Abstract
The term “endemic mycoses” refers to a group of fungi that maintains a baseline rate of infection only in certain geographical regions due to the hospitable enviormental conditions these regions offer. In the United States, Histoplasma capsulatum, Coccidioides spp, and Blastomyces dermatitidis are the three most prevalent endemic human fungal infections. The traditional endemic regions for these pathogens are defined based on data acquired many decades ago, and case detection is subject to diagnostic delays even in classically endemic areas, a problem that is likely to be magnified in areas less familiar with these fungal infections. The present series includes an example of each of these infections diagnosed in a medical center situated in the suburbs of New York City, a location not considered endemic for any of them. Likely routes of acquisition for the three patients are considered, and the history of encounters with these pathogens in New York State is briefly recounted. Altogether, this report is intended to serve as a reminder to clinicians that traditional distribution maps for the endemic mycoses are bound to be outdated in the face of modern trends in globalization, population dynamics, and ecological change.
Keywords: Endemic mycoses, Histoplasmosis, Blastomycosis, Coccidioidomycosis, New York state
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- AmB
amphotericin B
- BAL
bronchoalveolar lavage
- CAP
community acquired pneumonia
- CNS
central nervous system
- CT
computed tomography
- DOH
Department of Health
- EIA
enzyme immunoassay
- 18FDG
18fluorodeoxyglucose
- HIV
human immunodeficiency virus
- IGRA-MTB
interferon-gamma release assay for Mycobacterium Tuberculosis
- KOH
potassium hydroxide
- LUL
left upper lobe
- NYC
New York City
- NYS
New York State
- PET
positron emission tomography
- PN
pulmonary nodule
- RLL
right lower lobe
1. Introduction
Particular fungal pathogens are collectively termed “endemic mycoses” because they maintain a baseline rate of infection only in certain geographical regions due to the conducive natural conditions present there. In the United States, Histoplasma capsulatum, Coccidioides spp, and Blastomyces dermatitidis are the three most prevalent endemic fungal infections in that order [1]. On endemicity maps for these infections, New York State (NYS) is typically not identified as an endemic area with the exception of the northernmost reaches of the state bordering Canada when distribution of B. dermatitidis is depicted. However, in an increasingly globalized and environmentally dynamic world, traditional endemicity maps require frequent re-evaluation as cases are identified in unexpected locations [2,3]. Endemic mycoses are notorious for diagnostic delays in regions historically familiar with them [4], so recognition is likely to be an even greater challenge for clinicians in areas perceived to be non-endemic. To underscore the need for awareness of these infections in places not shaded on endemicity maps, we present and discuss a case of each of the aforementioned mycoses diagnosed at our institution located in the immediate suburbs of New York City (NYC). We specifically emphasize epidemiological considerations that may have led to the discovery of these pathogens in residents of southern NYS.
1.1. Case 1 – histoplasmosis
A 44-year-old woman, lifelong resident of the northern suburbs of NYC, with a past medical history of polycystic ovarian syndrome was referred to our institution after a 9mm right lower lobe (RLL) pulmonary nodule (PN) was detected incidentally on computed tomography (CT) of the abdomen performed for nephrolithiasis. Review of systems was negative for constitutional and respiratory symptoms. She was a never-smoker and denied relevant occupational exposures. Vital signs, physical examination, and routine laboratory evaluation were unremarkable. Testing for the human immunodeficiency virus (HIV) and an interferon-gamma release assay for Mycobacterium tuberculosis (IGRA-MTB) were both negative. Chest CT performed five months after the abdominal study revealed an increase in size of the solid RLL PN to 11mm (Fig. 1) along with mediastinal and right hilar lymph nodes all measuring less than 1cm in short axis. Neither the PN nor the intrathoracic lymph nodes demonstrated avidity for 18fluorodeoxyglucose (18FDG) on 18FDG-positron emission tomography (18FDG-PET). Cytology from endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) of the right hilar lymph node revealed a mixed cellular population consisting of lymphocytes and neutrophils against a background of extensive necrosis. No organisms were seen on lymph node cytology; bronchoscopic cultures returned negative for bacterial, mycobacterial, and fungal organisms. With the leading pre-operative diagnosis of neoplasia, the patient underwent wedge resection of the PN via video-assisted thoracoscopic surgery. Tissue examination revealed necrotizing granulomatous inflammation associated with small yeast-like forms exhibiting narrow-based budding, morphologically consistent with H. capsulatum (Fig. 2). Fungal cultures of the resection specimen yielded no growth. Antibodies against H. capsulatum and Coccidioides were not detected in the serum by either complement fixation or immunodiffusion. She was prescribed a six-week course of oral itraconazole. Upon further questioning, she reported having vacationed in Puerto Rico, an endemic region for histoplasmosis, within a year of her diagnosis. On that trip, she engaged in cave exploration.
Fig. 1.
Axial image from a CT scan of the chest set to lung window showing a solid 11mm nodule in the right lower lobe behind the diaphragm.
Fig. 2.
Histological section of lung tissue obtained at surgery and stained with Gomori methenamine silver highlights the presence of small yeast forms, some exhibiting narrow-based budding (black arrows) characteristic of H. capsulatum (original magnification x 400).
1.2. Case 2 – coccidioidomycosis
A 36-year-old woman residing in the northern suburbs of NYC was referred for outpatient pulmonary evaluation of a lung nodule. Her past medical history was significant for locally advanced endocervical adenocarcinoma, which had been managed with abdominal hysterectomy and bilateral salpingo-oophorectomy one year earlier. No adjuvant therapy was administered. Preoperatively, 18FDG-PET/CT revealed a 1.2cm hypermetabolic RLL PN with a maximal standardized uptake ratio (SUVmax) of 2.3 (Fig. 3A). Transthoracic needle biopsy of this lesion was performed and showed poorly defined granulomatous inflammation with negative stains for microorganisms. Tissue cultures were not obtained. On repeat 18FDG-PET/CT one year after the initial study, the PN was not appreciably changed in size but now had a cavitary appearance with an increase in SUVmax to 5.18 (Fig. 3B). At the time of her visit to the pulmonary clinic, she denied constitutional and respiratory symptoms. She was a never-smoker and had no relevant occupational exposures. Since arriving to suburban NYC from Ecuador three years prior to the visit, she had not traveled. Her vital signs, physical examination, and routine laboratory evaluation were unremarkable. HIV testing was negative, and antibodies against H. capsulatum were not detected. IGRA-MTB was positive, but serial sputum collection for acid fast bacillus smear and culture was negative. She then underwent bronchoscopy with bronchoalveolar lavage (BAL). No microorganisms were seen on cytological and potassium hydroxide (KOH) preparations of the fluid. Eventually, however, BAL fungal culture yielded growth identified as Coccidioides spp. Since she remained asymptomatic, clinical observation was chosen over antifungal therapy. On further questioning, the patient revealed that she had migrated to the United States from Ecuador through northern Mexico, entering into Texas and from there ultimately reaching New York by air. Both northern Mexico and Texas are locations in which Coccidioides spp are endemic.
Fig. 3.
A, Axial image from the CT portion of a18FDG-PET/CT study set to lung window showing a spherical, solid 1.2cm subpleural nodule in the right lower lobe. The nodule's maximal standardized uptake value on the PET portion (not shown) was 2.33. B, Axial image from the CT portion of a18FDG-PET/CT study obtained 12 months later than the one in panel A showing a similarly sized nodule but with interval development of cavitation. The maximal standardized uptake value on the PET portion (not shown) now measured 5.18.
1.3. Case 3 – blastomycosis
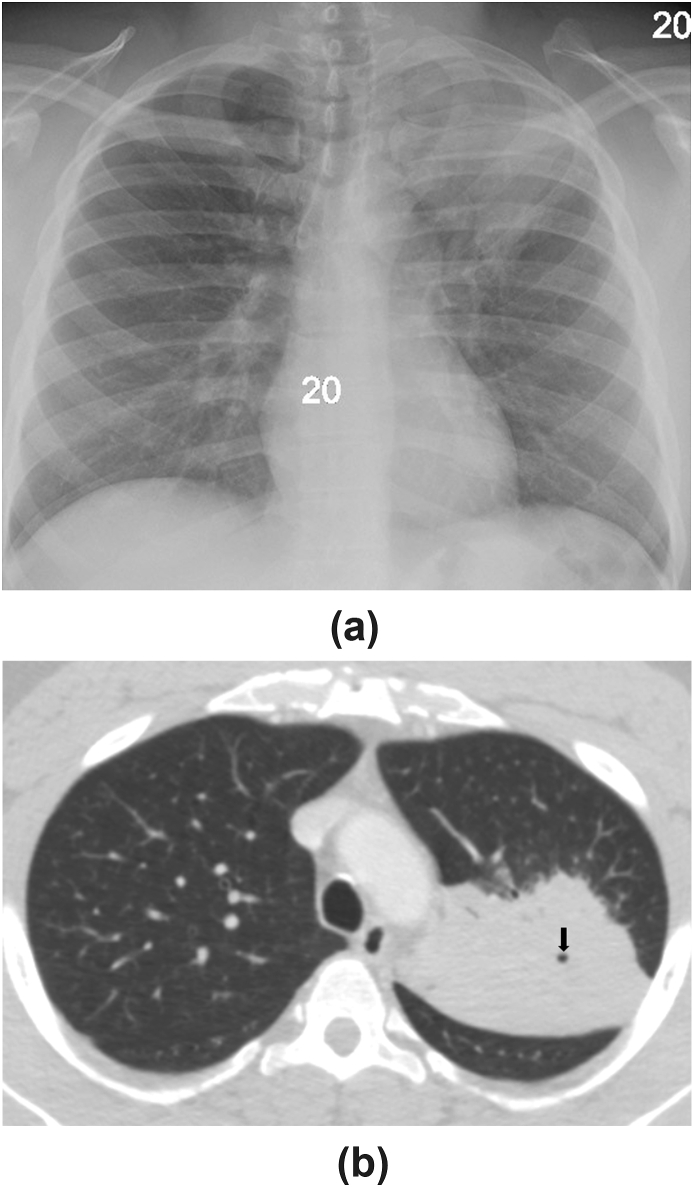
A 45-year-old African-American male was brought to our institution from the county correctional facility with fever, cough, and blood-streaked sputum of four weeks’ duration. The remainder of his review of systems was unrevealing. He was a never-smoker but a former user of multiple intranasal recreational drugs. On admission, he had a temperature of 38.3C and was tachycardic to 108 beats/min. His physical examination was notable only for crackles over the left upper chest anteriorly. Routine laboratory evaluation was unremarkable. HIV testing and IGRA-MTB were both negative. His chest radiograph revealed a dense left upper lobe (LUL) consolidation (Fig. 4A). Chest CT illustrated the same finding with no additional abnormalities present (Fig. 4B). Bronchoscopy with transbronchial biopsy and BAL of the LUL was performed. Biopsy material showed non-necrotizing granulomatous inflammation with negative stains for microorganisms. Rare fungal forms were visualized in the BAL fluid cytology specimen stained with Gomori methenamine silver (Fig. 5). KOH preparations of neither the BAL fluid nor lung tissue demonstrated the fungus, but both BAL fluid and tissue fungal cultures grew B. dermatitidis, an identification that was later confirmed by polymerase chain reaction in the NYS Department of Health (DOH). The patient responded well to a six-month course of oral itraconazole. When questioned about his incarceration history, he reported a recent stint at the Adirondack Correctional Facility in Rye Brook, NY located more than 200 miles north of our institution and about 60 miles from the Canadian border. His daily routine there included outdoor activities.
Fig. 4.
A, Frontal chest radiograph showing a dense consolidation in the left upper lobe. B, Axial image from a CT scan of the chest set to lung window demonstrating the consolidation to be located in the apicoposterior segment of the left upper lobe. A small focus of cavitation (black arrow) is evident within the consolidation.
Fig. 5.
Section of cell block prepared from the bronchoalveolar lavage fluid cytology specimen and stained with Gomori methenamine silver showing a representative fungal form of relatively large size that exhibits broad-based budding, making it morphologically consistent with B. dermatitidis (original magnification x 400).
2. Discussion
What follows is a brief review of the three endemic mycoses corresponding to the cases presented above with a special emphasis on their occurrence in NYS. All are associated with frequent misdiagnosis, especially in non-endemic areas, with community acquired pneumonia, tuberculosis (TB), and malignancy being the commonest initial considerations depending on disease tempo.
2.1. Histoplasmosis in NYS
Like Coccidioides and B. dermatitidis, H. capsulatum is a dimorphic fungus, meaning that it exists in its mold form in the environment, but upon inhalation and entry into the lungs it undergoes conversion to its yeast form, which is required for pathogenicity. It is the smallest of the three (2–4μm), reproduces by narrow-based budding, and flourishes in soil contaminated by bird and bat droppings. The latter makes cave exploration (spelunking) a particular risk factor for the acquisition of H. capsulatum [5]. Inhalation of a large spore inoculum, as might happen to a spelunker, can give rise to acute pulmonary histoplasmosis ranging from asymptomatic to life-threatening lung involvement, the latter usually when the inoculum is massive. Thoracic imaging in incidentally detected cases typically shows focal nodules or consolidations, whereas severe cases are characterized by diffuse nodular opacities. Diagnosis of acute pulmonary histoplasmosis can be established through direct visualization of H. capsulatum on cytopathology, its growth in culture, or presence of its antigen in serum or urine. Of these methods, examination of a KOH preparation yields the most immediate results. Immunosuppression and abrupt clinical presentation are likely to limit the sensitivity of antibody detection [6]. Treatment is reserved for symptomatic patients: Amphotericin B (AmB) is recommended for severe disease and itraconazole for non-severe cases [7]. Exposure to a smaller inoculum can give rise to slowly evolving infection known as subacute pulmonary histoplasmosis. Greater sensitivity of antibody-based assays is expected given the more indolent disease course. Itraconazole is the preferred therapy for persistent symptoms [7]. Chronic pulmonary histoplasmosis is a cavitary lung infection seen in patients with structural lung disease such as emphysema [8]. There is often an associated wasting illness. All of the aforementioned diagnostic tools for H. capsulatum are useful in this form of infection. Prolonged courses of itraconazole are the norm [7]. Especially in hosts with impaired cell-mediated immunity, failure to contain H. capsulatum at extrapulmonary sites of deposit can lead to infiltration of the reticuloendothelial system, central nervous system (CNS), skin, and bone marrow among other manifestations [9]. Disseminated histoplasmosis is often clinically aggressive, and patients can appear toxic due to systemic disease. Antigenemia and antigenuria are hallmarks of disseminated infection, which calls for treatment with AmB [10].
Of the three endemic mycoses under discussion, histoplasmosis is the most geographically diverse, though it has a particular predilection for the Ohio and Mississippi River valleys. NYS has traditionally been left off the endemicity map for histoplasmosis based on a study from the 1950s that estimated <10% reactivity to histoplasmin antigen in the population [11]. Reactivity rates vary across the state, being lowest in the NYC area (0–3%) and highest close to the great lakes of Erie and Ontario [12] (11–25%). Perhaps the state's first recorded outbreak occurred in 1938 when 23 workers contracted histoplasmosis after shoveling pigeon manure out of the attic of an old school in Lake Champlain [13]. In 1965, five adults and eight children were infected after cleaning out an old house inhabited by bats on Conesus Lake in the western part of the state [14]. In the 1970s, an outbreak originated in Auburn Correctional Facility located in the Finger Lakes Region near Lake Ontario. This outbreak lasted several years and was likely triggered by the excavation of soil contaminated with bird excreta [15]. In the following decade, NYC physicians encountered the unfamiliar entity of disseminated histoplasmosis in the context of treating patients with advanced acquired immunodeficiency syndrome [[16], [17], [18]] (AIDS). These patients were of Latin American or Caribbean origin and were believed to have harbored latent H. capsulatum infection acquired in their native countries that reactivated in the setting of immunosuppression. This theory was later substantiated by Keath and colleagues [19]. Since then, sporadic cases have periodically surfaced in NYC, with a common motif continuing to be former residence in Latin America or the Caribbean [9]. Our patient, born in suburban NYC, likely contracted asymptomatic histoplasmosis while exploring caves during a vacation in Puerto Rico, serving as a reminder of the role of travel in the appearance of endemic mycoses in unusual locations.
2.2. Coccidioidomycosis in NYS
In contrast to H. capsulatum and B. dermatitidis, Coccidioides spp, which consist of C. immitis and C. posadasii, inhabit arid, sandy lansdscapes found in the southwestern United States and bordering Mexican territories. Coccidioides spores, measuring 3–5μm, are easily dispersed by activities that disturb the local topography and, once inhaled, are extremely infectious. In lung tissue, hundreds of spores aggregate into characteristic spherules, much larger structures the diameter of which can reach 100μm [20]. The majority of infections appear to pass asymptomatically. Those with symptoms typically experience a self-limited, flu-like illness characterized by often disabling fatigue but relatively mild respiratory symptoms that commonly include pleuritic pain [21]. The thoracic imaging pattern of pulmonary coccidioidomycosis in its acute phase is that of parenchymal consolidation that notoriously creates diagnostic confusion with bacterial community acquired pneumonia (CAP). Prominent fatigue, reactive cutaneous lesions such as erythema nodosum, and peripheral eosinophilia favor coccidioidomycosis over bacterial CAP [4]. Resolution can leave behind a lung nodule resembling malignancy and prone to thin-walled cavitation. Pleural complications, a distinguishing feature of coccidioidomycosis relative to the other endemic mycoses, manifest as exudative pleural effusion and as pneumothorax secondary to cavity rupture [22]. In immunocompromised or otherwise susceptible patients, overwhelming lung involvement can occur, as can disseminated disease. Commonest sites of extrapulmonary infection are the CNS, skin, skeleton, and lymph nodes, a pattern reminiscent of the other endemic mycoses. The diagnostic gold standard for coccidioidomycosis is direct visualization or growth on culture, but both lack sensitivity. Serological testing is of particular utility in coccidioidomycosis because the widely available enzyme immunoassay (EIA) antibody detection is highly sensitive in immunocompetent hosts and is reliable evidence of current or recent infection, though positive results warrant confirmation with immunodiffusion or complement fixation, especially in cases of isolated IgM positivity [4]. Antigen testing also exists and can be complementary, but its limitations are availability and cross-reactivity [6]. Pharmacotherapy is restricted to symptomatic patients: in routine cases, the azole class of antifungals—fluconazole or itraconazole—is recommended, whereas AmB is reserved for the critically ill and those with dissemination of disease [10].
Coccidioidin antigen skin testing in the 1940s delineated the endemic region for Coccidioides in the United States as encompassing the states of California, Arizona, Nevada, New Mexico, Utah, and Texas [23]. Of these states, California and Arizona account for the vast majority of cases. In fact, hyperendemicity of C.immitis in the San Joaquin Valley of California is responsible for the term “valley fever” having become synonymous with pulmonary coccidioidomycosis. In the first decade of this millennium, Arizona experienced a further increase in the incidence of coccidioidal infection, coinciding with the surge in its popularity as a retirement destination for non-immune northeasterners, including residents of NYS [24]. In 2000, the NYS DOH reviewed cases of coccidioidomycosis recorded in the state's hospital discharge records [25] between 1992 and 1997. This investigation revealed a rate of approximately 30 hospitalizations per year; about 75% of cases were associated with risk factors such as HIV or travel history. Not to be overlooked is the endemic status of northern Mexico, including Baja California. A NYC case of coccidioidomycosis complicated by pneumothorax [26] published in 2011 and a multi-state outbreak in 2018 that involved NYS [27] were both traceable to exposures that had occurred in Mexico. Of note in regard to our patient, a child residing on Long Island developed hydrocephalus that was eventually found to be a sequela of CNS involvement by C. posadasii [28]. Phylogenomic analysis showed that the child's fungal strain correlated with isolates native to Texas, a location the child had visited. Our patient's land migration through Mexico and Texas during which she likely acquired coccidioidomycosis represents a possibly underestimated route of entry for endemic mycoses into non-endemic areas such as NYS.
2.3. Blastomycosis in NYS
At 8–20μm in diameter, B. dermatitidis is larger than H.capsulatum and individual Coccidioides spores. Its morphological hallmarks are a doubly refractile cell wall and broad-based budding [29]. Its ecological niche is characterized by soil enriched with decaying organic matter, and reservoirs have tended to cluster along waterways. As with the other endemic mycoses, the route of acquisition is inhalational; akin to Coccidioides, only about half of infected patients develop symptoms [30]. Cases with a rapid clinical tempo manifest disease features compatible with bacterial CAP, including consolidation on thoracic imaging, leading to frequent initial misdiagnosis. Subacute presentation with the same radiological picture can be preferentially attributed to TB or the more common histoplasmosis. Pulmonary nodules, often single and incidentally detected, may raise concern for neoplasia before fungal infection is invoked. Chronic pulmonary blastomycosis is a fibrocavitary process in a patient with vague symptoms that mimics other granulomatous lung diseases and malignancy. On the extreme end of severity are the rare life-threatening cases with diffuse lung involvement fulfilling ARDS criteria possibly linked to immune compromise [31]. Among the three endemic mycoses, dissemination of B. dermatitidis has the weakest correlation with immunosuppression and can befall overtly normal hosts. The most common (20%) and characteristic site of extrapulmonary spread is the skin [32]. Cutaneous blastomycosis assumes varied appearances and could serve as a valuable diagnostic clue to accompanying lung disease if the connection is recognized [33]. Metastatic infection of the CNS is well-described, albeit rare, and AIDS patients may be uniquely at risk [31]. Confident diagnosis of blastomycosis remains predicated on culture, yield of which is excellent [34] but indolent and therefore unconducive to rapid clinical decision-making. Direct visualization in stained cytopathological specimens is sensitive [35] but historically underused [34]. Urinary antigen detection is a recommended adjunct with reported sensitivity ranging from 76 to 93%, but cross-reactivity presents a problem [6]. Erratic performance has limited the utility of traditional antibody assays. While a promising EIA-based technique has been developed, it remains commercially unavailable [36]. Due to concerns about potential dissemination or lingering, treatment of blastomycosis is favored regardless of initial illness severity and immune status of the patient. Itraconazole is appropriate for most presentations with the exception of severe lung disease and CNS involvement, which call for AmB [10]. Underlying immunosuppression generally merits induction therapy with AmB followed by itraconazole maintenance in responders.
The understanding of blastomycosis epidemiology is comparatively imprecise owing to the absence of a skin test that could be used to define the geographic distribution. Lands with pockets of remarkably high incidence include the states of Wisconsin, Louisiana, and Mississippi as well as the province of Ontario. As mentioned, NYS has an endemic sector in its northernmost reaches where the St. Lawrence River Valley parallels the border with Canada. Of relevance to our patient, the St. Lawrence River Watershed drains the Adirondack Mountains. The earliest reported cluster of human blastomycosis cases detected in central NYS was a series of three patients who presented to a hospital in Cooperstown, located nearly 300 miles south of the St. Lawrence River basin [37]. Encounters with blastomycosis were documented by NYC physicians in 2015 [33] and 2016 [38], and then in 2017 Albany-area infectious disease specialists alerted the NYS DOH about the occurrence of multiple cases in the Capital District that lacked travel history [39]. Subsequent investigation identified 25 cases in the first half of 2017 alone diagnosed in a county along the Mohawk River, which corresponded to an incidence at that location (2.2. cases per 100,000 population) comparable to the most endemic parts of the United States. Since then, clinicians from Albany have published a notable case from their experience [40] and have also compiled a 20-patient series that confirms a recent incidence spike and reports striking diagnostic delays (unpublished data). Our patient's suggestive incarceration history adds another potential pathway for the movement of B. dermatitidis into areas unaccustomed to this pathogen.
3. Conclusion
In summary, distribution maps that were charted for the endemic mycoses in the middle of the 20th century would benefit from ongoing re-examination to correct for the dynamically changing epidemiology of these infections in areas of the country not classically associated with them. Using NYS as an illustration, the present series is meant to increase awareness of the fluid nature of “endemicity” in a world undergoing complex ecological change and subject to geographic movement of individuals. Physician awareness is critical to minimizing the diagnostic delays that are characteristic of endemic fungal infections and that can compromise clinical outcomes.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors have not received funding for any part of the study, and do not report any conflicts of interest.
Acknowledgements
None.
Contributor Information
Ravi Manglani, Email: ravipapu.manglani@wmchealth.org.
Liying Han, Email: Liying.Han@wmchealth.org.
References
- 1.Baddley J.W., Winthrop K.L., Patkar N.M. Geographic distribution of endemic fungal infections among older persons, United States. Emerg. Infect. Dis. 2011;17(9):1664–1669. doi: 10.3201/eid1709.101987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vallabhaneni S., Mody R.K., Walker T., Chiller T. The global burden of fungal diseases. Infect. Dis. Clin. 2016;30(1):1–11. doi: 10.1016/j.idc.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Ashraf N., Kubat R.C., Poplin V. Re-drawing the maps for endemic mycoses. Mycopathologia. 2020 doi: 10.1007/s11046-020-00431-2. [published online ahead of print, 2020 Feb 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hage C.A., Knox K.S., Wheat L.J. Endemic mycoses: overlooked causes of community acquired pneumonia. Respir. Med. 2012;106(6):769–776. doi: 10.1016/j.rmed.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Wheat L.J. Histoplasmosis: a review for clinicians from non-endemic areas. Mycoses. 2006;49(4):274–282. doi: 10.1111/j.1439-0507.2006.01253.x. [DOI] [PubMed] [Google Scholar]
- 6.Hage C.A., Carmona E.M., Epelbaum O. Microbiological laboratory testing in the diagnosis of fungal infections in pulmonary and critical care practice. An official American thoracic society clinical practice guideline [published correction appears in Am J respir crit care med. 2019 nov 15;200(10):1326] Am. J. Respir. Crit. Care Med. 2019;200(5):535–550. doi: 10.1164/rccm.201906-1185ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azar M.M., Hage C.A. Clinical perspectives in the diagnosis and management of histoplasmosis. Clin. Chest Med. 2017;38(3):403–415. doi: 10.1016/j.ccm.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Kauffman C.A. Histoplasmosis: a clinical and laboratory update. Clin. Microbiol. Rev. 2007;20(1):115–132. doi: 10.1128/CMR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi V., Ulyanovskiy P., Epelbaum O. Update on the spectrum of histoplasmosis among hispanic patients presenting to a New York City municipal hospital: a contemporary case series. Respir Med Case Rep. 2015;16:60–64. doi: 10.1016/j.rmcr.2015.07.009. Published 2015 Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Limper A.H., Knox K.S., Sarosi G.A. An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am. J. Respir. Crit. Care Med. 2011;183(1):96–128. doi: 10.1164/rccm.2008-740ST. [DOI] [PubMed] [Google Scholar]
- 11.Manos N.E., Ferebee S.H., Kerschbaum W.F. Geographic variation in the prevalence of histoplasmin sensitivity. Dis. Chest. 1956;29(6):649–668. doi: 10.1378/chest.29.6.649. [DOI] [PubMed] [Google Scholar]
- 12.Cronk G.A. Pulmonary calcification and histoplasmin sensitivity in New York State. N. Y. State J. Med. 1951;51(16):1919–1924. [PubMed] [Google Scholar]
- 13.Grayston J.T., Furcolow M.L. The occurrence of histoplasmosis in epidemics; epidemiological studies. Am. J. Public Health Nation's Health. 1953;43(6 Pt 1):665–676. doi: 10.2105/ajph.43.6_pt_1.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borja T.S., Vadde N.P., Leggiadro R.J. Histoplasmosis in a nonendemic area. Pediatr. Infect. Dis. J. 1996;15(10):923–924. doi: 10.1097/00006454-199610000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Morse D.L., Gordon M.A., Matte T., Eadie G. An outbreak of histoplasmosis in a prison. Am. J. Epidemiol. 1985;122(2):253–261. doi: 10.1093/oxfordjournals.aje.a114096. [DOI] [PubMed] [Google Scholar]
- 16.Mandell W., Goldberg D.M., Neu H.C. Histoplasmosis in patients with the acquired immune deficiency syndrome. Am. J. Med. 1986;81(6):974–978. doi: 10.1016/0002-9343(86)90390-6. [DOI] [PubMed] [Google Scholar]
- 17.Huang C.T., McGarry T., Cooper S., Saunders R., Andavolu R. Disseminated histoplasmosis in the acquired immunodeficiency syndrome. Report of five cases from a nonendemic area. Arch. Intern. Med. 1987;147(6):1181–1184. [PubMed] [Google Scholar]
- 18.Salzman S.H., Smith R.L., Aranda C.P. Histoplasmosis in patients at risk for the acquired immunodeficiency syndrome in a nonendemic setting. Chest. 1988;93(5):916–921. doi: 10.1378/chest.93.5.916. [DOI] [PubMed] [Google Scholar]
- 19.Keath E.J., Kobayashi G.S., Medoff G. Typing of Histoplasma capsulatum by restriction fragment length polymorphisms in a nuclear gene. J. Clin. Microbiol. 1992;30(8):2104–2107. doi: 10.1128/jcm.30.8.2104-2107.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guarner J., Brandt M.E. Histopathologic diagnosis of fungal infections in the 21st century. Clin. Microbiol. Rev. 2011;24(2):247–280. doi: 10.1128/CMR.00053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabe L.M., Malo J., Knox K.S. Diagnosis and management of coccidioidomycosis. Clin. Chest Med. 2017;38(3):417–433. doi: 10.1016/j.ccm.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Merchant M., Romero A.O., Libke R.D., Joseph J. Pleural effusion in hospitalized patients with Coccidioidomycosis. Respir. Med. 2008;102(4):537–540. doi: 10.1016/j.rmed.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Edwards P.Q., Palmer C.E. Prevalence of sensitivity to coccidioidin, with special reference to specific and nonspecific reactions to coccidioidin and to histoplasmin. Dis. Chest. 1957;31(1):35–60. doi: 10.1378/chest.31.1.35. [DOI] [PubMed] [Google Scholar]
- 24.Hector R.F., Rutherford G.W., Tsang C.A. The public health impact of coccidioidomycosis in Arizona and California. Int. J. Environ. Res. Publ. Health. 2011;8(4):1150–1173. doi: 10.3390/ijerph8041150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaturvedi V., Ramani R., Gromadzki S., Rodeghier B., Chang H.G., Morse D.L. Coccidioidomycosis in New York state. Emerg. Infect. Dis. 2000;6(1):25–29. doi: 10.3201/eid0601.000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiu C.T., Cook J., Pineros D.F. Pneumothorax in a young man in Brooklyn, New York. Clin. Infect. Dis. 2011;53(12):1255–1297. doi: 10.1093/cid/cir640. [DOI] [PubMed] [Google Scholar]
- 27.Toda M., Gonzalez F.J., Fonseca-Ford M. Notes from the field: multistate coccidioidomycosis outbreak in U.S. Residents returning from community service trips to Baja California, Mexico —. MMWR Morb. Mortal. Wkly. Rep. July–August 2018;68:332–333. doi: 10.15585/mmwr.mm6814a5. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barker B.M., Rajan S., De Melo Teixeira M. Coccidioidal meningitis in New York traced to Texas by fungal genomic analysis. Clin. Infect. Dis. 2019;69(6):1060–1062. doi: 10.1093/cid/ciz052. [DOI] [PubMed] [Google Scholar]
- 29.McBride J.A., Gauthier G.M., Klein B.S. Clinical manifestations and treatment of blastomycosis. Clin. Chest Med. 2017;38(3):435–449. doi: 10.1016/j.ccm.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein B.S., Vergeront J.M., Weeks R.J. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N. Engl. J. Med. 1986;314(9):529–534. doi: 10.1056/NEJM198602273140901. [DOI] [PubMed] [Google Scholar]
- 31.Pappas P.G., Pottage J.C., Powderly W.G. Blastomycosis in patients with the acquired immunodeficiency syndrome. Ann. Intern. Med. 1992;116(10):847–853. doi: 10.7326/0003-4819-116-10-847. [DOI] [PubMed] [Google Scholar]
- 32.Lemos L.B., Guo M., Baliga M. Blastomycosis: organ involvement and etiologic diagnosis. A review of 123 patients from Mississippi. Ann. Diagn. Pathol. 2000;4(6):391–406. doi: 10.1053/adpa.2000.20755. [DOI] [PubMed] [Google Scholar]
- 33.Gandhi V., Singh A., Woods G.L., Epelbaum O.A. 66-year-old woman with fever, cough. a tongue lesion. Chest. 2015;(4):147. doi: 10.1378/chest.14-1858. e140‐e147. [DOI] [PubMed] [Google Scholar]
- 34.Martynowicz M.A., Prakash U.B. Pulmonary blastomycosis: an appraisal of diagnostic techniques. Chest. 2002;121(3):768–773. doi: 10.1378/chest.121.3.768. [DOI] [PubMed] [Google Scholar]
- 35.Patel A.J., Gattuso P., Reddy V.B. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am. J. Surg. Pathol. 2010;34(2):256–261. doi: 10.1097/PAS.0b013e3181ca48a5. [DOI] [PubMed] [Google Scholar]
- 36.Richer S.M., Smedema M.L., Durkin M.M. Development of a highly sensitive and specific blastomycosis antibody enzyme immunoassay using Blastomyces dermatitidis surface protein BAD-1. Clin. Vaccine Immunol. 2014;21(2):143–146. doi: 10.1128/CVI.00597-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Permpalung N., Kaewpoowat Q., Prasidthrathsint K., Chongnarungsin D., Hyman C.L. Pulmonary blastomycosis: a new endemic area in New York state. Mycoses. 2013;56(5):592–595. doi: 10.1111/myc.12073. [DOI] [PubMed] [Google Scholar]
- 38.Gupta J., Patel G., Epelbaum O. Reversal of fortune: central nervous system blastomycosis. Am. J. Med. 2016;(8):129. doi: 10.1016/j.amjmed.2015.08.037. e109‐e112. [DOI] [PubMed] [Google Scholar]
- 39.McDonald R. Notes from the field: blastomycosis cases occurring outside of regions with known endemicity - New York, 2007-2017. MMWR Morb. Mortal. Wkly. Rep. 2018;67(38):1077–1078. doi: 10.15585/mmwr.mm6738a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Austin A., Jones D.M., Chopra A. A pregnant woman with anterior chest mass and respiratory failure: blastomycosis in a historically nonendemic area. Am. J. Med. 2019;132(11):1285–1288. doi: 10.1016/j.amjmed.2019.04.048. [DOI] [PubMed] [Google Scholar]