Abstract
Background:
Many older adults receive caregiving; however, less is known about how a change in a care recipient’s functional activity limitations (instrumental activities of daily living (IADL) and basic activities of daily living (ADL)) as well as their cognitive impairment influence the amount of caregiving received.
Methods:
Using the Health and Retirement Study (2002–2014) we identified community-dwelling respondents with Alzheimer’s disease and related dementias (ADRD; n=674), cognitive impairment no dementia (CIND; n=530), and no cognitive impairment (n=6,126). We estimated a series of two-part regression models to identify the association between care recipients’ level of cognitive impairment, change in total number of IADL/ADL limitations and amount of caregiving received.
Results:
Persons with ADRD received 235.8 (SD=265.6) monthly hours of care compared to 26.0 (SD=92.6) and 6.0 (SD=40.7) for persons with CIND and no cognitive impairment, respectively. An increase in one IADL/ADL limitation resulted in persons with ADRD and CIND receiving 4.90 (95%CI: 3.40–6.39) and 1.43 (95%CI: 0.17–2.69) more hours of caregiving than persons with no cognitive impairment. Increases in total IADL/ADL limitations were associated with persons with ADRD, but not CIND, receiving more days of caregiving and having more caregivers than persons with no cognitive impairment.
Conclusions:
Compared to persons with no cognitive impairment, increases in IADL/ADL limitations disproportionally increases the caregiving received for persons with ADRD. Policies and programs must pay attention to functional impairments among those living with ADRD.
Keywords: functional activity, dementia, long-term care and support, caregiving
INTRODUCTION
More than 7 million community-dwelling older adults receive care for instrumental activities of daily living (IADL; e.g., managing finances) and basic activities of daily living (ADL; e.g., help with dressing) from 14 million family and friends.1 Many of these older adults have Alzheimer’s disease and related dementias (ADRD).2,3 Although older adults desire to age in the community,4,5 helping individuals with ADRD and/or IADL/ADL limitations successfully live in the community can be costly for families.4,6
Among older adults that receive family caregiving, on average persons with ADRD receive more caregiving than older adults without ADRD.7–9 However, there are limited data on how a change in IADL/ADL limitations among persons with and without ADRD influence the amount of caregiving received. All else equal, an IADL/ADL limitation may differentially impact persons with ADRD compared to those without ADRD due to challenges with managing co-occurring ADRD clinical symptoms including cognitive impairment and behaviors.2,3,6,10–13 If the effect of a change in IADL/ADL limitations for a person with ADRD is greater than for a person without ADRD, then persons with ADRD and their caregivers may need additional or specialized support (e.g., interventions which provide strategies to manage IADL/ADL limitations as well as co-occurring ADRD behaviors).
Predicted reductions in the coming decades of the availability of family caregivers requires policy makers to better understand the need for caregiving following changes in IADL/ADL limitations.14,15 Currently, there is no national long-term care strategy. At the individual level, families with financial resources can offset caregiving by purchasing support (e.g., personal care aide). At the system level, Medicare fee-for-service does not pay for long-term care services.16 However, in 2018, the Centers for Medicare and Medicaid Services issued new regulations allowing Medicare Advantage plans (which cover ~1/3 of Medicare beneficiaries) the opportunity to offer some long-term care services.17 Of these newly available services, Medicare Advantage plans have most commonly elected to include coverage for caregiver supports. Also at the system level, accountable care organizations are exploring approaches to provide long-term care services.18,19 Finally, states are exploring providing public long-term care funding or have implemented programs to compensate family caregivers.20–22
Typically, service eligibility for system- or public-funded long-term care is determined by a beneficiary’s clinical (e.g., IADL/ADL limitations and/or cognitive impairment) and economic needs. The amount of caregiving support received/needed by a beneficiary does not formally factor into eligibility for services. Yet, changing payment models combined with predicted reductions in the availability of caregivers necessitates an overhaul of the traditional long-term care paradigm and adds relevance to understanding the need for caregiving following changes in IADL/ADL limitations.
Using the Health and Retirement Study (HRS), we examined the effect of a change in IADL/ADL limitations between HRS interviews on the combined total amount of caregiving (hours of caregiving, days of caregiving, and number of caregivers) provided by family, friends, and paid informal caregivers to community-dwelling older adults with ADRD, cognitive impairment no dementia (CIND), or no cognitive impairment.
METHODS
Data
We used data from 2002–2014 of the HRS (RAND HRS File 2014v2).23,24 The RAND HRS Files provide cleaned variables and imputations for many measures in the HRS. The HRS samples community-dwelling adults and follows all respondents until death. During HRS interviews, conducted approximately every two years, respondents or a proxy provide demographic, economic, family, and medical information. Respondents also report the amount of caregiving received from family, friends, and paid individuals.
Measures
Persons with ADRD, CIND, or No Cognitive Impairment
We used the validated Langa-Weir algorithm to identify community-dwelling HRS respondents predicted to have ADRD, CIND, or no cognitive impairment. The Langa-Weir ADRD algorithm generates a cognitive score for each respondent (scored 0–27) based on the individual’s answer to the immediate and delayed recall test (0–20 points), serial 7 subtraction test (0–5 points), and backward count from 20 test (0–2 points).25–28 The total cognitive score is used to predict if a respondent has ADRD (score 0–6), CIND (score 7–11) or no cognitive impairment (score 12–27). For respondents with a proxy, the algorithm generates a cognitive score (scored 0–11) using the proxy’s assessment of the respondent’s memory (excellent=0; very good=1, good=2, fair=3, and poor=4), physical function (0 to 5 limitations), and assessment of cognitive impairment (no cognitive impairment=0, may have cognitive impairment=1, and has cognitive impairment=2). The total score is used to predict if a respondent has ADRD (6–11 points), CIND (3–5 points), or no cognitive impairment (0–2 points). In a validation study, the Langa-Weir algorithm was compared against ADRD diagnoses from the Aging, Demographics, and Memory Study and correctly classified 76% of self-respondents and 84% of respondents with a proxy.26
To be included in our analytic sample, HRS respondents could not have any data linkage issues across HRS files that were merged for the analysis and they had to be >64 years of age. To ensure the accuracy of predicted cognitive status, we excluded respondents that had improvements in their level of cognitive impairment as predicted by the Langa-Weir algorithm. We also excluded respondents predicted to have ADRD or CIND when they were <65 years of age since the cause of their impairment may be different than for those ≥65, their clinical symptoms may be different than those ≥65, the role of caregivers may be different than those ≥65, and they generally do not qualify for Medicare meaning their options for health care may be different from those ≥65.29–31 To observe changes in IADL/ADL limitations, we required respondents to have at least two consecutive interviews in the community with the same cognitive status. We excluded respondents with missing data on covariates (described below). For respondents with ADRD, we excluded observations from HRS interview years where they were predicted to have CIND or no cognitive impairment. For respondents with CIND, but never predicted to have ADRD we exclude observations from interview years where they were predicted to have no cognitive impairment.
Instrumental Activities of Daily Living and Basic Activities of Daily Living
HRS respondents report if they have difficulty performing IADL (preparing hot meals, shopping for groceries, making telephone calls, and taking medication) and ADL tasks (getting across a room, dressing, bathing, toileting, eating, and getting in/out of bed). We used the RAND HRS version of these variables which recodes raw answers into a binary indicator (1=respondent has some difficulty; 0=respondent has no difficulty). Missing values were assumed to mean no difficulty with that activity. We calculated the total number of IADL/ADL limitations (0–10) and the change in number of limitations between HRS interviews. Few individuals had a change of <−4 (person observations=37) or >7 (person observations=35) total IADL/ADL limitations. Therefore, we bottom coded the measure of change in total IADL/ADL limitations to −4 and top coded the measure to greater than 7 IADL/ADL limitations.
Persons with ADRD, CIND, and no cognitive impairment may have different combinations of IADL/ADL limitations which may affect the amount of caregiving received. For example, three respondents may each have two functional activity limitations, but the combination of the limitations may differ which may affect the amount of caregiving received. We created a categorical variable that captured each respondent’s unique combination of IADL/ADL limitations in the current and previous interview. We grouped all combinations of IADL/ADL limitations with <15 individuals into the same category.
Caregiving Received - Hours Caregiving, Days Caregiving, and Number of Caregivers
If a respondent receives assistance for an IADL/ADL task (described above), then the HRS asks the respondent to report the individual(s) that provided caregiving and the hours and number of days they received caregiving in a month. Caregivers could include family members, friends, or paid caregivers. A paid caregiver was any individual employed by an organization or identified as a nonrelative that was paid for caregiving. For each respondent and year, we calculated the hours of caregiving received per month, number of days caregivers provided care per month, and number of caregivers per month. Monthly caregiving days were calculated as the sum of days caregivers provided care (e.g., spouse and friend each provide 25 days of care which is equivalent to 50 care days).
Respondent Characteristics
Using the RAND HRS, we obtained each respondent’s sociodemographic characteristics (age, gender, race, years of education, enrolled in Medicaid, long-term care insurance, proxy respondent), and number of chronic conditions (high blood pressure or hypertension, diabetes or high blood sugar, cancer except skin cancer, lung disease except asthma or emphysema, heart attack/coronary heart disease/angina/congestive heart failure/or other heart problems, stroke, psychiatric problems, or arthritis). As with IADL/ADL limitations, persons with ADRD, CIND, and no cognitive impairment may have different combinations of chronic conditions that may affect the amount of caregiving received.2,9,32 Thus, we created a measure that captured the combination of chronic conditions a respondent had in the current and previous interview. We assumed if a respondent had missing data on the presence of a chronic condition then it was not present. All chronic condition combinations with <15 individuals were grouped into the same category.
Finally, we obtained information on the respondent’s family characteristics (net worth, marital status, number of children, number of married children, and number of living siblings).
Statistical Analysis
We evaluated the sample characteristics stratified by cognitive status. Many respondents (n=21,950 [88%]) reported receiving no caregiving. Therefore, to determine the effect of a change in IADL/ADL limitations by cognitive status on caregiving received we estimated a series of two-part regression models.
We first estimated a logistic regression to model the probability a respondent received any caregiving (i.e., >0 hours of caregiving). We then estimated a generalized linear model with a log link and gamma distribution to determine the hours of caregiving received conditional on receiving >0 hours of caregiving. We adopted a similar modeling approach for the outcomes of days caregiving and number of caregivers. We used a log link and gamma distribution for the second part of all models after comparing the AIC/BIC and predicted values of different model distributional assumptions (e.g., negative binomial distribution).
Both parts of all models included main effects for a respondent’s cognitive impairment, change in total IADL/ADL limitations, and an interaction between cognitive impairment and change in total IADL/ADL limitations. We controlled for variables that we believed could confound the relationship between cognitive impairment, change in IADL/ADL limitations, and caregiving received. Control variables included the respondent’s demographic and family characteristics, if the respondent had a proxy, indicators for the combination of IADL/ADL limitations and chronic conditions, and the amount of caregiving received in the prior interview (e.g., in the model estimating hours of caregiving this represented hours of caregiving received in the respondent’s prior HRS interview).
In sensitivity analyses, we examined the independent effect of a change in total number of IADL limitations, total number of early ADL limitations (getting across a room, dressing, bathing, toileting), and total number of late ADL limitations (eating, and getting in/out of bed) on the amount caregiving received. We separated early ADL and late ADL limitations based on a hierarchy of decline.33
Models were estimated using cluster standard errors to account for repeated observations. Interpretation of coefficients in non-linear models with interaction terms is challenging, so we report unconditional average marginal effects (i.e., combined effects from both parts of the two-part model) and predicted values of caregiving received.34,35
RESULTS
Of the 29,759 persons in HRS (2002–2014), 674 met our definition of ADRD, 530 met our definition of CIND, and 6,126 met our definition of no cognitive impairment (Figure 1). On average, persons with ADRD were older, more racially diverse, and had more IADL/ADL limitations and chronic conditions than persons with no cognitive impairment and CIND (Table 1 and eTable 1). Between HRS interviews, persons with ADRD, CIND and no cognitive impairment accumulated on average 1.3 (SD=2.5), 0.3 (SD=1.3), and 0.09 (SD=0.8) additional IADL/ADL limitations, respectively.
Figure 1.
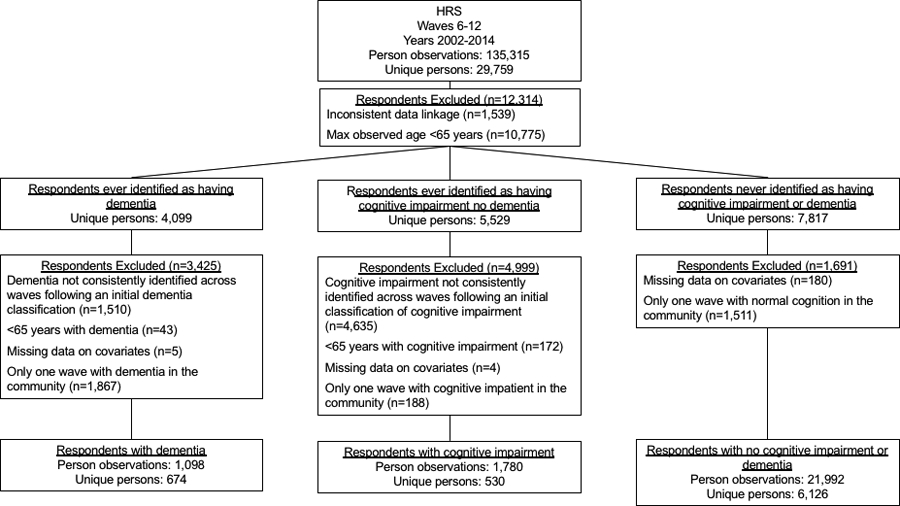
Sample
Table 1.
Sample Characteristics
| ADRD | CIND | No Cognitive Impairment | |
|---|---|---|---|
| Person Observations = 1,098 | Person Observations = 1,780 | Person Observations = 21,992 | |
| Age, mean (SD; 25th, 75th percentile), y | 82.8 (7.1; 78, 88)*** | 80.2 (6.6; 75, 85)*** | 74.4 (5.9; 70, 78) |
| Education, mean (SD; 25th, 75th percentile), y | 9.5 (4.1; 7, 12)*** | 11.8 (3.0; 10, 14)*** | 13.5 (2.5; 12, 16) |
| Female, n (%) | 676 (61.6) | 1,018 (57.2) | 12,886 (58.6) |
| Race, n (%) | |||
| White | 757 (68.9)*** | 1,497 (84.1)*** | 20,263 (92.1) |
| African American | 288 (26.2) | 235 (13.2) | 1,265 (5.8) |
| Othera | 53 (4.8) | 48 (2.7) | 464 (2.1) |
| Number of functional activity limitations | 4.4 (3.4; 1, 7)*** | 0.9 (1.7; 0, 1)*** | 0.3 (1; 0, 0) |
| Number of chronic conditions, mean (SD; 25th, 75th percentile) | 3.2 (1.6; 2, 4)*** | 2.6 (1.4; 1, 4)*** | 2.3 (1.4;1, 3) |
| Medicaid, n (%) | |||
| No | 790 (72.0)*** | 1,586 (89.1)*** | 21,255 (96.7) |
| Yes | 267 (24.3) | 157 (8.8) | 665 (3.0) |
| Unknown | 41 (3.7) | 37 (2.1) | 72 (0.3) |
| Long-term care insurance, n (%) | |||
| No | 967 (88.1)*** | 1,489 (83.7)*** | 17,576 (79.9) |
| Yes | 92 (8.4) | 238 (13.4) | 4,203 (19.1) |
| Unknown | 39 (3.6) | 53 (3.0) | 213 (1.0) |
| Proxy respondent, n (%) | 565 (51.5)*** | 68 (3.8)*** | 419 (1.9) |
| Net worth, mean (SD; 25th, 75th percentile), $ | 243,030 (649,966; 1,250, 253,000)*** | 376,638 (731,847; 32,500, 442,000)*** | 730,181 (1,650,414; 126,500, 787,750) |
| Marital status, n (%) | |||
| Married/partnered | 428 (39.0)*** | 958 (53.8)*** | 14,376 (65.4) |
| Separated /divorced | 83 (7.6) | 119 (6.7) | 1,813 (8.2) |
| Widowed | 558 (50.8) | 673 (37.8) | 5,293 (24.0) |
| Never married | 29 (2.6) | 30 (1.7) | 510 (2.3) |
| Number of children, mean (SD; 25th, 75th percentile) | 3.7 (2.4; 2, 5)*** | 3.4 (2.1; 2, 5)* | 3.3 (2.1; 2, 4) |
| Number of married children, mean (SD; 25th, 75th percentile) | 2.3 (1.8; 1, 3)* | 2.4 (1.7; 1, 3) | 2.4 (1.7; 1, 3) |
| Number of living siblings, mean (SD; 25th, 75th percentile) | 2.1 (2.3; 0, 3) | 2 (2.1; 1, 3)*** | 2.2 (2.0; 1, 3) |
| Hours of caregiving received, mean (SD; 25th, 75th percentile) | 235.8 (265.6; 7, 465)*** | 26.0 (92.6; 0, 0)*** | 6.0 (40.7; 0, 0) |
| Days of caregiving received, mean (SD; 25th, 75th percentile) | 33.6 (27.6; 4.3, 51)*** | 6.2 (14.8; 0, 0)*** | 1.8 (8.06; 0, 0) |
| Number of caregivers, mean (SD; 25th, 75th percentile) | 1.8 (1.5; 1, 3)*** | 0.4 (0.8; 0, 0)*** | 0.1 (0.5; 0, 0) |
ADRD = Alzheimer’s disease and related dementias
CIND = Cognitive impairment no dementia
All p-values are relative to persons with no cognitive impairment
p<0.05;
p<0.01;
p<0.001
Other race category includes American Indian, Alaskan Native, Asian, Native Hawaiian, and Pacific Islander.
Caregiving Received - Hours Caregiving, Days Caregiving, and Number of Caregivers
Persons with ADRD received 235.8 (SD=265.6) hours of caregiving per month compared to 26.0 (SD=92.6) hours of caregiving for persons with CIND, and 6.0 (SD=40.7) hours of caregiving for persons with no cognitive impairment. An increase in one total IADL/ADL limitation between HRS interviews was associated with receiving 5.06 (95%CI: 4.56, 5.55) more monthly hours of caregiving (Table 2; eTable 2 for full regression coefficients). On average, persons with ADRD and CIND received 16.95 (95%CI: 13.18, 20.72) and 4.35 (95%CI: 1.96, 6.73) more hours of caregiving per month than persons with no cognitive impairment, respectively. The effect of a change in IADL/ADL limitations varied by level of cognitive impairment. One additional IADL/ADL limitation between HRS interviews resulted in persons with ADRD and CIND receiving 4.90 (95%CI: 3.40, 6.39) and 1.44 (95%CI: 0.17, 2.69) more hours of caregiving per month compared to persons with no cognitive impairment. The greater the increase in the number of IADL/ADL limitations between HRS interviews, the more hours of caregiving per month received by all respondents (Figure 2a). At all levels of a change in IADL/ADL limitations, persons with ADRD received more hours of caregiving than persons with no cognitive impairment (Figure 3a).
Table 2.
Effect of Change in Total Functional Activity Limitations on Caregiving Outcomesa
| Hours of Caregiving Received | Days of Caregiving Received | Number of Caregivers | |
|---|---|---|---|
| Marginal Effect (95% CI) | Marginal Effect (95% CI) | Marginal Effect (95% CI) | |
| Total functional activity limitationsb | 5.06*** (4.56, 5.55) | 0.88*** (0.81, 0.94) | 0.05*** (0.05, 0.06) |
| Cognition impairment (ref = no cognitive impairment)c | |||
| CIND | 4.35*** (1.96, 6.73) | 0.43** (0.10, 0.76) | 0.02* (0.00, 0.04) |
| ADRD | 16.95*** (13.18, 20.72) | 1.85*** (1.31, 2.39) | 0.07*** (0.04, 0.19) |
| Total functional activity limitations × cognitive impairment (ref = no cognitive impairment)d | |||
| CIND | 1.43* (0.17, 2.69) | 0.04 (−0.12, 0.19) | 0.00 (−0.01, 0.01) |
| ADRD | 4.90*** (3.40, 6.39) | 0.44*** (0.20, 0.67) | 0.02** (0.01, 0.03) |
ADRD = Alzheimer’s disease and related dementias
CIND = Cognitive impairment no dementia
p<0.05;
p<0.01;
p<0.001
Unconditional marginal effect from the estimated two-part model. In the first part (n=24,530) a logistic regression was estimated to determine if a respondent received >0 caregiving. In the second part (n=2,920), the amount of caregiving received was estimated using a generalized linear model with gamma distribution and log link. In the first part of the two-part model, 340 observations were removed from analyses due to collinearity. All models control for age, gender, years of education, race, Medicaid enrollment, long-term care insurance, net worth, marital status, number of children, number of married children, number of living siblings, if a respondent had a proxy, number of functional activity limitations in previous HRS interview, an indicator for the combination of functional activity limitations in the current and previous interview, an indicator for the combination of chronic conditions in the current and previous interview, and amount of caregiving received (i.e., lag of the outcome) in the prior interview.
Marginal effect represents how a change in functional activity limitations, averaged over all persons, effects caregiving received.
Marginal effect represents the incremental difference in caregiving received, averaged over all persons, by levels of cognitive impairment.
Marginal effect represent the incremental difference in caregiving received by levels of cognitive impairment given a change in function activity limitations.
Figure 2.
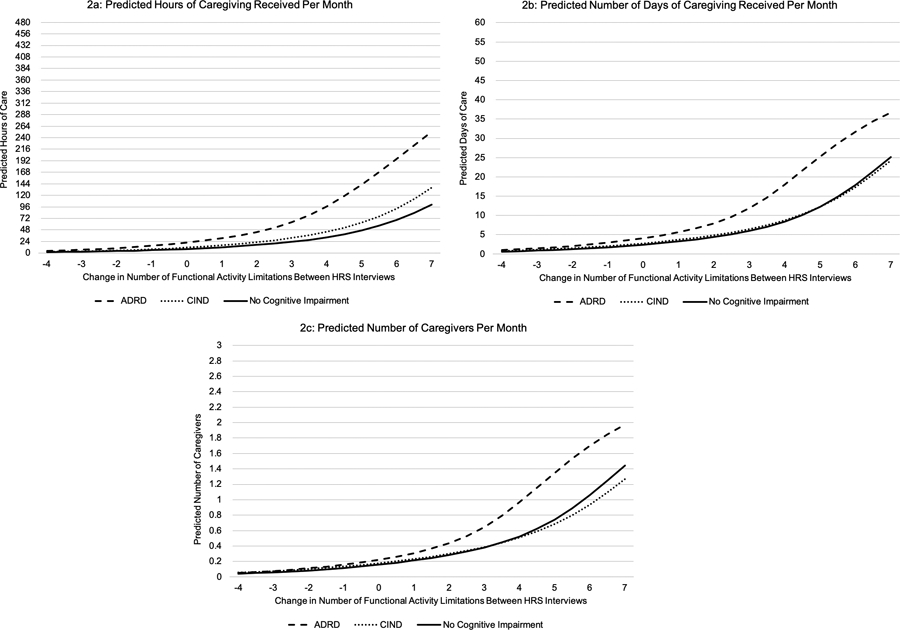
Predicted Values of Caregiving Received
Figure 3.
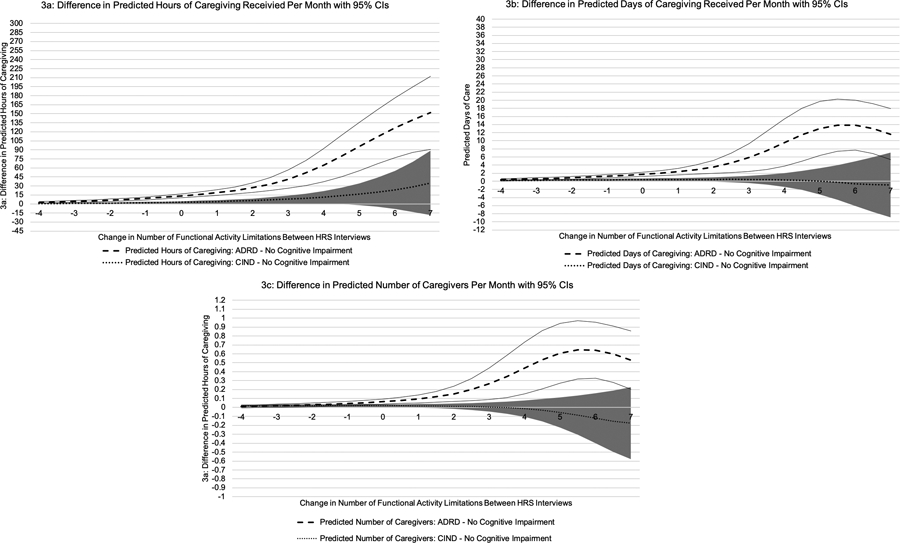
Difference in Predicted Value of Caregiving Received
Note: Sold lines represent 95% CI for ADRD – no cognitive impairment. Shaded region represents 95% CI for CIND – no cognitive impairment.
Persons with ADRD, CIND, and no cognitive impairment received on average 33.6 (SD=27.6), 6.2 (SD=14.8), and 1.8 (SD=8.1) days of caregiving per month, respectively. An increase in IADL/ADL limitations was associated with receiving 0.88 (95%CI: 0.81, 0.94) more days of caregiving per month, and persons with ADRD and CIND both received more days of caregiving per month than persons with no cognitive impairment (Table 2). On average, one additional IADL/ADL limitation between HRS interviews was associated with persons with ADRD and CIND receiving 0.44 (95%CI: 0.20, 0.67) and 0.04 (95%CI: −0.12, 0.19) more days of caregiving per month than persons with no cognitive impairment. As the number of IADL/ADL limitations increased between HRS interviews, the number caregiver days increased (Figure 2b), but the difference in caregiving days between levels of cognitive impairment was not constant (Figure 3b).
On average, persons with ADRD, CIND, and no cognitive impairment had 1.8 (SD=1.5), 0.4 (SD=0.8), and 0.1 (SD=0.5) caregivers per month, respectively. An increase in one IADL/ADL limitation resulted in respondents having 0.05 (95%CI: 0.05, 0.06) more monthly caregivers, and persons with ADRD and CIND had significantly more caregivers than persons with no cognitive impairment (Table 2). An increase in one IADL/ADL limitation between HRS interviews was associated with persons with ADRD having 0.02 (95%CI: 0.01, 0.03) more caregivers per month than persons with no cognitive impairment. An increase in IADL/ADL limitations did not differentially affect the number of caregivers of persons with CIND compared to persons with no cognitive impairment. At all levels of cognitive impairment, an increase in IADL/ADL limitations resulted in having more caregivers (Figure 2c), but the difference in number of caregivers between levels of cognitive impairment was not constant (Figure 3c).
In sensitivity analyses, an increase in IADL and early ADL limitations resulted in persons with ADRD receiving 7.26 (95%CI: 4.86, 9.66) and 2.73 (95%CI: 0.47, 4.99) more hours of caregiving per month than persons with no cognitive impairment, respectively (eTable 3). The effect of a change in late ADL limitations on hours of caregiving between persons with ADRD and no cognitive impairment was minimal. Changes in IADL limitations, but not early or late ADL limitations, were associated with persons with ADRD receiving more days of caregiving and having more caregivers than persons with no cognitive impairment. Changes in IADL and all ADL limitations were associated with minimal differences in the amount of caregiving received between persons with CIND and no cognitive impairment.
DISCUSSION
Persons with ADRD receive more hours and days of caregiving and have more caregivers than persons with CIND and no cognitive impairment. An increase in IADL/ADL limitations disproportionately results in persons with ADRD receiving more caregiving than persons with no cognitive impairment. These differences reflect need and challenges associated with ADRD caregiving including managing behaviors, and engaging in complex medical tasks.36,37 The findings emphasize the additive effect of ADRD on managing functional limitations both for the person with ADRD as well as their caregiving network, suggesting the need for particular attention to functional impairments among those living with dementia.
In the coming decades there are predicted to be increases in the number of Americans living with ADRD and declines in the availability of family caregivers.2,14 With fewer family caregivers, many older adults with cognitive impairment will face the prospect of living alone in the community with minimal support. Currently, few persons with ADRD have long-term care insurance and it is unlikely that there will be increases in the purchasing of policies. Without sufficient community support, persons with ADRD and concurrent functional limitations will need to move to a residential care facility. Solutions are needed to enhance affordable community-based supports for persons with ADRD and their families. These supports must account for the diverse characteristics of families. Already, African Americans are disproportionally represented among those with ADRD and this trend is expected to continue.2
State Medicaid programs have implemented strategies to help individuals remain in the community.38,39 Most Medicaid programs offer consumer directed care that enables beneficiaries to purchase paid community-based care, and in some states benefits include compensating family caregivers.21,40 All state level programs have eligibility requirements (e.g., functional limitations and income). Some programs also have exceptions for persons with cognitive impairment. Our results suggest that program exceptions for persons with ADRD are warranted given the excessive caregiving time associated with IADL/ADL decline.
Expanding Medicaid benefits to provide community based long-term care is important. However, only ~24% of community-dwelling persons with ADRD are enrolled in Medicaid.41 The remaining 75% still need support. There are additional avenues for states to implement policy that support caregivers. Almost 20% of caregivers are employed and with shifting demographics this proportion is likely to increase.42 Several states have enacted paid family leave laws that require employers to compensate employees who temporarily leave the workforce to provide caregiving.43 Most family leave policies only provide coverage for short durations and this may not be adequate to support the intense caregiving associated with ADRD. However, an early evaluation of the California paid family leave policy found it resulted in a reduction in nursing home utilization.44 Without expanding Medicaid coverage, states can continue to support caregivers by expanding paid family leave policies.
In sensitivity analyses, increases in IADL and early ADL limitations were associated with persons with ADRD receiving more caregiving than persons with no cognitive impairment. In addition, days and number of caregivers varied by change in IADL limitations and cognition but not ADL limitations. Severe ADL limitations are more likely to occur near the end of life at which point co-occurring ADRD clinical symptoms may create less additional burden. Furthermore, by the time an individual accumulates severe ADL limitations they are likely receiving large amounts of care, so the marginal effect of an additional limitation may be minimal. Similarly, at a certain level of IADL/ADL impairment persons with ADRD may have reached the maximum number of caregivers available to them. Thus, changes in ADL limitations, which typically occur after IADL limitations, may have a minimal effect on increasing the number of caregivers. These findings suggest once a person with ADRD starts exhibiting these IADL and early ADL limitations, they need additional supports.
Our study has limitations. We used a validated algorithm, which is subject to measurement error, to identify persons with ADRD, CIND, and no cognitive impairment. As this algorithm is unique to the HRS, our results may not be generalizable across surveys that employ different approaches to identify persons with ADRD. We required respondents to have at least two consecutive HRS interviews while alive and in the community with the same predicted cognitive impairment. This was necessary to understand how changes in functional activity impact caregiving. Persons with ADRD were less likely to have two consecutive interviews compared to persons with CIND and no cognitive impairment. In general, persons with ADRD who were excluded from analyses had either died or moved to a nursing home and had received more hours of caregiving in the interview prior to attrition from the community than persons with ADRD that had consecutive community-based interviews. For this reason, our results may underestimate the overall effect of a change in IADL/ADL limitations on caregiving received by persons with ADRD. We measured total community-based caregiving, which included care from family/friends and paid individuals. Separating the effect of changes in IADL/ADL limitations by cognitive status on unpaid and paid caregiving is an important issue but also a challenge, as these outcomes are endogenous. Finally, respondents reported the amount of caregiving received for IADL/ADL limitations. Yet, caregivers may provide care for a variety of other activities (e.g., communicating with physicians).
Conclusions
Increases in total IADL/ADL limitations result in persons with ADRD receiving significantly more hours of caregiving per month, more caregiving days per month and having more caregivers per month than persons with no cognitive impairment. The effect of a change in IADL/ADL limitations on the amount of caregiving received by persons with CIND relative to persons with no cognitive impairment is minimal. The presence of significant cognitive impairment or ADRD should qualify individuals for national, state, and health system level long-term care benefits.
Supplementary Material
Acknowledgements
The Health and Retirement Study is produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number NIA U01AG009740) Ann Arbor, MI.
Funding/Support: This work was supported by grants from National Institute on Aging (1R21AG059623-01 and 1R01AG060871-01 both to EJ) and from the Brown School of Public Health (EJ).
Footnotes
All authors report no conflicts of interest.
References
- 1.Wolff JL, Spillman BC, Freedman VA, et al. A National Profile of Family and Unpaid Caregivers Who Assist Older Adults With Health Care Activities. JAMA Intern Med 2016;176:372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement 2018;14:367–429. [Google Scholar]
- 3.Jutkowitz E, MacLehose RF, Gaugler JE, et al. Risk Factors Associated With Cognitive, Functional, and Behavioral Trajectories of Newly Diagnosed Dementia Patients. J Gerontol A Biol Sci Med Sci 2017;72:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Academies of Sciences Engineering, and Medicine. Families caring for an aging America In: Schulz R, Eden J, eds. Families Caring for an Aging America. Washington, DC; 2016. [PubMed] [Google Scholar]
- 5.Wiles JL, Leibing A, Guberman N, et al. The meaning of “aging in place” to older people. Gerontologist 2012;52:357–366. [DOI] [PubMed] [Google Scholar]
- 6.Jutkowitz E, Kane RL, Gaugler JE, et al. Societal and Family Lifetime Cost of Dementia: Implications for Policy. J Am Geriatr Soc 2017;65:2169–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasper JD, Freedman VA, Spillman BC, et al. The Disproportionate Impact Of Dementia On Family And Unpaid Caregiving To Older Adults. Health Aff (Millwood) 2015;34:1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman EM, Shih RA, Langa KM, et al. US Prevalence And Predictors Of Informal Caregiving For Dementia. Health Aff (Millwood) 2015;34:1637–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher GG, Franks MM, Plassman BL, et al. Caring for individuals with dementia and cognitive impairment, not dementia: findings from the aging, demographics, and memory study. J Am Geriatr Soc 2011;59:488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurd MD, Martorell P, Delavande A, et al. Monetary costs of dementia in the United States. N Engl J Med 2013;368:1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci 2009;11:217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jutkowitz E, Kane RL, Dowd B, et al. Effects of Cognition, Function, and Behavioral and Psychological Symptoms on Medicare Expenditures and Health Care Utilization for Persons With Dementia. J Gerontol A Biol Sci Med Sci 2017;72:818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jutkowitz E, Kuntz KM, Dowd B, et al. Effects of cognition, function, and behavioral and psychological symptoms on out-of-pocket medical and nursing home expenditures and time spent caregiving for persons with dementia. Alzheimers Dement 2017;13:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redfoot D, Feinberg L, Houser A. The Aging of the Baby Boom and the Growing Care Gap: A Look at Future Declines in the Availability of Family Caregivers [AARP web site]. August 2013. Available at: https://www.aarp.org/home-family/caregiving/info-08-2013/the-aging-of-the-baby-boom-and-the-growing-care-gap-AARP-ppi-ltc.html. Accessed January 26, 2019.
- 15.Gaugler JE, Kane RL. Family caregiving in the new normal. London: Academic Press; 2015. [Google Scholar]
- 16.Doty P, Spillman B. Chapter 10 - Help for Family Caregivers Available from Government Programs and Policies In: Gaugler JE, Kane RL, eds. Family caregiving in the new normal. Academic Press; 2015:153–190. [Google Scholar]
- 17.Meyers DJ, Mor V, Rahman M. Medicare Advantage Enrollees More Likely To Enter Lower-Quality Nursing Homes Compared To Fee-For-Service Enrollees. Health Aff (Millwood) 2018;37:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jutkowitz E, Scerpella D, Pizzi LT, et al. Dementia Family Caregivers’ Willingness to Pay for an In-home Program to Reduce Behavioral Symptoms and Caregiver Stress. Pharmacoeconomics 2019;37:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boustani M, Alder CA, Solid CA, et al. An Alternative Payment Model To Support Widespread Use Of Collaborative Dementia Care Models. Health Aff (Millwood) 2019;38:54–59. [DOI] [PubMed] [Google Scholar]
- 20.Simon-Rusinowitz L, Mahoney KJ, Loughlin DM, et al. Paying Family Caregivers: An Effective Policy Option in the Arkansas Cash and Counseling Demonstration and Evaluation. Marriage Fam Rev 2005;37:83–105. [Google Scholar]
- 21.McCarthy ES. A Policy Analysis of the Kupuna Caregivers Act Addressing the Needs of Working Caregivers in Hawaii. Theses and Dissertations--Public Health (M.P.H. & Dr.P.H.). 2018. Available at: https://uknowledge.uky.edu/cph_etds/186. Accessed February 5, 2019.
- 22.66th Legislature 2019 Regular Session Second Substitute House Bill 1087. Washington; 2019. Available at: https://lawfilesext.leg.wa.gov/biennium/2019-20/Pdf/Bills/Session%20Laws/House/1087-S2.SL.pdf. Accessed May 25, 2019. [Google Scholar]
- 23.Juster FT, Suzman R. An overview of the health and retirement study. J Hum Resour 1994;40:S7–S56. [Google Scholar]
- 24.Sonnega A, Faul JD, Ofstedal MB, et al. Cohort Profile: the Health and Retirement Study (HRS). Int J Epidemiol 2014;43:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langa KM, Larson EB, Crimmins EM, et al. A Comparison of the Prevalence of Dementia in the United States in 2000 and 2012. JAMA Intern Med 2017;177:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crimmins EM, Kim JK, Langa KM, et al. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci 2011;66 Suppl 1:i162–i171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace R, Herzog AR, Weir DR, et al. Documentation of Cognitive Functioning Measures in the Health and Retirement Study. HRS Documentation Report DR-006. March 2005. Available at: https://hrs.isr.umich.edu/sites/default/files/biblio/dr-006.pdf. Accessed March 23, 2019.
- 28.Langa KM, Weir DR, Kabeto M, et al. Langa-Weir Classification of Cognitive Function (1995 Onward). Survey Research Center Institute for Social Research University of Michigan. November 2018. Available at: http://hrsonline.isr.umich.edu/modules/meta/researcher-contributions/langa-weir-classifications/Data_Description_Langa_Weir_Classifications.pdf. Accessed April 23, 2019.
- 29.Koedam EL, Lauffer V, van der Vlies AE, et al. Early-versus late-onset Alzheimer’s disease: more than age alone. J Alzheimers Dis 2010;19:1401–1408. [DOI] [PubMed] [Google Scholar]
- 30.Toyota Y, Ikeda M, Shinagawa S, et al. Comparison of behavioral and psychological symptoms in early-onset and late-onset Alzheimer’s disease. Int J Geriatr Psychiatry 2007;22:896–901. [DOI] [PubMed] [Google Scholar]
- 31.Wawrziczny E, Pasquier F, Ducharme F, et al. Do spouse caregivers of young and older persons with dementia have different needs? A comparative study. Psychogeriatrics 2017;17:282–291. [DOI] [PubMed] [Google Scholar]
- 32.US Department of Health and Human Services; Office of the Assistant Secretary for Planning and Evaluation. A Profile of Older Adults with Dementia and Their Caregivers. September 2018. Available at: https://aspe.hhs.gov/basic-report/profile-older-adults-dementia-and-their-caregivers-issue-brief. Accessed March 3, 2019.
- 33.Jagger C, Arthur AJ, Spiers NA, Clarke M. Patterns of Onset of Diability in Activities of Daily Living with Age. J Am Geriatr Soc 2001l49:404–409. [DOI] [PubMed] [Google Scholar]
- 34.Karaca-Mandic P, Norton EC, et al. Interaction terms in nonlinear models. Health Serv Res 2012;47:255–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mize TD. Best Practices for Estimating, Interpreting, and Presenting Nonlinear Interaction Effects. Sociol. Sci 2019;6:81–117. Available at: https://www.sociologicalscience.com/download/vol-6/february/SocSci_v6_81to117.pdf. Accessed April 7, 2019. [Google Scholar]
- 36.Ory MG, Hoffman RR, Yee JL, et al. Prevalence and impact of caregiving: A detailed comparison between dementia and nondementia caregivers. The Gerontologist 1999;39:177–185. [DOI] [PubMed] [Google Scholar]
- 37.Schulz R, Martire LM. Family caregiving of persons with dementia: prevalence, health effects, and support strategies. Am J Geriatr Psychiatry 2004;12:240–249. [PubMed] [Google Scholar]
- 38.Pande A, Laditka SB, Laditka JN, et al. Aging in place? Evidence that a state Medicaid waiver program helps frail older persons avoid institutionalization. Home Health Care Serv Q 2007;26:39–60. [DOI] [PubMed] [Google Scholar]
- 39.Kaye HS. Gradual rebalancing of Medicaid long-term services and supports saves money and serves more people, statistical model shows. Health Aff (Millwood) 2012;31:1195–1203. [DOI] [PubMed] [Google Scholar]
- 40.Benjamin AE. Chapter 2 - Consumer-Directed Services at Home for Older People in the United States In: Carmel S, Morse CA, Torres-Gil FM, Hendricks H, eds. Lessons on Aging from Three Nations, The Art of Caring for Older Adults. 2018. [Google Scholar]
- 41.Garfield R, Musumeci M, Reaves EL, et al. Medicaid’s Role for People with Dementia [KPP web site]. 2015. Available at: http://files.kff.org/attachment/issue-brief-medicaids-role-for-people-with-dementia. Accessed December 8, 2017.
- 42.Nobel J, Weiss J, Sherman C, et al. Supporting Caregivers in the Workplace: A Practical Guide for Employers. September 2017. Available at: https://nebgh.org/wp-content/uploads/2017/11/NEBGH-Caregiving_Practical-Guide-FINAL.pdf. Accessed June 8, 2019.
- 43.Isaacs J, Healy O, Peters HE. Paid Family Leave in the United States, Time for a New National Policy. May 2017. Available at: https://www.urban.org/sites/default/files/publication/90201/paid_family_leave_0.pdf. Accessed April 3, 2019.
- 44.Arora K, Wolf DA. Does Paid Family Leave Reduce Nursing Home Use? The California Experience. J Policy Anal Manage 2018;37:38–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


