Graphical abstract
Keywords: Neural stem cells, Exosomes, Interferon gamma, Ischemic stroke, MicroRNA
Highlight
-
•
hNSC-Exo presented therapeutic roles in brain ischemic stroke model of rats.
-
•
IFN-γ preconditioning significantly altered the abilities and contents of hNSC-Exo.
-
•
IFN-γ-hNSC-Exo shown more therapeutic benefits than hNSC-Exo in vitro and in vivo.
-
•
Exosomal miRNAs in IFN-γ-hNSC-Exo mediated the potential effects on cell survival.
Abstract
Transplanted neural stem cells promote neural tissue regeneration and functional recovery primarily by releasing paracrine factors. Exosomes act as important secreted paracrine molecules to deliver therapeutic agents involved in cellular functions. Here, we focused on the role of exosomes (hNSC-Exo) derived from human neural stem cells (hNSCs). We utilized the pro-inflammatory factor interferon gamma (IFN-γ) to induce the generation of altered exosomes (IFN-γ-hNSC-Exo), and compared their roles with those of hNSC-Exo and explored the potential mechanism. Importantly, IFN-γ preconditioning did not affect the secretion, but significantly altered the ability of exosomes derived from hNSCs. Moreover, IFN-γ-hNSC-Exo was functionally superior to hNSC-Exo; showed increased cell proliferation and cell survival and decreased cell apoptosis in vitro. Furthermore, IFN-γ-hNSC-Exo further exerted therapeutic effects (showed better behavioral and structural outcomes) compared to those of hNSCs-Exo in an ischemic stroke rat model. Next-generation sequencing (NGS) revealed specific exosomal miRNAs (hsa-miR-206, hsa-miR-133a-3p and hsa-miR-3656) in IFN-γ-hNSC-Exo with important roles in cell survival. Thus, our findings demonstrate that the inflammatory factor IFN-γ can regulate the functions of exosomes and highlight its role in regulating the application of neural stem cell-derived exosomes.
Introduction
Cerebral stroke, one of the leading causes of death and disability, does not have an effective treatment currently. Tissue plasminogen activator (tPA) is the only effective drug when used within 6 h of stroke; thus, it is critical to develop new therapies for treating survivors of cerebral stroke [1], [2]. One promising advances in modern science is stem cell-based therapy [3]. In preclinical and clinical research, stem cell-based approaches, including the use of neural stem cells (NSCs), have shown potential for the regenerative treatment of various diseases including ischemic stroke [3], [4], [5], [6]. Stem cell-based therapy is based mainly on cell replacement and the induction of paracrine effects to replace damaged cells, reduce cell death, and provide trophic support for host cells [7], [8], [9]. However, preclinical studies have suggested that very few (<1% of injection) cells could survive over 4 weeks after transplantation [10], [11], because of the hostile, injured microenvironment. Furthermore, the association between the therapeutic benefits of transplantation and the paracrine effects of grafted cells is not yet known.
In recent years, studies have confirmed that paracrine signals are related to extracellular vesicles (EVs), which have been implicated as mediators of paracrine benefits and are involved in cell-cell communication [10], [12]. EVs, produced by all living cells, include microvesicles (50–1000 nm) and exosomes (40–200 nm). Exosomes are released into extracellular fluids by living cells; contain proteins, lipids, and genetic materials (mRNA, ncRNA etc.); and play essential roles in intercellular communication by transferring exosomal protein and RNA cargos between the source and target cells [13]. Emerging data have shown that exosomes have been successfully tested in preclinical models of stroke, myocardial infarction/reperfusion injury, and hind limb ischemia [14], [15], [16]. Furthermore, anti-tumor therapies based on EVs derived from dendritic cells have entered phase II human clinical trials [17].
Our previous studies [18], [19] showed that interferon gamma (IFN-γ) co-delivery with NSCs accelerated improvements in the behavioral performance of brain ischemic rats, and promoted the neuronal generation of grafted NSCs in the ischemic environment. IFN-γ increases the ability of NSCs to tolerate oxidative stress, regulate the paracrine effects of cells, and augment neuronal functions, enhancing the effectiveness of cell transplantation therapy. These results are similar to how interleukin 6 (IL-6) preconditioning protects grafted NSCs from ischemic reperfusion injury through STAT3-mediated upregulation of manganese superoxide dismutase, and transplantation of IL-6 preconditioned NSCs significantly attenuates infarct size and improves neurological performance [20]. Furthermore, IFN-γ plays an important role in mediating the communication between grafted stem cells and the immune system of the host through the EV-associated IFN-γ/Ifngr1 complex pathway [21].
Therefore, in this study, we focused on the role of exosomes derived from hNSCs stimulated by IFN-γ (IFN-γ-hNSC-Exo), compared their functions with the exosomes derived from human NSCs (hNSC-Exo), and explored their potential mechanism.
Materials and methods
Supplemental information
Supplemental Materials and methods are available from Online Library.
Animals
All animal procedures were conducted in accordance with the Care and Use of Laboratory Animals protocol approved by the Institutional Animal Care Committee of Southeast University, China (Ethical approval number: 2017ZDSYLL048-P01, Animal permit number: SYXK (Su) 2016–0013). All the male Sprague Dawley (SD) rats (Vital River Laboratory Animal Technology Co., Ltd. Beijing, China) were 8 weeks old and weighed 240–280 g, maintained on a 12 h light/dark cycle and given access to food and water ad libitum, and feed-restricted only before anesthetic events. All animal experiments were performed under general anesthesia using 2% isoflurane. The rectal temperature was controlled at 37 °C with a homeothermic blanket during experiments. Data analysis and examination were always carried out in a blinded manner by two investigators, randomized and blinded to the treatment.
Exosomes isolation and characterization
Human NSCs (hNSCs) were preserved in our laboratory (The cells were acquired from human fetal brain tissue with informed consent, under a protocol approved by the Institutional Review Board of Zhongda hospital Southeast University (Approval number: 2017ZDSYLL048-P01), as previously described and published [22]). Exosomes were isolated from hNSCs and stimulated by IFN-γ (concentration: 20 ng/mL) culture supernatants by ultracentrifugation or Exo-spin™ Exosomes Isolation and Exosomes Purification Kit (Cell Guidance Systems, Cambridge, UK) according to the manufacturer’s protocol. Briefly, conditioned media (CM) were collected and cell debris was removed by centrifugation, and then filtered through a 0.22 μm membrane. Ultracentrifugation was performed at 120,000g (Beckman) for 2 h at 4 °C. From the Exosomes Isolation and Purification Kit, ½ volume of Exo-spin™ Buffer was added and mixed, followed by centrifugation and purification. Finally, both of the pellets were resuspended in 100–200 μl of cold PBS. Then exosomes were identified by transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA) flow cytometry (FCM) and western blotting (WB).
H2O2 cell stress model and cell experiments
hNSCs were treated with 500 μM/L concentration of H2O2 (Sigma) to induce cell oxidative stress injury, leading to cell apoptosis and death in vitro, and then treated with exosomes (both hNSC-Exo and IFN-γ-hNSC-Exo) to evaluate their therapeutic abilities. Cell viability, survival and activity were measured by CCK-8 staining, Live-Dead cell staining, Ki67 proliferation assay, and cleaved Caspase-3 immunofluorescence analysis was performed in 96- or 24-well plates. The number of exosomes was 1 × 107 to 1 × 108 particles/well for either one or two days. For immunofluorescence staining method, in brief, cells were fixed and incubated with primary antibodies (rabbit anti-Caspase3 (1:600, Invitrogen) or anti Ki67 (1:400, Santa Cruz) diluted in blocking solution. After the secondary antibodies and nuclear marker, DAPI were added, the slides were observed under a fluorescence microscope.
Brain ischemic stroke rat model and exosome treatment
Adult male SD rats (240–280 g) were used to build a transient cerebral ischemia model according to the previously described MCAO method. hNSC-Exo and IFN-γ-hNSC-Exo (concentration 4 × 109 particles in 10 μl PBS/each rat) were then stereo-tactically transplanted into the striatum of the infarcted hemisphere at 24 h after stroke onset. Rats were randomly divided into four groups; sham operation group (n = 5 rats), PBS group (10 μl PBS), hNSC-Exo group (4 × 109 particles in 10 μl PBS), and IFN-γ-hNSC-Exo group (4 × 109 particles in 10 μl PBS), with n = 11 in the three experimental groups. 5-Bromo-2′-deoxyuridine (BrdU, 50 mg/kg, twice per day, Sigma) was intra-peritoneally administered into the rats after transplantation for 14 consecutive days.
Neurological functions and infarct volume analysis
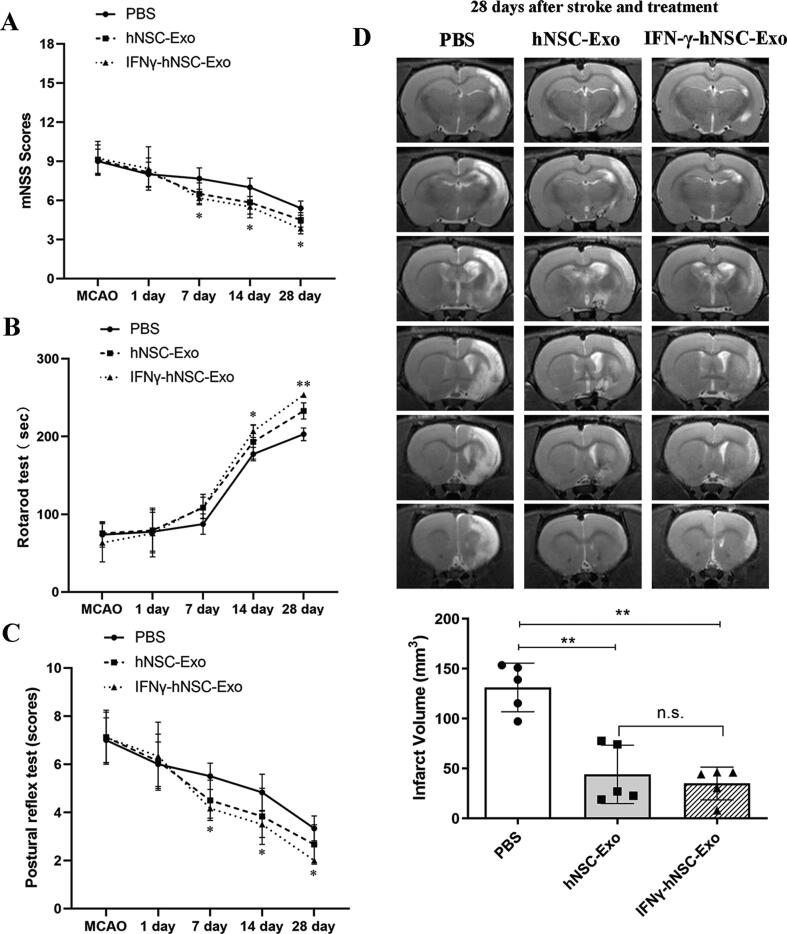
The neurobehavioral analysis was performed by determining the modified Neurological Severity Score (mNSS), Rotarod test and Postural reflex test before MCAO and on days 1, 7, 14 and 28 after stroke and transplantation, by two investigators who were blinded to the experimental groups. mNSS of the rats were graded on a scale of 0 to 18. For the Rotarod test, the rats were placed on the rotarod cylinder, and the test was ended if the animal fell off the rungs or gripped the device and spun around for two consecutive revolutions. The standing time was recorded and analyzed. Postural reflex test measured the sensitivity to cortex and striatum injuries, with scores ranging between 0 and 10. A higher score indicated a more serious behavioral disorder in the animal. The infarct size in the rats was measured using 7.0 Tesla animal magnetic resonance scanners (Brooke). Rats’ brains (n = 5 rats/group) were checked on day 28 after stroke and transplantation. Each layer of the infarct area was measured, and then the total infarct volume of rats per superposition was calculated.
High-throughput sequencing and data analysis
Three biological replicates of exosomes derived from hNSCs and IFN-γ preconditioning hNSCs were sequenced on an Illumina HiSeqTM 2500 desktop sequence. Differential miRNAs with fold change > 1 and p-value < 0.05 in expression between hNSC-Exo and IFN-γ-hNSC-Exo were considered for analysis. Gene Ontology (GO) (http://www.geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes pathway (KEGG) (http://www.genome.jp/kegg/) enrichment analyses were used according to fold change >2 and p-value < 0.05 of exclusion criteria.
Statistical analysis
Values were expressed as the mean with error bars representing standard deviation (SD). Statistical significance was performed using unpaired t test, one-way or two-way ANOVA via GraphPad Prism 8.0 Software. The significance of the differences between different groups was evaluated by variance analysis following by post hoc Tukey–Kramer test (P < 0.05 as significant, P < 0.01 as very significant).
Results
hNSC-Exo presented therapeutical ability in the brain ischemic stroke model of rats
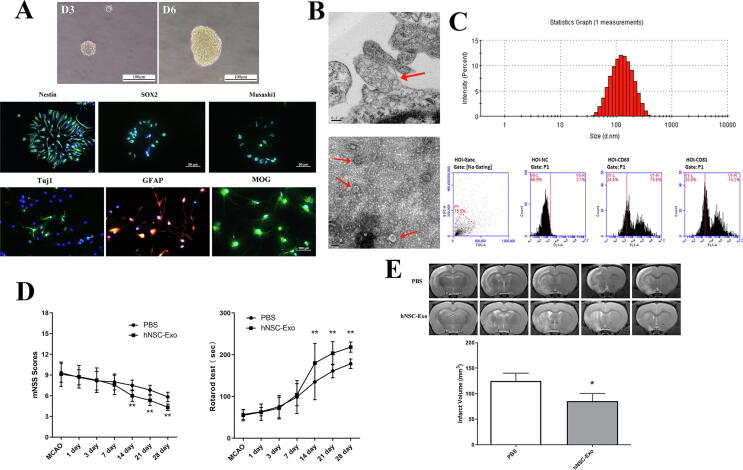
Exosomes are embraced by multivesicular endosomes or multivesicular bodies (MVBs) which are formed inside of cells, and then secreted via fusion with the plasma membrane. Exosomes were isolated from the cell medium of hNSCs. The characteristics and identity of hNSCs are shown in Fig. 1A which illustrates their morphological, markers and cell differentiation. Fig. 1B (TEM of hNSCs) shows that MVB was just released from the cell membrane, and was enriched with exosomal-like vesicles with sizes of approximately 50–200 nm in diameter. The exosomes were identified by TEM, NTA and FCM, their mean diameter was 115.3 ± 6.2 nm and significantly expressed protein markers CD63 and CD81 (Fig. 1B–C). We then assessed the therapeutic efficacy of isolated exosomes in the rats with brain ischemic stroke. The data (Fig. 1D–E) indicated that hNSCs-derived exosomes had behavioral and structural benefits in rats. Our results were consistent with those of Webb et al. [23], [24] which revealed that NSC EVs improved cellular, tissue, and functional outcomes in the middle-aged mouse thromboembolic (TE) stroke model, as well as significantly promoted neural tissue preservation and functional improvements in the pig of brain ischemic stroke model. Although these data suggest that EVs/exosomes derived from NSCs have therapeutic potential in stroke, but the harmful microenvironment associated with hypoxic, ischemic and oxidative stress may affect these functions. IFN-γ as a pro-inflammatory cytokine can increase cell tolerance to oxidative stress, and regulate the paracrine effects of cells [19], [21]. Thus, we performed IFN-γ preconditioning to evaluate the roles of isolated exosomes and examine their effects in vitro and in vivo.
Fig. 1.
The characteristics and functions of hNSCs and their exosomes. (A) The neurosphere morphology of hNSCs cultured in day 3 and 6, immunofluorescence shown hNSCs specific markers (Nestin, SOX2 and Musashi1 with green color), and hNSCs differentiated into neurons (Tuj1, green color), astrocytes (GFAP, red color) and oligodendrocytes (MOG, green color). Tuj1 = β-tubulin III, GFAP = glial fibrillary acidic protein, MOG = Myelin oligodendrocyte glycoprotein. (B) TEM of hNSCs shown that exosomal-like vesicles in MVBs were just released from cell memnbrane (Upper), and TEM of isolated exosomes from hNSCs presented small lipid bilayer membrane vesicles (Lower). Red arrows indicated exosomes. (C) The result of NTA shown the particle distribution of exosomes derived from hNSCs, and exosomes significantly expressed exosomal marker proteins CD63 and CD81 by flow cytometry. (D) The neurological functions of ischemic stroke rats were evaluated from 1 day to 28 days after exosomes treatment via mNSS and Rotarod tests. n = 5 rats/group (**p < 0.01). (E) The infarct volume of brain ischemia rats was examined by MRI at 28th day after stroke and transplantation. Analysis of T2-weighted images (T2WI) revealed a more reduction in infarct volume (ischemic lesion shown in white) in hNSC-Exo group relative to control group. n = 3 rats/group. (*p < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Characteristics of exosomes after IFN-γ preconditioning
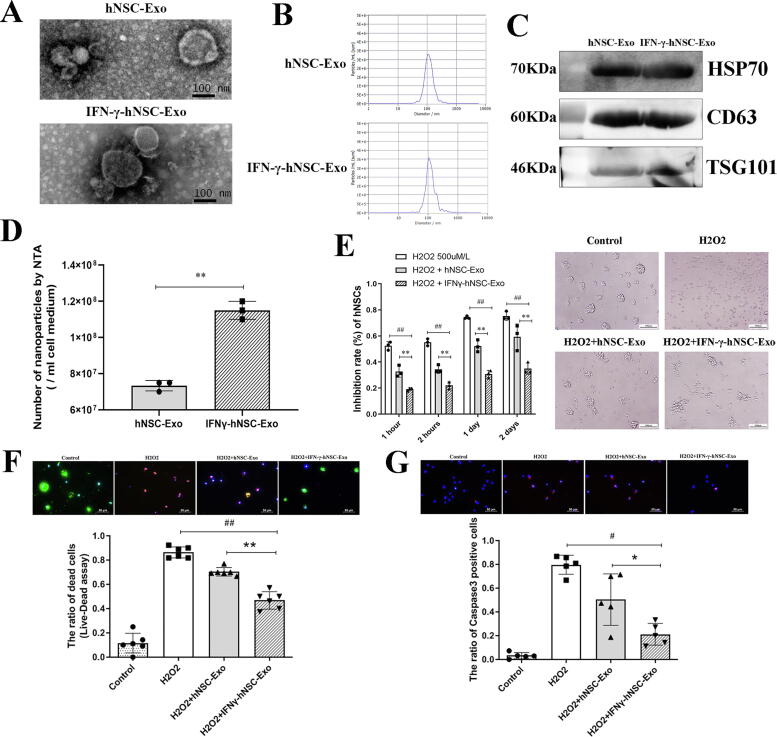
We collected the cell media and isolated exosomes before and after IFN-γ preconditioning. TEM, NTA, and WB analyses were performed to determine the characteristics of exosomes and compare IFN-γ-hNSC-Exo to hNSC-Exo (Fig. 2A–C). These results revealed that the two types of exosomes had the same morphology (small lipid bilayer membrane vesicles), size distribution (with mean diameter at 112.5 ± 4.8 nm vs 126.1 ± 7.2 nm), and exosomal marker proteins, CD63, Hsp70 and TSG101. Interestingly, NTA analysis further revealed that the yield of IFN-γ-hNSC-Exo was higher than that of hNSC-Exo in the same number of cultured cells and per unit volume of cell medium (Fig. 2D). Thus, the data confirmed that IFN-γ preconditioning did not affect the secretion and feature of exosomes derived from hNSCs.
Fig. 2.
The general features and cell activity experiments comparisons of IFN-γ-hNSC-Exo and hNSC-Exo. (A) TEM of isolated exosomes from hNSCs and IFN-γ-hNSCs, presented small lipid bilayer membrane vesicles. (B) The result of NTA analysis shown the particle distribution of exosomes derived from hNSCs and IFN-γ-hNSCs. (C) Both exosomes derived from hNSCs and IFN-γ-hNSCs significantly expressed exosomal marker proteins, CD63, Hsp70 and TSG101 by western blotting. (D) The number of exosomes derived from hNSCs stimulated by IFN-γ was much more than that of derived from hNSCs per unit volume of cell medium compared by NTA. (E) Exosomes significantly increased cell survival in the cell of H2O2 stress model, and IFN-γ-hNSC-exosomes have shown more positive effects by cytotoxicity assay, the phase-contrast images (Right side) presented bigger neurosphere formation in exosomes groups. (G) Live-dead cell assay indicated red-staining death cells and green-staining alive cells, the results shown IFN-γ-hNSC-Exo treatment upregulated much more green-staining alive cells expression than that of hNSC-Exo group. (F) Caspase-3 positive cells (Red staining) increased after treated cells by H2O2, and exosomes relieved the apoptotic state of cells, especially in the group of IFN-γ-hNSC-Exo. (Nuclei was blue by DAPI staining, *p < 0.05, #p < 0.05, and **p < 0.01, ##p < 0.01). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
IFN-γ-hNSC-Exo further increased cell activity in vitro cell H2O2 stress model
To determine whether exosomes affected on cell proliferation or survival under the hostile microenvironment, we prepared an H2O2 oxidative stress model of hNSCs to induce cell apoptosis and death. Fig. 2E reveals that most of the cells underwent apoptosis or death after H2O2 treatment. But after addition of exosomes to the cell medium, more living cells were detected, which could also form small neurospheres. Moreover, IFN-γ-hNSC-Exo had more positive effects on cells (as compared to hNSC-Exo). The results (Fig. 2E) of cytotoxicity assay revealed that exosomes significantly resisted the harmful role of H2O2 on cells and increased cell activity as compared to the H2O2 treatment group (P < 0.01). Moreover, the cell inhibition rate was lower in the IFN-γ-hNSC-Exo group than in the hNSC-Exo group.
Next, we further performed a live-dead cell assay (Fig. 2F) and also evaluated the expression of caspase-3 positive cells (Fig. 2G) in vitro. The results were consistent with those of the cytotoxicity assay, it showing that H2O2 treatment significantly induced cell death (red fluorescence cells indicated approximately 90% ± 3% dead cells). After exosomes were added to the cultured hNSCs for 3 days after plating, the number of dead cells was significantly decreased (Fig. 2F). Furthermore, in the IFN-γ-hNSC-Exo treatment group, the green fluorescence, which indicated live cells, was significantly upregulated. The number of alive cells in the IFN-γ-hNSC-Exo group was much higher than that in the hNSC-Exo group (Fig. 2F) (P < 0.01). The Fig. 2G shows that treatment with H2O2 showed rapidly increased the number of caspase-3 positive cells, and that the exosomes significantly relieved the apoptotic state of cells and augmented cell survival, particularly those derived from IFN-γ stimulated hNSCs (P < 0.05). Thus, the exosomes derived from hNSCs had some positive effect on cell viability, and IFN-γ preconditioned hNSCs further increased the ability of their exosomes to withstand H2O2 oxidative stress in vitro.
Exosomes affected cell proliferation and differentiation
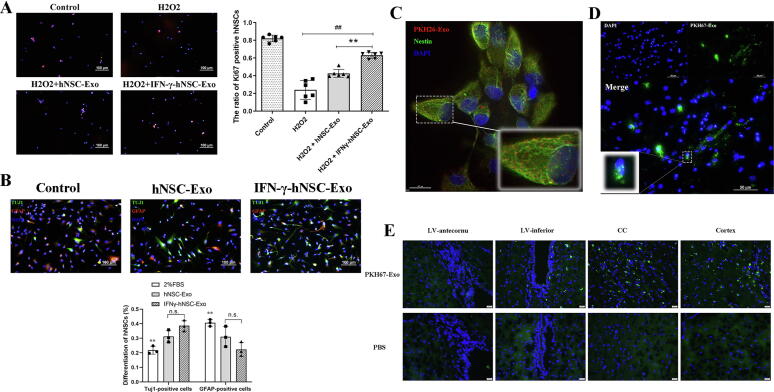
To explore whether the exosomes affected on hNSC proliferation and differentiation, they were added to the proliferation or differentiation medium to culture hNSCs. Exosomes groups showed significantly increased the proliferation of cells (Ki67 positive cells) compared to that in the H2O2 treatment group (Fig. 3A), and more Ki67 positive cells were detected in the IFN-γ-hNSC-Exo group, with significant difference between the hNSC-Exo and IFN-γ-hNSC-Exo group (P < 0.01). The results of the differentiation analysis of hNSCs showed (Fig. 3B) that exosomes stimulation increased the expression of Tuj1 positive cells (neuronal marker). But the number of neurons (Tuj1 positive expression) or astrocytes (GFAP positive expression) between hNSC-Exo and IFN-γ-hNSC-Exo treatment groups had no statistically significant difference (P > 0.05). These findings suggest that IFN-γ-hNSCs-Exo mainly affected cell proliferation compared to hNSCs-Exo.
Fig. 3.
The abilities of proliferation and migration of exosomes in cells. (A) IFN-γ-hNSC-Exo significantly further augmented the expression of Ki67 positive cells by compared to hNSC-Exo group in the cells of H2O2 model. (B) The differentiation of hNSCs was affected by exosomes treatment, but the number of neurons (Tuj1-green positive) and astrocytes (GFAP-red positive) between hNSC-Exo and IFN-γ-hNSC-Exo groups had no statistic difference (** vs. Exo groups). (**p < 0.01, ##p < 0.01 and n.s. was no significant) (C-D), PKH26-labeled (C, Red) or PKH67-labeled (D, Green) exosomes were co-incubated with hNSCs for 12 h checked by fluorescence microscopy. Internalization of exosomes was significantly confirmed visually as intracellular punctate red-fluorescence or green-fluorescence. (E) After PKH67-labled exosomes were injected into the striatum of ischemic rats, the green fluorescence-positive puncta (exosomes) was migrated from the injected site to the lateral ventricle, corpus callosum and cortex of brain. (Nuclei was blue by DAPI staining). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
PKH26/67-labled exosomes migrated and disseminated in vitro and in vivo
Exosomes are secreted by cells and then taken up by other cells, transferring their contents (such as protein or RNAs) into target cells that trigger intracellular signaling pathways to mainly mediate the biological characteristics of targeted cells or communication between the source and targeted cells. We used PKH26/67-labeled exosomes to incubate hNSCs and neurons in vitro. The internalization of PKH26-labeled exosomes was confirmed visually as intracellular punctate red-fluorescence in hNSCs (Fig. 3C). Furthermore, similar results were observed for PKH67-labeled exosomes through green-fluorescence was seen in neurons, especially around and inside the cell nucleus (Fig. 3D). Then we also examined the uptake of exosomes in vivo in the brain of ischemic rats. PKH67-Exos were stereo-tactically transplanted into the striatum of infarcted rats, after which green fluorescence-positive puncta exosomes migrated from the injected site to extensive regions of brain, such as around the lateral ventricle, corpus callosum and cortex (Fig. 3E).
Thus, these findings verified the internalization and migration of fluorescence-labeled exosomes in vitro and in vivo. Next we compared the therapeutic ability of IFN-γ-hNSC-Exo with hNSC-Exo in vivo brain ischemic rats.
IFN-γ-hNSC-Exo treatment shown more improved outcomes in brain ischemic rats
We next investigated the therapeutical effects of hNSC-Exo and IFN-γ-hNSC-Exo in vivo. We injected exosomes into the ischemic regions of brain ischemic stroke rats at 24 h after stroke onset. We mainly monitored neurological performance by mNSS, Rotarod and Postural reflex tests from day 1–28 after stroke and treatment (Fig. 4A–C). Both the hNSC-Exo and IFN-γ-hNSC-Exo groups showed significant neurological functional recovery improvement from days 7 to 28 via mNSS scores compared to that in the PBS control group, and the improved functional outcome in IFN-γ-hNSC-Exo group was superior to that in hNSC-Exo group (Fig. 4A) (n = 6–9 rats/group, P < 0.05). Rotarod and Postural reflex tests also showed significant functional improvement from days 7 or 14 to 28 in the exosomes groups compared to in the PBS control group, with greater improvements in outcome in the IFN-γ-hNSC-Exo group. The difference between the hNSC-Exo group and the IFN-γ-hNSC-Exo group had statistical significance at the 28th day after exosomes injection (Fig. 4B–C) (n = 6–9 rats/group, P < 0.05). Thus, IFN-γ-hNSC-Exo showed better efficient therapeutic effects in the brain ischemic rats and improved functional outcome.
Fig. 4.
IFN-γ-hNSC-Exo administration further improved behavioral outcomes in vivo. (A-C) To evaluate the neurological functions of ischemic stroke rats, mNSS, Rotarod and Postural reflex tests were used from 1 day to 28 days after exosomes treatment. Both hNSC-Exo and IFN-γ-hNSC-Exo groups promoted neurological recovery from days 7/14 to 28 compared to PBS control group, and the improved functional outcome in IFN-γ-hNSC-Exo group was superior to hNSC-Exo group. n = 6–9 rats/group (* and ** indicated IFN-γ-hNSC-Exo group vs. PBS group). (D) To evaluate the infarct volume of brain ischemia by MRI at 28th day after stroke and transplantation, the infarct size of exosomes groups decreased compared to PBS group. T2WI sequences of MRI revealed the infarct areas (ischemic lesion shown in white) of ischemic rats in PBS, hNSC-Exo and IFN-γ-hNSC-Exo groups. n = 5 rats/group. (*p < 0.05, **p < 0.01, and n.s. was no significant).
We further examined the brain infarct volume of ischemic rats after exosome treatment. We used MRI to evaluate the improved structural size or infarct volume of brain ischemia (Fig. 4D). The infarct size of exosomes groups significantly decreased compared to in the PBS group 28 days after stroke and exosome transplantation. Moreover, the reduction in infarct volume was much more in the IFN-γ-hNSC-Exo group than in the hNSC-Exo group, but the difference was not statistically significant (Fig. 4D) (n = 5 rats/group, P > 0.05).
Thus, the results indicate that after IFN-γ stimulation, IFN-γ-hNSC-Exo have neuro-functional therapeutical effects in brain ischemic stroke rats.
IFN-γ-hNSC-Exo treatment exerted better neuro-protection roles in brain ischemic rats
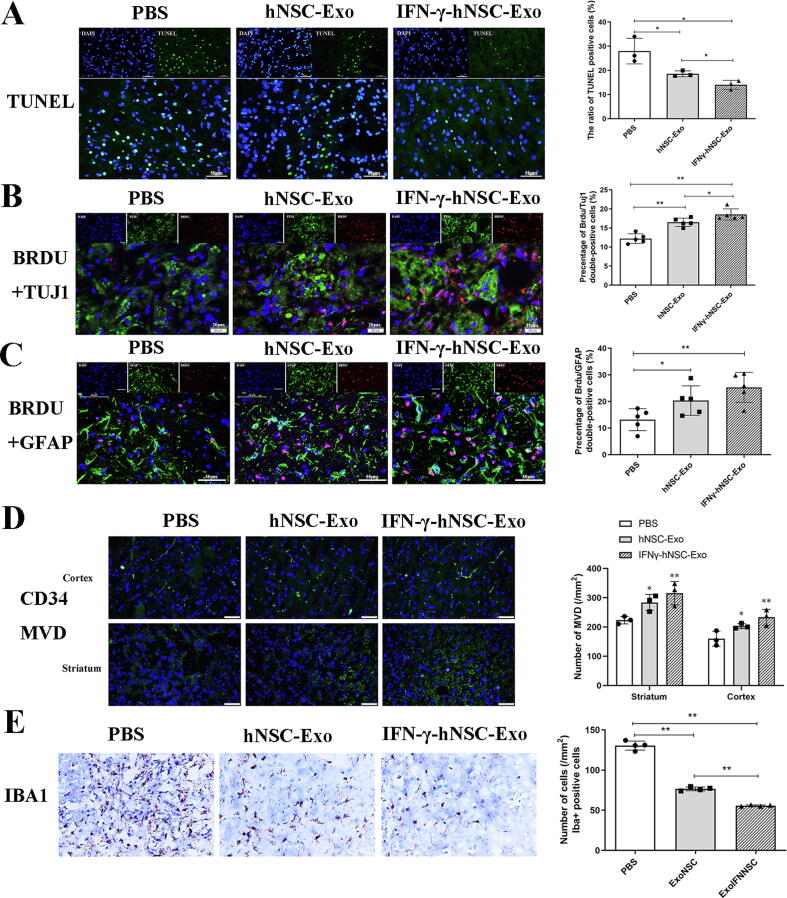
We further investigated whether exosomes could affect neural cell survival and neurogenesis in the ischemic injured regions in vivo. Exosomes were transplanted into the ischemic penumbra at 24 h after stroke onset, and the necrotic nerve cells were assessed around the ischemic area by TUNEL assay at 7 days after transplantation. The results (Fig. 5A) show that the number of TUNEL-positive necrotic cells in the exosomes groups was significantly lower than that in the PBS group, and apoptotic/necrotic cells in the IFN-γ-hNSC-Exo group were lower than those in the hNSC-Exo group (n = 3 rats/group, P < 0.05). This result suggests that the exosomes stimulated by IFN-γ further reduced the necrosis of ischemic nerve cells in vivo.
Fig. 5.
IFN-γ-hNSC-Exo treatment prompted neuro-protection and tissue repair in rats of ischemic model. (A) The number of TUNEL-positive necrotic nerve cells in the exosomes groups was decreased compared to PBS group, especially in the IFN-γ-hNSC-Exo group, by TUNEL assay 7 days after transplantation. n = 3 rats/group. (B-C) The double immunofluorescence staining revealed that the number of Brdu/Tuj1 and Brdu/GFAP dual-positive cells (indicated neurons and astrocytes, respectively) toward the ischemic lesion in the IFN-γ-hNSC-Exo group was much more than the hNSC-Exo group at 28th day after stroke and treatment. n = 5 rats/group. (D) Immunofluorescent staining of microvascular phenotype markers CD34 demonstrated that the microvessel density of exosomes groups was increased both in the cortex and striatum regions compared with PBS group at 28th day after stroke and treatment. n = 3 rats/group (* and ** vs. PBS group). (E) At 28th day after exosomes transplantation, Iba1-positive microglia were extensive scattered and accumulated around the ischemic area, but the density/intensity of Iba1-positive cells was much less in the IFN-γ-hNSC-Exo group compared to hNSC-Exo group. n = 4 rats/group. (Nuclei was blue by DAPI staining, *p < 0.05, and **p < 0.01). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Next, we examined the expression of neural cells around ischemic regions at 28 days after stroke and treatment, to evaluate cell or tissue regeneration. We checked the expression of BrdU/Tuj1 and BrdU/GFAP dual-positive cells which indicate neurons and astrocytes, respectively. The double immunofluorescence staining revealed many BrdU/Tuj1 and BrdU/GFAP dual-positive cells toward the ischemic lesion, especially in the exosomes groups (Fig. 5B–C). Importantly, there was statistical difference between PBS group and exosomes groups (n = 5 rats/group, P < 0.01). The number of BrdU/Tuj1 dual-positive cells in the IFN-γ-hNSC-Exo group was higher than that in the hNSC-Exo group (P < 0.05), but the number of BrdU/GFAP dual-positive cells between two exosomes groups had no statistical difference (n = 5 rats/group, P > 0.05). This indicates that IFN-γ-hNSC-Exo treatment generated and protected more functional neurons in vivo.
We further analyzed the micro-angiogenesis/microvessel proliferation capacity in ischemic regions of the brain. Immunofluorescent staining of microvascular phenotype markers CD34 demonstrated (Fig. 5D) that the micro-vessel density of exosomes groups was significantly increased compared to in the PBS group at 28 days after stroke and treatment (n = 3 rats/group, P < 0.05). Although a larger number of micro-vessels was detected in the IFN-γ-hNSC-Exo group than in the hNSC-Exo group, there was no significant difference between these groups (P > 0.05).
Finally, we evaluated the inflammatory immune response in the brain of rats after ischemia and exosomes treatment. The microglia in the ischemic lesion of brain was examined at 28 days after exosomes transplantation. In the PBS group, Iba1-positive microglia were extensively scattered and accumulated around the ischemic area. But after treatment with exosomes, the density/intensity of Iba1-positive staining was significantly reduced in comparison with the PBS control group, and IFN-γ-hNSC-Exo group showed further reduced Iba1-positive cells compared to the hNSC-Exo group (Fig. 5E) (n = 4 rats/group, P < 0.01). This suggests that IFN-γ-hNSC-Exo further reduce microglia proliferation or activation, and oppose the inflammatory hostile microenvironment in vivo.
Comparison of the miRNA expression profile of hNSC-Exo and IFN-γ-hNSC-Exo
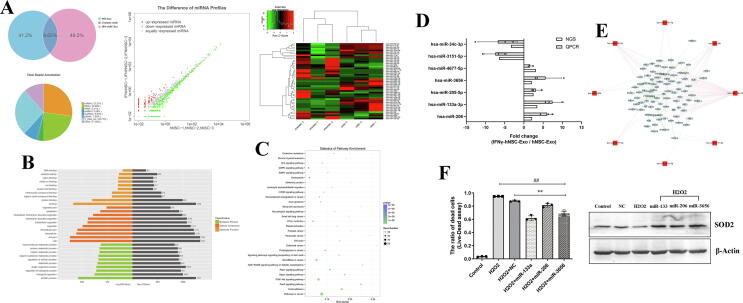
To explore the potential mechanism by which IFN-γ-hNSC-Exo had more positive roles than hNSCs–Exo in vitro and in vivo. We compared the miRNA expression profile by NGS. A total of three biological replicates for each type of exosomes was sequenced on the Illumina platform. We obtained 582 to 768 known miRNAs for each exosomes sample based on the public miRBase V21 database. Then to address the differences of in miRNA profiles between the exosomes derived from hNSCs and IFN-γ-hNSCs, the expression of each miRNA was quantified, after correcting for the number of reads per million (RPM) clean tags and quartile normalization (Fig. 6A). We identified 24 upregulated and 23 downregulated miRNAs (inclusion criteria: |log2(Fold Change)| ≥ 1 and p < 0.05). Moreover, after the inclusion criteria were adjusted to |log2(Fold Change)| ≥ 2 and p < 0.05, there were 14 upregulated and 16 downregulated miRNAs (Supplementary Table S1). Then after the criteria p < 0.01 was used, 7 prominent significant difference miRNAs were identified.
Fig. 6.
NGS and verification of special exosomal miRNAs of IFN-γ-hNSC-Exo. (A) Pie chart representation (Upper left) of the distribution of small RNA categories in exosomes of hNSC-Exo and IFN-γ-hNSC-Exo. Hierarchical clustering (Upper right) and scatter plot (Upper middle) of differentially expressed miRNAs between hNSC-Exo and IFN-γ-hNSC-Exo. Red indicates higher expression; green indicates lower expression. (B) The top 30 GO functions of predicted targets belong to the differentially expressed miRNAs. (C) The top 30 KEGG Pathway enrichment analysis of differential expression genes. (D) Special different exosomal 7 miRNAs (inclusion criteria, |log2(Fold Change)| ≥ 2 and p < 0.01) were validated by qRT-PCR assay, the results were consistent with NGS. (E) The potential targeted genes of special different exosomal 7 miRNAs in IFN-γ-hNSC-Exo were predicted by miRNA-gene network analysis, the hsa-miR-151a-5p was used to contrast. (F) Left: Transfected 3 overexpressed exosomal miRNAs (hsa-miR-206, hsa-miR-133a-3p and hsa-miR-3656) into hNSCs and then treated with H2O2, the proportion of cell survival was increased compared to the H2O2 and NC groups (##p < 0.01 vs·H2O2 group, **p < 0.01 vs. NC group). Right: The over-expression of exosomal miRNAs in cells upregulated anti-oxidative stress SOD2 expression by WB. β-Actin was used as the loading control. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Next target gene prediction and enrichment analysis of differential miRNAs were performed. Using the TargetScan, miRDB, miRWalk, and miRTarBase databases to predict the target genes of the differentially expressed miRNAs, 30 different miRNAs (inclusion criteria: |log2(Fold Change)| ≥ 2 and p < 0.05) were analyzed. GO was used to explore potential gene functions, and KEGG analysis was used to explore putative predominant pathways. Various GO categories were found, with the top 5 in 30 significant terms including binding, protein binding, Intracellular part, cellular process, and biological regulation (Fig. 6B). Furthermore, the top 5 in 30 KEGG signal pathways showing the most statistical difference (Fig. 6C) were MAPK signaling pathway, Endocytosis pathway, PI3K − Akt signaling pathway, FoxO signaling pathway, and Rap1 signaling pathway. These data suggest that the potential gene functions were most important in stem cell survival or exosomal transportation.
IFN-γ-hNSC-Exo may function via exosomal miRNAs
Then we chose the most significantly different 7 miRNAs (five upregulated and two downregulated) using the inclusion criteria of |log2(Fold Change)| ≥ 2 and p < 0.01, and validated these miRNAs via qRT-PCR, including hsa-miR-206, hsa-miR-133a-3p, hsa-miR-4677-5p, hsa-miR-205-5p, hsa-miR-3656, hsa-miR-34c-3p and hsa-miR-3151-5p. The results of qRT-PCR were consistent with those of NGS (Fig. 6D). These miRNAs were considered as specific to IFN-γ-hNSC-derived exosomal-miRNAs, and may play an important role in cell survival. And the key genes related to potential targeted pathways and functional categories were predicted by miRNA-gene network analysis (Fig. 6E), which were involved in the functions of IFN-γ-hNSC-derived exosomes.
Thus, to check the potential functions of these miRNAs, we performed cell live-dead assay to evaluate the ability of candidate miRNAs by cell H2O2 oxidative stress model in vitro. Of these, the results showed that the 3 miRNAs (hsa-miR-206, hsa-miR-133a-3p and hsa-miR-3656) were more important than the others and significantly increased cell survival (Fig. 6F) (P < 0.01). Furthermore, they significantly upregulated the level of the anti-oxidative-related protein SOD2, which is associated with cell viability (Fig. 6F).
Discussion
Although several hundred drugs have been tested for treating stroke, therapeutic options remain insufficient. Thus, the search for appropriate drugs for stroke is underway. EVs/exosomes derived from cells have recently emerged, and can provide information on multiple biological functions in adjacent or distant target cells[25]. Furthermore, EVs/exosomes are actively secreted by all cell types and have been identified in body fluids to have key roles in regulating immune responses [25], [26].
Recent studies [27], [28] showed that transplanted stem cells facilitate tissue regeneration through by releasing paracrine factor-EVs. EVs/exosomes act as important vehicles for paracrine delivery of therapeutic agents and are involved in complex intercellular communication systems. For example, NSC-derived small extracellular vesicles (NSC-sEVs) were used to treat spinal cord injury (SCI), the results showed that NSC-sEVs significantly reduced the extent of SCI, improved functional recovery, and reduced neuronal apoptosis, microglia activation, and neuro-inflammation in rats. This suggests that NSC-sEV can repair neural injury and improve the neuro-inflammatory microenvironment [29].
In the study, we focused on the role of IFN-γ-hNSCs-Exo (exosomes derived from hNSCs stimulated by IFN-γ), mainly by comparing their functions with those of hNSC-Exo. We revealed the characteristics of hNSC exosomes, and found that IFN-γ preconditioning did not affect the secretion and characteristics of exosomes derived from hNSCs, but altered their functions. We used cell proliferation and cell survival experiments in in vitro H2O2 stress model of cells to explore the abilities of IFN-γ-hNSC-Exo compared with those of hNSC-Exo, and further used these exosomes to treat brain ischemic stroke of rats to determine their therapeutic abilities in vivo.
Webb et al. [24] used EVs derived from hNSCs to treat mouse thromboembolic (TE) stroke model. They found that NSC EVs significantly improved cellular, tissue, and neurological functional outcomes in a middle-aged mouse model of stroke, but MSC EVs had fewer effects in mice. And the therapeutic effect of NSC EVs appeared to be mediated by alterations in the systemic immune response [24]. They also evaluated the role of NSC EVs in a pig ischemic stroke model induced by permanent middle cerebral artery occlusion [23]. Their results shown that NSC EV treatment led to significant improvements at the tissue and functional levels in pigs, including a decrease in the cerebral lesion volume, reduction in brain swelling and edema, increase in brain white matter integrity and corpus callosum fractional anisotropy values, and augmentation of exploratory behavior and spatiotemporal gait parameters restoration. Thus, these studies confirmed the therapeutic effects of NSC EVs on neural function in ischemic animals [23], [24].
However, central nervous system (CNS) inflammation mainly induced mononuclear phagocyte/microglial cell infiltration and oxidative stress activity, then leading to secondary CNS damage in vivo [30], [31]. Normally, the exosomes are also affected by CNS inflammation, and their abilities could be limited by the hostile microenvironment. Here we specifically focused on the effect of a cytokine (pro-inflammatory cytokine, IFN-γ) on the functional optimization of hNSC-Exo. Pluchino and Cossetti et al. [21], [32], [33] revealed that IFN-γ and the tumor necrosis factor alpha (TNF-α) regulate the phenotype of stem cells, release soluble factors from cells, and ultimately regulate the functions of stem cells. They confirmed that NSCs communicated with the microenvironment via their EVs. Furthermore, the IFN-γ pathway in NSCs exposed to pro-inflammatory cytokines was very important in these processes. IFN-γ can bind to EVs through Ifngr1, and activate Stat1 in target cells. Their results demonstrated that EV-associated IFN-γ/Ifngr1 complexes were critical for grafted stem cell communication with the host immune system [21]. Here, we shown that IFN-γ stimulation altered the ability of exosomes derived from hNSCs. Additionally, IFN-γ-hNSC-Exo significantly promoted the activity of hNSCs in the in vitro H2O2 oxidative stress model of cells, such as by augmenting stem cell survival, reducing cell apoptosis and exerting better protective abilities than hNSC-Exo. And in vivo, we found that IFN-γ-hNSC-Exo further facilitated the neurological functional recovery in the brain ischemic stroke model of rats compared to that in the hNSC-Exo group. Moreover, IFN-γ-hNSC-Exo further reduced nerve cell apoptosis, increased neuronal survival and diminished inflammatory responses in vivo. Thus, exosomes derived from IFN-γ-stimulation have more therapeutic advantage than naïve hNSC-Exo do.
Exosomes are involved in intercellular communication mainly by transferring exosomal RNA or protein cargos between cells. Zhang et al. [34] found that the hypothalamic stem cells controlled ageing speed partly through exosomal miRNAs. They disturbed the co-expression of Sox2 and Bmi1 in hypothalamic stem cells of mice and observed that each mouse model consistently displayed acceleration of ageing-like physiological changes or a shortened lifespan. Moreover, ageing retardation and lifespan extension were achieved in mid-aged mice that were locally implanted with healthy hypothalamic stem cells. The main reason was related to exosomal miRNAs derived from hypothalamic stem cells. These exosomal miRNAs declined during ageing, and hypothalamic stem cell-secreted exosomes which included those miRNAs led to the slowing of ageing [34]. Here we used NGS to compare the exosomal miRNA expression profile of IFN-γ-hNSC-Exo and hNSC-Exo. High-throughput miRNA analysis was used to reveal different gene changes in exosomes, mainly induced by pretreatment of cells with IFN-γ, containing the significantly different expression of cell functional regulators. The functional enrichment and signal pathways were closely correlated with stem cell survival and exosomal transportation. Further, we validated the expression of the most significantly different 7 miRNAs via qRT-PCR. Interestingly, the exosomal miRNAs (hsa-miR-206, hsa-miR-133a-3p and hsa-miR-3656) of IFN-γ-hNSC-Exo play important role in cell survival. Thus, these miRNAs may be functionally relevant in IFN-γ-hNSC-Exo.
However, there are some potential limitations associated with the development of exosomes remain [35], [36], [37], [38]: (1) although exosomes can have therapeutic effects in in vitro and in vivo ischemic models, their efficiency should be further explored, particularly compared with other drugs approved by the FDA; (2) the enrichment and purification of exosomes derived from stem cells should be uniform to avoid quality deviation; and (3) further studies are needed to determine how exosomes mediate functional interactions with immune cells—including macrophages or microglia cells, and to what extent they regulate immune effects.
Conclusion
In conclusion, we found that IFN-γ mediated the secretion and function of stem cell-derived exosomes, and induced specific exosomal miRNAs to promote cell survival. To clarify the direct role of exosomes stimulated by IFN-γ, we generated both IFN-γ-hNSC-Exo and naïve hNSC-Exo derived from the cell medium. The results revealed that IFN-γ-hNSC-Exo significantly increased the activity of hNSCs in in vitro cell H2O2 oxidative stress model compared to the hNSC-Exo. In the in vivo ischemic stroke model of rats, IFN-γ-hNSC-Exo further improved neurological function compared to hNSC-Exo. Through NGS, we found that IFN-γ-hNSC-Exo expressed specific exosomal miRNAs with greater therapeutic roles than hNSC-Exo. Thus, this study highlights an unexpected role for stem cell-derived exosomes stimulated by cytokine in the treatment of brain ischemic stroke and represents a significant advance in altering the functions of exosomes. Exosomes, as cell-derived bioactive molecules and next generation cell-free therapeutic candidates have numerous practical and conceptual advantages over stem cells, and how to optimize the efficacy of exosomes should further examined.
Compliance with ethics requirements
All the International and National Guidelines for the care and use of animals (fisheries) were followed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers 81671819) and China Postdoctoral Science Foundation (grant number 2019TQ0071).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2020.05.017.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Badhiwala J.H., Nassiri F., Alhazzani W., Selim M.H., Farrokhyar F., Spears J. Endovascular thrombectomy for acute ischemic stroke: a meta-analysis. JAMA. 2015;314(17):1832–1843. doi: 10.1001/jama.2015.13767. [DOI] [PubMed] [Google Scholar]
- 2.Fisher M. New approaches to neuroprotective drug development. Stroke. 2011;42(1 Suppl):S24–S27. doi: 10.1161/STROKEAHA.110.592394. [DOI] [PubMed] [Google Scholar]
- 3.Wei L., Wei Z.Z., Jiang M.Q., Mohamad O., Yu S.P. Stem cell transplantation therapy for multifaceted therapeutic benefits after stroke. Prog Neurobiol. 2017;157:49–78. doi: 10.1016/j.pneurobio.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao G., Wang Y., Guo X., Liu J., Zheng Z., Chen L. Neurorestorative effect of olfactory ensheathing cells and Schwann cells by intranasal delivery for patients with ischemic stroke: design of a multicenter randomized double-blinded placebo-controlled clinical study. J Neurorestoratol. 2018;6(1):74–80. [Google Scholar]
- 5.Kalladka D., Sinden J., Pollock K., Haig C., McLean J., Smith W. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388(10046):787–796. doi: 10.1016/S0140-6736(16)30513-X. [DOI] [PubMed] [Google Scholar]
- 6.Chen L., Zhang G., Khan A.A., Guo X., Gu Y. Clinical efficacy and meta-analysis of stem cell therapies for patients with brain ischemia. Stem Cells Int. 2016;2016:6129579. doi: 10.1155/2016/6129579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang H., Chen L., Zou Q., Han F., Sun T., Mao G. Clinical cell therapy guidelines for neurorestoration (China version 2016) J Neurorestoratol. 2017;5:39–46. [Google Scholar]
- 8.Bond A.M., Ming G.L., Song H. Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell. 2015;17(4):385–395. doi: 10.1016/j.stem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee S., Williamson D., Habib N., Gordon M., Chataway J. Human stem cell therapy in ischaemic stroke: a review. Age Ageing. 2011;40(1):7–13. doi: 10.1093/ageing/afq133. [DOI] [PubMed] [Google Scholar]
- 10.Marban E. The secret life of exosomes: what bees can teach us about next-generation therapeutics. J Am Coll Cardiol. 2018;71(2):193–200. doi: 10.1016/j.jacc.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malliaras K., Li T.S., Luthringer D., Terrovitis J., Cheng K., Chakravarty T. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125(1):100–112. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z.G., Chopp M. Exosomes in stroke pathogenesis and therapy. J Clin Invest. 2016;126(4):1190–1197. doi: 10.1172/JCI81133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallet R., Dawkins J., Valle J., Simsolo E., de Couto G., Middleton R. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017;38(3):201–211. doi: 10.1093/eurheartj/ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xin H., Li Y., Cui Y., Yang J.J., Zhang Z.G., Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33(11):1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Harane N., Kervadec A., Bellamy V., Pidial L., Neametalla H.J., Perier M.C. Acellular therapeutic approach for heart failure: in vitro production of extracellular vesicles from human cardiovascular progenitors. Eur Heart J. 2018;39(20):1835–1847. doi: 10.1093/eurheartj/ehy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lener T., Gimona M., Aigner L., Borger V., Buzas E., Camussi G. Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper. J Extracell Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang G., Chen L., Chen W., Li B., Yu Y., Lin F. Neural stem cells alleviate inflammation via neutralization of IFN-gamma negative effect in ischemic stroke model. J Biomed Nanotechnol. 2018;14(6):1178–1188. doi: 10.1166/jbn.2018.2568. [DOI] [PubMed] [Google Scholar]
- 19.Zhang G., Guo X., Chen L., Li B., Gu B., Wang H. Interferon-gamma promotes neuronal repair by transplanted neural stem cells in ischemic rats. Stem Cells Dev. 2018;27(5):355–366. doi: 10.1089/scd.2017.0225. [DOI] [PubMed] [Google Scholar]
- 20.Sakata H., Narasimhan P., Niizuma K., Maier C.M., Wakai T., Chan P.H. Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice. Brain. 2012;135(Pt 11):3298–3310. doi: 10.1093/brain/aws259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cossetti C., Iraci N., Mercer T.R., Leonardi T., Alpi E., Drago D. Extracellular vesicles from neural stem cells transfer IFN-gamma via Ifngr1 to activate Stat1 signaling in target cells. Mol Cell. 2014;56(2):193–204. doi: 10.1016/j.molcel.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang G., Chen L., Guo X., Wang H., Chen W., Wu G. Comparative analysis of microRNA expression profiles of exosomes derived from normal and hypoxic preconditioning human neural stem cells by next generation sequencing. J Biomed Nanotechnol. 2018;14(6):1075–1089. doi: 10.1166/jbn.2018.2567. [DOI] [PubMed] [Google Scholar]
- 23.Webb R.L., Kaiser E.E., Jurgielewicz B.J., Spellicy S., Scoville S.L., Thompson T.A. Human neural stem cell extracellular vesicles improve recovery in a porcine model of ischemic stroke. Stroke. 2018;49(5):1248–1256. doi: 10.1161/STROKEAHA.117.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webb R.L., Kaiser E.E., Scoville S.L., Thompson T.A., Fatima S., Pandya C. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl Stroke Res. 2018;9(5):530–539. doi: 10.1007/s12975-017-0599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bobrie A., Colombo M., Raposo G., Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 26.Tkach M., Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 27.Chen B., Li Q., Zhao B., Wang Y. Stem cell-derived extracellular vesicles as a novel potential therapeutic tool for tissue repair. Stem Cells Transl Med. 2017;6(9):1753–1758. doi: 10.1002/sctm.16-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran C., Damaser M.S. Stem cells as drug delivery methods: application of stem cell secretome for regeneration. Adv Drug Deliv Rev. 2015;82–83:1–11. doi: 10.1016/j.addr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rong Y., Liu W., Wang J., Fan J., Luo Y., Li L. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019;10(5):340. doi: 10.1038/s41419-019-1571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q., Sanai N., Jin W.N., La Cava A., Van Kaer L., Shi F.D. Neural stem cells sustain natural killer cells that dictate recovery from brain inflammation. Nat Neurosci. 2016;19(2):243–252. doi: 10.1038/nn.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dooley D., Vidal P., Hendrix S. Immunopharmacological intervention for successful neural stem cell therapy: new perspectives in CNS neurogenesis and repair. Pharmacol Ther. 2014;141(1):21–31. doi: 10.1016/j.pharmthera.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Drago D., Cossetti C., Iraci N., Gaude E., Musco G., Bachi A. The stem cell secretome and its role in brain repair. Biochimie. 2013;95(12):2271–2285. doi: 10.1016/j.biochi.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pluchino S., Cossetti C. How stem cells speak with host immune cells in inflammatory brain diseases. Glia. 2013;61(9):1379–1401. doi: 10.1002/glia.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y., Kim M.S., Jia B., Yan J., Zuniga-Hertz J.P., Han C. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017;548(7665):52–57. doi: 10.1038/nature23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai C.P., Kim E.Y., Badr C.E., Weissleder R., Mempel T.R., Tannous B.A. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat Commun. 2015;6:7029. doi: 10.1038/ncomms8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathivanan S., Fahner C.J., Reid G.E., Simpson R.J. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40(Database issue) doi: 10.1093/nar/gkr828. D1241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 38.Peruzzotti-Jametti L., Bernstock J.D., Vicario N., Costa A.S.H., Kwok C.K., Leonardi T. Macrophage-derived extracellular succinate licenses neural stem cells to suppress chronic neuroinflammation. Cell Stem Cell. 2018;22(3) doi: 10.1016/j.stem.2018.01.020. 355-68 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.