Abstract
Potential but unconfirmed risk factors for coronavirus disease 2019 in adults and children may include hypertension, cardiovascular disease, and chronic kidney disease, as well as the medications commonly prescribed for these conditions, angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers. Coronavirus binding to angiotensin-converting enzyme 2, a crucial component of the renin-angiotensin-aldosterone system, underlies much of this concern. Children are uniquely impacted by the coronavirus but the reasons are unclear. This review will highlight the relationship of coronavirus disease 2019 with hypertension, use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers, and lifetime risk of cardiovascular disease from the pediatric perspective. We briefly summarize the renin-angiotensin-aldosterone system and comprehensively review the literature pertaining to the angiotensin-converting enzyme 2/angiotensin-(1–7) pathway in children and the clinical evidence for how angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers affect this important pathway. Given the importance of the angiotensin-converting enzyme 2/angiotensin-(1–7) pathway and the potential differences between adults and children, it is crucial that children are included in coronavirus-related research, as this may shed light on potential mechanisms for why children are at decreased risk of severe coronavirus disease 2019.
Keywords: Angiotensin-(1–7), cardiovascular disease, children, chronic kidney disease, coronavirus, hypertension, renin-angiotensin-aldosterone system, SARS-CoV-2
Introduction
Early reports during the coronavirus disease 2019 (COVID-19) pandemic emphasized theoretical concerns that the continued use of medications that block the renin-angiotensin-aldosterone system (RAAS), including angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), may influence disease severity and mortality1, yet little attention has been paid to the potential unique aspects of this issue in pediatrics. Similar to adults, many children depend on these drugs to manage their chronic conditions. Unlike adults, children frequently have congenital anomalies, genetic defects, and distinct pathophysiology that underlie their elevated cardiovascular disease risk, highlighting their need for the targeted treatment these agents offer. Their lifetime-increased risk of cardiovascular disease mandates the continued use of evidence-based treatments.2 Given this, it is important to understand the pediatric data regarding the ACE2/angiotensin-(1–7) [Ang-(1–7)] pathway of the RAAS, including the effect ACE inhibitors and ARBs may have on this pathway. We will highlight the interaction of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with the RAAS and discuss the potential role of the RAAS in COVID-19 pathophysiology. Finally, we will discuss future directions, including implications for clinical management and the importance of including children in research that could inform why children appear to be largely protected against severe COVID-19.
COVID-19 and Hypertension
Early reports from the outbreak in Wuhan, China indicated that a large proportion of patients with severe COVID-19 had hypertension, raising the concern that hypertension was a risk factor for more-severe disease.3–5 Similar data have emerged from preliminary analyses from Italy and the United States.6, 7 However, in all of these reports, patients with severe COVID-19 were older – age 65 and above – making it challenging to separate the impact of age from comorbid conditions, such as hypertension. Indeed, the proportions of children and adolescents in these early reports were less than two percent. These early reports have been descriptive case series, describing one-way associations without employing appropriate epidemiological or statistical methods to estimate the independent association of hypertension with COVID-19 outcomes. It is well known that hypertension prevalence, and thus antihypertensive medication use, increases with age.8 Until the results of more-sophisticated analyses that account for age, comorbid conditions, baseline medication use, and other potential confounding factors become available, one cannot conclude that hypertension is an independent risk factor for SARS-CoV-2 infection or more-severe COVID-19.
There has been additional speculation that ACE inhibitors and ARBs may also increase the risk of SARS-CoV-2 infection and COVID-19.4 Like the original SARS-CoV described in 2003, data suggest that SARS-CoV-2 binds to ACE2 to gain entry to host cells on the respiratory tract epithelium. As select animal studies have demonstrated increased ACE2 expression with ACE inhibitor or ARB administration, a potential mechanistic link between SARS-CoV-2 infection and these medications has been proposed (see below).9, 10 However, studies in animals or humans have not demonstrated that this actually occurs in the lungs. Similar to the studies mentioned earlier that considered hypertension a risk factor for COVID-19, these early papers speculating on the link between RAAS-blocking agents and COVID-19 have not fully considered potential sources of bias, confounding, or mediation.11, 12 As such, it remains unclear if such an association exists.
Pediatric Significance
The distinct epidemiology of SARS-CoV-2 and COVID-19 in the pediatric population suggests that children may not only have a different response to the infection, but that management considerations for youth may need to be considered separately. One of the more-striking aspects of this pandemic is that, counter to the usual high incidence and prevalence of viral respiratory infections in the pediatric age group, children have not constituted a significant proportion of patients with COVID-19. Symptoms of SARS-CoV-2 infection and COVID-19 in children also appear to be different from those in adults, with fever, cough, and shortness of breath occurring less frequently.13 In the United States, children <18 years of age comprised 1.7% of cases in which age was known between February 12, 2020 and April 2, 2020.13 Three deaths were reported in this age group over the same timeframe.13 These reports are subject to a number of limitations, not the least of which is the lack of population-based screening for SARS-CoV-2. Despite this, the preponderance of data to-date suggest that children are at reduced risk of developing COVID-19 once infected with SARS-CoV-2. Reasons for this are unclear and require further study.
Pediatric-specific risk factors for COVID-19 have not been defined, thus there is no evidence that hypertension, cardiovascular disease, or chronic kidney disease per se are independent risk factors for COVID-19 in children.14, 15 Clarification of these risk factors is important, as an estimated 4% of children worldwide have hypertension, 50–75 per million of the age-related population have chronic kidney disease, and an even greater proportion either have or are at increased risk for cardiovascular disease.16, 17 ACE inhibitors and ARBs are often the first-line agents to treat these chronic conditions in children and are among the most-commonly prescribed antihypertensive medications to children.18, 19 Thus, the safety of continuing these medications during the pandemic is of particular interest to pediatricians.
SARS-CoV-2, ACE2, and the RAAS
Overview of the ACE2/Ang-(1–7) Pathway
ACE2, the SARS-CoV-2 binding site, is a critical component of the counter-regulatory pathway of the RAAS, which is one of the most-important regulators of blood pressure, inflammation, and fibrosis, and is crucial to the pathophysiology of hypertension, cardiovascular disease, and chronic kidney disease (Figure 1).20 ACE2 converts Ang II into Ang-(1–7) which acts at the Mas receptor to lower blood pressure and reduce inflammation and fibrosis.21 Ang-(1–7)’s primary actions are to promote vasodilation and sodium and water excretion, reduce sympathetic nervous system tone, and increase nitric oxide production.22–25 This counteracts the predominant effects of the ACE/Ang II/Ang II type 1 receptor pathway, namely vasoconstriction, sodium and water reabsorption, increased sympathetic nervous system tone, and increased oxidative stress leading to inflammation and fibrosis.21, 26, 27 Both pathways are co-expressed in a majority of tissues, including the lungs, heart, kidney, vasculature, and intestines. Thus the balance between the two major RAAS pathways, ACE2/Ang-(1–7) and ACE/Ang II, contributes to the development, progression, and regression of hypertension in children as well as adults, and plays a critical role in cardiovascular and kidney disease (Figure 1A–C).21, 28–33 Generally, in younger age groups, the RAAS is in balance and no disease develops (Figure 1A).
Figure 1.
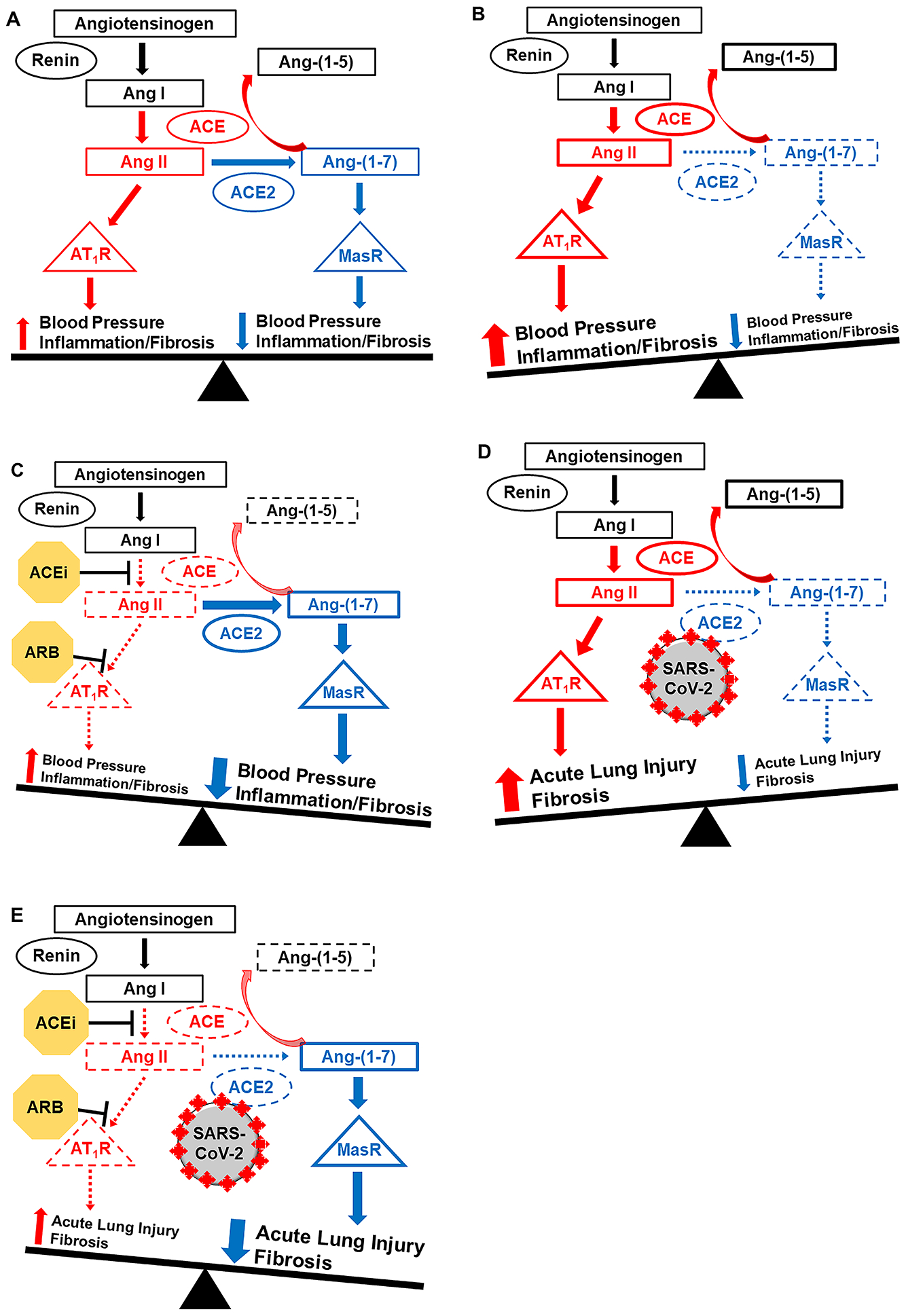
Balance of the RAAS and Impact of Age, Disease, SARS-CoV-2, and ACE inhibitors and ARBs
A: In younger and healthier individuals (e.g. children), the RAAS is in balance: blood pressure is appropriate and no disease develops.
B: With advancing age and in various disease states (e.g. hypertension), there is a shift towards the ACE/Ang II pathway.
C: ACEi and ARB block the ACE/Ang II pathway and shift the RAAS towards ACE2/Ang-(1–7).
D: SARS-CoV-2-induced ACE2 downregulation could stimulate a shift towards the ACE/Ang II pathway to propagate acute lung injury in COVID-19.
E: In patients with COVID-19, ACEi and ARB therapy could block the ACE/Ang II pathway and shift the RAAS towards the ACE2/Ang-(1–7) pathway to mitigate lung injury.
Pro-inflammatory and pro-fibrotic pathway in red, anti-inflammatory and anti-fibrotic pathway in blue. Dashed lines indicate decreased pathway activity. ACE, angiotensin-converting enzyme; ACEi, ACE inhibitors; Ang, angiotensin; ARB, angiotensin II receptor blocker; AT1R, Ang II type 1 receptor; COVID-19, coronavirus disease 2019; MasR, Mas receptor; RAAS, renin-angiotensin-aldosterone system; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
ACE2 Pathway in Children
Despite its importance, few pediatric-specific data exist on expression of either the ACE2/Ang-(1–7) or the ACE/Ang II pathways.34 Clinical studies in adults have demonstrated that in the kidney, for example, ACE2 is expressed on the epithelium and vascular endothelium of the glomeruli and tubulointerstitium, predominantly on the apical brush border of the proximal tubules.35 However, there are scant data specific to ACE2/Ang-(1–7) tissue expression in children.36 Studies predominantly report circulatory concentrations of the RAAS components, which are non-specific and do not correlate with local tissue expression, further limiting the clinical application of published results in children.34, 37
Most data in children come from clinical hypertension studies. In addition to describing that ACE2 gene single nucleotide polymorphisms may be associated with higher blood pressure in adolescence38, these studies have suggested that hypertension etiology is linked to plasma concentrations of the RAAS. In one cross-sectional study of children who had untreated hypertension, both primary hypertension and renovascular hypertension were associated with higher plasma Ang-(1–7) concentrations compared to normotensive controls, but only renovascular hypertension was associated with higher plasma Ang II concentrations compared to normotensive controls.30 Compared to primary hypertension, renovascular hypertension was associated with lower Ang-(1–7) and higher Ang II. In another cross-sectional analysis, children with chronic kidney disease and treated hypertension had higher plasma Ang-(1–7) concentrations compared to children with chronic kidney disease and normal blood pressure and to healthy controls.31 These data highlight the appropriate compensatory response to increased ACE/Ang II pathway activity that may occur in pediatric hypertension, consistent with adult data.39, 40 Indeed, the majority of clinical and animal studies support the notion of this compensatory increase in ACE2/Ang-(1–7) expression with increasing age and in the presence of various diseases, though the net effect is usually reduced expression relative to increased ACE/Ang II expression (Figure 1B).21, 34 Moreover, a hallmark of ACE inhibitor and ARB therapy in these diseases is to shift the RAAS back towards the ACE2/Ang-(1–7) pathway (Figure 1C).
Emerging data suggest that alterations to the RAAS can precede development of clinically apparent disease in children.21, 41 In a pilot study, decreasing urinary Ang-(1–7) levels over time significantly predicted biopsy-proven acute allograft injury in pediatric kidney transplant recipients (Andrew M. South and Paul C. Grimm, unpublished data, 2019; NCT03317925). Among healthy 14-year-olds participating in an ongoing longitudinal birth cohort (NCT04026776), those born preterm with very low birth weight had higher plasma Ang II concentrations relative to lower Ang-(1–7) compared to those born term.28 Furthermore, lower urinary Ang-(1–7)/creatinine in adolescence predicted higher blood pressure in young adulthood ~5 years later.21 However, these findings have not been consistently demonstrated, likely due to significant methodological differences between studies.42
Potential Role of the RAAS in COVID-19
The unique interaction of SARS-CoV-2 with the host cell is the putative link between COVID-19 and hypertension, cardiovascular disease, and kidney disease. As with SARS-CoV, the SARS-CoV-2 spike protein binds to membrane-bound ACE2 on the surface of respiratory epithelial cells to gain cell entry, though SARS-CoV-2 appears to do so with significantly greater affinity.9, 43, 44 Endocytosis of the SARS-CoV-2—ACE2 complex as well as viral-induced ACE2 cell surface shedding and ACE2 downregulation all may contribute to decreased ACE2 expression and activity in infected cells.45, 46 This overall loss of ACE2 functionality could be critically important to COVID-19 pathophysiology, as was suggested in SARS, though this has not yet been confirmed in humans.47, 48
There are two primary theories regarding the role of the RAAS in SARS-CoV-2 infection and COVID-19 pathophysiology. First, SARS-CoV animal models and other experimental data have demonstrated that Ang II-mediated inflammation is a major mediator of acute lung injury and progression to fibrosis.47, 49, 50 As with SARS-CoV, loss of ACE2 expression and activity could increase local Ang II concentration in the lungs and drive COVID-19 acute lung injury (Figure 1D). In patients with acute respiratory distress syndrome, ACE and ACE2 activity from bronchoalveolar lavage fluid correlated with inflammatory markers.51 One study demonstrated significantly higher circulating Ang II concentrations in patients with COVID-19 that correlated with viral load and indices of lung injury, though this study had significant methodological limitations.37, 48, 52 Data from the original SARS-CoV epidemic also suggest that SARS-CoV-2 may cause ACE2-dependent infection of the myocardium leading to decreased cardiac ACE2 expression, facilitating acute heart injury.53 While compelling, it is important to note that we do not yet have adequate clinical data to confirm this hypothesized pathophysiologic process.
Second, there is concern that ACE inhibitors and ARBs theoretically could increase ACE2 expression in the lungs, increasing the risk of acquiring SARS-CoV-2 infection.4, 54 While ACE inhibitors and ARBs can increase kidney and heart ACE2 expression in select experimental animal models10, 55, there are no data demonstrating that these agents definitively increase lung ACE2 expression in animals or humans. Similarly, there are no data to show that the downstream effects of these agents would increase viral infectivity or virulence.
The few clinical studies that have examined the effect of ACE inhibitors and ARBs on ACE2/Ang-(1–7) pathway expression and activity have not demonstrated any consistent association between ACE inhibitor and ARB use and increased ACE2/Ang-(1–7) expression, activity, or concentration in tissue, circulation, or urine.56–59 Interestingly, mineralocorticoid receptor antagonists block aldosterone-induced ACE2 downregulation, so the role of this drug class should be explored as well.60
The scant pediatric data exploring how these medications may affect the ACE2/Ang-(1–7) pathway are limited to children with specific disease processes.34 Among children with IgA nephropathy, RAAS blockade may attenuate increased ACE2 glomerular expression.36 ACE inhibitor use in hypertensive children with chronic kidney disease was associated with higher plasma Ang-(1–7) concentrations, while in patients with primary hypertension, treatment with a calcium channel blocker did not affect plasma Ang II or Ang-(1–7) concentrations.30, 31 Hopefully, the results of an ongoing observational study that is investigating the effect of an ACE inhibitor on circulatory and urinary ACE2/Ang-(1–7) and ACE/Ang II in children with primary hypertension (NCT03310684) will shed additional light on these complex relationships.
Despite the lack of evidence to support the role of ACE inhibitor/ARB use on ACE2 expression and SARS-CoV-2 infectivity, the majority of experimental evidence actually supports the notion that ACE inhibitors and ARBs may attenuate Ang II-driven acute lung injury and fibrosis by reducing the actions of Ang II relative to Ang-(1–7) (Figure 1E).48, 61, 62 As such, these agents offer promise as potential novel therapies to treat COVID-19.47, 49, 63, 64 Fortunately, several ongoing clinical trials in adults aim to inform these decisions by investigating the impact of withdrawal versus continuation of these medications in patients with COVID-19 (NCT04338009) and by determining the role of ARBs in treating COVID-19 (NCT04311177 and NCT04312009). While results from these trials and ongoing robust observational studies are not yet available, at a minimum the available data support the continued use of these pharmacologic agents among those who need them. In fact, in light of the many serious potential consequences of unnecessarily stopping ACE inhibitors and ARBs, numerous commentaries and hypertension/cardiovascular/kidney society statements have endorsed the continued use of these important medications until evidence emerges to the contrary.1, 65–69
Clinical Impact and Future Steps
At this time, there is no evidence that children with hypertension, cardiovascular disease, or chronic kidney disease, and/or those who are taking ACE inhibitors or ARBs, are at increased risk of SARS-CoV-2 infection or more-severe COVID-19. Given the proven benefits of these medications in all patients, and the unique circumstances that make these agents particularly beneficial for youth with these chronic conditions, we support the recommendations of many scientific societies affirming the continued use of ACE inhibitors and ARBs in accordance with current guidelines.68 Ongoing and planned research studies should include pediatric patients to both better characterize the important questions raised above and to identify any pediatric-specific nuances in the results, including the mechanisms behind why children may be at decreased risk of SARS-CoV-2 infection and COVID-19. Future research into RAAS expression in children, especially the ACE2/Ang-(1–7) pathway, is urgently needed as there remain significant knowledge gaps.
Conclusions
ACE2 is critical to SARS-CoV-2 infection, and an imbalance in the RAAS, with a shift towards ACE/Ang II and suppression of ACE2/Ang-(1–7), may be an important mediator of COVID-19 pathophysiology. Given its importance to cardiovascular and kidney physiology, the RAAS, and the differences therein between children and adults, could shed light on potential relationships between ACE inhibitor and ARB use and COVID-19. Further investigations in both children and adults to define risk factors and determine pathophysiology of SARS-CoV-2 and COVID-19 are of utmost importance given the high prevalence of hypertension and use of ACE inhibitors and ARBs.
Funding Source:
AMS has funding from NIH relevant to this work (R01HL146818 and Loan Repayment Program Award).
Abbreviations:
- ACE
angiotensin-converting enzyme
- ACE2
angiotensin-converting enzyme 2
- Ang II
angiotensin II
- Ang-(1–7)
angiotensin-(1–7)
- ARB
angiotensin II receptor blocker
- COVID-19
coronavirus disease 2019
- RAAS
renin-angiotensin-aldosterone system
- SARS-CoV
severe acute respiratory syndrome coronavirus
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Footnotes
Potential Conflicts of Interest: None.
References
- 1.South AM, Tomlinson L, Edmonston D, Hiremath S, Sparks MA. Controversies of renin–angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol. 2020; doi: 10.1038/s41581-020-0279-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Ferranti SD, Steinberger J, Ameduri R, Baker A, Gooding H, Kelly AS, Mietus-Snyder M, Mitsnefes MM, Peterson AL, St-Pierre J, Urbina EM, Zachariah JP, Zaidi AN. Cardiovascular Risk Reduction in High-Risk Pediatric Patients: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e603–e634. [DOI] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. J Am Med Assoc. 2020; doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019 — United States, February 12–March 28, 2020. Morb Mortal Wkly Rep. 2020;69:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Kibria GM, Nemirovsky A, Sharmeen A, Day B. Age-stratified prevalence, treatment status, and associated factors of hypertension among US adults following application of the 2017 ACC/AHA guideline. Hypertens Res. 2019;42:1631–1643. [DOI] [PubMed] [Google Scholar]
- 9.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol. 2020;doi: 10.1128/JVI.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. [DOI] [PubMed] [Google Scholar]
- 11.Yang G, Tan Z, Zhou L, Yang M, Peng L, Liu J, Cai J, Yang R, Han J, Huang Y, He S. Angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors usage is associated with improved inflammatory status and clinical outcomes in COVID-19 patients with hypertension [preprint]. medRxiv 2020;doi 10.1101/2020.03.31.20038935 %J medRxiv [DOI] [PubMed] [Google Scholar]
- 12.Bean D, Kraljevic Z, Searle T, Bendayan R, Pickles A, Folarin A, Roguski L, Noor K, Shek A, o’gallagher K, Zakeri R, Shah A, Teo J, Dobson RJ. Treatment with ACE-inhibitors is associated with less severe disease with SARS-Covid-19 infection in a multi-site UK acute Hospital Trust [preprint]. medRxiv. 2020;doi: 10.1101/2020.04.07.20056788 %J medRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coronavirus Disease 2019 in Children — United States, February 12–April 2, 2020. Morb Mortal Wkly Rep. 2020;69:422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, Zhang W, Wang Y, Bao S, Li Y, Wu C, Liu H, Liu D, Shao J, Peng X, Yang Y, Liu Z, Xiang Y, Zhang F, Silva RM, Pinkerton KE, Shen K, Xiao H, Xu S, Wong GWK. SARS-CoV-2 infection in children. N Engl J Med. 2020; doi: 10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tagarro A, Epalza C, Santos M, Sanz-Santaeufemia FJ, Otheo E, Moraleda C, Calvo C. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. 2020; doi: 10.1001/jamapediatrics.2020.1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song P, Zhang Y, Yu J, Zha M, Zhu Y, Rahimi K, Rudan I. Global prevalence of hypertension in children: a systematic review and meta-analysis. JAMA Pediatr. 2019;173:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012;27:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon EY, Cohn L, Rocchini A, Kershaw D, Freed G, Ascione F, Clark S. Antihypertensive prescribing patterns for adolescents with primary hypertension. Pediatrics. 2012;129:e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Binka E, Mendley S, Gaskin P, Himes C, Jinadu L, Baker-Smith CM. Description of antihypertensive medication use in a pediatric practice: Single and multiple antihypertensive medication therapy. J Clin Hypertens. 2017;19:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simões e Silva AC, Flynn JT. The renin-angiotensin-aldosterone system in 2011: role in hypertension and chronic kidney disease. Pediatr Nephrol. 2012;27:1835–1845. [DOI] [PubMed] [Google Scholar]
- 21.South AM, Shaltout HA, Washburn LK, Hendricks AS, Diz DI, Chappell Mark C. Fetal programming and the angiotensin-(1–7) axis: a review of the experimental and clinical data. Clin Sci. 2019;133:55–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49:185–192. [DOI] [PubMed] [Google Scholar]
- 23.Yousif MHM, Benter IF, Diz DI, Chappell MC. Angiotensin-(1–7)-dependent vasorelaxation of the renal artery exhibits unique angiotensin and bradykinin receptor selectivity. Peptides. 2017;90:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DelliPizzi A, Hilchey Sean D, Bell‐Quilley Caroline P. Natriuretic action of angiotensin(1–7). Br J Pharmacol. 1994;111:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold AC, Gallagher PE, Diz DI. Brain renin–angiotensin system in the nexus of hypertension and aging. Hypertens Res. 2013;36: 10.1038/hr.2012.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masi S, Uliana M, Virdis A. Angiotensin II and vascular damage in hypertension: role of oxidative stress and sympathetic activation. Vascul Pharmacol. 2019;115:13–17. [DOI] [PubMed] [Google Scholar]
- 27.Young CN, Davisson RL. Angiotensin-II, the brain, and hypertension: an update. Hypertension. 2015;66:920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.South AM, Nixon PA, Chappell MC, Diz DI, Russell GB, Jensen ET, Shaltout HA, O’Shea TM, Washburn LK. Association between preterm birth and the renin–angiotensin system in adolescence: influence of sex and obesity. J Hypertens. 2018;36:2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simões e Silva AC, Teixeira MM. ACE inhibition, ACE2 and angiotensin-(1–7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol Res. 2016;107:154–162. [DOI] [PubMed] [Google Scholar]
- 30.Simões e Silva AC, Diniz JSS, Regueira Filho A, Santos RAS. The renin angiotensin system in childhood hypertension: selective increase of angiotensin-(1–7) in essential hypertension. J Pediatr. 2004;145:93–98. [DOI] [PubMed] [Google Scholar]
- 31.Simões e Silva AC, Diniz JSS, Pereira RM, Pinheiro SVB, Santos RAS. Circulating renin angiotensin system in childhood chronic renal failure: marked increase of angiotensin-(1–7) in end-stage renal disease. Pediatr Res. 2006;60:734–739. [DOI] [PubMed] [Google Scholar]
- 32.South AM, Arguelles L, Finer G, Langman CB. Race, obesity, and the renin-angiotensin-aldosterone system: treatment response in children with primary hypertension. Pediatr Nephrol. 2017;32:1585–1594. [DOI] [PubMed] [Google Scholar]
- 33.Hamrahian SM, Falkner B. Hypertension in chronic kidney disease. Adv Exp Med Biol. 2017;956:307–325. [DOI] [PubMed] [Google Scholar]
- 34.Suessenbach FK, Burckhardt BB. Levels of angiotensin peptides in healthy and cardiovascular/renal-diseased paediatric population-an investigative review. Heart Fail Rev. 2019;24:709–723. [DOI] [PubMed] [Google Scholar]
- 35.Mizuiri S, Hemmi H, Arita M, Ohashi Y, Tanaka Y, Miyagi M, Sakai K, Ishikawa Y, Shibuya K, Hase H, Aikawa A. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis. 2008;51:613–623. [DOI] [PubMed] [Google Scholar]
- 36.Urushihara M, Seki Y, Tayama T, Nagai T, Kinoshita Y, Jamba A, Kondo S, Kagami S. Glomerular angiotensin-converting enzyme 2 in pediatric IgA nephropathy. Am J Nephrol. 2013;38:355–367. [DOI] [PubMed] [Google Scholar]
- 37.Chappell MC. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am J Physiol Heart Circ Physiol. 2016;310:H137–H152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malard L, Kakinami L, O’Loughlin J, Roy-Gagnon MH, Labbe A, Pilote L, Hamet P, Tremblay J, Paradis G. The association between the Angiotensin-Converting Enzyme-2 gene and blood pressure in a cohort study of adolescents. BMC Med Genet. 2013;14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epelman S, Shrestha K, Troughton RW, Francis GS, Sen S, Klein AL, Wilson Tang WH. Soluble angiotensin-converting enzyme 2 in human heart failure: relation with myocardial function and clinical outcomes. J Card Fail. 2009;15:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Li J, Hao P, Chen W, Meng X, Li H, Zhang Y, Zhang C, Yang J. Imbalance between angiotensin II and angiotensin-(1–7) in human coronary atherosclerosis. J Renin Angiotensin Aldosterone Syst. 2016;17:1470320316659618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chappell M, Marshall A, Al Zayadneh EM, Shaltout H, Diz D. Update on the angiotensin converting enzyme 2-angiotensin (1–7)-Mas receptor axis: fetal programing, sex differences, and intracellular pathways. Front Endocrinol (Lausanne). 2014;4:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paquette K, Fernandes RO, Xie LF, Cloutier A, Fallaha C, Girard-Bock C, Mian MOR, Lukaszewski M-A, Mâsse B, El-Jalbout R, Lapeyraque A-L, Santos RA, Luu TM, Nuyt AM. Kidney size, renal function, Ang (angiotensin) peptides, and blood pressure in young adults born preterm. Hypertension. 2018;72:918–928. [DOI] [PubMed] [Google Scholar]
- 43.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020; doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo H, Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G-F, Shi Z-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.South AM, Diz D, Chappell MC. COVID-19, ACE2 and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020; doi: 10.1152/ajpheart.00217.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu H, Xie Z, Li T, Zhang S, Lai C, Zhu P, Wang K, Han L, Duan Y, Zhao Z, Yang X, Xing L, Zhang P, Wang Z, Li R, Yu JJ, Wang X, Yang P. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci Rep. 2016;6:19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Molina-Molina M, Abdul-Hafez A, Uhal V, Xaubet A, Uhal BD. Angiotensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schouten LR, van Kaam AH, Kohse F, Veltkamp F, Bos LD, de Beer FM, van Hooijdonk RT, Horn J, Straat M, Witteveen E, Glas GJ, Wieske L, van Vught LA, Wiewel MA, Ingelse SA, Cortjens B, van Woensel JB, Bos AP, Walther T, Schultz MJ, Wösten-van Asperen RM. Age-dependent differences in pulmonary host responses in ARDS: a prospective observational cohort study. Ann Intensive Care. 2019;9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C, Zhang Z, Wang L, Peng L, Chen L, Qin Y, Zhao D, Tan S, Yin L, Xu J, Zhou C, Jiang C, Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watkins J. Preventing a covid-19 pandemic. Br Med J. 2020;368:m810. [DOI] [PubMed] [Google Scholar]
- 55.Ishiyama Y, Gallagher Patricia E, Averill David B, Tallant EA, Brosnihan KB, Ferrario Carlos M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. [DOI] [PubMed] [Google Scholar]
- 56.Epelman S, Tang WHW, Chen SY, Van Lente F, Francis GS, Sen S. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J Am Coll Cardiol. 2008;52:750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furuhashi M, Moniwa N, Mita T, Fuseya T, Ishimura S, Ohno K, Shibata S, Tanaka M, Watanabe Y, Akasaka H, Ohnishi H, Yoshida H, Takizawa H, Saitoh S, Ura N, Shimamoto K, Miura T. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2014;28:15–21. [DOI] [PubMed] [Google Scholar]
- 58.Hu XS, Xie XD, Wang XX, Zeng CL, Ni YM, Yu GW, Chen JZ. [Effects of angiotensin converting enzyme inhibitor on the expression of angiotensin converting enzyme 2 in atrium of patients with atrial fibrillation]. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:625–628. [PubMed] [Google Scholar]
- 59.Lely AT, Hamming I, van Goor H, Navis GJ. Renal ACE2 expression in human kidney disease. J Pathol. 2004;204:587–593. [DOI] [PubMed] [Google Scholar]
- 60.Keidar S, Gamliel-Lazarovich A, Kaplan M, Pavlotzky E, Hamoud S, Hayek T, Karry R, Abassi Z. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ Res. 2005;97:946–953. [DOI] [PubMed] [Google Scholar]
- 61.Supé S, Kohse F, Gembardt F, Kuebler WM, Walther T. Therapeutic time window for angiotensin-(1–7) in acute lung injury. Br J Pharmacol. 2016;173:1618–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zambelli V, Bellani G, Borsa R, Pozzi F, Grassi A, Scanziani M, Castiglioni V, Masson S, Decio A, Laffey JG, Latini R, Pesenti A. Angiotensin-(1–7) improves oxygenation, while reducing cellular infiltrate and fibrosis in experimental Acute Respiratory Distress Syndrome. Intensive Care Med Exp. 2015;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui C-C, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye R, Liu Z. ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp Mol Pathol. 2019;113:104350. [DOI] [PubMed] [Google Scholar]
- 65.Sparks MA, South A, Welling P, Luther JM, Cohen J, Byrd JB, Burrell LM, Batlle D, Tomlinson L, Bhalla V, Rheault MN, Soler MJ, Swaminathan S, Hiremath S. Sound science before quick judgement regarding RAS blockade in COVID-19. Clin J Am Soc Nephrol. 2020; doi: 10.2215/CJN.03530320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with COVID-19. N Engl J Med. 2020; doi: 10.1056/NEJMsr2005760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danser AHJ, Epstein M, Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020; doi: 10.1161/hypertensionaha.120.15082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.The Coronavirus Conundrum: ACE2 and Hypertension Edition. 2020. NephJC. Available at: http://www.nephjc.com/news/covidace2. [10 April 2020] [Google Scholar]
- 69.Kreutz R, Algharably EAE, Azizi M, Dobrowolski P, Guzik T, Januszewicz A, Persu A, Prejbisz A, Riemer TG, Wang JG, Burnier M. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovasc Res. 2020; doi: 10.1093/cvr/cvaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]


