Abstract
Background
National health-system hospitals of Lombardy faced a heavy burden of admissions for acute respiratory distress syndromes associated with coronavirus disease (COVID-19). Data on patients of European origin affected by COVID-19 are limited.
Methods
All consecutive patients aged ≥18 years, coming from North-East of Milan's province and admitted at San Raffaele Hospital with COVID-19, between February 25th and March 24th, were reported, all patients were followed for at least one month. Clinical and radiological features at admission and predictors of clinical outcomes were evaluated.
Results
Of the 500 patients admitted to the Emergency Unit, 410 patients were hospitalized and analyzed: median age was 65 (IQR 56–75) years, and the majority of patients were males (72.9%). Median (IQR) days from COVID-19 symptoms onset was 8 (5–11) days. At hospital admission, fever (≥ 37.5 °C) was present in 67.5% of patients. Median oxygen saturation (SpO2) was 93% (range 60–99), with median PaO2/FiO2 ratio, 267 (IQR 184–314). Median Radiographic Assessment of Lung Edema (RALE) score was 9 (IQR 4–16). More than half of the patients (56.3%) had comorbidities, with hypertension, coronary heart disease, diabetes and chronic kidney failure being the most common. The probability of overall survival at day 28 was 66%. Multivariable analysis showed older age, coronary artery disease, cancer, low lymphocyte count and high RALE score as factors independently associated with an increased risk of mortality.
Conclusion
In a large cohort of COVID-19 patients of European origin, main risk factors for mortality were older age, comorbidities, low lymphocyte count and high RALE.
Keywords: COVID-19, ARDS, Infection, RALE score
1. Introduction
Since the emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan, Hubei, China, in December 2019, its potential to become a serious public health threat worldwide was apparent. The SARS-CoV-2 causing the coronavirus 19 disease (COVID-19) spread very rapidly and took a heavy death toll, with nearly 10.000 confirmed cases and more than 200 deaths in the first month [1]. Extraordinary measures were established to control the outbreak in Wuhan exceeding by far the classic definition of local confinement, lockdown and isolation [2]. Nevertheless, the SARS-CoV-2 has spread globally, and on March 11th,2020 COVID-19 was declared a pandemic by the World Health Organization (WHO) [3]. The first cases in Europe were reported at the end of January 2020. Despite the time lapse since the outbreak in China, European healthcare systems were not prepared to cope with the steep increase in incidence and the large number of patients needing intensive care. The first confirmed case in Europe, a patient with no travel history to China was reported on February, 21st in the Lombardy region of northern Italy. Several more cases were reported in the following hours, with no apparent contact with the first patient nor with anyone known to have COVID-19. As of May 5th, 2020, 213.013 individuals were known having been infected with SARS-CoV-2 in Italy, of whom 29.315 died. Clinical manifestations of COVID-19 disease include a variety of presentations, spanning from asymptomatic disease to severe interstitial pneumonia with acute respiratory distress syndrome (ARDS), and death [[4], [5], [6]]. In the largest retrospective cohort study from China, hospitalized patients with COVID-19 were relatively young (median age 47 years), with males and females in similar proportions [7]. Older age and presence of comorbidities have been associated with increased mortality [5,7,8]. However, demographic and anthropometric characteristics differ between Asian and European populations [9,10] and these factors may impact clinical outcomes in patients with ARDS [11,12]. Of note, the unexpected rapid spread of the COVID-19 pandemic caused a dramatic overload of hospitals and intensive care unit (ICUs) in Western countries. Therefore, data on characteristics and outcomes of COVID-19 patients in Europe are crucial to the understanding of the disease, to develop specific treatment plans, and to potentially optimize resource allocation by allowing early prognostic stratification. To date, available data from Europe include those from a large retrospective cohort study of patients admitted to ICU in Italy [13], and a small case series of patients diagnosed with COVID-19 in France [14]. In this report we describe the demographical, clinical, radiological and laboratory characteristics, as well as the clinical outcomes and the risk factors for mortality, of the first 500 patients with COVID-19 admitted to San Raffaele Scientific Institute, a tertiary care academic hospital in Milan, Italy.
2. Methods
This series is part of the COVID-19 institutional clinical-biological cohort assessing patients with COVID-19 (Covid-BioB, ClinicalTrials.gov NCT04318366) at the Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) San Raffaele Hospital, a 1350-bed tertiary care hospital in Milan, Italy.
The study was approved by the Institutional Review Board (IRB), protocol number 34/int/2020). Data were analyzed and interpreted by the authors, who also reviewed the manuscript and vouch for the accuracy and completeness of the data and for the adherence of the study to the protocol. Informed consent was obtained according to the IRB guidelines.
2.1. Patient enrolment and follow-up
All consecutive patients aged ≥18 years admitted to the Emergency department (ED) at IRCCS San Raffaele with COVID-19 infection between February 25th and March 24th, 2020 were enrolled in this cohort. Patient data were censored at the time of data cut off, which occurred on May 1st, 2020. An infection case was defined as a SARS-CoV-2 positive real-time reverse-transcriptase polymerase chain reaction (RT-PCR) from a nasal and/or throat swab together with signs, symptoms, or radiological findings suggestive of COVID-19 pneumonia. The re-organization of the hospital to face the COVID-19 outbreak has been recently reported [15]. The hospital guidelines for the management of respiratory failure are listed in Table S2 Supplementary Appendix. Lopinavir/ritonavir, remdesivir, hydroxychloroquine, azithromycin, were prescribed following Italian recommendation (www.aifa.gov.it). In addition, we used immunomodulatory therapies with either anakinra (IL-1 receptor antagonist), tocilizumab or sarilumab (anti-IL6 receptor monoclonal antibodies), mavrilimumab (anti-human granulocyte macrophage colony-stimulating factor receptor monoclonal antibody), a novel anti-inflammatory agent that blocks complement (AMY101) [16], reparixin (IL8 inhibitor), or high-dose steroids in patients who displayed a hyper inflammatory laboratory profile in the context of expanded access programs or clinical trials.
2.2. Data collection
Data were collected from medical chart review or directly by patient interview, and entered in a dedicated electronic case record form (eCRF) specifically developed on site for the COVID-BioB study. The geographical distribution of patients place of residence has been evaluated to represent the areas of patients referral. Before analysis, data were data were cross-checked with medical charts and verified by data managers and clinicians, for accuracy.
2.3. Laboratory testing and X-ray analysis
Routine blood tests included: complete blood count (CBC) with differential, serum biochemical tests, including C-reactive protein (CRP), electrolytes, renal and liver function tests. In addition, coagulation profile with D-Dimer, lactate dehydrogenase (LDH), troponine, N-terminal prohormone of brain natriuretic peptide (NT-proBNP), interleukin-(IL-) 6 and procalcitonin (PCT) were available for a subgroup of patients.
Conventional chest X-ray (CXR) images were acquired in the postero-anterior (PA) or anteroposterior (AP) projections. CXR obtained at admission were blindly reviewed by consensus of two physicians (FdC and CM), with further review by an expert radiologist in case of disagreement. The following radiographic features were evaluated: ground glass opacities (GGO) and consolidation as defined by the Fleischner Society glossary of terms [17], hilar enlargement and pleural effusion. Furthermore, lung opacity distribution was assessed and categorized in peripheral predominance, peri-hilar predominance, or neither. Radiographic Assessment of Lung Edema (RALE) score [18] was used to quantify the extent and severity of lung opacities. Each radiographic quadrant was reviewed and assigned a score based on the extent of opacities (0–4) and their density (1–3); the final RALE score (maximum score 48) was then obtained by summing the product of the consolidation and density scores for each of the four quadrants. Lastly all CXRs were analyzed by the artificial intelligence (AI) software (qXR v2.1, Qure.ai Technologies, India) designed to interpret COVID-19 patients' plain radiographs and quantify the disease extension. Each lung involvement percentage (cut-off 3%) was reported from the AI software analysis.
2.4. Statistical analysis
Median values with respective inter-quartiles ranges (IQR), were used to express continuous variables while frequencies in percentages were used for categorical variables. Patient-related variables of survivors and non survivors were compared using the Chi-square or Fischer's exact test for categorical variables, and the Wilcoxon rank sum or Kruskal-Wallis test for continuous variables. Imputation for missing data was not performed.
The ability of the two radiological score in predicting death was determined by the area-under-the-curve (AUC) of receiver operating characteristics (ROC) curves. For each score, the optimal cut-off value, predicting death, was determined on the highest Youden index value (sensitivity + specificity −1). To evaluate the diagnostic accuracy of each radiological score, sensitivity, specificity, negative and positive predictive values with the corresponding 95% confidence intervals (95%CI) were estimated for both cut-off values. Total accuracy was also assessed by the percentage of patients that were correctly classified by each score according to the corresponding optimal cut-off value.
Repeated-measures analyses using univariate mixed linear models (allowing correlated errors for a patient's multiple determinations) were used to estimate and compare laboratory changes among survivors and non-survivors. These models were fitted for each laboratory parameter to the available values, in the raw scale, determined during hospitalization; the models were fitted with random slope and intercept for each patient and the crude mean changes (slopes) were reported with the corresponding 95% confidence interval.
Kaplan-Meier curves were used to estimate the probability of survival. The time-to- events was calculated from the date of hospital admission to the date of the event, or the date of last available visit, whichever occurred first. Kaplan-Meier curves on the time to death were estimated according to a number of covariates (on validated reference cut-offs or, if not available, on the overall median value) and compared by the log-rank test.
To evaluate the association between patients characteristic and in-hospital death univariable and multivariable Cox proportional hazards models were calculated. The effect estimates were reported as hazard ratio (HR) with the corresponding 95% CI, estimated according to the Wald approximation. To avoid overfitting in the multivariable model, and considering the total number of events, the following variables deemed conceptually important, and known risk factors of COVID-19 were included in the Cox model: age (median value), sex, hypertension, coronary heart disease, diabetes, kidney failure, RALE score, baseline lymphocyte count (median value) and C-reactive protein level (median value). The variables included in the models were fitted as time-fixed and measured at baseline. Although we acknowledge that some factors are subject to change over time (e.g. laboratory parameters) and affect the considered outcomes, we did not fit a model with time-updated variables, as laboratory changes over time would still be highly correlated with baseline values and because we were interested in assessing the impact of characteristics determined at the time of hospital admission on the considered outcomes. Finally, we conducted a sensitivity analysis, including people with available data, at hospital admission, of routine laboratory markers, related with organ distress (LDH (n = 308), D-Dimer (n = 189), NT Pro-BNP (n = 202). Two-tailed P values are reported for analyses, with p value <.05 considered to indicate statistical significance. All confidence intervals were two-sided and not adjusted for multiple testing. Statistical analyses were performed with the SPSS 25 (SPSS Inc./IBM, Armonk, NY, USA, SAS Software, release 9.4 (SAS Institute, Cary, NC) and R V.3.3.1.
3. Results
3.1. Patient characteristics
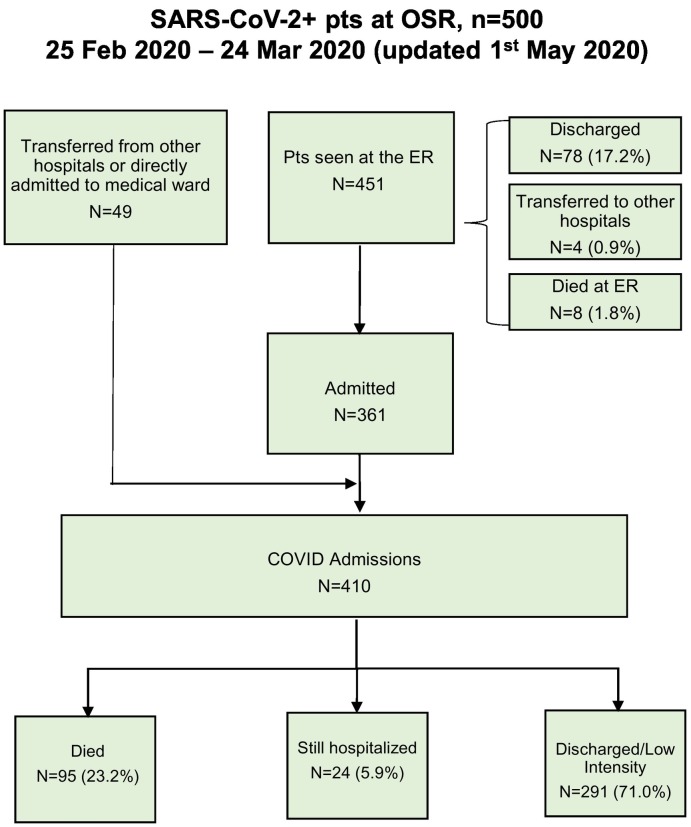
By 24th March 2020, 500 patients had been admitted to the ED of the IRCSS San Raffaele with COVID-19 pulmonary infection, as reported in Fig. 1 and table S3 of Supplementary Appendix. Geolocation of residence address was plotted using an on-line map software, showing the North East of Milano city and province as the main area of hospital referral (Fig. S1 Supplementary Appendix).
Fig. 1.
Study Flow chart.
A total of 410 patients were hospitalized and analyzed. All patients were followed for at least one month from the last patient admitted. The baseline characteristics, laboratory testing and CXR evaluation at admission of the 410 patients who were hospitalized into the general COVID-19 dedicated wards or into the ICU are summarized in Table 1 . Median (IQR) age was 65 (56–75) years, and the majority of patients were males (72.9%). Median (IQR) time from COVID-19 symptoms onset was 8 (5–11) days.
Table 1.
Characteristics of the 410 COVID-19 patients with severe bilateral pneumonia at Hospital admission.
| Characteristics (%) | Overall, n = 410 | Discharged (n = 291) | Still hospitalized (n = 24) | Dead (n = 95) | P-value§ |
|---|---|---|---|---|---|
| Age, years (IQR) | 65 (56–75) | 62 (54–72) | 60 (54–67) | 76 (67–82) | <0.001 |
| Age < 55 Age ≥ 55–65 Age ≥ 65–75 Age ≥ 75 |
90 (22) 113 (27.6) 104 (25.4) 103 (25.1) |
78 (26.8) 90 (30.9) 72 (24.7) 51 (17.5) |
7 (29.2) 10 (41.7) 4 (16.7) 3 (12.5) |
5 (5.3) 13 (13.7) 28 (29.5) 49 (51.6) |
<0.001 |
| Median days from COVID-19 symptoms onset | 8 (5–11) | 8 (6–11) | 7 (6–10) | 5 (3–9) | <0.001 |
| Median days from admission to last follow up⁎ | 14 (7–25) | 14 (8–24) | 43 (40–49) | 10 (5–15) | <0.001 |
| Sex, Male | 299 (72.9) | 207 (71.1) | 22 (91.7) | 70 (73.7) | 0.09 |
| Ethnicity European Asian Hispanic |
382 (93.2) 23 (5.6) 5 (1.2) |
274 (94.2) 13 (4.5) 4 (1.4) |
20 (83.3) 4 (16.7) 0 (0) |
88 (92.6) 6 (6.3) 1 (1.1) |
0.15 |
| Body Temperature°C | 38 (37.4–38.5) | 38 (37.4–38.5) | 37.9 (37.5–38.5) | 38 (37.1–38.7) | 0.96 |
| PaO2/FiO2 ratio | 267 (184–314) | 286 (227–323) | 145 (73–232) | 219 (105–287) | <0.001 |
| PaO2/FiO2 ≥ 300 mmHg PaO2/FiO2 < 300 mmHg |
115 (31.9) 245 (68.1) |
97 (38.2) 157 (61.8) |
1 (4.2) 23 (95.8) |
17 (20.7) 65 (79.3) |
<0.001 |
| Body Mass Index | 27 (24–29) | 27 (24–29) | 28 (24–31) | 26 (24–28) | 0.33 |
| Body Mass Index <18.5 Body Mass Index ≥18.5–25 Body Mass Index ≥25–30 Body Mass Index ≥30 |
5 (1.5) 85 (25) 172 (50.6) 78 (22.9) |
4 (1.6) 64 (24.8) 130 (50.4) 60 (23.3) |
0 (0) 6 (26.1) 9 (39.1) 8 (34.8) |
1 (1.7) 15 (25.4) 33 (55.9) 10 (16.9) |
0.72 |
| N of Comorbidities | <0.001 | ||||
| None | 160 (40.6) | 133 (46.3) | 13 (59.1) | 14 (16.5) | |
| 1 | 120 (30.5) | 91 (31.7) | 4 (18.2) | 25 (29.4) | |
| 2 | 70 (17.8) | 47 (16.4) | 4 (18.2) | 19 (22.4) | |
| 3 | 30 (7.6) | 15 (5.2) | 1 (4.5) | 14 (16.5) | |
| 4 | 12 (3) | 1 (0.3) | 0 (0) | 11 (12.9) | |
| 5 | 2 (0.5) | 0 (0) | 0 (0) | 2 (2.4) | |
| Hypertension | <0.001 | ||||
| No | 204 (50.1) | 160 (55) | 16 (69.6) | 28 (30.1) | |
| Yes | 203 (49.9) | 131 (45) | 7 (30.4) | 65 (69.9) | |
| Coronary artery disease | <0.001 | ||||
| No | 354 (87.4) | 265 (91.4) | 22 (95.7) | 67 (72.8) | |
| Yes | 51 (12.6) | 25 (8.6) | 1 (4.3) | 25 (27.2) | |
| Diabetes | <0.001 | ||||
| No | 337 (83) | 248 (85.5) | 19 (79.2) | 70 (76.1) | |
| Type 1 | 8 (2) | 2 (0.7) | 4 (16.7) | 2 (2.2) | |
| Type 2 | 61 (15) | 40 (13.8) | 1 (4.2) | 20 (21.7) | |
| Chronic Obstructive pulmonary disease | <0.001 | ||||
| No | 383 (94.6) | 284 (98.3) | 22 (95.7) | 77 (82.8) | |
| Yes | 22 (5.4) | 5 (1.7) | 1 (4.3) | 16 (17.2) | |
| Chronic Kidney disease | <0.001 | ||||
| No | 352 (88.2) | 270 (93.1) | 19 (86.4) | 63 (72.4) | |
| Yes | 47 (11.8) | 20 (6.9) | 3 (13.6) | 24 (27.6) | |
| Cancer | 0.005 | ||||
| No | 383 (94.6) | 279 (96.2) | 23 (100) | 81 (88) | |
| Yes | 22 (5.4) | 11 (3.8) | 0 (0) | 11 (12) | |
| Score of Radiographic assessment of lung edema | 9 (4–16) | 6 (3−12) | 15 (8–27) | 14 (8–20) | <0.001 |
| Laboratory findings at admission⁎ | |||||
| White Blood cell, x109/L Missing, n |
6.5 (4.9–9.3) (6) |
6.1 (4.8–8.2) 3 |
8.9 (5.8–11.2) 1 |
7.5 (5.3–11.6) 2 |
<0.001 |
| Lymphocyte count, x109/L Missing, n |
0.9 (0.7–1.2) (7) |
1.0 (0.7–1.2) 3 |
0.8 (0.6–1.1) 1 |
0.7 (0.6–0.9) 3 |
<0.001 |
| Neutrophil count, x109/L Missing, n |
5.0 (3.5–7.6) (7) |
4.5 (3.3–6.7) 3 |
7.8 (4.4–9.8) 1 |
6.3 (4.3–9.7) 3 |
<0.001 |
| Hemoglobin, g/dl Missing, n |
13.6 (12.3–14.7) (6) |
13.9 (12.6–14.8) 3 |
14.0 (12.9–14.6) 1 |
12.6 (10.9–14.1) 2 |
<0.001 |
| Platelet count, x109/L Missing, n |
192 (147–260) (6) |
195 (151–262) 3 |
182 (147–257) 1 |
182 (122–257) 2 |
0.23 |
| Neutrophil/ lymphocyte ratio | 5.7 (3.4–9.6) (7) |
4.9 (3–7.7) 3 |
10.3 (5.5–15.8) 1 |
8.7 (5.4–14) 3 |
<0.001 |
| Total bilirubin, mg/dl Missing, n |
0.54 (0.39–0.77) (65) |
0.53 (0.38–0.73) 51 |
0.53 (0.43–1.05) 1 |
0.56 (0.38–0.85) 13 |
0.28 |
| Alanine Amino Trasferase, U/L Missing, n |
36 (23–56) (4) |
37 (24–56) 2 |
47 (30–63) 0 |
32 (20–54) 2 |
0.04 |
| Aspartate Transaminase, U/L Missing, n |
46 (33–66) (18) |
44 (31–62) 10 |
49 (38–84) 3 |
50 (34–82) 5 |
0.26 |
| Creatinine, mg/dl Missing, n |
1.02 (0.84–1.25) (2) |
0.96 (0.81–1.18) 1 |
1.08 (0.87–1.32) | 1.19 (0.94–1.79) 1 |
<0.001 |
| Glucose, mg/dl Missing, n |
108 (98–131) (17) |
104 (96–119) 10 |
119 (108–165) 1 |
128 (107–151) 6 |
<0.001 |
| Sodium, mmol/L Missing, n |
137 (134–139) (5) |
137 (134–139) 4 |
136 (133–139) 0 |
136 (133–139) 1 |
0.80 |
| Lactate dehydrogenase, U/L Missing, n |
392 (304–496) (102) |
368 (298–447) 66 |
443 (388–637) 6 |
521 (333–630) 30 |
<0.001 |
| C-reactive protein, mg/L Missing, n |
83 (41–151) (1) |
69 (35–119) 1 |
250 (128–328) 0 |
126 (57–217) 0 |
<0.001 |
| Lactate, mmol/L Missing, n |
1.33 (1.01–1.77) (31) |
1.19 (0.97–1.59) 26 |
1.68 (1.23–2.19) 0 |
1.62 (1.26–2.38) 5 |
<0.001 |
| Prothrombin time Missing, n |
0.99 (0.94–1.06) (71) |
0.98 (0.93–1.05) 59 |
|
1.03 (0.97–1.12) 12 |
<0.001 |
| D-Dimer, μg/mL Missing, n |
1.54 (0.84–3.28) (221) |
1.10 (0.68–2.22) 165 |
2.72 (1.87–13.20) 7 |
3.15 (1.21–18.16) 49 |
<0.001 |
| Interleukin-6 Missing, n |
48.2 (23.3–121.7) (244) |
37.6 (20.8–83.3) 170 |
177 (71.4–798) 13 |
86.6 (45.4–195.0) 61 |
<0.001 |
| Serum Ferritin, ng/mL Missing, n |
1236 (712–2588) (154) |
1113 (658–1822) 109 |
3046 (1502–4385) 4 |
1570 (764–3273) 41 |
<0.001 |
| Creatine kinase, U/L Missing, n |
113 (66–260) (135) |
104 (64–204) 104 |
148 (73–564) 5 |
165 (77–478) 26 |
0.28 |
| Procalcitonin, ng/mL Missing, n |
0.52 (0.30–1.18) (165) |
0.43 (0.28–0.79) 122 |
1.02 (0.69–2.35) 4 |
1.18 (0.44–3.99) 39 |
<0.001 |
| N-terminal prohormone of brain natriuretic peptide, pg/mL Missing, n |
205 (88–780) (207) |
150 (60–409) 165 |
177 (130–754) 5 |
1150 (331–3268) 37 |
<0.001 |
| Cardiac troponin, ng/L Missing, n |
12.35 (6.60–27.42) (168) |
9.85 (5.57–20.25) 135 |
9.05 (7.3–24.6) 4 |
25.20 (13.80–62.05) 29 |
<0.001 |
Results reported as median (IQR) or frequency (%).
§ by chi-square or Fisher exact test (categorical variables) or or Kruskal-Wallis test (continuous variables).
Laboratory findings reported at the date of admission at ED or 1st day of Hospitalization.
At hospital admission, fever (≥ 37.5 °C, ≥ 99.5 °F) was present in 67.5% of patients. Median oxygen saturation (SpO2) was 93% (range 60–99), with median (IQR) PaO2/FiO2 ratio, 267 (184–314). The median (IQR) values for white blood cell count, lymphocytes count, creatinine, lactate and CRP, were 6.5 (4.9–9.3) x109/L, 0.9 (0.7–1.2)x109/L, 1.02 (0.84–1.25) mg/dl, 1.33 (1–1.77) mmol/L and 83 (41–151) mg/dl, respectively.
For the subgroup of patients with available information at baseline (Table 1), the median (IQR) values of LDH, NT-pro BNP, and D-dimer, were 392 (304–496) U/L, 205 (88–780) pg/mL, 1.54 (0.84–3.28) μg/mL, respectively.
CXR evaluation showed ground glass opacities and diffuse area of consolidations with bilateral involvement, and a RALE score of 13 was determined by the ROC curves as the accurate predictor of COVID19 lung involvement and mortality ( Table 1 , Fig. S2 Supplementary Appendix).
Overweight or obesity was reported in 75% of the patients. More than half of the patients (56.3%) had comorbidities, with hypertension, coronary heart disease, diabetes and chronic kidney failure being the most common. Overall, 21 patients were under treatment for malignant diseases.
3.2. Main outcomes
As of May 1st, 2020, 95 (23.1%) patients had died (19 in ICU), 24 (5.9%) were still hospitalized and 291 (71%) had been discharged and no one was readmitted in the entire follow up time. Main causes of death were refractory hypoxia, massive pulmonary thrombosis and multiple organ failure (MOF).
The median (IQR) length of stay from hospitalization to discharge was 14 (8–24) days, while the median (IQR) time from hospitalization to death was 10 (5–15) days. Overall seventy patients (17%) were admitted to ICU in a median (IQR) time from hospitalization to ICU of 2 (0−11) days, 19 patients died (23%). For the 24 patients who were still hospitalized (22 in ICU) the median (IQR) time from hospitalization to last follow up was 43 (40–49) days. Median (IQR) age of the 24 patients still hospitalized is 60 (54–67) years, with 70% aged less than 65 years.
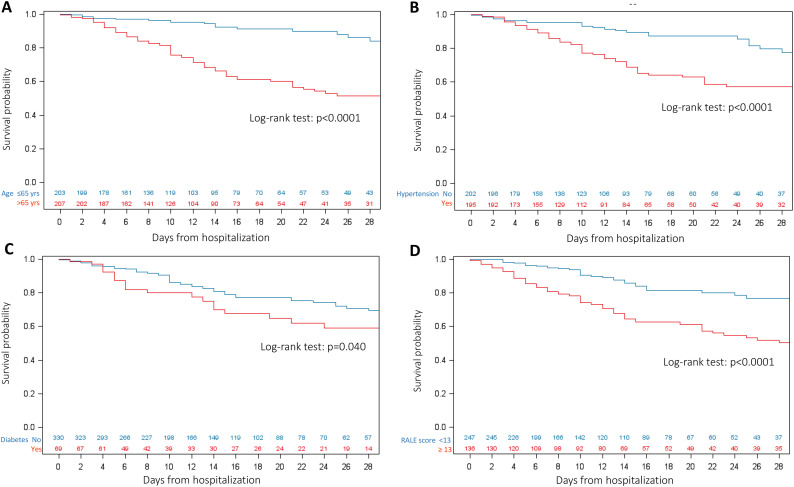
The probability of day- 28 survival was 66% (95% CI, 59.2%–72.2%) (Fig. S3 Supplementary Appendix). Results of univariable analysis for survival according to pre-treatment characteristics are reported in Table 2 , and Fig. 2 .
Table 2.
– Univariable and multivariable Cox proportional hazard models on the risk of death.
| Characteristics | Univariable, HR (95%CI) | p value | Multivariable*, HR (95%CI) | p value |
|---|---|---|---|---|
| Gender (Female vs Male) | 1.09 (0.69–1.72) | 0.70 | ||
| Age | 1.07 (1.05–1.09) | <0.001 | 3.17 (1.84–5.44) | <0.001 |
| Ethnicity, European vs other |
0.93(0.49–1.86) | 0.91 | ||
| Body Mass Index | 0.96(0.90–1.02) | 0.25 | ||
| Presence of comorbidity (vs none) | 3.91 (2.26–6.84) | <0.001 | ||
| Hypertension | 2.60 (1.67–4.05) | <0.001 | ||
| Diabetes | 1.51 (0.96–2.05) | 0.06 | ||
| Coronary artery disease | 3.21 (2.02–5.10) | <0.001 | 2.93 (1.77–4.86) | <0.001 |
| Chronic kidney failure | 2.75 (1.47–4.40) | <0.001 | ||
| Cancer | 2.77 (1.47–5.22) | 0.002 | 2.32 (1.15–4.67) | 0.01 |
| Radiographic assessment of lung edema score | 1.04 (1.02–1.06) | <0.001 | 1.05 (1.03–1.07) | <0.001 |
| White blood cell count, x109/L, (median) | 1.71 (1.11–2.62) | 0.001 | ||
| Lymphocyte count, x109/L, (median) | 2.24 (1.42–3.52) | <0.001 | 1.83 (1.14–2.95) | 0.01 |
| Hemoglobin, g/dl, (median) | 0.52 (0.33–0.80) | 0.003 | ||
| Platelets, x109/L, (median) | 0.89 (0.59–1.34) | 0.59 | ||
| Glucose, mg/dl, (median) | 2.63 (1.62–4.28) | <0.001 | ||
| ASpartate Transaminase, U/L, (median) | 1.28 (0.84–1.95) | 0.23 | ||
| C-Reactive protein, mg/L, (median) | 1.53 (1.03–2.35) | 0.04 | ||
| Lactate deydrogenase, U/L, (median) | 2.28 (1.32–3.94) | 0.003 | ||
| D-Dimer, microgr/ml, (median) | 2.02 (1.03–3.96) | 0.03 | ||
| Lactate, mmol/L, (median) | 2.00 (1.27–3.15) | <0.001 | ||
| Creatin Kinase, U/L, (median) | 1.16 (0.72–1.88) | 0.52 | ||
| Serum Ferritin, ng/ml, (median) | 0.88 (0.50–1.53) | 0.65 | ||
| Procalcitonine, ng/ml, (median) | 2.09 (1.15–3.80) | 0.01 | ||
| N-terminal prohormone of brain natriuretic peptide, pg/mL, (median) | 4.82 (2.43–9.54) | <0.001 |
Fig. 2.
Kaplan-Meier estimates on survival by A: age; B: Hypertension; C: Diabetes; D: RALE score (stratified according to best cut-off).
Multivariable analysis ( Table 2 ) showed age older than 65 years (HR 3.17, 95%CI 1.84–5.44, p < .001), history of coronary artery disease (HR 2.93, 95%CI 1.77–4.86, p < .001), active cancer (HR 2.32, 95%CI 1.15–4.67, p = .001), low lymphocyte count (<0.9 × 109/L) (HR 1.83, 95%CI 1.14–2.95, p = .01) and high RALE score (HR 1.05, 95%CI 1.03–1.07, p < .001), as factors independently associated with an increased risk of mortality.
With the aim of evaluating the impact of routine laboratory markers, related with organ distress we performed a further multivariable model (Table S4 Supplementary Appendix) in the subgroup of patients with available data at hospital admission. An LDH level above the median (HR 2.95, p = .01) and increased D-Dimer above the median (HR 2.54, p = .01), were independently associated with increased risk of death.
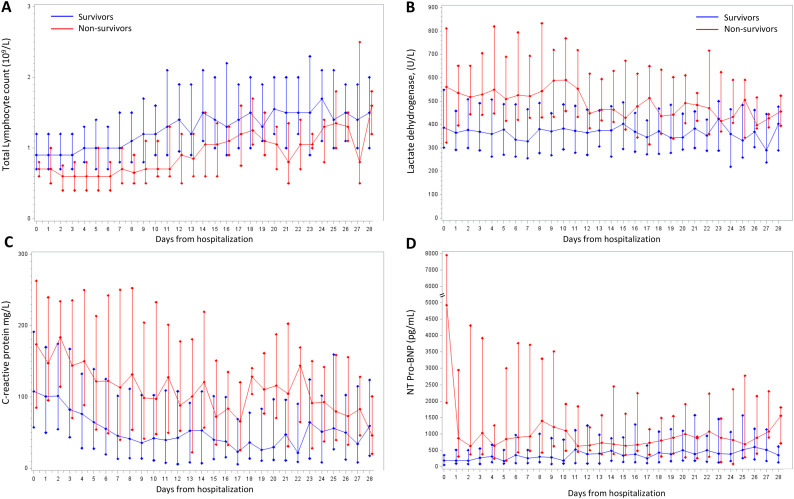
Repeated-measures analyses of relevant laboratory markers were analyzed from day of hospitalization to the last available follow up as showed in Fig. 3 . Non survivors had marked severe lymphopenia from hospital admission until death, whereas levels of CRP, LDH were and remained higher throughout the entire hospitalization compared to surviving patients.
Fig. 3.
Temporal trends in laboratory markers during hospitalization. A: Total Lymphocyte count (109/L), B: Lactate dehydrogenase, (U/L), C: C-reactive protein (mg/L), D: N-terminal prohormone of brain natriuretic peptide, (pg/mL). Solid lines connect median values estimated on raw data; bars are quartiles.
4. Discussion
Our study describes a large series from an academic center reporting the clinical characteristics of patients from Milano, Lombardy, Italy, one of the regions most affected by the COVID-19 outbreak in Europe. With a clinical observation longer than one months from the last patient admitted, we were able to identify early predictors of mortality related to patient characteristics, radiological and laboratory findings at hospital admission for COVID-19.
The results presented in this analysis reflect the first attempt to cope with a new dramatic disease as the COVID-19, with its atypical ARDS features [19]. We aimed to provide a real life picture of the initial pandemic wave of the infection breakthrough which imposed a radical reshaping of the clinical activity at our tertiary care academic hospital [15]. We increased the hospital capacity for treating COVID-19 patients, moving rapidly from regular 32 ICU beds to 56 COVID-19 dedicated ICU beds and repurposing a total of 270 beds to COVID-19 dedicated units. In this perspective, these results will be a reference to evaluate the potential benefits of further developments, new drugs and additional therapeutic measures in the near future.
Currently, the vast majority of clinical reports available on the COVID-19 are from Asian populations [6,7]. Our study is the first addressing early risk factors associated with mortality in a population of European origin. We confirmed findings previously observed in patients from China and United States, including older age, associated comorbidities such as coronary artery disease, history of hypertension, diabetes, chronic obstructive lung disease and chronic renal failure [20]. Some other comorbidities, like cancer, were also identified as associated with increased mortality [21].
The identification of underlying conditions of risk of vascular diseases is the first hallmark of our series and may reflect the increased risk of severity of the COVID-19 spectrum in the Caucasian cohort. These results translate into practical implications, on the targeting of such very high-risk population, and establishment of dedicated policies of social and work measures. In view of the upcoming post-pandemic long-wave with recurrent infection outbreaks [22], these findings are also of utmost importance for reducing the burden of the general health system, targeting the effort for adequate screening of the patients at risk.
With regard to gender, we observed a large prevalence of male patients in our cohort, as already reported by others [23], but the mortality risk was not different, and this may be in part explained by the stronger effect of older age in our population. Furthermore, we recognize a significant mortality in a cluster of elderly patients (70% of non survivors were 70 years aged or more) with high burden of comorbidities and admitted with an advanced phase of respiratory distress. However the mortality in our elderly population is in line with the general lethality rate currently reported in Italy [24], and highlights the need for early hospital referrals for this very high risk patients.
A novel finding from our report is the high predictive value for mortality by the chest X-ray quantitative RALE score at the admission, a marker of lung edema with high accuracy in the diagnosis of ARDS. RALE score maintained a strong hazard ratio for mortality also in the multivariable analysis, suggesting this chest X-ray quantitative assessment as a simple tool for predicting clinical outcome very early, at the first patient evaluation at the ED [18].
A large number of laboratory parameters were abnormal at hospital admission; among them lymphopenia was a significant marker of the severity of the COVID-19 clinical course. One of the possible mechanisms underlying might be functional exhaustion of the cytotoxic lymphocytes [25], and it may reflect a specific hallmark of the SARS-CoV-2 pathogenesis.
Similarly, we have also demonstrated on a relevant proportion of patients, the prognostic impact of hyper-inflammation in COVID-19. At clinical presentation, non survivors had increased inflammatory markers which did not normalize throughout the entire hospitalization, suggesting a possible specific mechanism related with the pathogenesis of COVID-19. SARS-CoV-2 infection is likely responsible for a direct cellular damage and it also seems to enhance the host innate immune responses towards further tissue and vascular endothelial injuries [26]. Our data support the hypothesis of a COVID-19 associated trombo-inflammatory syndrome, involving the endothelium and vessels primarily in the lung (microCLOTS) [27], as one possible mechanism of the severe SARS-CoV-2 disease manifestations. Of interest, the analysis of these markers may drive specific therapeutic interventions targeting selected inflammatory pathways [28,29].
One strength of our single center study is the observation time of more than one month that allowed us to capture the history of this new disease and describe in details the relevant events. This translates into a public interest in making our results and experience available, to contribute to a better targeting of patients at risk. Importantly, we have set up a plan of systematic intervention of outpatient follow up visits, which includes sample collection for a biobanking in order to ensure a better understanding of the natural history and the biology of this new disease and to warrant the highest level of care for these patients.
Although we are confident that the major confounders were considered, we cannot exclude that residual confounding factors may still be present. We were unable to analyze the impact of socio-economic status as a potential indirect factor for mortality. Indeed, we can affirm that its effect in our results would have been mitigated by the national care system-based access for people living in Italy.
In conclusion, our results clearly identify risk factors for mortality in patients from a definite territorial area treated at the time of the COVID-19 pandemia breakthrough in Italy. We identified older age, comorbidities, RALE score and biomarkers of systemic hyper inflammation, namely lymphopenia below 0.9 × 109/L, elevated LDH, and elevated D-dimer, as the predictors of early death in a large cohort of European origin.
Acknowledgment
The authors thank the entire staff of the San Raffaele Scientific Institute, working every day to ensure the best quality of care to patients and their families. We dedicate this work to the memory of the Italian health care workers who have given their lives in the care of patients with COVID-19.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2020.108509.
Appendix A. Supplementary data
Supplementary material.
References
- 1.WHO Director-General's opening remarks at the media briefing on COVID-19. 2020. https://www.who.int/dg/speeches/detail/who-director-general-sopening-remarks-at-the-media-briefing-on-covid19
- 2.Prem K., Liu Y., Russell T.W. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020 May;5(5):e261–e270. doi: 10.1016/S2468-2667(20)30073-6. Epub 2020 Mar 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Novel Coronavirus(2019-nCoV) Situation Report - 11. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200131-sitrep-11-ncov.pdf?sfvrsn=de7c0f7_4 Available at. Last accessed May 4, 2020.
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C. China; JAMA: 2020. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 Apr 30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. Epub 2020 Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020 May;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. Epub 2020 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Central Intelligence Agency The World Factbook. https://www.cia.gov/library/publications/resources/the-world-factbook/fields/343rank.html Available at. Last accessed April 07, 2020.
- 10.Dulloo A.G., Jacquet J., Solinas G., Montani J.P., Schutz Y. Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int. J. Obes. 2010;34(Suppl. 2):S4–17. doi: 10.1038/ijo.2010.234. [DOI] [PubMed] [Google Scholar]
- 11.De Jong A., Verzilli D., Jaber S. ARDS in obese patients: specificities and management. Crit. Care. 2019;23(1):74. doi: 10.1186/s13054-019-2374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthay M.A., Zemans R.L., Zimmerman G.A. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grasselli G., Zangrillo A., Zanella A. JAMA; Italy: 2020. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lescure F.X., Bouadma L., Nguyen D. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 2020 Jun;20(6):697–706. doi: 10.1016/S1473-3099(20)30200-0. Epub 2020 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zangrillo A., Beretta L., Silvani P. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. Crit Care Resusc. 2020 Apr 1 doi: 10.51893/2020.2.pov1. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Risitano A.M., Mastellos D.C., Huber-Lang M. Complement as a Target in COVID-19? Nat. Rev. Immunol. 2020 Jun;20(6):343–344. doi: 10.1038/s41577-020-0320-7. Epub 2020 Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Muller N.L., Remy J. Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 18.Warren M.A., Zhao Z., Koyama T. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax. 2018;73(9):840–846. doi: 10.1136/thoraxjnl-2017-211280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. JAMA. 2020 Apr 22;323(20):2052–2059. doi: 10.1001/jama.2020.6775. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganatra S., Hammond S.P., Nohria A. The novel coronavirus disease (COVID-19) threat for patients with cardiovascular disease and Cancer. JACC CardioOncol. 2020 Apr 10 doi: 10.1016/j.jaccao.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. JACC CardioOncol. 2020 Apr 10 doi: 10.1016/j.jaccao.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen N.Z.M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Task force COVID-19 del Dipartimento Malattie Infettive e Servizio di Informatica, Istituto Superiore di Sanità . 2020. Epidemia COVID-19. [Google Scholar]
- 25.Zheng M., Gao Y., Wang G. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020 May;17(5):533–535. doi: 10.1038/s41423-020-0402-2. Epub 2020 Mar 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020 Jun 10;27(6):992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciceri F., Beretta L., Scandroglio A.M. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020 Apr 15 doi: 10.51893/2020.2.pov2. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 Apr 13 doi: 10.1001/jama.2020.6019. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Mastaglio S., Ruggeri A., Risitano A.M. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin. Immunol. 2020 Apr 29;215:108450. doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.