Abstract
Wound is among the most common injuries. A suitable wound dressing has a significant effect on the healing process. In this study, a porous wound dressing was prepared using poly (lactic acid) (PLA) and two plasticizers, polyethylene glycol (PEG) and triacetin (TA), through solvent casting method. For antibacterial activities, metronidazole was incorporated in the structure. The morphology was investigated by scanning electron microscopy (SEM). In addition, the effect of plasticizers ratio on porosity growth was evaluated. It was also observed that each had a unique effect on the structure’s porosity. The mechanical properties confirmed the effect of both plasticizers on increasing polymer softness and flexibility, and the most similar formulations to human skin in terms of mechanical properties were introduced. According to the results, TA had stronger effect on mechanical properties. The differential scanning calorimetry (DSC) showed the effect of increasing plasticizer concentration on crystalline structure and Tm reduction of PLA. The water contact angle measurement showed that both plasticizers enhanced hydrophilic characteristics of PLA, and this effect was weaker in PEG-containing formulations. The in vitro degradation study showed biodegradability, as a desirable property in wound dressing. Results suggested that higher degradation can be obtained by both plasticizers at the same time. The results also showed that PEG was more effective in enhancing water absorbency. In vitro drug release study indicated an explosive release and the highest amount was 85% over 186 h. The antibacterial activity test confirmed the effectiveness of the drug in preventing bacterial growth in the drug-containing formulations, while it showed the antibacterial property of TA. MTT assay was performed and the cellular toxicity of the formulations was checked and those that revealed the least toxicity were introduced.
Keywords: Wound dressing, Antibacterial, Drug release, PLA, PEG, TA
Introduction
Wound is an injury or disturbance in living organs, that causes damages or loss of their function. Skin is more exposed to damages than other tissues, and slow and incorrect healing can result in serious complications (Boateng and Catanzano 2015). Wound healing is a complex dynamic process, which depends on different factors, including age, nutrition, alcohol consumption, wound size and infection (Martin and Nunan 2015; Guo and DiPietro 2010). The basic need for all wounds is a clean environment without any infection, necrotic tissue and foreign body. Selection of a suitable wound dressing is one of the most important factors in wound healing (Vowden and Vowden 2017). Some of the ideal wound dressing properties include the absorption of wound exudate, non-adhesiveness, non-toxicity, preventing bacterial penetration, desirable mechanical properties, maintaining adequate moisture, low cost and ease of use (Chowdhary and Rathore 2015; Gallagher 2012; Mogoşanu and Grumezescu 2014). Traditional wound dressings are often made of natural materials, such as wool, cellulose, cotton fibers, or synthetic materials, such as polyamides and polyesters. Despite cost-effectiveness, problems such as adhesion, bacterial penetration, and painful dressing removal have resulted in deeper studies to replace traditional wound dressings with new ones (Boateng and Catanzano 2015; Boateng and Matthews 2008). So far, various polymeric materials have been used to produce wound dressings, some of which are described below. Hydrogels have a significant amount of water in their structure and are suitable for maintaining moisture in the environment for use in dry wounds and wounds with necrotic tissue. Polymers such as polymethyl methacrylate, polyvinyl alcohol, and gelatin are used in the structure of hydrogels (Vowden and Vowden 2017; Boateng and Matthews 2008; Madaghiele and Demitri 2014). Hydrocolloid dressings contain polymers such as carboxymethyl cellulose and pectin, which have high absorption capacity. This type of wound dressing is not recommended to use for infectious wounds, due to the presence of an external coating, which acts as a barrier to gas exchange (Mogoşanu and Grumezescu 2014; Boateng and Matthews 2008). Alginate-based dressings are highly absorbent and can be used in deep wounds (Vowden and Vowden 2017; Mogoşanu and Grumezescu 2014; Boateng and Matthews 2008). Polyurethane and silicone dressings are non-adhesive and do not cause any pain when removed (Vowden and Vowden 2017; Boateng and Matthews 2008). Other polymers used in wound dressings, include chitin and chitosan (Jayakuma and Prabaharan 2011), agar and agarose (Mogoşanu and Grumezescu 2014), collagen (Moraes and Saska 2016), gelatin, keratin and fibrin (Mogoşanu and Grumezescu 2014). One of the most likely problems that may occur after a wound, is infection. Infection occurs when microorganisms (often bacteria) invade the host body, compete with the natural immune system and begin to reproduce (Xiong and Bao 2014). Uncontrolled wound infection poses serious risks, even death. Almost, 75% of burn wounds are exposed to infection. The presence of bacteria greatly affects the healing process and duration (Boateng and Catanzano 2015; Edwards and Harding 2004). As a result, the use of wound dressings, capable of preventing or treating infection, is very helpful. To this end, various factors can be used in wound dressing structure, including antibiotics as the most important ones.
Antibiotics are compounds that kill bacteria or prevent their growth. Due to the toxicity of high doses of these drugs for oral or intravenous administration, it is often not possible to use effective doses with these two methods, and therefore, the treatment process will not be complete. However, topical use of antibiotics in the wound dressing structure allows for the administration of effective dose (Boateng and Catanzano 2015; Xiong and Bao 2014). The soluble or ointment antibiotics can quickly absorb the exudates, turn into fluid and lose efficiency. Therefore, after a short period of time, wound dressings need to be replaced. In drug containing dressings, topical drug delivery is made in a long time and thus reduces the need for continuous replacement of dressings (Sofokleous et al. 2013).
Among the studies in this field is the use of bismuth subgallate drug in paraffin-based dressings, gentamicin in collagen sponges, ofloxacinin silicone sheets and tetracycline in fibrin dressings (Boateng and Catanzano 2015; Boateng and Matthews 2008). Silver nanoparticles are also one of the antibacterial agents that can be used in wound dressing, especially to overcome with pathogens that are resistant to common drugs (Boateng and Catanzano 2015; Kim et al. 2010; Tian and Wong 2007). Other antibacterial agents include honey, polyhexamethylene biguanide, hydrogen peroxide, and acetic acid release agents (Boateng and Catanzano 2015; Mogoşanu and Grumezescu 2014). In this study, poly lactic acid (PLA) was used for making wound dressing. This is a lactic acid-based, biodegradable, biocompatible and low cost polymer with renewable resources. The polymer degradation occurs through hydrolytic degradation (by water) and biological degradation (by the enzyme). The polymer degradation by-products, such as lactic acid, are also biocompatible (Averous 2013; Pelegrini 2016; Nor Azowa 2010; Tyler and Gullotti 2016; Dzikowski and Castanie 2017). One of the weak points of PLA for making wound dressing is its low elasticity and toughness (Hamad 2015 Farah et al. 2016). In this study, two plasticizers, namely triacetin (TA) and polyethylene glycol (PEG), were used to improve the mechanical and biodegradability properties of the polymer. PEG with molecular weight of 300 is a non-toxic, neutral, and viscous liquid (Dsouza and Shegokar 2016). TA, a triester of glycerol and acetic acid, is a hydrophilic plasticizer and a solvent of PLA. This material is used in the pharmaceutical industry as an antifungal agent and plasticizer of polymeric capsule coatings (Nor Azowa 2010; Fiume 2003; Bailey et al. 1991; Opdyke 1979). According to previous works, in this study, TA is used for the first time, along with PLA, to make a wound dressing, and its different properties were investigated. Moreover, for the first time, various ratios of two different plasticizers were used with PLA, and their properties were compared to find the best formulation.
To achieve the antibacterial properties, metronidazole was used in the wound dressing structure. After preparing the wound dressing, morphology, mechanical properties, hydrophilicity, in vitro degradation rate, in vitro drug release study, antibacterial activity and MTT assay were evaluated. Moreover, the effects of each plasticizer were investigated in each test and the results were compared together.
Based on other studies, in this work, TA is used for the first time, along with PLA, to make a wound dressing, and to investigate its different properties. Moreover, for the first time, various ratios of two different plasticizers were used with PLA, and their properties were evaluated to find the best formulation.
Materials and methods
Materials
PLA with the density of 1.25 g/cm3, molecular weight: 90,000 g/mol, and melting point of 170 °C (Zhejiang Hisun Biomaterials, China), PEG with molecular weight of 300 g/mol and density of 1.13 g/cm3 (Merck, Germany), TA with CAS no.102-76-1 (PROLABO, France), dichloromethane (Dr Mojallali Industrial Chemical Complex Company, Iran), PBS solution (Bioidea Company, Iran) and pure metronidazole powder (Dr Abidi Pharmaceuticals Company, Iran) (Fig. 1).
Fig. 1.
Structure of materials used in this study
Preparation of porous films
Liquid PEG and TA were used to improve mechanical properties of PLA and increase its degradation rate. The first step in making a wound dressing film is to obtain a polymer solution. To this end, the total mass of the system (polymer and plasticizers) was taken as 3 g. According to studies, dichloromethane was selected and used as the solvent (Chitrattha and Phaechamud 2016; Zilberman and Egozi 2015).
To prepare each sample, the masses of PEG, PLA, and TA were calculated based on Table 1 and weighed using a digital scale. Then, 50 mL of the solvent was added to 3 g of the initial materials and mixed by a magnetic mixer at 40 °C. As a result, a homogenous and clear solution without air bubbles was obtained. Then, this solution was placed in glass petri dishes (10 cm in diameter) and maintained at room temperature for 24 h. After that, they were placed in a vacuum oven at 50 °C for 24 h to dry completely. After preparation of the samples, 10 various films with more desired physical properties, specifically better plasticizing properties, were selected for drug loading (5% w/w). Metronidazole is capable of absorbing moisture but due to high-temperature degradation, it is recommended to stay under vacuum for several hours before use. To prepare metronidazole-containing samples, after preparing the polymer solution, 150 g of the drug was added to the polymer solution and then mixed for 20 min using a magnetic mixer. These film samples were dried in the same manner as drug-free films.
Table 1.
Weight percentage of plasticizers in polymer films
| Sample | PEG (wt%) | TA (wt%) |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 10 | 0 |
| 3 | 20 | 0 |
| 4 | 30 | 0 |
| 5 | 40 | 0 |
| 6 | 50 | 0 |
| 7 | 60 | 0 |
| 8 | 0 | 30 |
| 9 | 0 | 40 |
| 10 | 0 | 50 |
| 11 | 0 | 60 |
| 12 | 10 | 40 |
| 13 | 20 | 30 |
| 14 | 30 | 20 |
| 15 | 40 | 10 |
Morphological characterization
To investigate the effect of plasticizers and their concentration on the structure and morphology of PLA, SEM images of some samples were obtained by a Philips/XL30 scanning electron microscope. First, samples of 1 cm2 cross-section were prepared and coated with gold before their microscopic images were acquired. The mean diameter of the porosities was measured using the Microstructure Measurement software.
Mechanical properties
The mechanical properties of all 15 samples (Table 1) were evaluated according to ISO1926, using Universal Testing Machine (GOTECH, Taiwan). According to this standard, the machine was set with the grip spacing of 50 mm and grip travel speed of 50 mm/min interval. The experiment was repeated three times for each sample.
Differential scanning calorimetry (DSC)
Differential scanning calorimetry (DSC 200 F3 Maia, NETZSCH, Germany) was applied on seven samples (designated as 1, 5, 6, 7, 9, 10 and 11; Table 1). The aim was to investigate the effect of plasticizers and their concentration on thermal properties, crystalline structure and mechanical properties of PLA.
The temperature range and temperature increase rate were set at 20–220 °C and 5 °C/min, respectively. This method is reported based on heating and cooling processes.
Hydrophilicity of film samples
Among the formulations in Table 1, Samples 5, 7, 9, and 11 were selected for investigating the effect of plasticizers on wettability of the polymer. First, the water drops (1 µL) were placed at three points of each sample surface. Then, the images of water droplets on sample surface were obtained using a digital microscope (Dino-Lite, Taiwan). Finally, the contact angle was measured using Dinocapture 2.0 software. The experiment was repeated three times for each sample.
In vitro degradation study
First, specified dimensions (15 mm × 20 mm) of all 15 samples (Table 1) were prepared. Then, they were placed in an oven at 50 °C for 4 h to dry completely. Each sample was weighed (W0), then placed in PBS solution (20 mL). A shaking incubator (gamma ELISA, PSI-600G, Pouya Electronic Yaran, Iran) was used in this assay (rate of 80 rpm). After a week, the samples were collected from the PBS solution, their surface moisture was removed with a filter paper, and then reweighed (W1). Next, they were placed in the oven at 50 °C to dry completely and weighed again (W2). This process was repeated once per week for 8 weeks. The water absorbency and weight loss (degradation) of samples were calculated, using the following equations:
| 1 |
| 2 |
These experiments were repeated three times for each sample.
In vitro drug release study
To determine the release rate of the drug, a standard diagram is provided and then, by comparing the amount of drug release from the sample with the standard diagram, the unknown concentration is calculated.
Drug release from metronidazole-containing polymer films
The solubility of metronidazole in PBS media is 2 mg/mL (Maxey 2018). To achieve this level, 200 mg of drug, along with 100 mL of PBS solution was first placed on a magnetic stirrer for 2 h. Then, this solution was diluted to prepare 15 samples with different concentrations. After the preparation of the solutions, the absorbance of each sample was measured at 320 nm (Chitrattha and Phaechamud 2016), using Microplate Reader (ELx 800, Bio Tek, USA). The standard diagram and the respective line's equation were obtained.
To determine the drug release pattern of the samples, they were placed in dialysis tubing before being transferred to the PBS solution (250 mL). After that, the sample-containing solutions were placed in an incubator at 37 °C and the shaking speed of 80 rpm. The sampling was carried out at the 2nd, 6th, 12th, 18th, 42nd, 66th, 90th, 138th and 186th h. Each time, 1 mL of the solution was taken for the analysis and replaced with 1 mL of fresh PBS solution. This experiment was repeated three times for each sample.
Antimicrobial activity test
Bacteria culture
In this study, two facultative anaerobic bacteria were used, namely Escherichia coli (Gram-negative) and Staphylococcus aureus (Gram-positive), which exist in infected skin and wounds (Simoes and Moguel 2018). After overnight culture of bacteria in an autoclaved liquid LB media (containing 10 g/L of tryptone, 5 g/L of yeast extract, and 10 g/L of sodium chloride dissolved in distilled water), they were cultured on the LB-Agar plate. After the bacteria culture in the liquid media, their absorbance number was obtained at the wavelength of 600 nm. To achieve a relatively uniform concentration of both bacteria, they were diluted with culture media until reached the absorbance value of 0.8.
Preparation of samples and evaluation
To perform this test, samples with desired mechanical properties and different amounts of plasticizers were selected with/without drug according to Table 2. The polymer films were first cut into circles with the surface area of 1 cm2, and then sterilized with UV radiation for 1 h. After the bacteria preparation and cultivation on the plate surface, the samples were placed on the plate surface and then in the incubator at 37 °C. After 48 h, the plates were removed from the incubator and the effectiveness of the sample was compared by calculating the radius of the bacterial growth inhibition zone around each one.
Table 2.
Samples used in antibacterial activity test
| Sample | PEG (wt%) | TA (wt%) | Drug (wt%) |
|---|---|---|---|
| 1A | 40 | 0 | 0 |
| 1B | 40 | 0 | 5 |
| 2A | 60 | 0 | 0 |
| 2B | 60 | 0 | 5 |
| 3A | 0 | 40 | 0 |
| 3B | 0 | 40 | 5 |
| 4A | 0 | 50 | 0 |
| 4B | 0 | 50 | 5 |
| 5A | 30 | 20 | 0 |
| 5B | 30 | 20 | 5 |
| 6A | 20 | 30 | 0 |
| 6B | 20 | 30 | 5 |
Cytotoxicity assay
Proliferation of fibroblastic cells
The human fibroblast cell line was purchased from the “Iranian Biological and Genetic Resource Center” and cultured in a DMEM media with the serum concentration of 10% inside the cell culture flask. Then, it was transferred to the incubator with moist environment and CO2 concentration of 5% at 37 °C to prevent bacteria growth, penicillin and streptomycin with the ratio of 1% and FBS with the ratio of 10% were added to the culture media and maintained at 4 °C.
Extraction
To investigate the toxicity of samples and their effect on the cell growth, 10 samples with drug (Table 3), and same samples without drug, were selected. For sterilization, all 20 samples were exposed to UV radiation for 1 h. The extraction process was done according to the ISO10993-5 (adding 1 mL of culture media per 6 cm2 of each sample). Samples of 1 × 1 cm2 cross-section were first prepared. Then, 160 µL of the culture medium was added to each sample. Finally, the plate containing sample and culture media were placed in the incubator at 37 °C and 50 rpm for 24 h. After this time, the culture medium was removed and added to each cell.
Table 3.
Components of polymer films containing metronidazole drug
| Sample | PEG (wt%) | TA (wt%) | drug (mg) |
|---|---|---|---|
| 5 M | 40 | 0 | 150 |
| 6 M | 50 | 0 | 150 |
| 7 M | 60 | 0 | 150 |
| 9 M | 0 | 40 | 150 |
| 10 M | 0 | 50 | 150 |
| 11 M | 0 | 60 | 150 |
| 12 M | 10 | 40 | 150 |
| 13 M | 20 | 30 | 150 |
| 14 M | 30 | 20 | 150 |
| 15 M | 40 | 10 | 150 |
MTT assay
The MTT assay was used to investigate the samples' toxicity. First, the cells were cultured in a 96-well plate with 10,000 cells in each well. This process was repeated three times for each sample. Then, the cells were incubated for 24 h to fully adhere to the plate. After this period and ensuring that the cells were fully attached, the culture media were removed and the extracts with different dilution rates were added to each well. After 24 h of cells exposure to the extract, MTT (5 mg/mL) was added to each well as long as it did not exceed the volume of culture media by 10%. The cell-containing plate was placed once again in the incubator for 4 h at 37 °C. Then, the culture media were removed and 100 µL of DMSO was added into each well. The plate was subjected to low shaking frequency for 15 min to dissolve the formed formazan, and the solution absorbency of each well was recorded at 570 nm (the control cell wells only contained the culture media). The optical density was higher in wells with more cells. As a result, the cell viability was obtained from the following equation:
| 3 |
Results and discussion
Morphological study
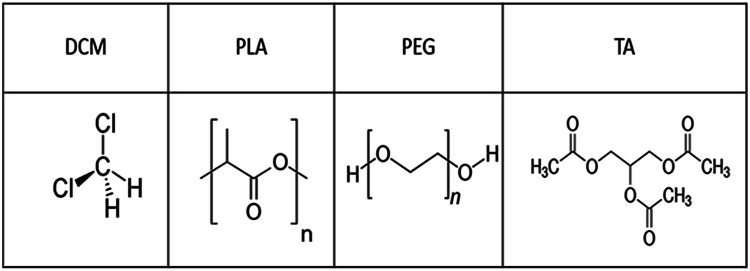
According to Fig. 2, the pore size increased with increasing the PEG concentration. According to Table 4, the pore size increased from approximately 5 µm with PEG concentration of 10% to 73 µm in the sample with PEG concentration of 60%. PEG is a plasticizer that leads to an increase in the chain movement, reduction of chain entanglement, creation of empty space in polymer, and finally an increase in pore size by infiltrating into PLA chains (Chen and Wang 2014).
Fig. 2.
SEM images: a PLA90/PEG10, b PLA60/PEG40, c PLA50/PEG50, d PLA40/PEG60
Table 4.
Average pore size of film samples
| Sample | Pore size (µm) |
|---|---|
| PLA90/PEG10 | 5.7 ± 0.8 |
| PLA60/PEG40 | 16.7 ± 2.3 |
| PLA50/PEG50 | 41.3 ± 5.1 |
| PLA40/PEG60 | 73.2 ± 7.2 |
| PLA70/TA30 | 0 |
| PLA60/TA40 | 1.02 ± 0.17 |
| PLA40/TA60 | 0.96 ± 0.13 |
| PLA50/PEG10/TA40 | 4.5 ± 0.9 |
| PLA50/PEG40/TA10 | 14.2 ± 1.8 |
The hydrogen bonding interaction is formed between PLA and PEG. The intramolecular interaction of the hydrogen bonding occurs between oxygen attached to carbonyl (C=O) in PLA structure and hydroxyl (–OH) end group in the PEG molecular chain. The addition of lower Mw (PEG 300) can raise chain mobility between PLA and PEG through hydrogen bonding (Septevani and Bhakri 2017). This is why PEG is a suitable plasticizer for PLA.
Moreover, increased concentration of plasticizer contributed to the infiltration of solvent into the polymer bulk, resulting in larger pore size after the solvent evaporation.
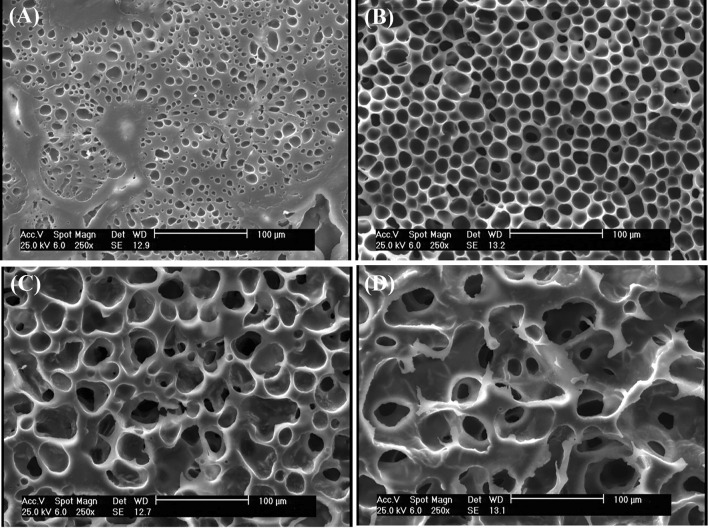
PEG is a plasticizer and TA is a solvent of PLA (Chang and Garripelli 2015). As a result, TA separates PLA particles from each other. In all images (Fig. 3), separation of PLA particles and their boundaries is observable. Despite their high porosity, the samples containing only PLA and PEG have integrated structure and the polymer is like fully entangled network. In samples containing PLA and TA, the material structure is not integrated and micro-sized particles are separated from each other. Triacetin is a relatively less hydrophobic solvent (50 mg/mL aqueous phase solubility), but can dissolve a hydrophobic polymer such as PLA (Chang and Garripelli 2015). TA is a triester and PLA is a polyester. TA has similar molecular structure to PLA, and they are expected to be well compatible. By placing TA between the PLA chains, there would be changes in molecular arrangement and reducing the intermolecular force of the polymer.
Fig. 3.
SEM images: a PLA70/TA30, b PLA60/TA40, c PLA40/TA60
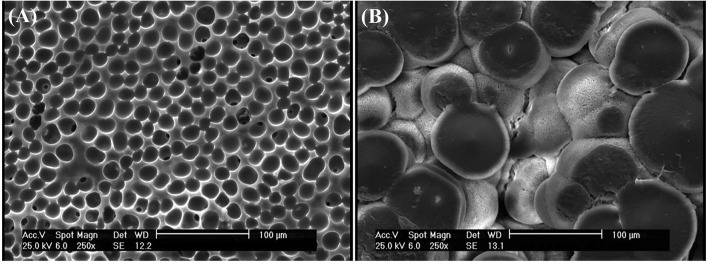
According to Fig. 4 with a 1000 × magnification, the PEG-containing sample had larger pores, but the polymer surface was almost smooth. In TA-containing sample, the polymer revealed a completely non-uniform surface with very small porosities on its surface (approximately 1 µm) (Fig. 5).
Fig. 4.
SEM images: aPLA40/TA60, bPLA40/PEG60with magnification 1000 ×
Fig. 5.
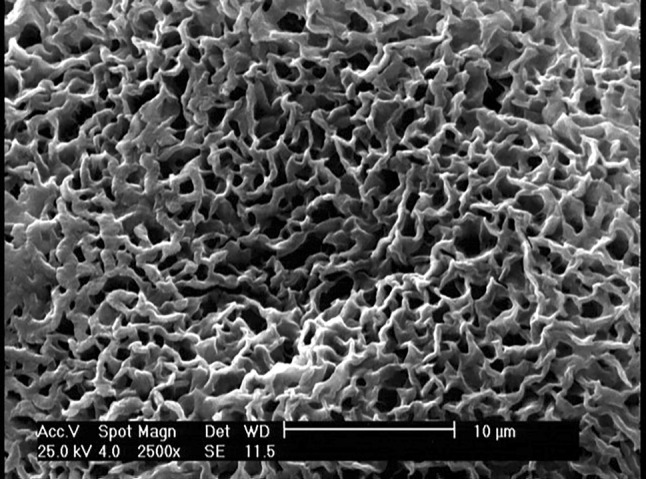
SEM image of PLA60/TA40with magnification 2500 × , to observe porosities completely
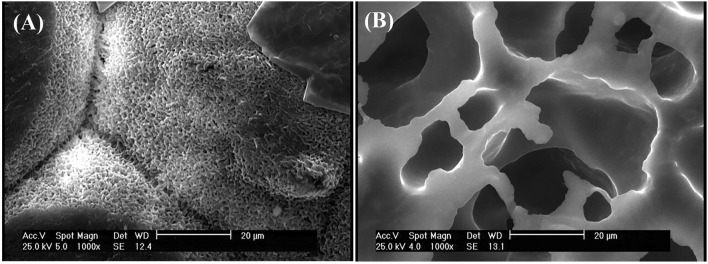
Figure 6 compares the samples containing both TA and PEG. TA is a solvent for PLA, so the sample containing 40% TA had smaller pores, but the pale area represents an incomplete separation of different PLA areas. PEG is a plasticizer for PLA, so the sample containing 40% PEG had larger pores, but the polymer maintained its integration and no separations are evident in its different areas. Therefore, as it is explained in the mechanical properties section, the sample containing 40% TA (Fig. 6a) has weaker mechanical properties than the sample containing 40% PEG (Fig. 6b). Therefore, the solvent has a greater role in weakening the mechanical properties than the plasticizer.
Fig. 6.
SEM images of a PLA50/PEG10/TA40, b PLA50/PEG40/TA10
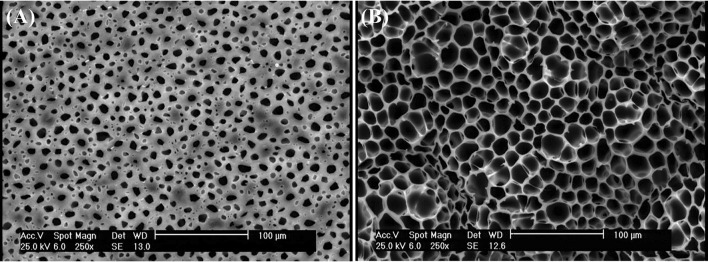
Figure 7 shows that there is no significant difference between the microstructure of samples with the drug and those without the drug.
Fig. 7.
SEM images of a PLA60/PEG40/MZ, b PLA60/TA40/MZ
Mechanical properties
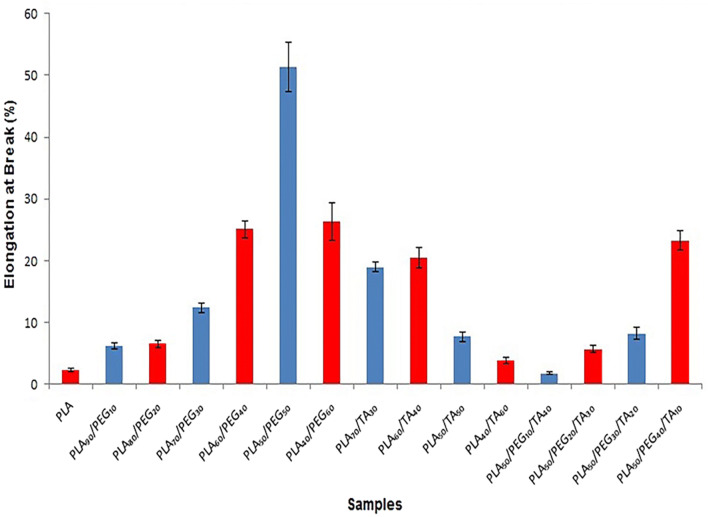
For accurate evaluation of mechanical properties, the “stress-at-break” and “elongation-at-break” were represented in different diagrams and compared together. According to Fig. 8, the elongation-at-break increased with increasing PEG ratio. It was 2.29 ± 0.2% in pure PLA and 51.33 ± 4% in the sample containing PLA50/PEG50. However, it reduced to 26.32 ± 3% with 60% increase in PEG concentration. In other words, the flexibility of the sample increased with increasing the concentration of PEG to 50%. In the sample containing 60% PEG, the flexibility reduced because of the strength reduction. As a result, an increase in PEG concentration to 50% can improve the flexibility of the samples; however, a further increase may cause structural breakdown. This can be seen in microscopic images of the samples. The elongation-at-break increased with increasing TA concentration from 30 to 40%. However, the elongation-at-break significantly reduced with increasing the PEG concentration to 50% and 60%. In other words, the strength, thereby flexibility, reduced with increasing TA concentration to above 40%. As a result, TA accelerated the breakdown of polymer structure (faster than PEG), confirmed by microscopic images. Comparison of samples containing both plasticizers with a constant PLA concentration showed that the elongation was greater in samples containing higher amount of PEG.
Fig. 8.
Diagram of elongation-at-break of samples
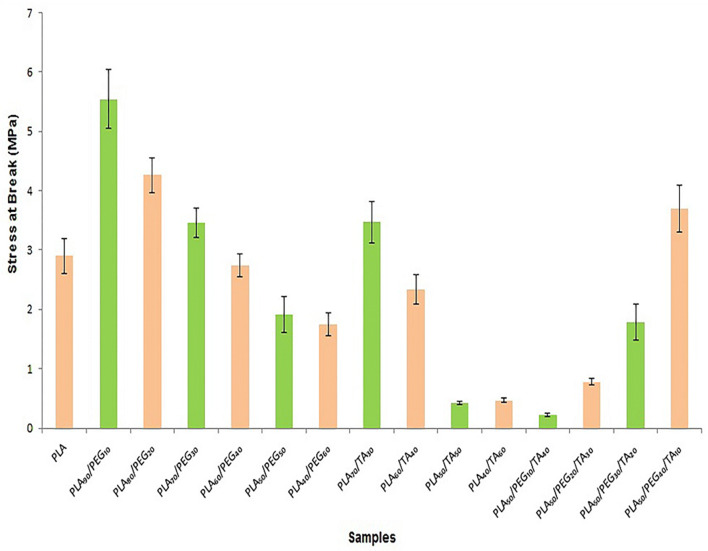
According to Fig. 9, stress-at-break and strength of the samples reduced with increasing PEG from 10 to 60%. The same trend can be observed by increases in TA concentration. Although the sample containing PLA70/TA30 was as strong as the sample containing PLA70/PEG30, the elongation was higher in the film containing TA with the concentration of 30%. As a result, TA-containing film was more flexible. The elongation and stress-at-break were lower in film samples containing PLA60/TA40, PLA50/TA50 and PLA40/TA60than those containing the same PEG concentration. This indicated lower flexibility and strength of the film samples containing TA. Regarding the stress-at-break of film samples containing both plasticizers at constant PLA concentration, this parameter reduced with increasing TA. Therefore, this plasticizer had a greater influence in reducing the polymer strength, as the SEM images showed the separation of PLA particles and its structural breakdown with increasing TA. Therefore, TA has shown a greater plasticizing impact when the plasticizer concentration is lower than 40%. Whereas, PEG has a greater plasticizing impact when the plasticizer concentration is higher than 40%. Because TA acts as a solvent as it is evident in SEM images, it separates the polymer chains and disrupts the structure. Therefore, and according to Figs. 8 and 9, high ratio of TA results in structural breakdown and polymer disintegration.
Fig. 9.
Diagram of stress-at-break of samples
Differential scanning calorimetry (DSC)
According to Fig. 10, increasing PEG concentration leads to reduction of Tm, which may confirm its plasticizing characteristics. Increasing the plasticizer infiltration between PLA chains enhances the free movement of the chains, reduces crystallinity of the polymer, and finally it lowers Tm. With increasing the plasticizer, crystalline regions become more in number and shrink in volume. As a result, the Tm range reduces with reducing Tm, representing sharper peaks in the diagram. As previously mentioned, TA is a PLA solvent (Chang and Garripelli 2015). So, PLA particles are dissolved in TA and cannot form crystalline regions. As a result, Tm reduces with increasing TA concentration. The results of DSC test confirmed the findings of mechanical properties test.
Fig. 10.
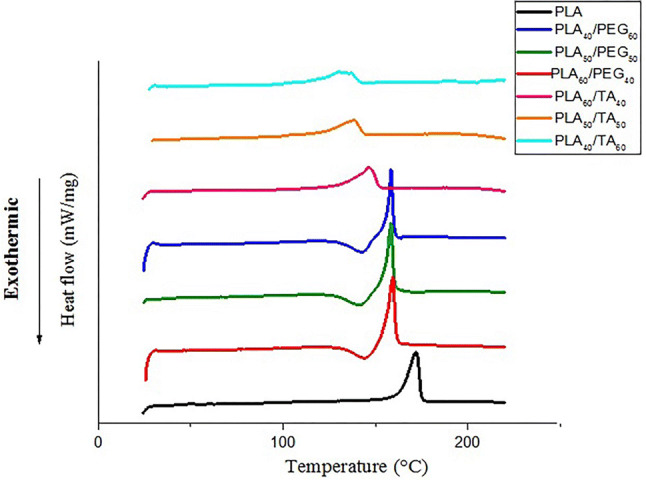
Effect of plasticizers on DSC curve
The area under the DCS curve within the range of Tm indicates the reaction enthalpy. Its multiplication by PLA concentration gives the melting enthalpy of PLA in each sample. According to Table 5, reduced polymer melting enthalpy due to the increase in the plasticizer concentration reflects polymer softness and free movement of the chains. According to this Table, TA has a greater impact than PEG in reducing the melting point of the compound. Because plasticizers with smaller molecular weight, are able to penetrate into PLA chains more easily. As a result, TA (218 g mol−1) has a greater plasticizing effect than PEG (300 g mol−1).
Table 5.
PLA melting enthalpy and melting temperature in samples
| Sample | Δhm° of PLA (J) | Tm (°C) |
|---|---|---|
| PLA | 6.02 | 172 |
| PLA60/PEG40 | 3.096 | 160 |
| PLA50/PEG50 | 2.69 | 158 |
| PLA40/PEG60 | 1.83 | 157 |
| PLA60/TA40 | 3.39 | 146 |
| PLA50/TA50 | 2.82 | 138 |
| PLA40/TA60 | 2.3 | 130 |
Contact angle measurement
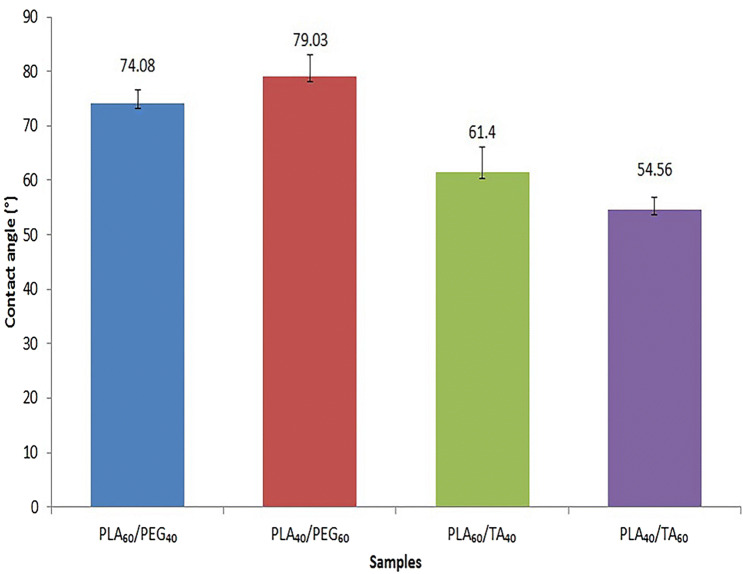
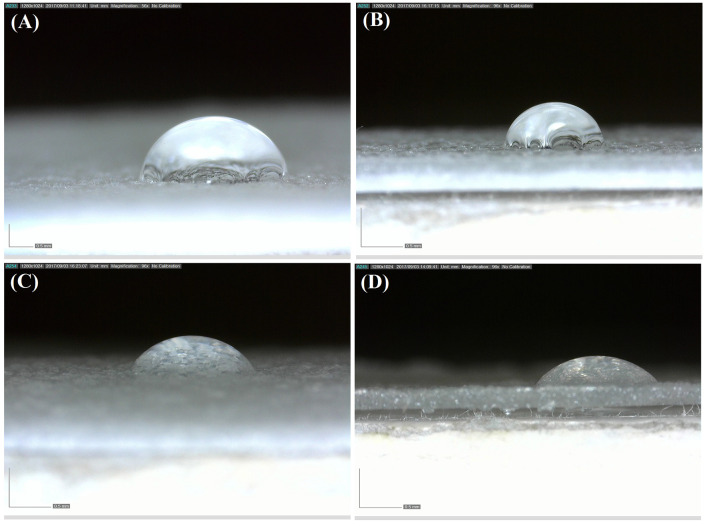
The contact angle of water-PLA is reported 82° in the literature. The increased hydrophilicity of this polymer results in enhanced bioactivity, biodegradability, and its interaction with body tissues (Janorkar et al. 2004). According to Fig. 11, both plasticizers have increased PLA hydrophilicity. Comparison of the compounds containing PLA at constant concentration showed smaller contact angle in TA-containing than PEG-containing film samples. So greater role of TA in increasing the polymer hydrophilicity is proved. An increase in contact angle with increasing the ratio of PEG (in samples containing PLA and PEG) represents reduced hydrophilicity. This phenomenon is due to increased porosity with increasing PEG. Since air hydrophobicity overcomes PEG hydrophilicity, the hydrophobicity of samples containing PEG 60% is greater than that of samples containing PEG 40% (Fig. 12).
Fig. 11.
Water contact angle of films containing different amounts of plasticizers
Fig. 12.
Images of water contact: a PLA60/PEG40, b PLA40/PEG60, c PLA60TA40, d PLA40/TA60
In vitro degradation study
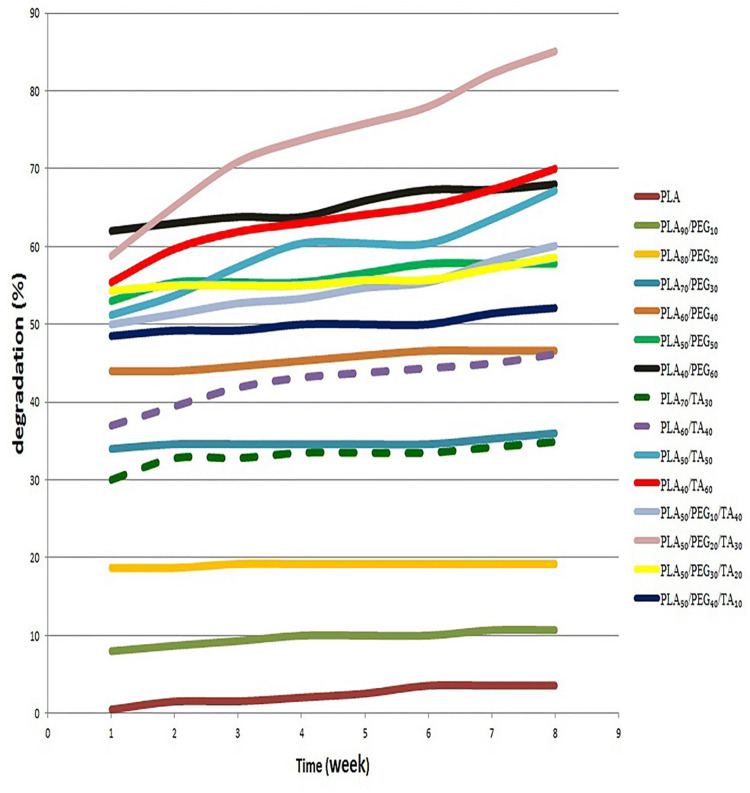
According to Fig. 13, the degradation rate in a film sample containing pure PLA was about 0.5% in the first week and 3.5% in the eighth week. This indicates an increase in PLA degradation with time; however, this increase was very slow and negligible. The PLA90/PEG10 and PLA80/PEG20 degraded, respectively, to 10.7% and 19.2% in 8 weeks; however, the major degradation occurred in the first week and remained almost constant in the next seven weeks. Regarding the degradation of these two samples, the mass loss was due to the dissolution of PEG in the media in the first week. Therefore, the concentration of PEG in these two samples was not able to increase PLA degradation, and only its dissolution in the media resulted in mass reduction of the sample.
Fig. 13.
In vitro degradation diagram (at PBS)
The PLA70/PEG30, PLA60/PEG40, PLA50/PEG50, and PLA40/PEG60 degraded, respectively, to 36%, 47%, 58% and 68% in 8 weeks, indicating higher mass reduction than the PEG mass. As a result, PLA degradation increases with increasing PEG, which can be attributed to the pore size of the samples. According to microscopic images, the density and pores sizes increased with increasing PEG ratio, which resulted in an increase in contact area of the material with PBS solution, thereby increasing degradation.
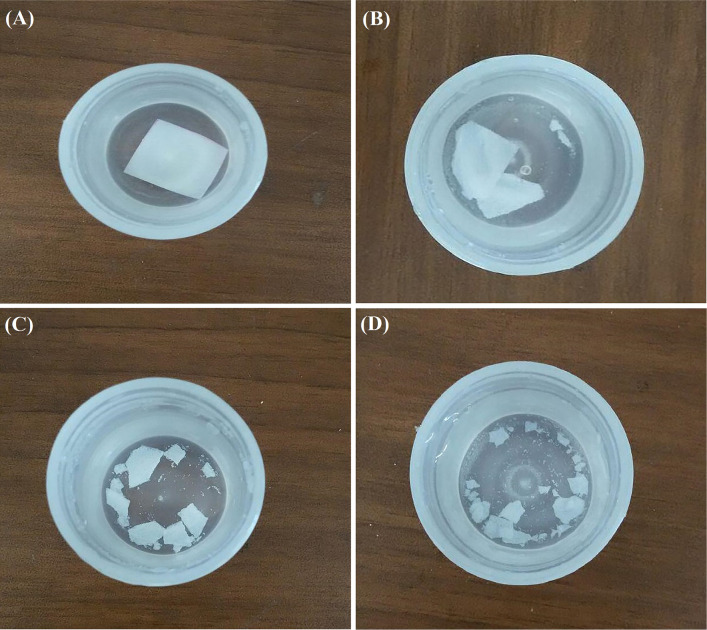
The PLA70/TA30, PLA60/TA40, PLA50/TA50, and PLA40/TA60 degraded, respectively, to 35%, 46%, 67% and 70% in 8 weeks. As a result, degradation was increased and accelerated with increasing TA concentration. The PLA70/PEG30 and PLA70/TA30, as well as PLA60/PEG40 and PLA60/TA40showed approximately similar degradation rate. As a result, the effect of PEG and TA on the polymer film degradation remains similar until their concentration is about 40% or lower. The PLA50/TA50 degradation was 51% in the first week and 67% in the eighth week. This degradation trend indicates that in addition to water-dissolved TA in this sample, part of the PLA was also degraded. Regarding the degradation rate of PLA50/PEG50 in 8 weeks (58%), it can be concluded that in contrast to similar effect of PEG and TA with the concentration of lower than 50%, TA showed a greater effect than PEG by 10% at the concentration of 50%. Degradation rate increased only 3% with increasing TA concentration to 60%. As a result, PLA50/TA50 and PLA40/TA60 had almost similar degradation. In samples containing PLA 50% and varying concentrations of TA and PEG, the highest degradation rate of 85% was observed in PLA50/PEG20/TA30 in 8 weeks. Comparison of sample degradation from the first to eighth week showed that 60% of degradation occurred in the first week and the rest in the following 7 weeks. According to Fig. 13, the highest curve with the greatest slope was observed in this sample. In this sample, in addition of both plasticizers, 35% of PLA was also degraded, indicating the increasing effect of plasticizers on polymer film degradation when both of them are used together. Since this sample has good mechanical properties and does not collapse rapidly outside the body (in contrast to PLA40/TA60 with the lower degradation rate), it can be regarded as a good sample in terms of biodegradability. The expected time frame to replace biological wound dressings is lower than one week. As a result, samples 6, 7, 10, 13, and 14 with the degradation rate higher than 50% in the first week seem desirable (Fig. 14).
Fig. 14.
Samples in PBS during the degradation study: a PLA, b PLA70/PEG30, c PLA50/TA50, d PLA50/PEG20/TA30
Water absorbency
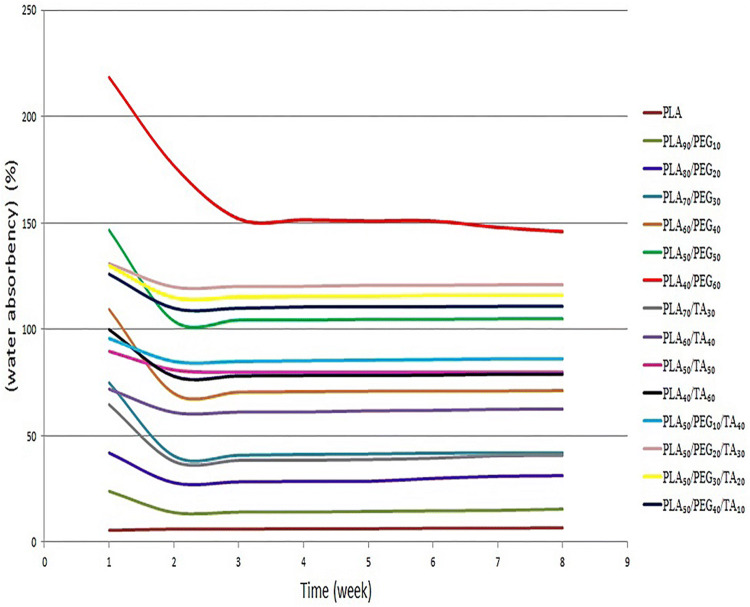
According to Fig. 15, the water absorbency of all samples is at their maximum in the first week. After the first week when almost the major part of the sample plasticizers was dissolved in the aquatic media and they were no longer hydrophilic, the water absorbency almost remained unchanged by the end of the eighth week.
Fig. 15.
Water absorption diagram of samples in 8 weeks
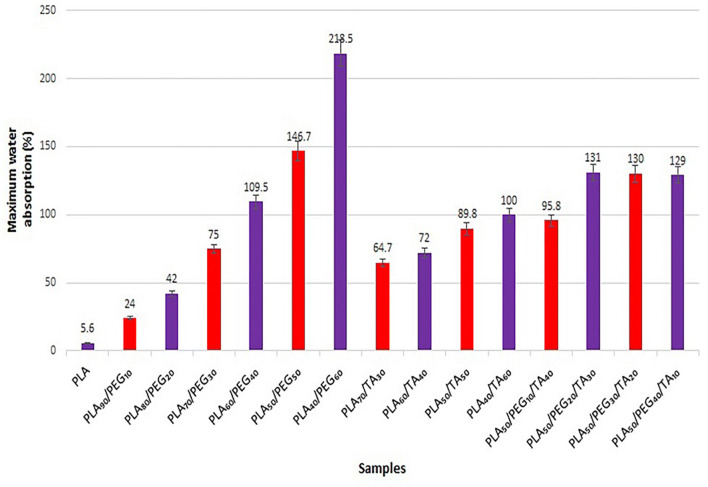
According to Fig. 16, the water absorbency in samples containing PLA and PEG increases with increasing PEG concentration, which can be attributed to the presence of larger pores, deeper infiltration of water into the polymer and increased polymer hydrophilicity. Similar phenomenon has been observed in the samples containing PLA and TA, that the water absorbency increases with increasing TA. The difference in water absorbency in the samples containing PLA and TA was lower than the samples containing PLA and PEG. This indicates the more effective role of PEG than TA in water absorbency, which can be attributed to higher hydrophilicity of PEG than TA. In the samples containing both plasticizers, an increase in PEG from 10 to 20% increased water absorbency to approximately 35%, but no significant difference was observed in other samples.
Fig. 16.
Water absorption of film samples in the first week (maximum)
In vitro drug release study
After putting the drug-containing film samples in the PBS solution, the absorbance of each one was measured. Using the standard diagram, the drug concentration in each sample was calculated at certain hours.
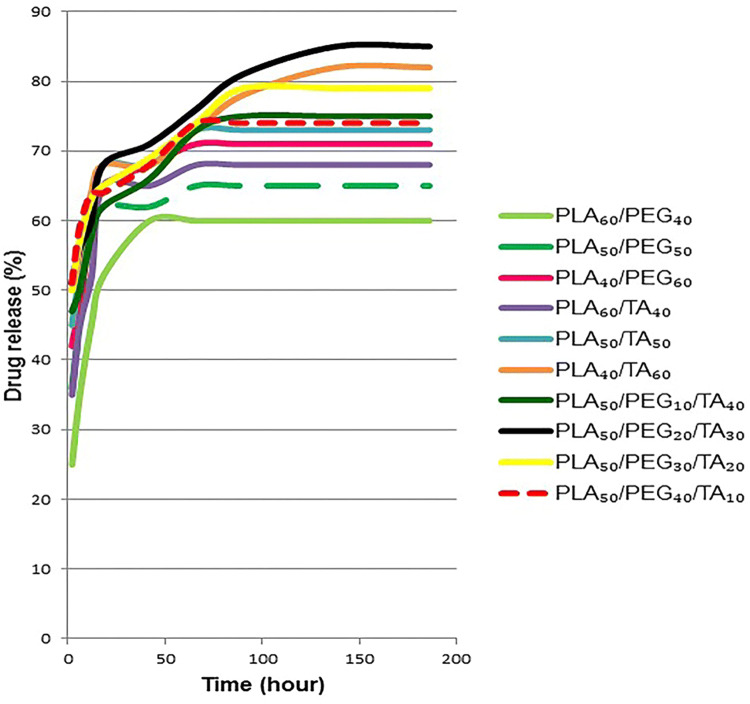
According to Fig. 17, the minimum and maximum drug release rates in different samples over 186 h were 60% and 85%, respectively. In all samples, drug release was stopped after about 42 h and its concentration in the sample-containing solution reached a constant level. As a result, after using the wound dressing, drug release continued for 42 h.
Fig. 17.
In vitro drug release profiles from samples at pH 7.4 and 37 °C in PBS
With increasing PEG concentration in PEG-containing samples, drug release increased as well. The difference in these values was higher in the initial hours. The risk of infection is higher in the early minutes and hours after wounding. Because after time passage and wound healing, the chance of bacteria infiltration into the wound reduces. Similar to PEG-containing samples, the drug release increased with increasing plasticizer ratio in TA-containing samples. Since degradation increases with increasing the plasticizer concentration, this phenomenon can be completely justified. Almost half of the drug in samples containing both plasticizers were released during the first 2 h. In addition, the maximum release, 85%, in PLA50/PEG20/TA30 was observed during 186 h. High solubility of both plasticizers in water, facilitated water infiltration into the polymer film and release of the drug. This is also the reason for further release from films containing higher concentration of plasticizers.
Findings showed that using of wound dressing for a time range of 18–48 h resulted in relatively similar drug release from all samples. Therefore, it seems that the degradation time should be considered in selecting the wound dressing type, whereas when the wound dressing is used for less than 12 h, there is a significant difference between various samples in drug release during this time frame. In this case, using samples with higher release rate and thus higher degradability seems more logical.
Regarding that the highest number of granulocytes appears after 12–24 h at the injury site and their key responsibility is to control microbial growth and inhibit infection, the first 24 h after wounding is the most important time frame to create optimal conditions and prevent infection (Orsted and Keast 2011). As a result, the drug release rate is a significant factor in preventing infection.
Antibacterial activities
After 48 h of exposure of drug-containing samples to the applied bacteria, the bacteria inhibition zone was measured.
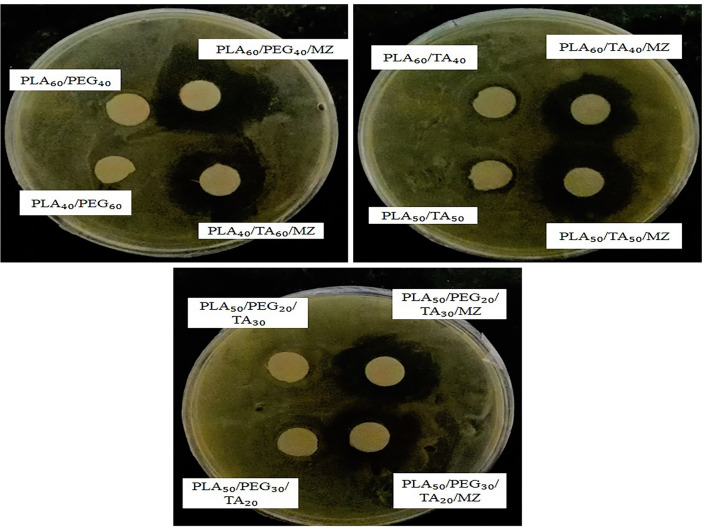
Figure 18 shows the effect of all drug-containing samples on E. coli growth inhibition. According to Table 6, they had almost a similar effect without any significant difference in the inhibition zone, which seems logical considering relatively similar drug release in 48 h. In addition, PLA60/TA40 and PLA40/TA60 had, even slight, impact on E. coli growth inhibition, indicating antibacterial effect of TA. The mutagenesis of this material (TA) in bacteria is shown in the literature (PUBLICATIONS O.S.U 2002).
Fig. 18.
Antibacterial activity of samples against Escherichia coli
Table 6.
Radius of bacterial inhibition zone
| Sample | Escherichia coli inhibition zone (mm) | Staphylococcus aureus inhibition zone (mm) |
|---|---|---|
| PLA60/PEG40 | 0 | 0 |
| PLA40/PEG60 | 0 | 0 |
| PLA60/TA40 | 1 | 0 |
| PLA50/TA50 | 1 | 0 |
| PLA50/PEG50/TA30 | 0 | 0 |
| PLA50/PEG30/TA20 | 0 | 0 |
| PLA60/PEG40/MZ | 7 | 2 |
| PLA40/PEG60/MZ | 6.5 | 2 |
| PLA60/TA40/MZ | 6 | 2.5 |
| PLA50/TA50/MZ | 7 | 4 |
| PLA50/PEG50/TA30/MZ | 6.5 | 2 |
| PLA50/PEG30/TA20/MZ | 6 | 3 |
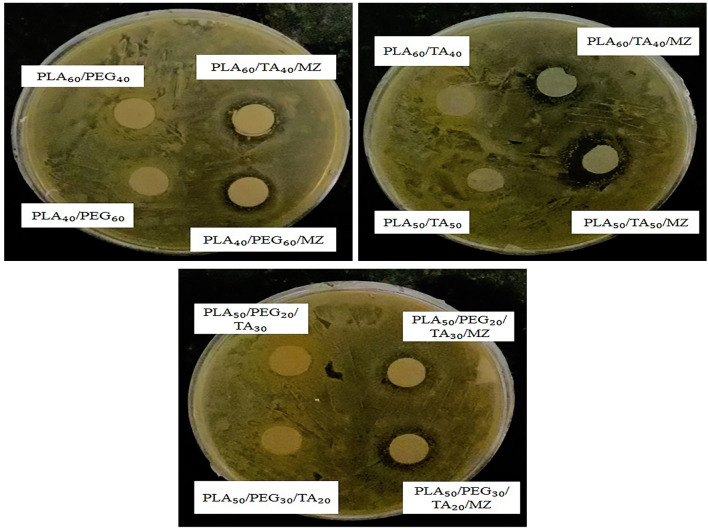
Figure 19 shows the effect of all drug-containing samples on the prevention of Staphylococcus aureus growth. However, it has a smaller inhibition zone than E. coli. This can be attributed to the difference in the nature of bacteria and their resistance to different drug types. According to these observations, bacteria growth inhibition in the vicinity of drug-containing samples indicates the effectiveness of these polymer films when used as wound dressing.
Fig. 19.
Antibacterial activity of samples against Staphylococcus aureus
Cytotoxicity results
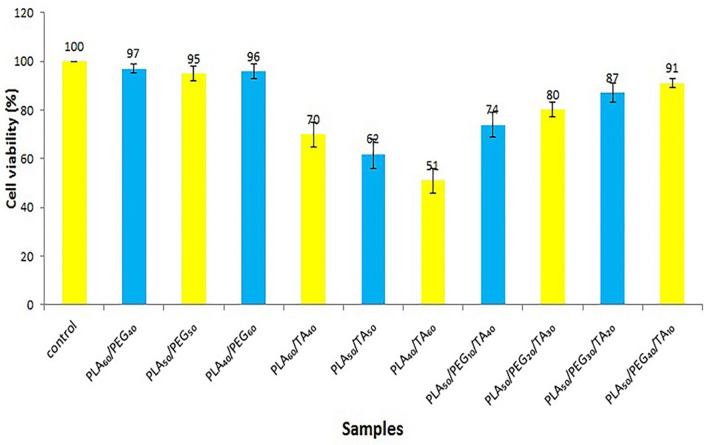
According to Fig. 20, samples containing PLA and PEG displayed almost no cellular toxicity. Samples containing TA (due to acetic acid release in the aqueous medium and the creation of an acidic environment) have shown more cellular toxicity.
Fig. 20.
Cytotoxicity of samples without drug
The acidic environment controls wound infection, increases antimicrobial activity, releases oxygen, reduces the toxicity of final bacterial products, and enhances epithelialization, and plays an important role in wound healing (Persival and McCarty 2014).
A study used TA for treating Canavan disease, as one of the most common degenerative brain diseases. Results reported the effective dose of 4.5 g/kg body weight with no toxicity. According to this study, TA is a biocompatible material (Segal and Anikster 2011).
Due to these and MTT test results, the use of TA in amounts less than 40% is acceptable.
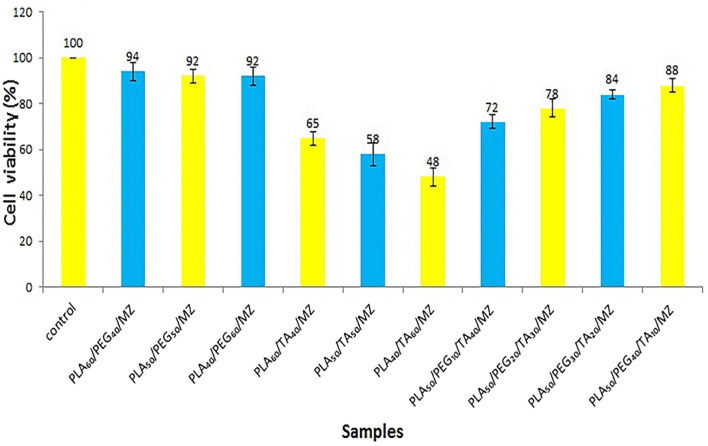
According to Fig. 21, and a comparison between the drug-free and drug-containing samples, it is clear that the addition of the drug does not cause significant changes in the charts (p > 0.05). Therefore, the dose of the drug used is acceptable and has no toxicity.
Fig. 21.
Cytotoxicity of samples with drug
Conclusion
The porous PLA films were successfully made through solvent casting method. According to the SEM images, PEG and TA changed polymer structure by making pores and separating PLA particles in a microscopic scale, respectively. According to the results from mechanical test, adding PEG (with a concentration to 50%) and TA (with a concentration to 40%) increased polymer softness and flexibility. Whereas, adding more plasticizer weakened the material structure and strength. The samples containing PLA60/PEG40, PLA50/PEG50, PLA70/TA30, PLA60/TA40 and PLA50/PEG40/TA10 were introduced as the most similar compounds to human skin in terms of flexibility. The DSC test results confirmed the effectiveness of both plasticizers in reducing Tm and increasing polymer softness. In addition, due to a greater impact on the melting point, TA was introduced as a more powerful plasticizer. Hydrophilicity is among the desired characteristics of wound dressing, that both plasticizers have positive effect on the polymer hydrophilicity. Although both plasticizers accelerated polymer degradation, their usage together showed a greater impact. The highest degradation (85%) over the eight weeks was observed in PLA50/PEG20/TA30. Both plasticizers, specifically PEG, increased water absorbency of polymer, that the highest water absorption (218%) was observed in PLA40/PEG60. According to the drug release test, 50–60% of drug release occurred in the first 12 h (explosive release); however, the release reached a constant rate after 42 h. The minimum and maximum drug release during the test were observed in PLA60/PEG40 with 60% and PLA50/PEG20/TA30 with 85%. The effect of bacteria growth inhibition in all drug-containing samples was clearly observed. In addition, this effect was observed in TA-containing samples (without drug), indicating its antibacterial properties. Cell toxicity was investigated by MTT assay. All film samples containing PLA and PEG were non-toxic.The films containing less than 40% TA were also reported without toxicity. Based on overall results, the following samples are introduced for further study in future researches: PLA60/PEG40–PLA50/PEG50–PLA70/TA30–PLA50/PEG40/TA10.
Author contributions
HB: designed and performed experiments; BD: performed experiments and co-wrote the paper: SM: co-wrote the paper.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Avérous L. Synthesis, Properties, Environmental and Biomedical Applications of Polylactic Acid A2 - Ebnesajjad, Sina, in Handbook of Biopolymers and Biodegradable Plastics. Boston: William Andrew Publishing; 2013. pp. 171–188. [Google Scholar]
- Bailey JW, Haymond MW, Miles JM. Triacetin: a potential parenteral nutrient. J Parenter Enter Nutr. 1991;15:32–36. doi: 10.1177/014860719101500132. [DOI] [PubMed] [Google Scholar]
- Boateng J, Catanzano O. Advanced therapeutic dressings for effective wound healing—a review. J Pharm Sci. 2015;104:3653–3680. doi: 10.1002/jps.24610. [DOI] [PubMed] [Google Scholar]
- Boateng JS, Matthews KH, et al. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97:2892–2923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- Chang DP, Garripelli VK, et al. Investigation of fragment antibody stability and its release mechanism from poly(Lactide-co-Glycolide)-triacetin depots for sustained-release applications. J Pharm Sci. 2015;104:3404–3417. doi: 10.1002/jps.24546. [DOI] [PubMed] [Google Scholar]
- Chen BY, Wang YS, et al. Effect of poly(ethylene glycol) on the properties and foaming behavior of macroporous poly(lactic acid)/sodium chloride scaffold. J Appl Polym Sci. 2014;131:1–10. [Google Scholar]
- Chitrattha S, Phaechamud T. Porous poly(DL-lactic acid) matrix film with antimicrobial activities for wound dressing application. Mater Sci Eng C. 2016;58:1122–1130. doi: 10.1016/j.msec.2015.09.083. [DOI] [PubMed] [Google Scholar]
- Chowdhary K, Rathore SPS. Biopolymers for wound healing. Res Reinf J. 2015;3:1–8. [Google Scholar]
- Dsouza A, Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv. 2016;13:1257–1275. doi: 10.1080/17425247.2016.1182485. [DOI] [PubMed] [Google Scholar]
- Dzikowski M, Castanie N, et al. Antibiotic incorporation in jet-sprayed nanofibrillar biodegradable scaffolds for wound healing. Int J Pharm. 2017;532:802–812. doi: 10.1016/j.ijpharm.2017.08.117. [DOI] [PubMed] [Google Scholar]
- Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis. 2004;17:91–96. doi: 10.1097/00001432-200404000-00004. [DOI] [PubMed] [Google Scholar]
- Farah S, Anderson DG, Langer R. Physical and mechanical properties of PLA, and their functions in widespread applications—a comprehensive review. Adv Drug Deliv Rev. 2016;107:367–392. doi: 10.1016/j.addr.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Fiume MZ. Final report on the safety assessment of triacetin. Int J Toxicol. 2003;22:1–10. doi: 10.1177/1091581803022S203. [DOI] [PubMed] [Google Scholar]
- Gallagher AJ, Anniadh AN, Bruyere K (2012) Dynamic tensile properties of human skin. In IRCOBI conference: Ireland. p 494–502
- Guo S, DiPietro LA. Factors affecting wound healing. Crit Rev Oral Biol Med. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad K, et al. Properties and medical applications of polylactic acid: a review. Express Polym Lett. 2015;9:435–455. doi: 10.3144/expresspolymlett.2015.42. [DOI] [Google Scholar]
- Janorkar AV, Metters AT, Hirt DE. Modification of poly(lactic acid) films: enhanced wettability from surface-confined photografting and increased degradation rate due to an artifact of the photografting process. Macromolecules. 2004;37:9151–9159. doi: 10.1021/ma049056u. [DOI] [Google Scholar]
- Jayakuma R, Prabaharan M, et al. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29:322–337. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Kim ES, Kim SH, Lee CH. Electrospinning of polylactide fibers containing silver nanoparticles. Macromol Res. 2010;18:215–221. doi: 10.1007/s13233-010-0316-4. [DOI] [Google Scholar]
- Madaghiele M, Demitri C, et al. Polymeric hydrogels for burn wound care: advanced skin wound dressings and regenerative templates. Polymer Hydrogels Burn Wound Care. 2014;2:153–161. doi: 10.4103/2321-3868.143616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Brit J Derma. 2015;173:370–378. doi: 10.1111/bjd.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogoşanu GD, Grumezescu AM. Natural and synthetic polymers for wounds and burns dressing. Int J Pharm. 2014;463:127–136. doi: 10.1016/j.ijpharm.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Moraes PRFdS, Saska S, et al. Bacterial cellulose/collagen hydrogel for wound healing. Mater Res. 2016;19:106–116. doi: 10.1590/1980-5373-MR-2015-0249. [DOI] [Google Scholar]
- Nor Azowa I, et al. Poly(lactic acid) (PLA)-reinforced kenaf bast fiber composites: the effect of triacetin. J Reinf Plast Compos. 2010;29:1099–1111. doi: 10.1177/0731684409344651. [DOI] [Google Scholar]
- Opdyke DLJ. Monographs on fragrance raw materials. Food Cosmet Toxicol. 1979;16:879–882. doi: 10.1016/S0015-6264(78)80155-2. [DOI] [PubMed] [Google Scholar]
- Orsted HL, Keast D, et al. Basic principles of wound healing. Wound Care Can. 2011;9:4–12. [Google Scholar]
- Pelegrini K, Donazzolo I, et al. Degradation of PLA and PLA in composites with triacetin and buriti fiber after 600 days in a simulated marine environment. J Appl Polym Sci. 2016;133:43290. doi: 10.1002/app.43290. [DOI] [Google Scholar]
- Percival SL, McCarty S, et al. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014;22:174–186. doi: 10.1111/wrr.12125. [DOI] [PubMed] [Google Scholar]
- PUBLICATIONS, OSU (2002) OECD SIDS Initial Assessment Report For Triacetin 2002
- Segal R, Anikster Y, et al. A safety trial of high dose glyceryl triacetate for Canavan disease. Mol Genet Metab. 2011;103:203–206. doi: 10.1016/j.ymgme.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Septevani AA, Bhakri S. Plasticization of poly(lactic acid) using different molecular weight of Poly(ethylene glycol) AIP Conf Proc. 2017;1904:020038. doi: 10.1063/1.5011895. [DOI] [Google Scholar]
- Simões D, Moguel SP, et al. Recent advances on antimicrobial wound dressing: a review. Eur J Pharm Biopharm. 2018;127:130–141. doi: 10.1016/j.ejpb.2018.02.022. [DOI] [PubMed] [Google Scholar]
- Sofokleous P, Stride E, Edirisinghe M. Preparation, characterization, and release of amoxicillin from electrospun fibrous wound dressing patches. Pharm Res. 2013;30:1926–1938. doi: 10.1007/s11095-013-1035-2. [DOI] [PubMed] [Google Scholar]
- Tian J, Wong KKY, et al. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem. 2007;2:129–136. doi: 10.1002/cmdc.200600171. [DOI] [PubMed] [Google Scholar]
- Tyler B, Gullotti D, et al. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv Drug Deliv Rev. 2016;107:163–175. doi: 10.1016/j.addr.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Vowden K, Vowden P. Wound dressings: principles and practice. Surgery (Oxford) 2017;35:489–494. doi: 10.1016/j.mpsur.2017.06.005. [DOI] [Google Scholar]
- Maxey K (2018) https://www.caymanchem.com/pdfs/9002409.pdf
- Xiong MH, Bao Y, et al. Delivery of antibiotics with polymeric particles. Adv Drug Deliv Rev. 2014;78:63–76. doi: 10.1016/j.addr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Zilberman M, Egozi D, et al. Hybrid wound dressings with controlled release of antibiotics: Structure-release profile effects and in vivo study in a guinea pig burn model. Acta Biomater. 2015;22:155–163. doi: 10.1016/j.actbio.2015.04.029. [DOI] [PubMed] [Google Scholar]