethnic differences in physical pain sensitivity: Role of acculturation. PAIN®. 2013. January 31;154(1):119–23. [DOI] [PubMed] [Google Scholar]
- Chicago Tribune. (2019). Retrieved April 20, 2019: https://www.chicagotribune.com/classified/realestate/ct-re-0603-housing-segregation-20180525-story.html
- Cook SW, Aten JD, Moore M, Hook JN, & Davis DE (2013). Resource loss, religiousness, health, and posttraumatic growth following Hurricane Katrina. Mental Health, Religion & Culture, 16(4), 352–366. [Google Scholar]
- Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. Morbidity and Mortality Weekly Report. 2018. September 14;67(36):1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KA, Silman AJ, Macfarlane GJ, Nicholl BI, Dickens C, Morriss R, Ray D, McBeth J. The association between neighbourhood socio‐economic status and the onset of chronic widespread pain: Results from the EPIFUND study. European Journal of Pain. 2009. July 1;13(6):635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day MA, Thorn BE. The relationship of demographic and psychosocial variables to pain-related outcomes in a rural chronic pain population. PAIN®. 2010. November 30;151(2):467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CL, Fillingim RB, Keefe F. Race, ethnicity and pain. Pain. 2001. November 30;94(2):133–7. [DOI] [PubMed] [Google Scholar]
- El Tumi H, Johnson MI, Dantas PBF, Maynard MJ, & Tashani OA (2017). Age‐related changes in pain sensitivity in healthy humans: A systematic review with meta-analysis. European Journal of Pain, 21(6), 955–964. [DOI] [PubMed] [Google Scholar]
- Elliott AM, Smith BH, Penny KI, Smith WC, Chambers WA. The epidemiology of chronic pain in the community. The lancet. 1999. October 9;354(9186):1248–52. [DOI] [PubMed] [Google Scholar]
- Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001. November 30;94(2):149–58. [DOI] [PubMed] [Google Scholar]
- Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. The Journal of Pain. 2013. December 1;14(12):1539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe LP, Thorn B, Day M, Shelby G. Race and sex differences in primary appraisals, catastrophizing, and experimental pain outcomes. The Journal of Pain. 2011. May 31;12(5):563–72. [DOI] [PubMed] [Google Scholar]
- Fuentes M, Hart-Johnson T, Green CR. The association among neighborhood socioeconomic status, race and chronic pain in black and white older adults. Journal of the National Medical Association. 2007. October;99(10):1160. [PMC free article] [PubMed] [Google Scholar]
- Geisser ME, Roth RS, Bachman JE, & Eckert TA (1996). The relationship between symptoms of post-traumatic stress disorder and pain, affective disturbance and disability among patients with accident and non-accident related pain. PAIN®, 66(2–3), 207–214. [DOI] [PubMed] [Google Scholar]
- Grandner MA, Patel NP, Gehrman PR, Xie D, Sha D, Weaver T, Gooneratne N. Who gets the best sleep? Ethnic and socioeconomic factors related to sleep complaints. Sleep medicine. 2010. May 31;11(5):470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CR, Hart-Johnson T. The association between race and neighborhood socioeconomic status in younger Black and White adults with chronic pain. The Journal of Pain. 2012. February 29;13(2):176–86. [DOI] [PubMed] [Google Scholar]
- Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007. September 1;30(9):1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale L, Hill TD, Friedman E, Nieto FJ, Galvao LW, Engelman CD, … & Peppard PE (2013). Perceived neighborhood quality, sleep quality, and health status: evidence from the Survey of the Health of Wisconsin. Social Science & Medicine, 79, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TD, Burdette AM, Hale L. Neighborhood disorder, sleep quality, and psychological distress: testing a model of structural amplification. Health & place. 2009. December 31;15(4):1006–13. [DOI] [PubMed] [Google Scholar]
- Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, … & European Palliative Care Research Collaborative (EPCRC. (2011). Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. Journal of pain and symptom management, 41(6), 1073–1093. [DOI] [PubMed] [Google Scholar]
- Hobfoll SE (1989). Conservation of resources: A new attempt at conceptualizing stress. American psychologist, 44(3), 513. [DOI] [PubMed] [Google Scholar]
- Holley AL, Wilson AC, Noel M, & Palermo TM (2016). Post‐traumatic stress symptoms in children and adolescents with chronic pain: A topical review of the literature and a proposed framework for future research. European journal of pain, 20(9), 1371–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou WK, Law CC, Yin J, & Fu YT (2010). Resource loss, resource gain, and psychological resilience and dysfunction following cancer diagnosis: a growth mixture modeling approach. Health Psychology, 29(5), 484. [DOI] [PubMed] [Google Scholar]
- Jenewein J, Wittmann L, Moergeli H, Creutzig J, & Schnyder U (2009). Mutual influence of posttraumatic stress disorder symptoms and chronic pain among injured accident survivors: a longitudinal study. Journal of Traumatic Stress: Official Publication of The International Society for Traumatic Stress Studies, 22(6), 540–548. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Brown DL, Morgenstern LB, Meurer WJ, & Lisabeth LD (2015). The association of neighborhood characteristics with sleep duration and daytime sleepiness. Sleep Health, 1(3), 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Yang GS, Greenspan JD, Downton KD, Griffith KA, Renn CL, Johantgen M, Dorsey SG. Racial and ethnic differences in experimental pain sensitivity: systematic review and meta-analysis. Pain. 2017. February 1;158(2):194–211. [DOI] [PubMed] [Google Scholar]
- Koffel E, Kroenke K, Bair MJ, Leverty D, Polusny MA, & Krebs EE (2016). The bidirectional relationship between sleep complaints and pain: Analysis of data from a randomized trial. Health Psychology, 35(1), 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. American journal of epidemiology. 2009. March 18;169(9):1052–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal AD, Edinger J, Wohlgemuth W, & Marsh GR (1998). Sleep in peri-menopausal and post-menopausal women. Sleep medicine reviews, 2(4), 243–253. [DOI] [PubMed] [Google Scholar]
- Lillis TA, Gerhart J, Bouchard LC, Cvengros J, O’mahony S, Kopkash K, … & Burns J (2018). Sleep disturbance mediates the association of post-traumatic stress disorder symptoms and pain in patients with cancer. American Journal of Hospice and Palliative Medicine®, 35(5), 788–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis TA, Burns J, Aranda F, Purim-Shem-Tov YA, Bruehl S, Beckham JC, & Hobfoll SE (2018). PTSD Symptoms and Acute Pain in the Emergency Department. The Clinical journal of pain, 34(11), 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhaupt SE, Tak S, & Calvert GM (2010). The prevalence of short sleep duration by industry and occupation in the National Health Interview Survey. Sleep, 33(2), 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh HW, Hau KT, Wen Z. In search of golden rules: Comment on hypothesis-testing approaches to setting cutoff values for fit indexes and dangers in overgeneralizing Hu and Bentler’s (1999) findings. Structural equation modeling. 2004. July 1;11(3):320–41. [Google Scholar]
- McLaughlin KA, Alvarez K, Fillbrunn M, Green JG, Jackson JS, Kessler RC, … & Alegría M (2018). Racial/ethnic variation in trauma-related psychopathology in the United States: a population-based study. Psychological medicine, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanic D (2002). Disadvantage, inequality, and social policy. Health Affairs, 21(2), 48–59. [DOI] [PubMed] [Google Scholar]
- Meints SM, Miller MM, & Hirsh AT (2016). Differences in pain coping between black and white Americans: a meta-analysis. The Journal of pain, 17(6), 642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus. Statistical analysis with latent variables. Version 8, 2017. [Google Scholar]
- Nobles J, Weintraub MR, Adler NE. Subjective socioeconomic status and health: relationships reconsidered. Social Science & Medicine. 2013. April 30;82:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Tolge M, & Hall M (2014). Low socioeconomic status negatively affects sleep in pregnant women. Journal of Obstetric, Gynecologic & Neonatal Nursing, 43(2), 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. Journal of behavioral medicine. 2000. August 1;23(4):351–65. [DOI] [PubMed] [Google Scholar]
- Ostrom C, Bair E, Maixner W, Dubner R, Fillingim RB, Ohrbach R, Slade GD, et al. (2017). Demographic predictors of pain sensitivity: Results from the OPPERA study. The Journal of Pain, 18, 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JD, Keane TM, Kerns RD. An examination of the relationship between chronic pain and post-traumatic stress disorder. Journal of rehabilitation research and development. 2003. September 1;40(5):397–406. [DOI] [PubMed] [Google Scholar]
- Phifer J, Skelton K, Weiss T, Schwartz AC, Wingo A, Gillespie CF, … & Ressler KJ (2011). Pain symptomatology and pain medication use in civilian PTSD. PAIN®, 152(10), 2233–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigeon WR, Moynihan J, Matteson-Rusby S, Jungquist CR, Xia Y, Tu X, Perlis ML. Comparative effectiveness of CBT interventions for co-morbid chronic pain & insomnia: a pilot study. Behaviour research and therapy. 2012. November 30;50(11):685–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesh O, Crawford PB, Gansky SA. Chronic pain in a biracial population of young women. Pain. 2002. October 31;99(3):515–23. [DOI] [PubMed] [Google Scholar]
- Poleshuck EL, Green CR. Socioeconomic disadvantage and pain. Pain. 2008. June;136(3):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portenoy RK, Ugarte C, Fuller I, Haas G. Population-based survey of pain in the United States: differences among white, African American, and Hispanic subjects. The Journal of Pain. 2004. August 31;5(6):317–28. [DOI] [PubMed] [Google Scholar]
- Präg P, Mills MC, Wittek R. Subjective socioeconomic status and health in cross-national comparison. Social Science & Medicine. 2016. January 31;149:84–92. [DOI] [PubMed] [Google Scholar]
- Rahim‐Williams B, Riley JL, Williams AK, Fillingim RB. A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter?. Pain Medicine. 2012. April 1;13(4):522–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond I, Nielsen TA, Lavigne G, Manzini C, & Choinière M (2001). Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. PAIN®, 92(3), 381–388. [DOI] [PubMed] [Google Scholar]
- Riskowski JL. Associations of socioeconomic position and pain prevalence in the United States: findings from the National Health and Nutrition Examination Survey. Pain Medicine. 2014. September 1;15(9):1508–21. [DOI] [PubMed] [Google Scholar]
- Roberts AL, Gilman SE, Breslau J, Breslau N, Koenen KC. Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychological medicine. 2011. January;41(1):71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rona RJ, Li L, Gulliford MC, Chinn S. Disturbed sleep: effects of sociocultural factors and illness. Archives of disease in childhood. 1998. January 1;78(1):20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CL, & Siebens J (2012). Educational Attainment in the United States: 2009 Population Characteristics. Current Population Reports. P20–566. US Census Bureau. [Google Scholar]
- Sachs-Ericsson N, Schatschneider C, Blazer DG. Perception of unmet basic needs as a predictor of physical functioning among community-dwelling older adults. Journal of aging and health. 2006. December;18(6):852–68. [DOI] [PubMed] [Google Scholar]
- Schneider S, Schmitt H, Zoller S, Schiltenwolf M. Workplace stress, lifestyle and social factors as correlates of back pain: a representative study of the German working population. International archives of occupational and environmental health. 2005. May 1;78(4):253–69. [DOI] [PubMed] [Google Scholar]
- Schumm JA, Hobfoll SE, Keogh NJ. Revictimization and interpersonal resource loss predicts PTSD among women in substance-use treatment. J Trauma Stress. 2004;17:173–181. [DOI] [PubMed] [Google Scholar]
- Sharp TJ, & Harvey AG (2001). Chronic pain and posttraumatic stress disorder: mutual maintenance?. Clinical psychology review, 21(6), 857–877. [DOI] [PubMed] [Google Scholar]
- Shavers VL. Measurement of socioeconomic status in health disparities research. Journal of the national medical association. 2007. September;99(9):1013. [PMC free article] [PubMed] [Google Scholar]
- Sherman JJ, Turk DC, & Okifuji A (2000). Prevalence and impact of posttraumatic stress disorder-like symptoms on patients with fibromyalgia syndrome. The Clinical journal of pain, 16(2), 127–134. [DOI] [PubMed] [Google Scholar]
- Shi L, Stevens GD. Vulnerability and unmet health care needs. Journal of general internal medicine. 2005. February 1;20(2):148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep medicine reviews. 2004. April 30;8(2):119–32. [DOI] [PubMed] [Google Scholar]
- Stamatakis KA, Kaplan GA, Roberts RE. Short sleep duration across income, education, and race/ethnic groups: population prevalence and growing disparities during 34 years of follow-up. Annals of epidemiology. 2007. December 31;17(12):948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingrímsdóttir ÓA, Landmark T, Macfarlane GJ, & Nielsen CS (2017). Defining chronic pain in epidemiological studies: a systematic review and meta-analysis. Pain, 158(11), 2092–2107. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychological assessment. 1995. December;7(4):524. [Google Scholar]
- Urwin M, Symmons D, Allison T, Brammah T, Busby H, Roxby M, Simmons A, Williams G. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Annals of the rheumatic diseases. 1998. November 1;57(11):649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau, American Community Survey, 2017. Retrieved December 11, 2019, from: https://www.census.gov/acs/www/data/data-tables-and-tools/data-profiles/.
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The ptsd checklist for dsm-5 (pcl-5). Scale available from the National Center for PTSD; at www.ptsd.va.gov.2013. [Google Scholar]
- Williams DR, Mohammed SA, Leavell J, & Collins C (2010). Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Annals of the New York Academy of Sciences, 1186(1), 69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Priest N, & Anderson NB (2016). Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychology, 35(4), 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Buysse DJ, Germain A, et al. Development of Short Forms from the PROMIS Sleep Disturbance and Sleep-Related Impairment Item Banks. Behavioral Sleep Medicine. 2011;10(1):6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]