Abstract
Air pollution is a major contributor to cardiovascular morbidity and mortality. Fine particulate air pollution (PM2.5) may be a modifiable risk factor for hypertension. The benefits of in-home air filtration on systolic blood pressure and diastolic blood pressure are unclear. To examine the effects of in-home personal air cleaner use on fine particulate exposure and blood pressure, we queried PubMed, Web of Science, Cochrane Central Register, Inspec, and EBSCO GreenFILE databases for relevant clinical trials. Included studies were limited to non-smoking participants in smoke-free homes with active or sham filtration on indoor fine particulate concentrations and changes in systolic and diastolic blood pressure. Of 330 articles identified, ten trials enrolling 604 participants met inclusion criteria were considered. Over a median 13.5 days, there was a significant reduction of mean systolic blood pressure by nearly 4 mmHg (−3.94 mmHg; 95% CI [−7.00,−0.89]; p = 0.01), but a non-significant difference in mean DBP (−0.95mmHg; 95% CI: [−2.81, 0.91]; p=0.32). Subgroup analyses indicated no heterogeneity of effect by age, level of particulate exposure, or study duration. Given the variation in study design, additional study is warranted to confirm and better quantify the observed benefits in systolic blood pressure found with personal air cleaner use.
Keywords: Fine particulate matter, PM2.5, Personal air cleaner, Air filtration, Systolic blood pressure, Home intervention
Graphical Abstract

Summary
This is the first meta-analysis to assess the effect of personal air cleaner use on reduction of indoor PM2.5 and reduction of blood pressure. Our findings indicate that, across ten trials enrolling 604 participants, personal air cleaners are associated with a significant decrease in systolic blood pressure.
INTRODUCTION
Background:
Ambient air pollution is linked with poor health outcomes and is a major contributor to the global burden of disease. Fine particulate matter less than 2.5 µm in diameter (PM2.5) is strongly associated with cardiovascular morbidity and mortality. In 2016, ambient and household air pollution were together responsible for an estimated 6.1 million deaths globally, the majority of which were due to cardiovascular disease (CVD).1–3 Short-term PM2.5 exposure (hours to weeks) increases the likelihood of adverse cardiovascular events, including myocardial infarction, stroke, and heart failure, and longer-term exposure magnifies that risk. One pathway through which long-term PM exposures may contribute to CVD is by potentiating chronic cardiovascular risk factors, including hypertension.4 Hypertension is a well-established risk factor for CVD.5 Given that individuals spend roughly 80–90% of their time indoors,6,7 improving the indoor environment may be an effective CVD prevention strategy. Outside and indoor PM2.5 levels are correlated, particularly at high levels of PM 2.5 exposure, and the indoor environment contributes substantially to human pollutant exposure.8–11 High-efficiency indoor air filters, or “personal air cleaners” (PACs), have been proposed as an intervention to decrease indoor PM2.5 exposure. Although some trials have demonstrated improvements in systolic and diastolic blood pressure (SBP and DBP, respectively) with indoor air filtration,12,13 evidence remains mixed overall.14–16 Therefore, to evaluate the utility of indoor air filtration on SBP and DBP, we conducted a systematic review and meta-analysis of published trials evaluating effects of in-home PACs on: 1) indoor particulate matter concentrations; and, 2) systolic and diastolic blood pressure in non-smoking adults.
METHODS
The authors declare that all supporting data are available within the article and its online supplementary files.
Search strategy
We conducted a systematic search of the literature in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria17. The search Strategy was developed in consultation with an experienced Medical Librarian (TR) and run by co-author (DW). The search was performed on July 9 2019, and updated on July 23, 2019. We queried the following databases: PUBMED, Web of Science Core Collection, Inspec, EBSCO GreenFILE, and Cochrane Central Register of Controlled Trials (which includes published and unpublished records from ClinicalTrials.gov and the WHO’s International Clinical Trials Registry Platform). The search was not limited by language or publication date. An example of the PubMed Search Strategy is included as an appendix to this article.
Study Inclusion & Exclusion Criteria
We included studies that met all of the following criteria: (1) were randomized controlled trials in humans; (2) evaluated PACs, including high efficiency particulate air (HEPA) filters and electrostatic precipitators; 3) compared indoor PM2.5 during air filtration versus no air filtration (defined as active vs sham filtration); (4) compared blood pressure during active versus sham filtration; (5) took place in a home or residential setting; and, (6) were published in English. One included study reported only levels of Particle Number Concentration (PNC, a measure of ultrafine particles with diameter < 100 nm) in the absence of PM2.5 measurements. Given the large quantity of particulate matter delivered by a single cigarette,18 we excluded studies that enrolled current cigarette smokers, or that were performed in cigarette smoking households.
Article selection
Two authors (DW and JN) screened all articles, independently and in duplicate. Based on the title and abstract, eligible articles were selected if they fulfilled all inclusion and exclusion criteria. If a title and abstract did not provide sufficient detail for a determination, the article was included and progressed to a further round of screening. All selected articles underwent a more rigorous second round of screening, involving a full-text review and assessment of eligibility. At this stage, the reviewers recorded a rationale to justify the exclusion of any article. Discrepancies between the reviewers (DW and JN) were resolved through verbal discussion and consensus (n=2). All final articles were determined to meet all eligibility criteria.
Data extraction
Data were extracted manually and stored in a dedicated spreadsheet. The following study-level data points were extracted: participant characteristics (sex, age, medical history, medications, intervention duration), pre- and post-intervention levels of PM2.5 (or PNC if PM2.5 was not reported), SBP and DBP at baseline and post-intervention, setting and location of air filtration, mode of intervention, and “sham” or control air filtration. If a washout period occurred between intervention and control conditions its duration was recorded.
Outcomes
The primary health outcomes assessed were changes in SBP or DBP in association with PAC use.
Statistical Analysis
The effects of PAC on BP reduction and their 95% confidence intervals (CIs) were estimated using random-effects meta-analysis of study-level data. Heterogeneity in the study estimates were assessed using I2 statistics. Publication bias was assessed by visual inspection of funnel plots, and with Egger’s statistic.19 A sensitivity analysis was performed excluding studies with longer duration to determine consistency of the general findings. Statistical analyses were performed in Review Manager (RevMan) version 5.3 (Cochrane Community) and R version 3.5.2 (R Project for Statistical Computing).
RESULTS
Database Search Strategy
A systematic search of the literature was conducted on July 9 2019, and updated on July 23, 2019. The search retrieved 330 total database records, of which 29 duplicates were removed. Unique studies (n = 301) were initially screened by title and abstract. At this stage, 272 articles were deemed ineligible for failing to meet the inclusion criteria, and one ongoing study20 was excluded due to lack of published data (Figure 1). The remaining 29 articles underwent a full-text review. One study was discarded for including participants who smoked or lived in smoking homes16. Ultimately, we identified 10 studies, enrolling a total of 604 participants, meeting inclusion criteria.14,15,21–28
Figure 1.

Flow Diagram Illustrating the Stages of the Literature Search
Studies’ baseline PM2.5 concentrations were categorized using WHO criteria as summarized in Table 1. Two studies14,21 reported low air pollution exposure levels as defined by WHO air pollution standards 29 (baseline PM2.5 <10 μg/m3); four studies 24,26–28 enrolled participants at “high” levels of exposure, or between WHO recommended levels and WHO Interim Target-1 (IT-1) (baseline PM2.5 between 10–35 μg/m3); and three studies22,23,25 enrolled participants at “extreme” levels of exposure, or above WHO IT-1 levels (baseline PM2.5 >35 μg/m3).29 One study was classified as indeterminate, as PM2.5 was not quantified. Four studies enrolled only healthy college-aged subjects;22,24–26 four studies enrolled older adults age > 50 years (many with cardiopulmonary risk factors, medical comorbidities, or receiving vasoactive medications); 14,21,23,28 and two studies enrolled healthy participants and did not limit enrollment based on age.15,27 Characteristics of the ten included studies are summarized in Table 2. Methods of blood pressure measurement are further synthesized in Supplementary Table S1.
Table 1:
Criteria for Categorizing Study Air Pollution Levels
| PM2.5 Exposure | PM2.5 Range |
|---|---|
| Low | <10 μg/m3 |
| High | 10–35 μg/m3 |
| Extreme | > 35 μg/m3 |
| Indeterminant | Not Reported |
For each study, air pollution levels were categorized as “low”, “high”, “extreme,” or “indeterminate” based on participants’ baseline PM2.5 exposure levels. The WHO air quality guidelines were used to define PM2.5 range levels for each category, as shown above. Studies that did not report PM2.5 levels but instead reported other measures of air pollution such as particle number concentration (PNC) were classified as indeterminant.
Table 2:
Characteristics of Included Studies
| Study ID | n | Population | Intervention | Outcomes | Baseline Air Pollution | Indoor Pollution Decrease (%) | ||
|---|---|---|---|---|---|---|---|---|
| Filter Type | Duration | PM2.5 μg/m3 | PNC #/cm3 | |||||
| Brauner et al, 2008 | 41 | Healthy elderly adults, 60–75 years Copenhagen, Denmark |
HEPA Air purifier: Airshower; Airsonett AB, Ångelholm, Sweden |
2 × 48h; no washout | - SBP, DBP - PM2.5 - PNC |
Low | 7.9 (63%) | 6810 (61.7%) |
| Chen et al, 2015 | 35 | Healthy college-aged (23±2 years) students Shanghai, China |
Electret (model FAP04, 3M Filtrete, 3M Inc.) | 2 × 48h; 14d washout | - SBP, DBP - PM2.5 |
Extreme | 54.9 (57.1%) | ND |
| Chuang et al, 2017 | 200 | Healthy homemakers (30–65 years, mean: 43.4 ± 7.6 years) Taipei, Taiwan | Electret (3M Filtrete, 3M Inc.) | 2 × 12 months; no washout | - SBP, DBP - PM2.5 |
High | 8.6μg (40%) | ND |
| Cui et al, 2018 | 70 | Healthy young adults (22.0 ± 1.6 years) in dormitory housing, Shanghai, China | HEPA + activated carbon + polypropylene mesh Air purifier: Atmosphere®, Amway |
2 × overnight; 14d washout | - SBP, DBP - PM2.5 - PNC |
High | 23.3 (72.4%) | 3622 (59.2%) |
| Karottki et al, 2013 | 48 | Adults (67 ± 6.5 years) living <350 meters from major roadways; 23% taking vasoactive drugs Copenhagen, Denmark |
HEPA | 2 × 14d; no washout | - SBP, DBP - PM2.5 - PNC |
Low | 3.8 (49%) | 2317 (30.2%) |
| Li et al, 2017 | 55 | Healthy college students (mean 20.2±1.3 years) in dormitory housing Shanghai, China |
Electret (3M Filtrete, 3M Inc.) | 2 × 9d; 12d washout | - SBP, DBP - PM2.5 |
Extreme | 38.2 (81.6%) Personal exposure: 28.8 (54.2%) |
ND |
| Lin et al, 2011 | 60 | Healthy college students (median 25 ± 2.3 years) Taipei, Taiwan |
Electret (3M Filtrete, 3M Inc,) | 4 × 48h; 14d washout | - SBP, DBP - PM2.5 |
High | 55.6 (23%) | ND |
| Morishita et al, 2018 | 40 | Older residents of government-subsidized housing, most with chronic comorbidities (79% hypertensive, 25% diabetic) Detroit, USA |
HEPA-type low efficiency filter (air purifier model HAPF30D-U2; Holmes) OR true HEPA (air purifier model HAPF300D-U2; Holmes) | 3 × 3d; 7d washout | - SBP, DBP - PM2.5 |
High | 9.8 (56%) Personal exposure: 6.4 (41%) |
ND |
| Padro-Martinez et al, 2015 | 20 | Middle-aged (≥40y; mean 53.9±9.2 years) publjc housing residents living <200m from an interstate highway. Abundant comorbidities (55% hypertensive). Somerville MA, USA |
HEPA Air purifier: HEPAirX®, Air Innovations, Inc. |
2 × 21d; no washout | - SBP, DBP - PNC |
ND | 4900 (47%) | |
| Shao et al, 2017 | 35 | Seniors with COPD (60%; mean 66.8±7.9 years) and non-COPD partners (40%; mean 65.9±6.9 years) Beijing, China |
HEPA & activated carbon Air purifier: AC4374 and AC4016, Philips Lifestyle Ltd. |
2 × 14d; no washout | - SBP, DBP - PM2.5 |
Extreme | 36 (60%) | - |
Characteristics of all included studies. Per the WHO air quality guidelines, “low” air pollution levels are here defined as PM2.5<10 μm/m3; “high” air pollution levels are defined as 10 μm/m3 < PM2.5 < 35 μm/m3; and “extreme” air pollution levels are defined as PM2.5 > 35 μm/m3.
(Abbreviations: HEPA = High Efficiency Particulate Air; SBP = Systolic Blood Pressure; DBP = Diastolic Blood Pressure; PNC = Particle Number Concentration; ND = No Data)
Outcomes recorded prior to and immediately after intervention
Outcomes of Air Filtration on PM2.5 and Blood Pressure:
For 9 out of 10 included studies, the median ± standard deviation (S.D.) study duration was 9 ± 14.1 days. One longer-term study was included that randomized subjects to one year of sham or air filtration intervention.27 Among all trials, PAC use yielded an absolute PM2.5 reduction of 20.9 μg/m3 (S.D. ± 18.2 μg/m3), and a relative reduction of 55.9% (S.D. ± 17.0%). Across all 10 trials, the use of PACs was associated with a mean SBP reduction of nearly 4 mmHg (−3.94 mmHg; 95% CI [−7.00,−0.89]; p = 0.01), no evidence of a difference in mean DBP (−0.95mmHg; 95% CI: [−2.81, 0.91]; p=0.32), Figure 2. Sensitivity analyses (results not shown) showed consistency with these findings when analysis was restricted to studies designed with SBP as a primary outcome, or which only used ABPM results alone. In a separate sensitivity analysis excluding the one longer-term study of air filtration intervention,30 similar effects were observed on SBP (−3.02mmHg; 95% CI: [−5.90, −0.14]; p = 0.04) and DBP (p=0.96). The finding of SBP reduction with home air filtration was consistent by health status at baseline (no comorbidities vs. chronic comorbidities or risk factors), P interaction = 0.36 (Supplementary Figure S1). Subgroup analyses revealed no heterogeneity of effect when participants were stratified by age (Supplementary Figure S2a), level of PM2.5 exposure (Supplementary Figure S2b), or study duration (data not shown). Due to lack of observed effect for mean reduction in DBP in the primary analysis, subgroup analyses were not performed for effect on DBP.
Figure 2.
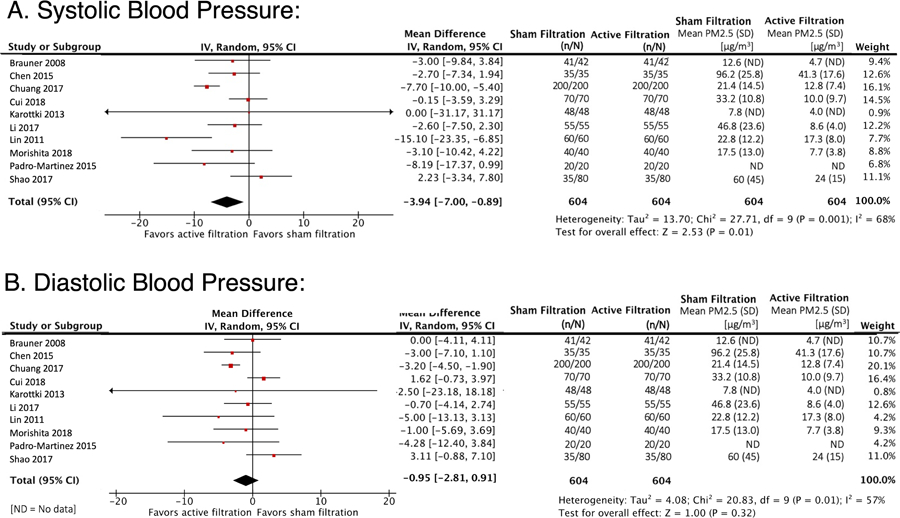
Meta-Analysis of the Effects of Air Filtration on SBP and DBP
Meta-analysis of the effects of indoor air filtration on blood pressure. The mean difference estimates are shown for A) Systolic blood pressure (SBP) and B) Diastolic blood pressure (DBP).
ND = No Data.
Results of Bias Assessment
Using the Cochrane Collaboration Risk of Bias criteria, 8 of 10 studies were classified as having low risk of bias, and 2 of 10 studies as having unclear risk of bias (Supplementary Table S2). Egger’s test did not provide evidence of publication bias among studies assessing the impact of air filtration interventions on SBP (p = 0.74) or DBP (p=0.65). Funnel plots were not markedly asymmetric for either SBP or DBP. Methodologic heterogeneity was present for both SBP (I2 = 68%) and DBP (I2 = 57%).
DISCUSSION
To our knowledge, this is the first meta-analysis to evaluate the effect of in-home PACs on blood pressure. Among ten randomized control trials (RCTs) enrolling over 600 non-smoking participants, the use of PACs over a median 13.5 day duration was associated with a nearly 4 mmHg reduction in SBP, and no evidence of an effect on DBP. This observation was consistent across categories of cardiopulmonary risk factors, medication categories, age, or levels of PM2.5 exposure, suggesting the results of our meta-analysis are similar across different population subgroups. The findings of this meta analysis are notable, as even a small decrease in the distribution of blood pressure in a population may significantly reduce cardiovascular morbidity and mortality. For example, it is estimated that shifting the distribution of SBP in a population downward by 5 mmHg could reduce mortality from stroke by 14%, coronary heart disease by 9%, and all-cause mortality by 7%.31 Thus, although modest, a short-term decrease in SBP of 4mmHg through the use of PACs may have important health benefits if sustainable over the long-term.
While we included studies with a wide range of participants and categories of exposure, studies that included current cigarette smokers as participants were excluded. The quantity of PM2.5 delivered by cigarette smoke vastly exceeds environmental PM2.5 concentrations; therefore, we concluded that the effects of particles and gases produced by smoking may confound any potential benefits from home air filtration.18 Importantly, our finding of a significant reduction in SBP was observed despite inclusion of participants with hypertension, diabetes, and vasoactive medication regimens, suggesting that short-term health effects of in-home air filtration may be widely applicable.
PACs are a low-cost and simple intervention to lower PM2.5 exposure. Individuals in post-industrial societies spend disproportionate amounts of time indoors, and indoor PM2.5 often significantly exceeds outdoor concentrations.32 The mechanisms through which PM2.5 increases blood pressure are not well elucidated. Evidence from human and animal controlled exposure studies suggests that PM2.5 raises blood pressure through 1) increasing inflammation and oxidative stress; 2) impairing endothelial function or; 3) increasing sympathetic activation, while decreasing parasympathetic tone.33 Putative changes in sympathetic and parasympathetic tone may be one pathway for the differential effects of in-home air filtration on SBP compared with DBP observed in this analysis. Further analysis of the study presented Li 2017 showed an association between indoor air filtration and reduction in circulating stress hormones, markers of inflammation, and metabolic activity. These findings suggests that PM2.5 likely influences autonomic signaling to affect HPA axis activation and, subsequently, blood pressure.25
Limitations:
Our study is limited by the relatively small number of published RCTs investigating the effects of home air filtration on blood pressure in non-smokers. We also observed heterogeneity in study characteristics, including intervention duration, participant age and risk factors, composition and concentration of air pollution, proportion of time participants spent in the home, and indoor filtration method. Additionally, only one study (Morishita, 2018) quantified personal-level PM2.5 exposure in addition to absolute indoor PM2.5 concentrations, and many studies restricted participants’ mobility outside the home. Both these factors limit our ability isolated the effect of air filtration interventions on actual exposure reduction in a “real-world” setting. However, sensitivity analysis on the aforementioned parameters demonstrated consistency of effect of indoor air filtration for reductions in SBP. Also, one large study (Chuang, 2017) was very influential in the analysis, but when excluding that study a significant reduction of SBP was still observed (p=0.04). Our analysis found no significant effect on DBP, and it is unclear if this lack of effect on DBP is due to limitations in power, study design or a true biological effect.
Furthermore, only a portion of included trials were designed with blood pressure as a primary outcome; and among these, most were powered to detect changes in SBP specifically (as opposed to DBP). It is probable that several studies were not adequately powered to detect significant reduction of SBP and especially DBP. We also recognize that variations in the method of blood pressure assessment may be confounding in this relationship; Supplementary Table S2 shows these parameters in further detail.
Finally, the majority of included studies assess only the immediate impact of short-term (≤2 weeks) air filtration interventions. It is unknown whether these effects are sustained following termination of the intervention. Finally, we can only speculate on the effects of longer-term interventions on blood pressure and reduction in cardiovascular risk.
PERSPECTIVES
Although improving outdoor air quality is the first priority against ambient air pollution health impacts, this meta-analysis found that in-home air filtration significantly reduced indoor exposures to PM2.5 air pollution and SBP. PACs, a low-cost and generally accessible intervention, may represent an effective cardioprotective intervention for physicians to recommend to populations especially at risk. However, further investigation is needed to identify the optimal populations, durations and conditions for sustained use of indoor air filtration as a potential novel strategy to reduce cardiovascular risk.
Supplementary Material
Novelty and Significance.
What is new?
To our knowledge, this is the first meta-analysis to examine the effect of personal air cleaners on blood pressure.
Personal air cleaner use is associated with a significant decrease in systolic blood pressure.
No change in diastolic blood pressure associated with personal air cleaner use was observed.
What is relevant?
Indoor exposure to fine particulate matter may be a modifiable risk factor for hypertension.
Acknowledgments
Funding Sources: NONE.
Disclosures:
Dalia Walzer, BS: NONE.
Terry Gordon, PhD: NONE.
Lorna Thorpe, PhD was supported in this work in part by the National Cancer Institute award R01CA22059.
George Thurston, ScD was supported in this work in part by the NYU-NIEHS Center of Excellence (NIH Grant ES00260).
Yuhe Xia, MS: NONE.
Hua Zhong, PhD was supported in this work in part by the NIH awards R01AG054467 and UL1TR001445.
Timothy R. Roberts, MPH: NONE.
Judith S. Hochman, MD: NONE.
Jonathan D. Newman, MD MPH reports funding from the National Institute of Health K23HL125991.
REFERENCE SECTION
- 1.Babatola SS. Global burden of diseases attributable to air pollution. J Public Health Afr. 2018;9(3):813–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen AJ, Brauer M, Burnett R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389(10082):1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadley MB, Baumgartner J, Vedanthan R. Developing a Clinical Approach to Air Pollution and Cardiovascular Health. Circulation. 2018;137(7):725–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brook Robert D, Rajagopalan S, Pope CA, et al. Particulate Matter Air Pollution and Cardiovascular Disease. Circulation. 2010;121(21):2331–2378. [DOI] [PubMed] [Google Scholar]
- 5.Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371(9623):1513–1518. [DOI] [PubMed] [Google Scholar]
- 6.Matz CJ, Stieb DM, Davis K, et al. Effects of age, season, gender and urban-rural status on time-activity: CanadianHuman Activity Pattern Survey 2 (CHAPS 2). Int J Environ Res Public Health. 2014;11(2):2108–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klepeis NE, Nelson WC, Ott WR, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11(3):231–252. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Zhao B. Review of relationship between indoor and outdoor particles: I/O ratio, infiltration factor and penetration factor. Atmospheric Environment. 2011;45(2):275–288. [Google Scholar]
- 9.Morawska L, Ayoko GA, Bae GN, et al. Airborne particles in indoor environment of homes, schools, offices and aged care facilities: The main routes of exposure. Environ Int. 2017;108:75–83. [DOI] [PubMed] [Google Scholar]
- 10.Samet JM, Bahrami H, Berhane K. Indoor Air Pollution and Cardiovascular Disease: New Evidence From Iran. Circulation. 2016;133(24):2342–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundell J On the history of indoor air quality and health. Indoor Air. 2004;14 Suppl 7:51–58. [DOI] [PubMed] [Google Scholar]
- 12.Maestas MM, Brook RD, Ziemba RA, et al. Reduction of personal PM2.5 exposure via indoor air filtration systems in Detroit: an intervention study. J Expo Sci Environ Epidemiol. 2019;29(4):484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajagopalan S, Brook RD. Personalizing your airspace and your health. J Am Coll Cardiol. 2015;65(21):2288–2290. [DOI] [PubMed] [Google Scholar]
- 14.Karottki DG, Spilak M, Frederiksen M, et al. An indoor air filtration study in homes of elderly: cardiovascular and respiratory effects of exposure to particulate matter. Environmental Health: A Global Access Science Source. 2013;12(1):3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padro-Martinez LT, Owusu E, Reisner E, et al. A Randomized Cross-over Air Filtration Intervention Trial for Reducing Cardiovascular Health Risks in Residents of Public Housing near a Highway. International Journal of Environmental Research and Public Health. 2015;12(7):7814–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weichenthal S, Mallach G, Kulka R, et al. A randomized double-blind crossover study of indoor air filtration and acute changes in cardiorespiratory health in a First Nations community. Indoor air. 2013;23(3):175–184. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535–b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghio AJ, Hilborn ED, Stonehuerner JG, et al. Particulate matter in cigarette smoke alters iron homeostasis to produce a biological effect. Am J Respir Crit Care Med. 2008;178(11):1130–1138. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J The Use of Air Cleaners to Mitigate Cardiopulmonary Health Impact of Indoor Exposure to Particles and Phthalates. Https://clinicaltrialsgov/show/nct03500614 2018.
- 21.Brauner EV, Forchhammer L, Moller P, et al. Indoor particles affect vascular function in the aged - An air filtration-based intervention study. American Journal of Respiratory and Critical Care Medicine. 2008;177(4):419–425. [DOI] [PubMed] [Google Scholar]
- 22.Chen R, Zhao A, Chen H, et al. Cardiopulmonary benefits of reducing indoor particles of outdoor origin: a randomized, double-blind crossover trial of air purifiers. J Am Coll Cardiol. 2015;65(21):2279–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao D, Du Y, Liu S, et al. Cardiorespiratory responses of air filtration: A randomized crossover intervention trial in seniors living in Beijing: Beijing Indoor Air Purifier StudY, BIAPSY. Sci Total Environ. 2017;603–604:541–549. [DOI] [PubMed] [Google Scholar]
- 24.Lin L-Y, Chen H-W, Su T-L, Hong G-B, Huang L-C, Chuang K-J. The effects of indoor particle exposure on blood pressure and heart rate among young adults: An air filtration-based intervention study. Atmospheric Environment. 2011;45(31):5540–5544. [Google Scholar]
- 25.Li H, Cai J, Chen R, et al. Particulate Matter Exposure and Stress Hormone Levels A Randomized, Double-Blind, Crossover Trial of Air Purification. Circulation. 2017;136(7):618-+. [DOI] [PubMed] [Google Scholar]
- 26.Cui X, Li F, Xiang J, et al. Cardiopulmonary effects of overnight indoor air filtration in healthy non-smoking adults: A double-blind randomized crossover study. Environment International. 2018;114:27–36. [DOI] [PubMed] [Google Scholar]
- 27.Chuang H-C, Ho K-F, Lin L-Y, et al. Long-term indoor air conditioner filtration and cardiovascular health: A randomized crossover intervention study. Environment International. 2017;106:91–96. [DOI] [PubMed] [Google Scholar]
- 28.Morishita M, Adar SD, D’Souza J, et al. Effect of Portable Air Filtration Systems on Personal Exposure to Fine Particulate Matter and Blood Pressure among Residents in a Low-Income Senior Facility: a Randomized Clinical Trial. JAMA internal medicine. 2018:E1–E8. [DOI] [PMC free article] [PubMed]
- 29.WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide: global update 2005: summary of risk assessment. https://apps.who.int/iris/handle/10665/69477: World Health Organization;2006. [PubMed]
- 30.Liu C, Fuertes E, Tiesler CM, et al. The associations between traffic-related air pollution and noise with blood pressure in children: results from the GINIplus and LISAplus studies. Int J Hyg Environ Health. 2014;217(4–5):499–505. [DOI] [PubMed] [Google Scholar]
- 31.Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. Jama. 2002;288(15):1882–1888. [DOI] [PubMed] [Google Scholar]
- 32.Batterman S, Du L, Mentz G, et al. Particulate matter concentrations in residences: an intervention study evaluating stand-alone filters and air conditioners. Indoor Air. 2012;22(3):235–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giorgini P, Di Giosia P, Grassi D, Rubenfire M, Brook RD, Ferri C. Air Pollution Exposure and Blood Pressure: An Updated Review of the Literature. Curr Pharm Des. 2016;22(1):28–51. [DOI] [PubMed] [Google Scholar]
- 34.Muntner P, Shimbo D, Carey RM, et al. Measurement of Blood Pressure in Humans: A Scientific Statement From the American Heart Association. Hypertension. 2019;73(5):e35–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pucci G, Cheriyan J, Hubsch A, et al. Evaluation of the Vicorder, a novel cuff-based device for the noninvasive estimation of central blood pressure. J Hypertens. 2013;31(1):77–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


