Highlights
-
•
The clinical manifestations of COVID-19 children are milder than those of influenza A children under 5 years.
-
•
The laboratory tests of COVID-19 children are milder than those of influenza A children under 5 years.
-
•
Imaging results more commonly presented as ground-glass opacities in COVID-19 patients.
Keywords: COVID-19, SARS-CoV-2, Influenza A, Pneumonia, Child
Abstract
Background
Since the outbreak of Coronavirus Disease 2019 (COVID-19) in Wuhan, considerable attention has been paid to its epidemiology and clinical characteristics in children. However, it is also crucial for clinicians to differentiate COVID-19 from other respiratory infectious diseases, such as influenza viruses.
Methods
This was a retrospective study. Two groups of COVID-19 patients (n = 57) and influenza A patients (n = 59) were enrolled. We analyzed and compared their clinical manifestations, imaging characteristics and treatments.
Results
The proportions of cough (70.2%), fever (54.4%) and gastrointestinal symptoms (14.1%) in COVID-19 patients were lower than those of influenza A patients (98.3%, P < 0.001; 84.7%, P < 0.001; and 35.6%, P = 0.007; respectively). In addition, COVID-19 patients showed significantly lower levels of leukocytes (7.87 vs. 9.89 × 109 L–1, P = 0.027), neutrophils (2.43 vs. 5.16 × 109 L–1, P < 0.001), C-reactive protein (CRP; 3.7 vs. 15.1 mg/L, P = 0.001) and procalcitonin (PCT; 0.09 vs. 0.68 mm/h, P < 0.001), while lymphocyte levels (4.58 vs. 3.56 × 109 L–1; P = 0.006) were significantly higher compared with influenza A patients. In terms of CT imaging, ground-glass opacification in chest CT was more common in COVID-19 patients than in influenza A patients (42.1% vs. 15%, P = 0.032). In contrast, consolidation was more common in influenza A patients (25%) than in COVID-19 patients (5.2%, P = 0.025).
Conclusion
The clinical manifestations and laboratory tests of COVID-19 children are milder than those of influenza A children under 5 years. Additionally, imaging results more commonly presented as ground-glass opacities in COVID-19 patients.
Introduction
Since December2019, a novel coronavirus has broken out in Wuhan, and spread rapidly worldwide. On February 11, 2020, the World Health Organization (WHO) officially named this novel coronavirus pneumonia as Coronavirus Disease 2019 (COVID-19), whereas the International Committee on Taxonomy of Viruses has named it as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). On 11 March, WHO declared that COVID-19 should be characterized as a pandemic. Because of the highly contagious nature of SARS-CoV-2, the entire population was generally susceptible, including young children. Data from China showed young children were vulnerable to SARS-CoV-2 infection (Dong et al., 2020). In addition, WHO estimated that more than 2 million children under 5 years of age die from pneumonia, accounting for almost one in five under-5 deaths worldwide in 2004 (Wardlaw et al., 2006). Pneumonia was the leading infectious cause of death in children younger than 5 years (Wardlaw et al., 2006). Therefore, close attention should be paid to children with pneumonia less than 5 years old during the COVID-19 pandemic.
Influenza viruses have precipitated pandemics several times over the past 100 years, specifically in 1918, 1957, 1968 and 2009. Influenza A was a common cause of pneumonia in young children (Jain et al., 2015). Recently, Kong et al. retrospectively investigated the presence of SARS-CoV-2 among local patients with influenza like illness (ILI) from 6 October 2019 to 21 January 2020 and found SARS-CoV-2 RNA was detected in nine ILI patient specimens (Kong et al., 2020). In addition, ILI data for the 2019-2020 winter was significantly higher in comparison to previous years regarding children, suggesting that it was necessary to distinguish the difference between influenza A and COVID-19 pneumonia in young children. Therefore, the aim of this study was to compare the different clinical presentations between patients infected with COVID-19 pneumonia versus influenza A pneumonia, to provide some recommendations for their differential diagnosis.
Methods
Study design and participants
Subjects of the study were consecutive children with either confirmed COVID-19 pneumonia (admitted between 28 January and 11 March 2020) or influenza A pneumonia (admitted between 14 December 2019 and 30 February 2020) in Wuhan Children's hospital. The study was approved by the Research Ethics Board of Wuhan Children's Hospital (No. 2020003). Consent of the patients’ legal guardians was obtained.
Definitions
Children were included in the study if they had evidence consistent with pneumonia as assessed by means of chest radiography or CT within 72 h before or after admission. The virus nucleic acid detection kit confirmed COVID-19 patients through detecting the RNA of SARS-CoV-2 in throat swab samples based on the manufacturer's protocol (Shanghai BioGerm Medical Biotechnology Co., Ltd.). Influenza A was detected by direct immunofluorescence assay. The diagnostic criteria for severe pneumonia caused by SARS-CoV-2 and influenza A conform to guidelines (Society of Pediatrics, 2020, Harper et al., 2009).
Data collection
A COVID-19 or influenza A case report form was designed to document primary data regarding demographic, clinical and laboratory characteristics from electronic medical records. The following information was extracted from each patient: gender, age, medical history, chief complaints, laboratory findings and computed tomography (CT) imaging on admission.
Statistical analysis
Categorical data were described as percentages, and continuous data as median with standard deviation (SD). Nonparametric comparative test for continuous data and χ 2 test for categorical data were used to compare variables between groups. The statistical analyses were performed using SPSS Statistics version 25.0 software. P < 0.05 was considered statistically significant.
Results
Baseline characteristics of COVID-19 and influenza A patients
A total of 57 COVID-19 patients and 59 influenza A patients were included (Table 1 ). No significant differences were found in the median age between COVID-19 patients and influenza A patients (18.7 months vs. 21.8 months, P = 0.121). The proportion of males to females was also not significantly different between the two groups (61.4% vs. 66.1%, P = 0.599). The most common symptoms and signs were cough (84.5%), fever (69.8%) and gastrointestinal symptoms (25%), whereas dyspnea (6.0%) and convulsions (3.4%) were less common. Moreover, the proportions of cough (70.2%), fever (54.4%) and gastrointestinal symptoms (14.1%) in COVID-19 patients were lower than those of influenza A patients (98.3%, P < 0.001; 84.7%, P < 0.001; and 35.6%, P = 0.007; respectively). Additionally, the highest body temperature of influenza A patients was also significantly higher than that of COVID-19 patients (38.5 °C vs. 39.3 °C, P < 0.001). The proportion of severe pneumonia in COVID-19 patients was also lower than that in patients infected with influenza A (3.5% vs. 18.6%, P = 0.016) (Table 1).
Table 1.
Characteristics of patients with COVID-19 or Influenza A.
| Variable | No. (%) or median ± SD |
P value | ||
|---|---|---|---|---|
| Total (n = 116) | COVID-19 (n = 57) | Influenza A (n = 59) | ||
| Male | 74 (63.8) | 35 (61.4) | 39 (66.1) | 0.599 |
| Age (months) | 20.2 ± 16.7 | 18.7 ± 16.7 | 21.8 ± 16.7 | 0.121 |
| Severe pneumonia | 13 (11.2) | 2 (3.5) | 11 (18.6) | 0.016 |
| Symptoms and signs | ||||
| Fever | 81 (69.8) | 31 (54.4) | 50 (84.7) | <0.001 |
| Highest temperature (°C) | 38.9 ± 0.7 | 38.5 ± 0.7 | 39.3 ± 0.6 | <0.001 |
| Cough | 98 (84.5) | 40 (70.2) | 58 (98.3) | <0.001 |
| Dyspnea | 7 (6.0) | 2 (3.5) | 5 (8.5) | 0.439 |
| Gastrointestinal symptoms | 29 (25) | 8 (14.1) | 21 (35.6) | 0.007 |
| Convulsions | 4 (3.4) | 1 (1.7) | 3 (5.1) | 0.619 |
| Hospitalized treatment | ||||
| Oxygen inhalation | 8 (6.9) | 1 (1.7) | 7 (11.8) | 0.061 |
| ICU | 6 (5.2) | 2 (3.5) | 4 (6.7) | 0.679 |
COVID-19, coronavirus disease 2019; No., number; SD, standard deviation; ICU, Intensive Care Unit.
Laboratory and imaging findings between COVID-19 and influenza A patients
For blood inflammatory indictors, lower levels of C-reactive protein (CRP) and procalcitonin (PCT) were observed in COVID-19 patients than in influenza A patients (3.7 vs. 15.1 mg/L, P = 0.001; 0.09 vs. 0.68 ng/ml, P < 0.001). For routine blood tests, COVID-19 patients showed significantly lower levels of leukocytes and neutrophils, while lymphocyte levels were significantly higher compared with influenza A patients (7.87 vs. 9.89 × 109 L–1, P = 0.027; 2.43 vs. 5.16 × 109 L–1, P < 0.001; 4.58 vs. 3.56 × 109 L–1; P = 0.006). For biochemistry testing, no significant differences were found in the alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), bilirubin, blood urea nitrogen (BUN) and blood creatinine between the two groups of patients. However, lower levels of d-dimer and prothrombin time (PT) and potassium ion were found in COVID-19 patients than influenza A patients (0.34 vs. 1.94, P < 0.001; 10.8 vs. 11.2 mm/h, P = 0.014; 5.14 vs. 7.07 mmol/L, P = 0.001) (Table 2 ).
Table 2.
Laboratory findings of COVID-19 and Influenza A patients.
| Variable | No. (%) or median ± SD |
P value | ||
|---|---|---|---|---|
| Total (n = 116) | COVID-19 (n = 57) | Influenza A (n = 59) | ||
| Blood routine test | ||||
| Leukocytes (×109 L–1) | 8.88 ± 4.07 | 7.87 ± 2.87 | 9.89 ± 4.84 | 0.027 |
| Neutrophils (×109 L–1) | 3.81 ± 3.69 | 2.43 ± 1.92 | 5.16 ± 4.46 | <0.001 |
| Lymphocytes (×109 L–1) | 4.06 ± 2.09 | 4.58 ± 2.06 | 3.56 ± 2.01 | 0.006 |
| Platelets (×109 L–1) | 304 ± 110 | 298 ± 107 | 311 ± 113 | 0.455 |
| Hemoglobin((g/L)) | 117 ± 11 | 117 ± 11 | 117 ± 12 | 0.932 |
| Coagulation function | ||||
| PT (s) | 10.9 ± 0.8 | 10.8 ± 0.7 | 11.2 ± 0.8 | 0.014 |
| APTT (s) | 33.6 ± 5.4 | 33.4 ± 5.2 | 34.1 ± 5.9 | 0.650 |
| d-Dimer (ng/ml) | 0.8 ± 1.7 | 0.34 ± 0.29 | 1.94 ± 2.88 | <0.001 |
| Inflammatory indictors | ||||
| CRP (mg/dl) | 9.5 ± 24.1 | 3.7 ± 6.85 | 15.1 ± 32.2 | 0.001 |
| PCT (ng/ml) | 0.41 ± 1.35 | 0.09 ± 0.09 | 0.68 ± 1.82 | <0.001 |
| Biochemical test | ||||
| ALT (U/L) | 31 ± 57 | 36 ± 78 | 26 ± 25 | 0.595 |
| AST (U/L) | 54 ± 64 | 57 ± 86 | 50 ± 30 | 0.591 |
| TIBL (umol/L) | 7.44 ± 4.53 | 7.35 ± 4.58 | 7.51 ± 4.52 | 0.799 |
| ALB (g/L) | 43.1 ± 5.5 | 42.7 ± 6.9 | 43.5 ± 3.6 | 0.964 |
| CK (U/L) | 138 ± 106 | 147 ± 89 | 130 ± 121 | 0.042 |
| LDH (U/L) | 338 ± 128 | 319 ± 92 | 357 ± 154 | 0.340 |
| CK-MB (U/L) | 42 ± 32 | 38 ± 20 | 46 ± 41 | 1.000 |
| Creatinine (umol/L) | 28.6 ± 29.9 | 26.5 ± 24.7 | 30.6 ± 34.2 | 0.939 |
| BUN (mmol/L) | 3.3 ± 1.7 | 3.48 ± 2.24 | 3.24 ± 1.15 | 0.122 |
COVID-19, coronavirus disease 2019; No., number; SD, standard deviation; CRP, C-reactive protein; PCT, procalcitonin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TIBL Total-bilirubin;ALB, albumin; PT, prothrombin time; APTT, activated partial thromboplastin time; LDH, lactate dehydrogenase; CK, Creatinine kinase; CK-MB, MB isoenzyme of creatine kinase; BUN, blood urea nitrogen.
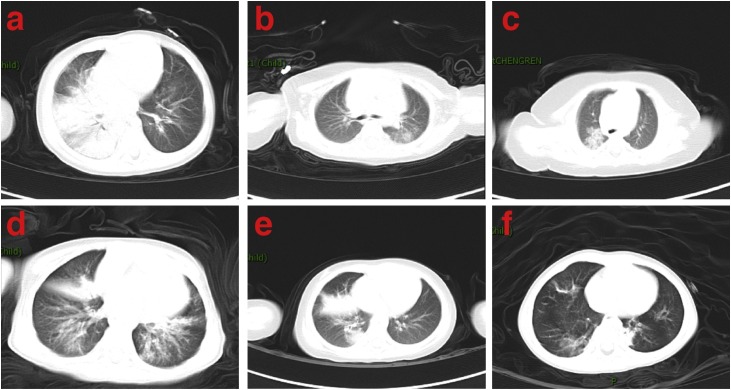
In terms of CT imaging, ground-glass opacification in chest CT was more common in COVID-19 patients than in influenza A patients (42.1% vs. 15%, P = 0.032). In contrast, consolidation was more common in influenza A patients (25%) than that in COVID-19 patients (5.2%, P = 0.025) (Figure 1 and Table 3 ).
Figure 1.
Imaging characteristics of chest CT from COVID-19 and influenza A patients. (a) A 1-year-old boy with COVID-19 exhibited ground-glass opacity mixed consolidation in both lungs. (b) A 7-month-old girl with COVID-19 exhibited ground-glass opacities in left lung. (c) A 1-month-old boy with COVID-19 exhibited consolidation in right lung. (d) A 1-year-old boy with influenza A exhibited exudation and consolidation distributed diffusely in both lungs. (e) A 2-year-old girl with influenza A exhibited consolidation in right lung. (f) A 2-year-old boy with influenza A exhibited consolidation in left lung and ground-glass opacity in right lung.
Table 3.
CT Imaging findings of patients with COVID-19 or H1N1 at presentation
| Variable | No. (%) |
P value | ||
|---|---|---|---|---|
| Total (n = 77) | COVID-19 (n = 57) | H1N1 (n = 20) | ||
| Patterns of the lesion | ||||
| Ground glass opacification | 24 (20.7) | 24 (42.1) | 3 (15) | 0.032 |
| Consolidation | 8 (6.9) | 3 (5.2) | 5 (25) | 0.025 |
COVID-19, coronavirus disease 2019; CT, computed tomography; No., number.
Discussion
Since December 2019, an outbreak of coronavirus disease 2019 (COVID-19) has spread globally. The outbreak of COVID-19 began in December 2019, which also corresponded with the flu season in China (Yu et al., 2013). It is important to differentiate these two respiratory infectious diseases via early clinical presentation in consideration of their different therapies, prognoses, and protective measures. In this study, we first compared the different clinical courses of pneumonia caused by SARS-CoV-2 and influenza A virus. We found that COVID-19 patients were mild in clinical symptoms and laboratory examinations in children under 5 years. Additionally, imaging results more commonly presented as ground-glass opacity in COVID-19 patients.
As we found before, cough and fever were common symptoms in COVID-19 (Lu et al., 2020), which is similar with influenza A. Our present study revealed that COVID-19 manifested as mild; severe pneumonia was less than influenza A patients. Some COVID-19 patients only presented as fever or cough. Meanwhile, influenza A patients were more likely to have fever with higher temperature. Gastrointestinal symptoms were common in patients with COVID-19 (Cheung et al., 2020), however, they were less than compared with influenza A. Convulsions could be found in both patients, but the reason was different, as only one in COVID-19 was secondary to pneumonia and three in influenza A were confirmed as febrile convulsion.
Lymphopenia and increase in d-dimer were common laboratory abnormalities in COVID-19 adults (Huang et al., 2020, Wang et al., 2020) and were proven as a caution for severity in COVID-19 (Ruan et al., 2020, Yang et al., 2020, Zheng et al., 2020). In our study, lymphocyte count and d-dimer were lower in influenza A patients than in COVID-19, which could further prove that COVID-19 is milder than influenza A. Similarly, CRP (C-reactive protein) and PCT (procalcitonin), which was the severity index of pneumonia, were lower in COVID-19 than influenza A.
In our study, we found that ground-glass opacity was more common in COVID-19 patients than in influenza A patients, whereas consolidation was more frequent in influenza A patients, which was consistent with previous studies. The radiological findings of children with COVID-19 pneumonia from our team and other studies showed that ground-glass opacities were the most common pattern of abnormalities in chest CT (Chang et al., 2020). Additionally, studies on influenza A-associated pneumonia showed that consolidation was common on CT (Guo et al., 2012). Therefore, these differential pathological changes may contribute to distinguish imaging characteristics during clinical assessments.
There were some limitations of our present study. First, this was a retrospective study that included data from a single-center cohort. We hope for a prospective cohort and multi-center study. Second, influenza A was diagnosed by direct immunofluorescence assay and failed to be typed.
In conclusion, COVID-19 cases were mild not only in clinical symptoms but also in laboratory examination results which included lymphocyte, CRP, PCT, and d-dimer in the children under 5 years. Additionally, imaging results more commonly presented as ground-glass opacity in COVID-19 patients.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding source
None declared.
Ethical approval
This study was approved by the Research Ethics Board of Wuhan Children's Hospital (No. WHCH 2020003).
Authors’ contribution
All authors collected the clinical data. YL and HZW drafted the manuscript. XXL revised the final manuscript. FW, HD and XRL were responsible for summarizing all data related to this study.
References
- Society of Pediatrics, Chinese Medical Association; Editorial Board, Chinese Journal of Pediatrics Recommendations for the diagnosis, prevention and control of the 2019 novel coronavirus infection in children (first interim edition)] Zhonghua er ke za zhi. 2020;58(3):169–174. doi: 10.3760/cma.j.issn.0578-1310.2020.03.001. [DOI] [PubMed] [Google Scholar]
- Chang T.H., Wu J.L., Chang L.Y. Clinical characteristics and diagnostic challenges of pediatric COVID-19: a systematic review and meta-analysis. J Formos Med Assoc. 2020;119(5):982–989. doi: 10.1016/j.jfma.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K.S., Hung I.F., Chan P.P., Lung K.C., Tso E., Liu R. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong Cohort and systematic review and meta-analysis. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6) doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- Guo W., Wang J., Sheng M., Zhou M., Fang L. Radiological findings in 210 paediatric patients with viral pneumonia: a retrospective case study. Br J Radiol. 2012;85(1018):1385–1389. doi: 10.1259/bjr/20276974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper S.A., Bradley J.S., Englund J.A., File T.M., Gravenstein S., Hayden F.G. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(8):1003–1032. doi: 10.1086/604670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Self W.H., Wunderink R.G., Fakhran S., Balk R., Bramley A.M. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W.H., Li Y., Peng M.W., Kong D.G., Yang X.B., Wang L. SARS-CoV-2 detection in patients with influenza-like illness. Nat Microbiol. 2020;5(5):675–678. doi: 10.1038/s41564-020-0713-1. [DOI] [PubMed] [Google Scholar]
- Lu X., Zhang L., Du H., Zhang J., Li Y.Y., Qu J. SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw T., Salama P., Johansson E.W., Mason E. Pneumonia: the leading killer of children. Lancet (London, England) 2006;368(9541):1048–1050. doi: 10.1016/S0140-6736(06)69334-3. [DOI] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Alonso W.J., Feng L., Tan Y., Shu Y., Yang W. Characterization of regional influenza seasonality patterns in China and implications for vaccination strategies: spatio-temporal modeling of surveillance data. PLoS Med. 2013;10(11):e1001552. doi: 10.1371/journal.pmed.1001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]