Abstract
Objective
Patients with chronic obstructive pulmonary disease (COPD) are known to be at risk of osteoporosis. The purpose of this study was to evaluate the association between thoracic vertebral bone density measured on chest CT (DThorax) and clinical variables, including survival, in patients with COPD.
Materials and Methods
A total of 322 patients with COPD were selected from the Korean Obstructive Lung Disease (KOLD) cohort. DThorax was measured by averaging the CT values of three consecutive vertebral bodies at the level of the left main coronary artery with a round region of interest as large as possible within the anterior column of each vertebral body using an in-house software. Associations between DThorax and clinical variables, including survival, pulmonary function test (PFT) results, and CT densitometry, were evaluated.
Results
The median follow-up time was 7.3 years (range: 0.1–12.4 years). Fifty-six patients (17.4%) died. DThroax differed significantly between the different Global Initiative for Chronic Obstructive Lung Disease stages. DThroax correlated positively with body mass index (BMI), some PFT results, and the six-minute walk distance, and correlated negatively with the emphysema index (EI) (all p < 0.05). In the univariate Cox analysis, older age (hazard ratio [HR], 3.617; 95% confidence interval [CI], 2.119–6.173, p < 0.001), lower BMI (HR, 3.589; 95% CI, 2.122–6.071, p < 0.001), lower forced expiratory volume in one second (FEV1) (HR, 2.975; 95% CI, 1.682–5.262, p < 0.001), lower diffusing capacity of the lung for carbon monoxide corrected with hemoglobin (DLCO) (HR, 4.595; 95% CI, 2.665–7.924, p < 0.001), higher EI (HR, 3.722; 95% CI, 2.192–6.319, p < 0.001), presence of vertebral fractures (HR, 2.062; 95% CI, 1.154–3.683, p = 0.015), and lower DThorax (HR, 2.773; 95% CI, 1.620–4.746, p < 0.001) were significantly associated with all-cause mortality and lung-related mortality. In the multivariate Cox analysis, lower DThorax (HR, 1.957; 95% CI, 1.075–3.563, p = 0.028) along with older age, lower BMI, lower FEV1, and lower DLCO were independent predictors of all-cause mortality.
Conclusion
The thoracic vertebral bone density measured on chest CT demonstrated significant associations with the patients' mortality and clinical variables of disease severity in the COPD patients included in KOLD cohort.
Keywords: Chest CT, Chronic obstructive pulmonary disease, Osteoporosis, Bone density, Vertebral body
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a condition that primarily affects the lung (1,2,3). COPD also has adverse effects on extrapulmonary organs and osteoporosis is one of the most significant comorbidities (4). The prevalence of osteoporosis is reported to be 4–59% in COPD patients (5). Patients with osteoporosis are at a higher risk of bone fractures that may result in increased morbidity and mortality. The rib fractures or vertebral compression fractures and the resultant kyphosis have been reported to reduce pulmonary function in COPD patients and could contribute to patients' mortality in those with COPD exacerbation (6,7,8). The current gold standard for diagnosing osteoporosis is dual-energy X-ray absorptiometry (DXA) and DXA of the lumbar spine and hip is currently the most commonly used reference standard (9,10).
Recently, computed tomography (CT) covering various body parts has been suggested to be a useful method for identifying patients with osteoporosis (11,12,13). These studies have shown that the simple measurement of CT attenuation of the trabecular regions of the vertebral bodies is well-correlated with the bone mineral density (BMD) measured using DXA. Moreover, Romme et al. (14) demonstrated that CT-measured thoracic vertebral bone density on chest CT correlated strongly with BMD on DXA in patients with COPD. Considering that chest CT is widely used for assessing structural changes in COPD patients, chest CT may be a useful and safe strategy for simultaneously assessing bone health status by measuring the CT attenuation of the thoracic spine in COPD patients who are at high risk of osteoporosis. However, most studies with epidemiologic data suggesting an association between COPD and osteoporosis have evaluated bone health status using the BMD on DXA (6,15,16,17).
Therefore, in this study, we aimed to assess the association between thoracic vertebral bone density measured on chest CT (DThorax), and clinical variables and patient mortality in patients with COPD.
MATERIALS AND METHODS
Patients
This retrospective study was approved by the Institutional Review Board of our medical center and written informed consent was obtained from all patients. All patients were selected from the Korean Obstructive Lung Disease (KOLD) cohort. The details of the design of the KOLD study have been documented previously (18); this cohort included patients with COPD prospectively recruited from 17 hospitals in South Korea between June 2005 and October 2013. From this cohort, 322 patients enrolled between May 2005 and October 2011 were included in this study. Patients were excluded if they had co-existing significant illnesses (e.g., malignancy, congestive heart failure, chronic renal failure, diabetes with severe complications, and significant inflammatory diseases such as rheumatoid arthritis), a recent exacerbation, or other respiratory illness (18).
Clinical Characteristics
The baseline clinical information including smoking history, body mass index (BMI), and use of inhaled corticosteroid (ICS) and oral corticosteroid, as well as survival information, were documented in all patients. The pulmonary function test (PFT) with spirometry was performed as recommended by the American Thoracic Society (Vmax 22, Sensor-Medics, Yorba Linda, CA, USA or PFDX machine, MedGraphics, St Paul, MN, USA) (19). The diffusing capacity of the lung for carbon monoxide corrected with hemoglobin (DLCO) and a six-minute walk distance (6MWD) were also determined (20,21). To assess the effect of systemic inflammation on DThorax, the blood platelet count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) level were determined as biomarkers of systemic inflammation (4,22,23). These examinations were performed on the same day or within two weeks of the chest CT scan.
CT Acquisition
All patients underwent volumetric chest CT scans during full inspiration using one of the following scanners: Somatom Sensation 16 (Siemens Healthineers, Erlangen, Germany) (n = 239), Brilliance 16 (Philips Healthcare, Best, Netherlands) (n = 52), or Brilliance CT 40-channel (Philips Healthcare) (n = 31). Scan parameters were 100–133 mAs, 140 kVp, 0.75 or 0.625 collimation, and pitch 1.0. CT data were reconstructed at a 0.75-mm slice thickness and 0.7-mm increment using a B30f kernel for the Somatom Sensation 16 CT and a 0.8-mm slice thickness and 0.8-mm increment using a standard reconstruction algorithm for the other two CT scanners. CT scanners were calibrated every week using an The American Association of Physicists in Medicine (AAPM) standard phantom. Patients were scanned in the supine position from the thoracic inlet to the lung base.
Measurement of CT Attenuation of the Thoracic Spine
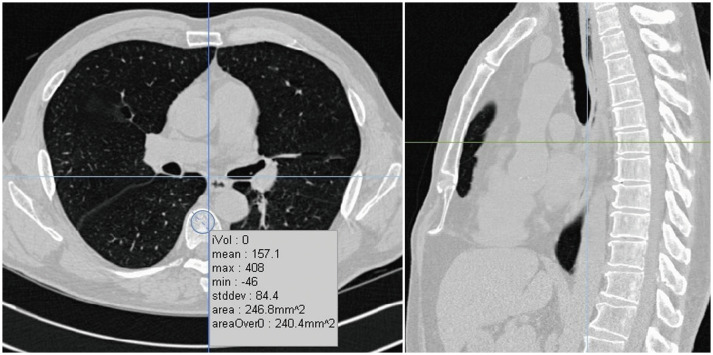
To obtain DThorax, the CT attenuations of three consecutive vertebral bodies were measured at the level of the left, main coronary artery as described previously (24,25,26) from the chest CT images using an in-house software. After inspecting the sagittal and coronal reconstructed images with a 5-mm slice thickness, the central section of axial CT images with a 3-mm slice thickness at each vertebral level were selected. In this selected slice, a round region of interest (ROI) as large as possible was manually set to encompass the anterior portion of each vertebral body (Fig. 1). The center of the ROI was located at the center of the vertebrae 2–3 mm from the spinal cortical bone. The cortical bone area, large veins, bone islands, and calcified herniated disks were excluded from the ROI using manual tracing. Measurement of DThorax was performed by a radiographer with more than 10 years of experience in CT image analysis. The appropriateness of the location of the ROI in the vertebral body was checked by two radiologists. DThorax was calculated by averaging the measured CT attenuations of three consecutive vertebral bodies. If there were compression fractures among the three consecutive vertebral bodies, CT attenuation was measured in the vertebral bodies following the vertebral bodies with compression fractures. Additionally, the DThorax of a random sample of 89 patients was measured by a radiologist to evaluate the interobserver variability of the measurements.
Fig. 1. Measurement of DThorax.
After inspecting sagittal and coronal reconstructed images, central section of axial CT images at each vertebral level were selected. In this selected slice, round region of interest as large as possible was manually set to encompass anterior portion of each vertebral body. Cortical bone area, with large veins, as well as calcified herniated disks, were excluded from region of interest using manual free tracing. DThorax = thoracic vertebral bone density measured on chest CT
Assessment of Vertebral Fractures and Other CT Density Variables
Vertebral compression fracture and other CT density variables were evaluated using commercial software (Aview; Coreline Soft, Seoul, Korea). An observer blinded to the DThorax results assessed the presence of vertebral compression fractures in the T1-L1 vertebrae in the sagittal reconstructed CT images using Genant's method (27). For CT density variables related to COPD, the volume fraction of the lung below -950 HU was calculated automatically and termed the emphysema index (EI). CT-based metrics of airway disease, i.e., wall thickness, wall area (WA), lumen area (LA), and wall area percentage (WA%), were measured at the right, apical, subsegmental bronchus using a full-width-half-maximum method. WA% was defined as WA / (WA + LA) × 100 (28).
Statistical Analysis
The interobserver variation in the measurement of DThorax was assessed using the intra-class correlation coefficient (ICC). Differences in measured DThorax, according to the global initiative for obstructive lung disease (GOLD) stage were analyzed using one-way analysis of variance with a post-hoc analysis (Tukey's honestly significant difference). Pearson correlation coefficients were calculated in order to assess the correlations between the DThorax and other CT and clinical variables. To determine the predictors of DThorax, multiple regression analysis with stepwise regression was performed. Univariate and multivariate Cox proportional hazards regression analyses were performed to investigate the association between DThorax and mortality in COPD patients with forward stepwise selection. As there are no generally accepted optimal cut-off values for risk factors involving continuous variables for differentiating these patient groups, the optimal cut-off values of DThorax and BMI that yielded the greatest statistical difference in survival were generated (29). For other variables, we stratified patients according to methods used in previous studies (30,31). A survival analysis was performed using Kaplan-Meier estimates. A p value < 0.05 was defined as being statistically significant. All statistical analyses were performed using SPSS software version 21.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Baseline Characteristics
The baseline characteristics are summarized in Table 1. The median follow-up time was 7.3 years (range: 0.2–12.4 years). When classified using the GOLD criteria for COPD severity, most patients were categorized as GOLD stage II. Fifty-six patients (17.4%) died and in 31 of those 56 patients, death was lung-related, including respiratory failure (13 patients), pneumonia (10 patients), lung cancer (six patients), sepsis (one patient), and pneumothorax (one patient). The other causes of death were non-lung cancer malignancy (seven patients), cardiovascular disease (six patients), suicide (one patient), and unknown (11 patients). The median patient survival time was 4.9 years. ESR values were available in 198 patients and CRP values were available in 116 patients. The mean laboratory data values were within the normal range. ESR was elevated in 14 of 198 patients, CRP in five of 116 patients, and platelet count in two patients. CRP and ESR were both elevated in two patients.
Table 1. Patient Characteristics and Summary of PFTs, and Laboratory Data, and CT Indices in Patients with COPD.
| Variables | Values |
|---|---|
| Patient characteristics | |
| No. of patients | 322 |
| Sex ratio (M/F) (n %) | 298/24 (92.5/7.5) |
| Age (years) | 65.6 ± 7.7 |
| BMI (kg/m2) | 22.9 ± 4.3 |
| Smoking history (pack/year) | 44.7 ± 26.9 |
| PFT | |
| FEV1 (% predicted) | 61.9 ± 20.3 |
| FVC (% predicted) | 91.0 ± 19.4 |
| FEV1/FVC | 48.2 ± 12.4 |
| DLCO (% predicted) | 74.3 ± 26.2 |
| 6MWD (m) | 438.4 ± 92.2 |
| GOLD COPD stage (n %) | |
| I | 56 (17.4) |
| II | 167 (51.9) |
| III | 82 (25.5) |
| IV | 17 (5.3) |
| Baseline use of steroid | |
| Current use of ICS (n %) | 95 (29.5) |
| Current use of OCS for respiratory disease (n %) | 7 (2.2) |
| Duration of use of ICS (days) | 255.0 ± 438.7 (range: 0−1981) |
| Use of ICS last 6 months (n %) | 28 (8.7) |
| Thoracic vertebral compression fracture (n %) | 63 (19.6) |
| Laboratory data | |
| Platelet (x 103/μL) | 241.34 ± 61.99 |
| ESR (mm/hr)* | 9.79 ± 8.93 |
| CRP (mg/dL)† | 0.73 ± 2.01 |
| CT index | |
| DThorax (HU) | 128.38 ± 49.89 |
| EI (%) | 22.1 ± 15.4 |
| WA% | 66.8 ± 6.2 |
All data are given as mean ± standard deviation except for sex ratio and GOLD stage. *ESR was available in 198 of 322 patients, †CRP was available in 116 of 322 patients. BMI = body mass index, COPD = chronic obstructive pulmonary disease, CRP = C-reactive protein, DLCO = diffusing capacity of lung for carbon monoxide corrected with hemoglobin, DThorax = thoracic vertebral bone density measured on chest CT, EI = emphysema index, ESR = erythrocyte sedimentation rate, FEV1 = forced expiratory volume in one second, FVC = forced vital capacity, GOLD = global initiative for obstructive lung disease, HU = Hounsfield unit, ICS = inhaled corticosteroid, OCS = oral corticosteroid, PFT = pulmonary function test, WA = wall area, WA% = wall area percentage, 6MWD = six-minute walk distance
Correlations between DThorax and Other CT and Clinical Variables
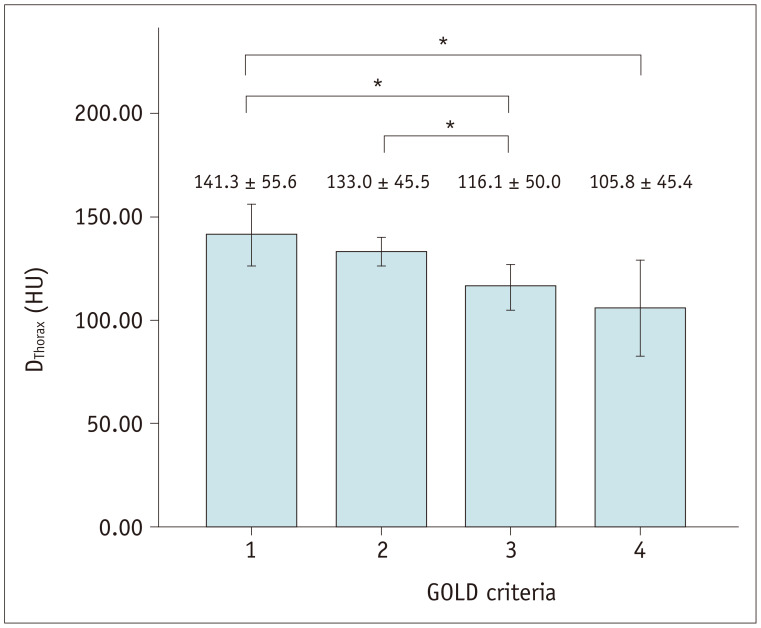
The mean DThorax on baseline chest CT was 128 ± 50 HU. The inter-observer ICC for the measurement of DThorax was good at 0.970 (95% confidence interval [CI], 0.955–0.981). DThorax differed significantly between different GOLD stages (p = 0.003) and patients with a higher GOLD stage had a lower DThorax (Fig. 2). The mean DThorax was not significantly different between baseline ICS users and non-ICS users (128 ± 47 vs. 129 ± 50, p = 0.835) and was significantly lower in the patients with vertebral fractures (99 ± 45 vs. 136 ± 47, p < 0.001). DThorax was negatively correlated with patient age (r = −0.247) and positively correlated with BMI (r = 0.176; all p < 0.05). DThorax showed a significant positive correlations with some PFT results, including forced expiratory volume in one second (FEV1) (r = 0.179), FEV1/forced vital capacity (r = 0.213), DLCO (r = 0.183), and 6MWD (r = 0.299; all p ≤ 0.001). DThorax was negatively correlated with some blood laboratory data, including platelet count (r = −0.143) and ESR (r = −0.221), as well as a quantitative CT variable, EI (r = −0.139; all p < 0.05) (Table 2). In the multiple regression analysis of DThorax using patient age, BMI, PFT, platelet count, and CT variables, older patient age and lower 6MWD were significantly associated with lower DThorax (Table 3).
Fig. 2. DThorax according to GOLD severity stage.
Mean DThorax was significantly different between different GOLD stages. Patients with higher GOLD stage had lower DThorax (*). Post-hoc analysis showed statistically significant difference. GOLD = global initiative for obstructive lung disease
Table 2. Correlation between CT Thoracic Vertebral Bone Density and Clinical Characteristics, PFT, Six-Minute Walk Test, Blood Laboratory Data, and Other CT Measurements.
| Variables | r Value | P |
|---|---|---|
| Patient age | -0.247 | < 0.001 |
| BMI (kg/m2) | 0.176 | 0.002 |
| Smoking history (pack-year) | 0.018 | 0.763 |
| PFT | ||
| FEV1 (% predicted) | 0.179 | 0.001 |
| FVC (% predicted) | 0.066 | 0.567 |
| FEV1/FVC | 0.213 | < 0.001 |
| DLCO (% predicted) | 0.183 | < 0.001 |
| 6MWD | 0.299 | < 0.001 |
| Duration of use of ICS | -0.006 | 0.912 |
| Blood laboratory data | ||
| Platelet | -0.143 | 0.011 |
| ESR* | -0.221 | 0.002 |
| CRP† | -0.117 | 0.211 |
| CT measurements | ||
| EI (%) | -0.139 | 0.013 |
| WA% | -0.038 | 0.495 |
*ESR was available in 198 of 322 patients, †CRP was available in 116 of 322 patients.
Table 3. Multivariable Predictor of CT Thoracic Vertebral Bone Density.
| Variables | Standardized β-Coefficients | P | r2 |
|---|---|---|---|
| Patient age | -0.167 | 0.010 | 0.159 |
| BMI (kg/m2) | 0.066 | 0.345 | |
| Smoking history (pack-year) | 0.027 | 0.661 | |
| FEV1 (% predicted) | 0.065 | 0.460 | |
| FEV1/FVC | 0.102 | 0.310 | |
| DLCO (% predicted) | 0.118 | 0.155 | |
| 6MWD | 0.149 | 0.033 | |
| EI (%) | 0.093 | 0.367 | |
| Platelet | -0.109 | 0.069 |
ESR and CRP were excluded in multiple regression analysis due to missing value.
Associations between DThorax and Patient Mortality
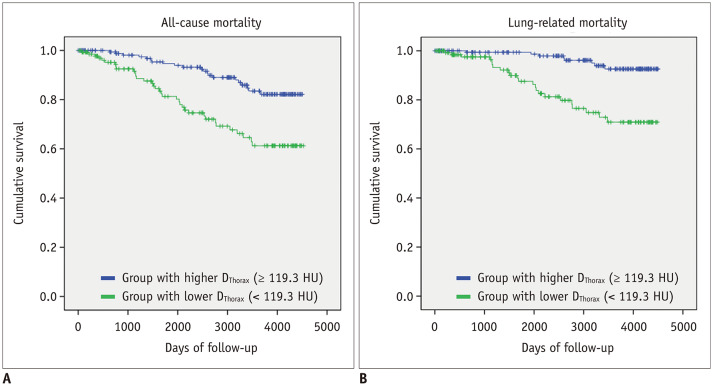
The mean DThorax was significantly different between survivors (133 ± 49 HU) and non-survivors (109 ± 51 HU; p = 0.001). In the univariate Cox regression analysis, older patient age (hazard ratio [HR], 3.617; 95% CI, 2.119–6.173), lower BMI (HR, 3.589; 95% CI, 2.122–6.071), lower FEV1 (HR, 2.975; 95% CI, 1.682–5.262), lower DLCO (HR, 4.595; 95% CI, 2.665–7.924), higher EI (HR, 3.722; 95% CI, 2.192–6.319), thoracic vertebral fracture (HR, 2.062; 95% CI, 1.154–3.683), and lower DThorax (HR, 2.773; 95% CI, 1.620–4.746) were significantly associated with all-cause patient mortality (Table 4). The same variables also significantly predicted lung-related mortality (Table 5). Patient sex was not a significant predictor of mortality. In the multivariate Cox regression analysis, DThorax (HR, 1.957; 95% CI, 1.075–3.563, p = 0.028) along with patient age (HR, 3.186; 95% CI, 1.788–5.679, p < 0.001), BMI (HR, 2.167; 95% CI, 1.171–4.012, p = 0.014), FEV1 (HR, 2.200; 95% CI, 1.170–4.137, p = 0.014), and DLCO (HR, 2.499; 95% CI, 1.391–4.489, p = 0.002) remained significant predictors of all-cause mortality, and DThorax (HR, 4.345; 95% CI, 1.707–11.060, p = 0.002), patient age, FEV1, EI, and DLCO remained significant predictors of lung-related mortality. Patients with a lower DThorax exhibited significantly inferior survival due to all-cause mortality and lung-related mortality according to the Kaplan-Meier test (log-rank test, p < 0.001, respectively) (Fig. 3).
Table 4. Univariate and Multivariate Cox Regression Analysis of Prognostic Factors for All-Cause Mortality in Patients with COPD.
| Variables | No. | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (years) | |||||
| > 70 | 81 | 3.617 (2.119–6.173) | < 0.001 | 3.186 (1.788–5.679) | < 0.001 |
| ≤ 70 | 241 | ||||
| Sex | |||||
| Female | 24 | 0.595 (0.145–2.441) | 0.471 | ||
| Male | 298 | ||||
| BMI (kg/m2) | |||||
| < 22.7 | 90 | 3.589 (2.122–6.071) | < 0.001 | 2.167 (1.171–4.012) | 0.014 |
| ≥ 22.7 | 232 | ||||
| FEV1 (% predicted) | |||||
| < 50 | 148 | 2.975 (1.682–5.262) | < 0.001 | 2.200 (1.170–4.137) | 0.014 |
| ≥ 50 | 174 | ||||
| DLCO (% predicted) | |||||
| < 51 | 80 | 4.595 (2.665–7.924) | < 0.001 | 2.499 (1.391–4.489) | 0.002 |
| ≥ 51 | 242 | ||||
| EI (%) | |||||
| > 34 | 80 | 3.722 (2.192–6.319) | < 0.001 | ||
| 0–34 | 242 | ||||
| Vertebral fracture | |||||
| Presence | 63 | 2.062 (1.154–3.683) | 0.015 | ||
| Absence | 259 | ||||
| DThorax (HU) | |||||
| < 119.3 | 142 | 2.773 (1.620–4.746) | < 0.001 | 1.957 (1.075–3.563) | 0.028 |
| ≥ 119.3 | 180 | ||||
CI = confidence interval, HR = hazard ratio
Table 5. Univariate and Multivariate Cox Regression Analysis of Prognostic Factors for Lung-Related Mortality in Patients with COPD.
| Variables | No. | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (years) | |||||
| > 70 | 81 | 3.746 (1.759–7.977) | 0.001 | 2.961 (1.310–6.693) | 0.009 |
| ≤ 70 | 241 | ||||
| Sex | |||||
| Female | 24 | 0.045 (0.000–51.809) | 0.389 | ||
| Male | 298 | ||||
| BMI (kg/m2) | |||||
| < 22.7 | 90 | 5.276 (2.467–11.282) | < 0.001 | ||
| ≥ 22.7 | 232 | ||||
| FEV1 (% predicted) | |||||
| < 50 | 148 | 4.805 (1.946–11.862) | 0.001 | 3.912 (1.485–10.309) | 0.006 |
| ≥ 50 | 174 | ||||
| DLCO (% predicted) | |||||
| < 51 | 80 | 7.023 (3.270–15.083) | < 0.001 | 2.551 (1.003–6.484) | 0.049 |
| ≥ 51 | 242 | ||||
| EI (%) | |||||
| > 34 | 80 | 8.585 (3.755–19.628) | < 0.001 | 2.408 (0.844–6.870) | 0.100 |
| 0–34 | 242 | ||||
| Vertebral fracture | |||||
| Presence | 63 | 2.472 (1.118–5.467) | 0.025 | ||
| Absence | 259 | ||||
| DThorax (HU) | |||||
| < 119.3 | 142 | 5.491 (2.331–12.932) | < 0.001 | 4.345 (1.707–11.060) | 0.002 |
| ≥ 119.3 | 180 | ||||
Fig. 3. Kaplan-Meier survival analysis showing outcomes of patients with chronic obstructive pulmonary disease classified using DThorax for all-cause death (A) and lung-related death (B).
Patients were classified into two groups, as follows: group with lower DThorax (< 119.3 HU) (n = 142) (green line) and group with relatively higher DThorax (≥ 119.3 HU) (n = 180) (blue line). Patients with lower DThorax had poorer prognosis than those with higher DThorax for both all-cause death and lung-related death.
DISCUSSION
Assessing bone health condition by measuring thoracic vertebral bone density on chest CT is straightforward, easily accessible, and not highly time-consuming. Our study demonstrated that thoracic vertebral bone density measured on chest CT was significantly different according to the severity of COPD and was mildly correlated with the clinical variables and quantitative CT variables of COPD. Moreover, the lower value of the thoracic vertebral bone density measured on chest CT was a significant predictor of all-cause mortality and lung-related mortality in patients with COPD.
Lower BMD on DXA has been reported to be significantly associated with an increased risk of all-cause and cardiovascular mortality in the general population (32). Osteoporosis and osteoporotic fractures are two major comorbidities of COPD. The effect of lower BMD or osteoporosis on the increased risk of mortality in the COPD population is still not clear (33,34,35). However, in the study by Campos-Obando et al. (34), the low BMD on DXA was associated with mortality in patients with chronic lung disease, including COPD. Moreover, osteoporosis leads to a greater risk of bone fractures, including hip and vertebral fractures; therefore, osteoporosis may affect mortality in the COPD population. Vertebral compression fractures, with osteoporosis being the leading cause, have been significantly associated with mortality in patients with COPD exacerbation (8). In our study, we attempted to assess the association between DThorax and mortality in patients with COPD; lower DThorax was a significant predictor of mortality in the patients with COPD. Our study did not allow us to demonstrate a causal relationship between DThorax and patient mortality; however, it did indicate that both elements are closely related, similar to findings in previous studies using DXA. Further studies are needed with a larger sample size to validate this finding.
However, in the studies from the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints cohort, the presence of osteoporosis or CT-measured thoracic vertebral bone density was not associated with mortality in patients with COPD, although CT-measured thoracic vertebral bone density correlated with COPD exacerbation or hospitalization rates (33,35). We cannot definitively explain this difference, although there are several possible explanations for it. First, the previous studies included a small non-Caucasian population; however, all of the patients in our study were Asian. Clinical risk factors, such as osteoporosis or lower bone density, for patient mortality may be ethnicity-specific and might differ between Asians and Caucasians. Second, osteoporosis or low BMD is greatly under-diagnosed and under-treated, even among high-risk groups in East Asia (36) and may affect the patients' mortality. Therefore, future studies should evaluate the relationship between genetic factors and the osteoporosis-related increased risk of patient mortality.
In COPD, various systemic effects may also contribute to the severity of the disease. Our results showed that osteoporosis is proportional to the degree of airflow limitation (FEV1) and parenchymal destruction (EI). These results are concordant with those of previous studies (35,37,38,39) and may suggest a common pathogenesis connecting parenchymal destruction and bone loss. A systemic inflammatory mediator, such as interleukin-6, tumor necrosis factor-α, and CRP, has been implicated as a possible mediator of COPD-related osteoporosis according to the characteristics of the systemic inflammatory response in COPD (4,22,23). We tried to assess the association between systemic inflammation reflected by elevated CRP, ESR, or platelet count and DThorax in COPD patients in our study. Although the platelet count and ESR showed a negative correlation with DThorax, the multiple regression analysis showed no significant association between platelet count and DThorax. The precise role of systemic inflammation in COPD-related osteoporosis remains to be determined.
Among the various variables, older patient age and lower 6MWD were significant predictors for lower DThorax in our study. Older patient age is a well-stablished risk factor for osteoporosis in the general population (40) and the association of age and osteoporosis has also been reported in the COPD population (38,41). In line with the results of our study, the association between decreased exercise capacity and acceleration of bone mass loss has also been reported (42,43). Dynamic hyperinflation contributes to the weakness and fatigue of respiratory muscles and this induces a worsening of physical condition and adversely affects daily physical activity (44,45). This vicious cycle of inactivity, deconditioning, and dyspnea has been reported to induce the development of osteoporosis in COPD patients (42,46).
Our data did not demonstrate a relationship between DThorax and the baseline use of ICSs or the duration of use of ICS, although previous studies have shown that the use of ICS was associated with an increased risk of fractures and significant decrease of BMD (47,48). The lack of a relationship between DThorax and use of ICS in our study may be because of the small number of patients with long-term ICS use. Regarding the effects of ICS on bone density in patients with COPD, treatment duration may play a role. The Lung Health Study Research Group observed patients receiving ICS; while significant declines in BMD were observed after 3 years of use, there was no effect on BMD after 1 year (49). Moreover, the effect of ICS on bone density may be dose-dependent. However, the exact cumulative dose of ICS before joining our cohort was not available.
Our study has several limitations. First, BMD on DXA was not compared with DThorax. However, chest CT-measured thoracic vertebral bone attenuation has been reported to be strongly correlated with BMD on DXA in COPD patients (14). Second, we used various CT machines for the CT scans. This could create slightly different CT attenuation values which could affect the CT-measured bone density. However, the CT scanners were regularly calibrated using an AAPM standard phantom. Third, DThorax was only assessed using the baseline CT. Therefore, longitudinal changes in DThorax and its association with clinical outcomes were not investigated. Fourth, information on the use of medication for osteoporosis was not documented in our patients and the possible effects of antiosteoporosis drugs on DThorax and mortality were not investigated. However, a recent meta-analysis reported that osteoporosis treatment was not associated with a reduction in overall mortality (50). Finally, the study population was only Korean. External validation that includes multinational and multi-ethnic patients with COPD may be required.
In conclusion, the thoracic vertebral bone density measured on chest CT demonstrated significant associations with patients' mortality and other disease severity-related variables in COPD patients included in the KOLD cohort. These data suggest that radiologists and clinicians should be aware of the risk of poor bone health in COPD patients and CT-measured bone density may be evaluated in COPD patients who underwent chest CT.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 3.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 4.Agustí AGN. Systemic effects of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:367–370. doi: 10.1513/pats.200504-026SR. discussion 371–372. [DOI] [PubMed] [Google Scholar]
- 5.Lehouck A, Boonen S, Decramer M, Janssens W. COPD, bone metabolism, and osteoporosis. Chest. 2011;139:648–657. doi: 10.1378/chest.10-1427. [DOI] [PubMed] [Google Scholar]
- 6.Graat-Verboom L, van den Borne BE, Smeenk FW, Spruit MA, Wouters EF. Osteoporosis in COPD outpatients based on bone mineral density and vertebral fractures. J Bone Miner Res. 2011;26:561–568. doi: 10.1002/jbmr.257. [DOI] [PubMed] [Google Scholar]
- 7.Harrison RA, Siminoski K, Vethanayagam D, Majumdar SR. Osteoporosis-related kyphosis and impairments in pulmonary function: a systematic review. J Bone Miner Res. 2007;22:447–457. doi: 10.1359/jbmr.061202. [DOI] [PubMed] [Google Scholar]
- 8.Pascual-Guardia S, Badenes-Bonet D, Martin-Ontiyuelo C, Zuccarino F, Marín-Corral J, Rodríguez A, et al. Hospital admissions and mortality in patients with COPD exacerbations and vertebral body compression fractures. Int J Chron Obstruct Pulmon Dis. 2017;12:1837–1845. doi: 10.2147/COPD.S129213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi ML, et al. International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone. 2008;43:1115–1121. doi: 10.1016/j.bone.2008.08.106. [DOI] [PubMed] [Google Scholar]
- 10.Schousboe JT, Shepherd JA, Bilezikian JP, Baim S. Executive summary of the 2013 International Society for Clinical Densitometry position development conference on bone densitometry. J Clin Densitom. 2013;16:455–466. doi: 10.1016/j.jocd.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Pickhardt PJ, Lee LJ, del Rio AM, Lauder T, Bruce RJ, Summers RM, et al. Simultaneous screening for osteoporosis at CT colonography: bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J Bone Miner Res. 2011;26:2194–2203. doi: 10.1002/jbmr.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158:588–595. doi: 10.7326/0003-4819-158-8-201304160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyabara Y, Holmes D, 3rd, Camp J, Miller VM, Kearns AE. Comparison of calibrated and uncalibrated bone mineral density by CT to DEXA in menopausal women. Climacteric. 2012;15:374–381. doi: 10.3109/13697137.2011.618566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romme EA, Murchison JT, Phang KF, Jansen FH, Rutten EP, Wouters EF, et al. Bone attenuation on routine chest CT correlates with bone mineral density on DXA in patients with COPD. J Bone Miner Res. 2012;27:2338–2343. doi: 10.1002/jbmr.1678. [DOI] [PubMed] [Google Scholar]
- 15.Biskobing DM. COPD and osteoporosis. Chest. 2002;121:609–620. doi: 10.1378/chest.121.2.609. [DOI] [PubMed] [Google Scholar]
- 16.Jørgensen NR, Schwarz P, Holme I, Henriksen BM, Petersen LJ, Backer V. The prevalence of osteoporosis in patients with chronic obstructive pulmonary disease: a cross sectional study. Respir Med. 2007;101:177–185. doi: 10.1016/j.rmed.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 17.Bon J, Fuhrman CR, Weissfeld JL, Duncan SR, Branch RA, Chang CC, et al. Radiographic emphysema predicts low bone mineral density in a tobacco-exposed cohort. Am J Respir Crit Care Med. 2011;183:885–890. doi: 10.1164/rccm.201004-0666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park TS, Lee JS, Seo JB, Hong Y, Yoo JW, Kang BJ, et al. Study design and outcomes of Korean Obstructive Lung Disease (KOLD) cohort study. Tuberc Respir Dis (Seoul) 2014;76:169–174. doi: 10.4046/trd.2014.76.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152:653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 20.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 21.Crapo RO, Hankinson JL, Irvin C, MacIntyre NR, Voter KZ, Wise RA. American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique--1995 update. Am J Respir Crit Care Med. 1995;152:2185–2198. doi: 10.1164/ajrccm.152.6.8520796. [DOI] [PubMed] [Google Scholar]
- 22.Bai P, Sun Y, Jin J, Hou J, Li R, Zhang Q, et al. Disturbance of the OPG/RANK/RANKL pathway and systemic inflammation in COPD patients with emphysema and osteoporosis. Respir Res. 2011;12:157. doi: 10.1186/1465-9921-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang B, Feng Y. The association of low bone mineral density with systemic inflammation in clinically stable COPD. Endocrine. 2012;42:190–195. doi: 10.1007/s12020-011-9583-x. [DOI] [PubMed] [Google Scholar]
- 24.Budoff MJ, Hamirani YS, Gao YL, Ismaeel H, Flores FR, Child J, et al. Measurement of thoracic bone mineral density with quantitative CT. Radiology. 2010;257:434–440. doi: 10.1148/radiol.10100132. [DOI] [PubMed] [Google Scholar]
- 25.Budoff MJ, Khairallah W, Li D, Gao YL, Ismaeel H, Flores F, et al. Trabecular bone mineral density measurement using thoracic and lumbar quantitative computed tomography. Acad Radiol. 2012;19:179–183. doi: 10.1016/j.acra.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Mao SS, Li D, Syed YS, Gao Y, Luo Y, Flores F, et al. Thoracic quantitative computed tomography (QCT) can sensitively monitor bone mineral metabolism: comparison of thoracic QCT vs Lumbar QCT and dual-energy X-ray absorptiometry in detection of age-relative change in bone mineral density. Acad Radiol. 2017;24:1582–1587. doi: 10.1016/j.acra.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 28.Lee YK, Oh YM, Lee JH, Kim EK, Lee JH, Kim N, et al. Quantitative assessment of emphysema, air trapping, and airway thickening on computed tomography. Lung. 2008;186:157–165. doi: 10.1007/s00408-008-9071-0. [DOI] [PubMed] [Google Scholar]
- 29.Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S, et al. Cutoff finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haruna A, Muro S, Nakano Y, Ohara T, Hoshino Y, Ogawa E, et al. CT scan findings of emphysema predict mortality in COPD. Chest. 2010;138:635–640. doi: 10.1378/chest.09-2836. [DOI] [PubMed] [Google Scholar]
- 31.Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173:1326–1334. doi: 10.1164/rccm.200510-1677OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qu X, Huang X, Jin F, Wang H, Hao Y, Tang T, et al. Bone mineral density and all-cause, cardiovascular and stroke mortality: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;166:385–393. doi: 10.1016/j.ijcard.2011.10.114. [DOI] [PubMed] [Google Scholar]
- 33.Miller J, Edwards LD, Agustí A, Bakke P, Calverley PM, Celli B, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med. 2013;107:1376–1384. doi: 10.1016/j.rmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Campos-Obando N, Castano-Betancourt MC, Oei L, Franco OH, Stricker BH, Brusselle GG, et al. Bone mineral density and chronic lung disease mortality: the Rotterdam study. J Clin Endocrinol Metab. 2014;99:1834–1842. doi: 10.1210/jc.2013-3819. [DOI] [PubMed] [Google Scholar]
- 35.Romme EA, Murchison JT, Edwards LD, van Beek E, Jr, Murchison DM, Rutten EP, et al. CT-measured bone attenuation in patients with chronic obstructive pulmonary disease: relation to clinical features and outcomes. J Bone Miner Res. 2013;28:1369–1377. doi: 10.1002/jbmr.1873. [DOI] [PubMed] [Google Scholar]
- 36.Kung AW, Fan T, Xu L, Xia WB, Park IH, Kim HS, et al. Factors influencing diagnosis and treatment of osteoporosis after a fragility fracture among postmenopausal women in Asian countries: a retrospective study. BMC Womens Health. 2013;13:7. doi: 10.1186/1472-6874-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva DR, Coelho AC, Dumke A, Valentini JD, de Nunes JN, Stefani CL, et al. Osteoporosis prevalence and associated factors in patients with COPD: a cross-sectional study. Respir Care. 2011;56:961–968. doi: 10.4187/respcare.01056. [DOI] [PubMed] [Google Scholar]
- 38.Ohara T, Hirai T, Muro S, Haruna A, Terada K, Kinose D, et al. Relationship between pulmonary emphysema and osteoporosis assessed by CT in patients with COPD. Chest. 2008;134:1244–1249. doi: 10.1378/chest.07-3054. [DOI] [PubMed] [Google Scholar]
- 39.Bon J. Does radiographic emphysema correlate with low bone mineral density? Curr Opin Pulm Med. 2012;18:125–130. doi: 10.1097/MCP.0b013e32834f8194. [DOI] [PubMed] [Google Scholar]
- 40.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graat-Verboom L, Spruit MA, van den, Smeenk FW, Martens EJ, Lunde R, et al. Correlates of osteoporosis in chronic obstructive pulmonary disease: an underestimated systemic component. Respir Med. 2009;103:1143–1151. doi: 10.1016/j.rmed.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Rio F, Lores V, Mediano O, Rojo B, Hernanz A, López-Collazo E, et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med. 2009;180:506–512. doi: 10.1164/rccm.200812-1873OC. [DOI] [PubMed] [Google Scholar]
- 43.Nishimura Y, Nakata H, Tsutsumi M, Maeda H, Yokoyama M. Relationship between changes of bone mineral content and twelve-minute walking distance in men with chronic obstructive pulmonary disease: a longitudinal study. Intern Med. 1997;36:450–453. doi: 10.2169/internalmedicine.36.450. [DOI] [PubMed] [Google Scholar]
- 44.O'Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:180–184. doi: 10.1513/pats.200508-093DO. [DOI] [PubMed] [Google Scholar]
- 45.Folgering H, von Herwaarden C. Exercise limitations in patients with pulmonary diseases. Int J Sports Med. 1994;15:107–111. doi: 10.1055/s-2007-1021029. [DOI] [PubMed] [Google Scholar]
- 46.Watz H, Waschki B, Boehme C, Claussen M, Meyer T, Magnussen H. Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional study. Am J Respir Crit Care Med. 2008;177:743–751. doi: 10.1164/rccm.200707-1011OC. [DOI] [PubMed] [Google Scholar]
- 47.Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax. 2011;66:699–708. doi: 10.1136/thx.2011.160028. [DOI] [PubMed] [Google Scholar]
- 48.Langhammer A, Forsmo S, Lilleeng S, Johnsen R, Bjermer L. Effect of inhaled corticosteroids on forearm bone mineral density: the HUNT study, Norway. Respir Med. 2007;101:1744–1752. doi: 10.1016/j.rmed.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 49.Wise R, Connett J, Weinmann G, Scanlon P, Skeans M Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902–1909. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- 50.Cummings SR, Lui LY, Eastell R, Allen IE. Association between drug treatments for patients with osteoporosis and overall mortality rates: a meta-analysis. JAMA Intern Med. 2019;179:1491–1500. doi: 10.1001/jamainternmed.2019.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]