Summary
Systemic sclerosis (SSc) is a severe autoimmune fibrotic disease characterized by fibrosis, vasculopathy, and immune dysregulation. Dendritic cells (DCs) are the most potent antigen‐presenting cells, specialized in pathogen sensing, with high capacity to shape the immune responses. The most recent technological advances have allowed the discovery of new DC subsets with potential implications in inflammatory conditions. Alterations of DC distribution in circulation and affected tissue as well as impaired DC function have been described in SSc patients, pointing towards a crucial role of these cells in SSc pathogenesis. In particular, recent studies have shown the importance of plasmacytoid DCs either by their high capacity to produce type I interferon or other inflammatory mediators implicated in SSc pathology, such as chemokine C‐X‐C motif ligand 4 (CXCL4). In‐vivo models of SSc have been vital to clarify the implications of DCs in this disease, especially DCs depletion and specific gene knock‐down studies. This review provides these new insights into the contribution of the different DCs subsets in the pathogenesis of SSc, as well as to the novel developments on DCs in in‐vivo models of SSc and the potential use of DCs and their mediators as therapeutic targets.
Keywords: conventional dendritic cells, dendritic cells, fibrosis, inflammation, plasmacytoid dendritic cells, systemic sclerosis
Dendritic cells play a crucial role in the pathogenesis of systemic sclerosis. Herein, we summarized very recent findings on the role of classical , plasmacytoid and monocyte‐derived inflammatory dendritic cells, in both, human and mouse studies in the disease. Furthermore, we discussed current and potential treatment options targeting dendritic cells function in systemic sclerosis.

Introduction
Systemic sclerosis (SSc), also known as scleroderma, is an immune‐mediated rheumatic disease characterized by vasculopathy, inflammation and fibrosis of the skin and internal organs. The aetiology of SSc is largely unknown, and its pathogenesis is complex and poorly understood 1.
Cell types prominently implicated in the disease process include endothelial cells, platelets, structural cells such as pericytes, vascular smooth muscle cells, fibroblasts and myofibroblasts; both innate and adaptive immunity also play an important contribution in SSc. Additionally, highly specific circulating autoantibodies are present in nearly all the patients 2. Immune mediators such as transforming growth factor (TGF)‐β, platelet‐derived growth factor (PDGF), interleukin (IL)‐6, IL‐13, endothelin 1, angiotensin II, lipid mediators and reactive oxygen species (ROS) have been shown to be relevant in SSc pathology, as well as Toll‐like receptors (TLRs) and NOD‐like receptor protein 3 (NLRP3) inflammasome dysfunction 3, 4, 5, 6. Moreover, the chemokine C‐X‐C motif ligand 4 (CXCL4) also plays an important role in SSc, as it was found increased in circulation and in the skin of SSc patients and correlated with the presence and progression of complications such as lung fibrosis and pulmonary arterial hypertension 7, 8.
The role of dendritic cells (DCs) in SSc have gained particular interest not only due to their capability to regulate the immune responses, but also the vasculature cell and fibroblast‐like cells 9, 10. Significant progress has been made in comprehending the pathogenesis of SSc, mainly by an increasing number of studies using advanced molecular technologies and a more complete representation of the features of SSc in preclinical mouse models of the disease 11.
DC role and function
DCs represent the most potent antigen‐presenting cells in promoting activation of naive T cells, being crucial in initiating and shaping immune responses. They express an array of pathogen recognition receptors such as TLRs, C‐type lectin receptors (CLRs), RIG‐I‐like receptors (RLRs) and NOD‐like receptors (NLRs) to recognize pathogen‐ or danger‐associated molecules in the extracellular and intracellular environment 12. Besides their important role as effector cells, fighting against pathogens and controlling adaptive immunity, DCs are also important for maintaining peripheral T cell homeostasis and preventing inappropriate T cell activation 13, 14.
In autoimmune diseases, it has been shown that DCs have a critical role contributing to the breakdown of tolerance. For instance, in systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA), alteration in numbers of circulating cells and also in affected tissues has been reported. Additionally, changes in their function, with impaired phagocytosis of apoptotic cells, enhanced cross‐presentation of autoantigens derived from apoptotic cells and altered cytokine secretion, have been found. Therefore, these functional alterations are responsible for a harmful imbalance between T helper type 1 (Th1), Th2, Th17 and regulatory T cells (Tregs). DCs are also able to shape B cell responses through the secretion of B cell stimulatory and survival factors and through the contribution to the formation and maintenance of ectopic lymphoid structures in target tissues 15.
DC population in humans and mice
Despite the low numbers of DCs in circulation of healthy individuals 16, DCs can be broadly subdivided into plasmacytoid DCs (pDCs) and conventional or classical DCs (cDCs). As suggested by Guilliams et al., cDCs can be further subdivided into cDC type 1 (cDC1s) and cDC type 2 (cDC2s), as their development depends upon distinct sets of transcription factors and because they arise from discrete committed precursors 17.
The complexity of these cell subsets leads to a continuous demand for specific cell markers to identify and characterize these cells more clearly. As summarized by Collin et al., currently there are markers that perform consistently across species, such as CD141, C‐type lectin domain family 9 (CLEC9A), cell adhesion molecule 1 (CADM1), B and T lymphocyte associated (BTLA) and CD26 for cDC1s; and CD1c, CD2, high‐affinity immunoglobulin (Ig)E receptor (FcεR1) and signal regulatory protein α (SIRPα) for cDC2s. pDCs express CD303, CD304, CD123 in humans and B220, sialic‐acid‐binding immunoglobulin‐like lectin‐H (Siglec‐H) and bone marrow stromal cell antigen 2 (BST2) in mouse in the absence of other myeloid and lymphoid markers 18.
However, in recent years highly sensitive and high‐throughput technologies at the transcriptional and proteomic level have revealed a remarkable heterogeneity among the DC subsets. DCs are under a continuous process of differentiation that starts in the bone marrow with common DC progenitors, diverges at the point of emergence of pre‐DC and pDC potential, and culminates in maturation of both lineages in the blood and spleen. The pre‐DC compartment contains functionally and phenotypically distinct lineage‐committed subpopulations, including one early uncommitted CD123+ pre‐DC subset and two CD45RA+CD123low lineage‐committed subsets 19.
pDCs are characterized by their capacity in the production of type I IFNs, mainly in response to TLR‐7 and TLR‐9 activation, and translate into their importance in anti‐viral immune responses; conversely, pDCs have also been implicated in the pathogenesis of autoimmune diseases that are characterized by a type I IFN signature. Moreover, pDCs are also known to induce tolerogenic immune responses 20. However, recent studies have highlighted pDC heterogeneity in mice and humans (Fig. 1) 19, 21, 22, 23. Conversely, using the integration of high‐dimensional single‐cell protein and RNA expression, it also becomes possible to identify several cDC2 subsets in mice and humans (Fig. 1) 21, 24, 25. Additionally, Dutertre et al. data allowed us to identify distinct markers to differentiate monocytes from cDC2s. CD88, together with CD89, were used to identity monocytes, while HLA‐DQ and FcεRIα were used for cDC2s, allowing their specific identification in blood and tissues 25.
Figure 1.
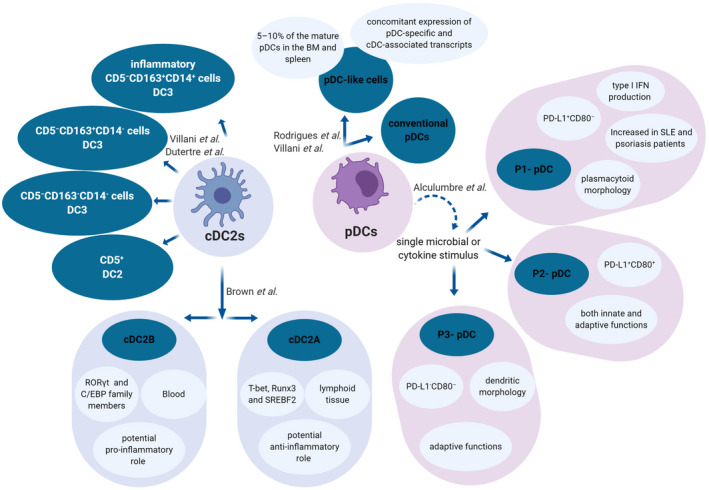
Plasmacytoid dendritic cell (pDC) and type 2 conventional dendritic cell (cDC2) heterogeneity. Recently, two subsets of mature pDCs were described: conventional pDCs and a small subset of pDC‐like cells. Although both subsets secreted type I interferons (IFNs) in response to cytosine–phosphate–guanosine (CpG)‐A stimulation, pDC‐like cells were unable to do it when stimulated with CpG‐B 22. This pDC‐like cell population has also been described in human circulation 19, 21. Additionally, it was reported that the activation of human pDCs with a single microbial or cytokine stimulus triggers pDC diversification into three stable subpopulations that were described as P1, P2 and P3–pDC 23. Recently, two different cDC2 subsets were identified in mice: cDC2A and cDC2B. These findings were extended to humans, but the cDC2B population was only present in blood, whereas a cDC2A population found in mice were also present in human spleen. Interestingly, bone marrow DC progenitors lacked the expression of T‐bet and RAR‐related orphan receptor gamma t (RORγt), suggesting that cDC2s acquire expression of the respective transcription factors in response to environmental signals 24. Other cDC2 were subdivided into different subsets based on CD5, CD163 and CD14 expression, which were phenotypically and functionally different 25 and related to previously described DC3s 22. Nevertheless, the role of the new DC subsets needs still to be clarified in the pathogenesis of systemic sclerosis (SSc).
These recent findings point towards the necessity of exploring these newly described pDCs and cDC2s subsets in the context of SSc, given their potential inflammatory role, as they were already found disturbed in conditions such as SLE and psoriasis 23, 25.
DCs in the affected tissues of SSc patients
The skin is frequently affected in SSc patients and, based on the extent of skin fibrosis, SSc patients can be classified as limited cutaneous SSc (lcSSc) or diffuse cutaneous SSc (dcSSc). In lcSSc, skin fibrosis is restricted to the distal areas of the elbows and knees, while in dcSSc skin fibrosis is more extensive and accompanied by internal organ involvement 26, 27, 28. These patients often present lung fibrosis or interstitial lung disease, as well as pulmonary arterial hypertension. Other affected organs may include the gastrointestinal tract, kidney and heart 1, 2.
In barrier tissues such as skin and lung, DCs play a major role in determining the severity of inflammatory response and consequently the severity of inflammatory diseases. The different DCs subsets previously described, cDC1s, cDC2s and pDCs, were not only present in blood but also in different tissues, especially in draining lymph nodes and mucosal sites 29, and these DCs subsets were also found in the lung. Albeit cDC2 proinflammatory role, they have also been associated with tolerogenic properties; for instance, isolated lung CD1c+ DCs from patients with chronic obstructive pulmonary disease (COPD) benefited the differentiation of IL‐10‐secreting CD4+ T cells. pDCs are distributed throughout the lung, in the airways, parenchyma and alveolar septa and are essential during anti‐viral responses for their ability to produce type I IFNs 30, 31. Lung tissues from SSc patients and non‐SSc controls were tested for the presence of pDCs, which were detected at low levels in the control lung tissues, while in SSc lungs, pDCs were found to be increased not only in the interstitial tissue and bronchi, but also on their bronchoalveolar lavage (BAL) samples 32.
In skin under steady‐state conditions, there are at least three well‐described major DC subsets: epidermal Langerhans cells (LCs), dermal cDC1 and dermal cDC2. Under inflammatory conditions, however, additional subtypes of DCs arise in the inflamed skin, such as pDCs, inflammatory myeloid DCs and monocyte‐derived DCs 33, 34. In SSc skin samples, CD1a+ survivin+ DCs were shown to infiltrate into dermal lesions 35.
Plasmacytoid DCs in SSc
In SSc patients, genetic risk factors studies have found several genes involved in the IFN type I signalling pathway to be highly associated with the risk of SSc [36, 37, 38, 39]. Moreover, the IFN signature present in blood and affected skin of SSc patients is indicative of the presence of aberrant pDCs 40, 41. In a proteomic profiling study, CXCL4 has been identified to be largely produced by pDCs from SSc patients. Increased numbers of pDCs co‐localized with CXCL4 in the skin of SSc patients indicate that pDCs are also the main source of CXCL4 in the affected tissue 7, 42. In the same study, CXCL4 was shown to induce the production of IFN‐α in pDCs stimulated with TLR‐9. Also, blocking CXCL4 abrogated the production of IFN‐α in SSc pDCs 7. Another recent study has confirmed that pDCs largely infiltrate the skin of SSc patients and release high quantities of CXCL4 and IFN‐α. It was also reported that the expression of TLR‐8 is increased in SSc pDCs, and that TLR‐8 signalling is responsible for the high production of CXCL4 and IFN‐α in SSc pDCs 43. Moreover, CXCL4 was shown to potentiate TLR‐8 and TLR‐9 activation of SSc pDCs 43. Another study found that CXCL4 forms liquid crystalline complexes with human and bacterial DNA that amplify TLR‐9‐mediated IFN‐α production in SSc pDCs. In the same study, it was also shown that CXCL4–DNA complexes activates pDCs in a TLR‐9‐dependent manner but independent of CXCR3, a known CXCL4 receptor. Interestingly, CXCL4–DNA complexes were found to be present in vivo and to correlate with type I IFN in the blood of SSc patients 42.
In vivo, in the bleomycin‐induced SSc mouse model, depleting pDCs attenuated fibrosis of the skin and lung, highlighting the importance of pDCs in SSc pathogenesis 32, 43. Furthermore, imatinib treatment in SSc patients reduced pDC numbers in the lung and improved skin and lung disease 44, 45.
Conventional DCs and inflammatory DCs (inflDCs) in SSc
The role of cDCs is less studied in the pathogenesis of SSc than the role of pDC. It has been shown that cDCs from early limited and diffused patients produce higher amounts of different proinflammatory cytokines, such as IL‐6 and TNF‐α, in response to TLR‐2, ‐4 and ‐6 activation than the cDCs from SSc patients with a long disease history or healthy controls 46. This emphasizes a possibly higher impact of cDCs in the early and progressive phase of the disease.
Recently, it has been shown that cDC2s of lcSSc patients spontaneously produce higher amounts of CXCL10 ex vivo than cDC2s from healthy individuals 47. In addition, they also produce more CXCL8 upon in‐vitro stimulation with lipopolysaccharide (LPS) plus IFN‐γ, which activate signalling pathways involved in SSc pathology. Moreover, dcSSc cDC2s were found to produce more CCL4 than cDC2 from healthy individuals 47. Differences in the cytokine production patterns may suggest differences in the molecular mechanism in two disease subtypes.
The frequency of the newly characterized CD14+CD163+ inflDC population has been studied in a small SSc cohort, but was not found to be altered compared to healthy controls 25. Nevertheless, the scavenger receptor CD163, mainly considered as a marker for M2 macrophages, has been found to be increased in the serum of SSc patients 48, 49, 50. Therefore, the role of CD163+ DCs in SSc could be studied more extensively using a larger patient cohort with distinguished SSc subsets. A new role has been described for phospholipase phospholipase D family member 4 (PLD4), linked to SSc genome‐wide association studies 51. The study by Gavin et al. on PLD4‐deficient mice suggested that PLD4 functions as 5′ exonuclease, which breaks down single‐stranded oligonucleotide (ODN), thereby limiting TLR‐9 stimulatory capacity. Importantly, PLD4‐deficient DCs were indirectly responsible for up‐regulation of major histocompatibility complex (MHC) class II on macrophages and, in general, enhanced responsiveness to TLR‐9 ligands 52. As TLR‐9 signalling has an important role in SSc pathogenesis, aberrant PLD4 activity, specifically in DCs, might contribute to the increased immune responses in SSc.
Another factor which might be involved in the development of SSc is P‐selectin glycoprotein ligand‐1 (PSGL‐1) 53. Increased expression of PSGL‐1 specifically in SSc cDCs was associated with the presence and severity of interstitial lung disease (ILD), although the precise role of DCs in ILD development is not clear.
Taken together, the evidence indicates that cDCs do not contribute to SSc only by proinflammatory cytokine production, but also by dysregulated antigen processing, T cell activation and probably other mechanisms to be elucidated further.
As a result of systemic autoimmune activation in SSc, monocytes are likely to be recruited to the affected lesions, consequently differentiating into inflDC, similar to that described to occur during inflammation 54. In humans, an in‐vitro model of monocyte differentiation into DC (moDC) is often exploited to describe the potential role of inflDC. Importantly, there is a high correlation between cytokine production capacity of cDCs and moDCs of the same SSc patients 46. Recently, several studies have focused on understanding how SSc‐related factors modulate DC differentiation and function. For instance, CXCL4, in combination with granulocyte–macrophage colony‐stimulating factor (GM‐CSF) and IL‐4, was shown to skew monocytes to differentiate into more proinflammatory and profibrotic moDCs 55, 56, 57. This suggests that under the effect of an inflammatory environment and the presence of CXCL4, monocytes might differentiate into inflDC that could potentially contribute to the fibrotic process. However, the presence of these cells in the affected tissues of SSc patients still needs to be confirmed.
The role of DCs in mouse models
The importance of DCs in SSc has been studied in several mice models for many years 58, 59, 60, 61, 62, 63, 64, 65. Recent studies have focused on the role of DCs in the bleomycin induced‐SSc mouse model. The bleomycin model is the most commonly used model to study the pathogenesis of SSc 66. Using this mouse model, several recent studies have highlighted the importance of pDCs in SSc. pDCs depletion resulted in attenuation of skin fibrosis in two independent studies, but also reduced lung fibrosis, chemotaxis, inflammation and differentiation of DC in the skin and lung of animals 32, 43.
Kioon et al. have reported high expression of TLR‐8 in SSc pDCs, and to understand more clearly whether TLR‐8 can directly promote fibrosis in vivo they used bleomycin model in huTLR‐8Tg mice (mice with a single copy of the human TLR‐8 gene under the control of human TLR‐8 genomic regulatory regions). They found that TLR‐8 exacerbates skin fibrosis in the bleomycin‐induced fibrosis model, leading to an increased number of pDCs in the skin of bleomycin‐injected mice, confirming an important role of TLR‐8 signalling and pDCs in skin fibrosis developments 43.
A recent study by Affandi et al. focused on the identification of pathways underlying pDCs aberrances in SSc. This study identified down‐regulation of runt‐related transcription factor 3 (Runx3) in SSc pDCs, which correlated with skin severity in SSc patients. Using mice with DC‐specific deletion of Runx3, they showed increased skin inflammation and fibrosis, together with an enhanced pDC infiltration and increased expression of CD86. Low Runx3 expression in SSc pDCs further highlights the pathological role of pDCs in SSc pathogenesis 67.
Conversely, no reports are available on the pathological role of cDCs in SSc pathogenesis. For instance, depletion of non‐pDCs worsened bleomycin‐induced skin fibrosis. However, further analysis at different stages of the disease is required to identify different roles of DC subsets in the development of fibrosis in SSc 62.
DC‐targeted therapies for SSc
The main therapies for SSc involve immunosuppression, treatment of skin and lung fibrosis and separate treatment of other complications. So far, there is no cure to reverse the disease, therefore there is a serious demand for more specific and efficient therapies. Although there are no clinical trials for specifically targeting DCs in SSc, most of the available and potential treatment options affect DC activity.
Autologous haematopoietic stem cell transplantation (HSCT) has been shown to be a promising solution for severe and therapy‐refractory forms of SSc. HSCT aims to re‐establish the normal immune system, including self‐tolerant DCs. Results have been published for several recent clinical trials 68, 69, showing better patient long‐term survival. The clinical evidence supporting HSCT usage has been recently reviewed by Ng et al. 70. HSCT has been suggested recently as the standard of care for rapidly progressive dcSSc patients 71, 72.
Another promising cellular‐based treatment to alter fibrosis is mesenchymal stem cell therapy, recently discussed by Peltzer et al. 73. Mesenchymal stromal cells (MSCs) possess anti‐inflammatory, anti‐proliferative, anti‐fibrotic and immunomodulatory properties. There is evidence that dermal white adipose tissue DCs could contribute to potentially reversing fibrosis by sustaining the viability of adipose tissue stem cells 62. In an ongoing clinical trial (NCT03060551 ClinicalTrials.gov), adipose tissue stromal vascular fraction (SVF)‐containing stem cells are subjected to subcutaneous injection of SVF in the fingers in SSc.
Recent evidence suggests that tolerized dendritic cells (tolDC) could become a treatment option for autoimmune conditions 74. This includes differentiating autologous monocytes into DCs, loading them with disease‐specific autoantigens and injecting them back into the patients. A small‐scale clinical trial has been conducted to treat patients with inflammatory arthritis, with promising data of autoantigen‐loaded tolDCs stability and effect on disease symptoms when injected into the inflamed knee 75. No study has yet been conducted in SSc; however, considering the more recognized role of DCs in SSc pathogenesis, it might be a particularly beneficial treatment approach for early SSc in which the immune disbalance plays a bigger role than in the fibrosis phase.
A few biologicals targeting DC–T cell interactions and DC‐produced cytokines have been or are being tested in clinics 76, 77 (NCT01284322 ClinicalTrials.gov). For instance, Abatacept, an immune checkpoint inhibitor, cytotoxic T lymphocyte antigen 4 (CTLA‐4) and Ig fusion protein binds to co‐stimulatory molecules CD80/CD86 on DCs and thereby inhibits DC–T cell interaction and T cell activation 76. Targeting IL‐6 by tocilizumab has been shown to moderately improve skin and fibrosis in SSc patients; however, it is accompanied by an augmented risk for severe infections 77, 78. Targeting IL‐23/IL‐17 axis by ustekinumab, also implicated in SSc pathogenesis 79, 80, has been shown to be promising in other autoimmune diseases 81, 82.
In addition, results from clinical trials in other autoimmune diseases, in‐vitro experiments and mouse models support the idea of inhibiting certain signalling pathways downstream from PRR and cytokine receptor activation of DCs and other immune cells, such as the Janus kinase–signal transducer and activator of transcription (JAK–STAT) pathway by tofacitinib (clinical trial, Phase I/II, NCT03274076 ClinicalTrials.gov) and mitogen‐activated protein kinase (MAPK) p38 inhibition 56, 83.
Conclusion and future perspectives
SSc pathogenesis is currently perceived as a complex condition with a strong link between impaired inflammatory and fibrotic processes. In this study, we show that DCs are an essential link between these processes. In disturbed conditions such as SSc, different DC subsets have the capacity to produce a large array of inflammatory mediators with the ability either to activate other immune cells or to skew different structural and stromal cells towards activated myofibroblasts (Fig. 2). The role of DCs in SSc pathology has recently been reinforced by several studies using in‐vivo models of SSc (Fig. 3). Nevertheless, more studies will be imperative to unveil the role of the most recent described DCs subsets in SSc and their potential use as therapeutic targets.
Figure 2.
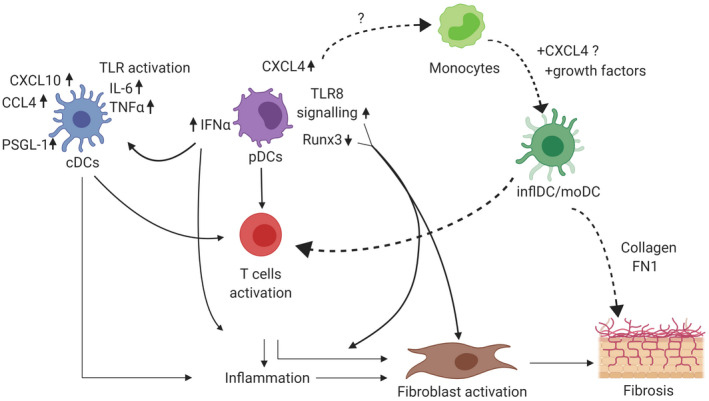
Plasmacytoid dendritic cells (pDCs) and conventional dendritic cells (cDCs) have a crucial role in the inflammatory and fibrotic processes in systemic sclerosis (SSc). pDCs produce a large amount of type I interferon (IFN)‐α and chemokine C‐X‐C motif ligand 4 (CXCL4) due to dysregulated mechanisms such as runt‐related transcription factor 3 (RUNX3) and Toll‐like receptor (TLR)‐8 signalling. IFN‐α has a strong capacity to induce inflammation and to activate other innate immune cells such as cDCs. Exacerbated TLR activation in SSc cDCs lead to an increased production of cytokines and chemokines as, for example, CXCL10, chemokine (C‐C motif) ligand 4 (CCL4), IL‐6, TNF‐α and cell adhesion molecules such as P‐selectin glycoprotein ligand 1 (PSGL‐1). Activated cDCs display a higher ability to induce T cell activation. Additionally, exacerbated CXCL4 production by pDCs might modulate monocytes differentiation into monocyte‐derived inflammatory DCs (inflDC) with an enhanced cytokine production capacity upon TLR (Toll‐like receptor) stimulation, a superior T cell stimulation and a profibrotic phenotype. As a result of these dysregulated mechanisms, DCs promote inflammation, myofibroblast transformation and extracellular matrix (ECM) deposition in the affected tissue of SSc patients.
Figure 3.
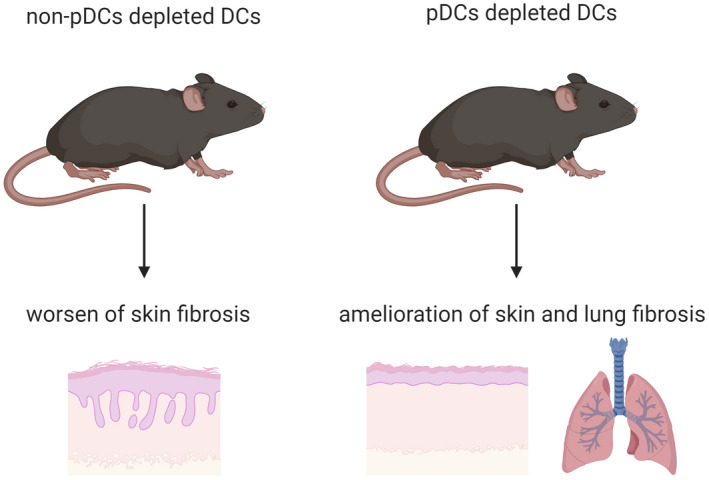
In‐vivo models of systemic sclerosis (SSc) have been fundamental in unravelling the role of dendritic cells (DCs) in systemic sclerosis. In the bleomycin‐induced SSc mouse model, plasmacytoid DC (pDC) depletion has improved the clinical score, skin and lung fibrosis 32, 43. Therefore, these findings point towards a crucial role for DCs on SSc pathogenesis and makes DCs potential targets in this disease.
Disclosures
All authors have no conflicts of interest to declare.
Author contributions
T. C., M. Z. and W. M. performed the literature review, wrote the manuscript and prepared the figures. T. R. D. J. R. critically reviewed and discussed the manuscript content. All authors edited and approved the final version of the manuscript.
Acknowledgements
T. C. was supported by a grant from the Portuguese national funding agency for science, research and technology: Fundação para a Ciência e a Tecnologia (SFRH/BD/93526/2013).
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Innate immunity in systemic sclerosis. Clinical and Experimental Immunology 2020, 201: 12–13.
Toll‐like receptors in mediating pathogenesis in systemic sclerosis. Clinical and Experimental Immunology 2020, 201: 14‐24.
The role of innate immune cells in systemic sclerosis in the context of autologous hematopoietic stem cell transplantation. Clinical and Experimental Immunology 2020, 201: 34‐39.
References
- 1. Denton CP, Khanna D. Systemic sclerosis. Lancet 2017; 390:1685–99. [DOI] [PubMed] [Google Scholar]
- 2. Allanore Y, Simms R, Distler O et al Systemic sclerosis. Nat Rev Dis Primers 2015; 1:15002. [DOI] [PubMed] [Google Scholar]
- 3. Bhattacharyya S, Wei J, Varga J. Understanding fibrosis in systemic sclerosis: shifting paradigms, emerging opportunities. Nat Rev Rheumatol 2011; 8:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown M, O’Reilly S. The immunopathogenesis of fibrosis in systemic sclerosis. Clin Exp Immunol 2019; 195:310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Henderson J, Bhattacharyya S, Varga J, O’Reilly S. Targeting TLRs and the inflammasome in systemic sclerosis. Pharmacol Ther 2018; 192:163–9. [DOI] [PubMed] [Google Scholar]
- 6. O’Reilly S. Pound the alarm: danger signals in rheumatic diseases. Clin Sci 2015; 128:297–305. [DOI] [PubMed] [Google Scholar]
- 7. van Bon L, Affandi AJ, Broen J et al Proteome‐wide analysis and CXCL4 as a biomarker in systemic sclerosis. N Engl J Med 2014; 370:433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Volkmann ER, Tashkin DP, Roth MD et al Changes in plasma CXCL4 levels are associated with improvements in lung function in patients receiving immunosuppressive therapy for systemic sclerosis‐related interstitial lung disease. Arthritis Res Ther 2016; 18:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu TT. Dendritic cells: novel players in fibrosis and scleroderma. Curr Rheumatol Rep 2012; 14:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Affandi AJ, Carvalheiro T, Radstake T, Marut W. Dendritic cells in systemic sclerosis: advances from human and mice studies. Immunol Lett 2018; 195:18–29. [DOI] [PubMed] [Google Scholar]
- 11. Yue X, Yu X, Petersen F, Riemekasten G. Recent advances in mouse models for systemic sclerosis. Autoimmun Rev 2018; 17:1225–34. [DOI] [PubMed] [Google Scholar]
- 12. Boltjes A, van Wijk F. Human dendritic cell functional specialization in steady‐state and inflammation. Front Immunol 2014; 5:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Audiger C, Rahman MJ, Yun TJ, Tarbell KV, Lesage S. The importance of dendritic cells in maintaining immune tolerance. J Immunol 2017; 198:2223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Waisman A, Lukas D, Clausen BE, Yogev N. Dendritic cells as gatekeepers of tolerance. Semin Immunopathol 2017; 39:153–63. [DOI] [PubMed] [Google Scholar]
- 15. Coutant F, Miossec P. Altered dendritic cell functions in autoimmune diseases: distinct and overlapping profiles. Nat Rev Rheumatol 2016; 12:703–15. [DOI] [PubMed] [Google Scholar]
- 16. Orsini G, Legitimo A, Failli A, Massei F, Biver P, Consolini R. Enumeration of human peripheral blood dendritic cells throughout the life. Int Immunol 2012; 24:347–56. [DOI] [PubMed] [Google Scholar]
- 17. Guilliams M, Ginhoux F, Jakubzick C et al Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 2014; 14:571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology 2018; 154:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. See P, Dutertre CA, Chen J et al Mapping the human DC lineage through the integration of high‐dimensional techniques. Science 2017; 356:eaag3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol 2015; 15:471–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Villani AC, Satija R, Reynolds G et al Single‐cell RNA‐seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017; 356:eaah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodrigues PF, Alberti‐Servera L, Eremin A, Grajales‐Reyes GE, Ivanek R, Tussiwand R. Distinct progenitor lineages contribute to the heterogeneity of plasmacytoid dendritic cells. Nat Immunol 2018; 19:711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alculumbre SG, Saint‐Andre V, Di Domizio J et al Diversification of human plasmacytoid predendritic cells in response to a single stimulus. Nat Immunol 2018; 19:63–75. [DOI] [PubMed] [Google Scholar]
- 24. Brown CC, Gudjonson H, Pritykin Y et al Transcriptional basis of mouse and human dendritic cell heterogeneity. Cell 2019; 179:846–63.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dutertre CA, Becht E, Irac SE et al Single‐cell analysis of human mononuclear phagocytes reveals subset‐defining markers and identifies circulating inflammatory dendritic cells. Immunity 2019; 51:573–89.e8. [DOI] [PubMed] [Google Scholar]
- 26. van den Hoogen F, Khanna D, Fransen J et al 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2013; 72:1747–55. [DOI] [PubMed] [Google Scholar]
- 27. LeRoy EC, Black C, Fleischmajer R et al Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988; 15:202–5. [PubMed] [Google Scholar]
- 28. LeRoy EC, Medsger TA Jr. Criteria for the classification of early systemic sclerosis. J Rheumatol 2001; 28:1573–6. [PubMed] [Google Scholar]
- 29. Granot T, Senda T, Carpenter DJ, Matsuoka N, Weiner J, Gordon CL, et al. Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity. 2017;46:504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Freeman CM, Curtis JL. Lung dendritic cells: shaping immune responses throughout chronic obstructive pulmonary disease progression. Am J Respir Cell Mol Biol 2017; 56:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsoumakidou M, Tousa S, Semitekolou M et al Tolerogenic signaling by pulmonary CD1c+ dendritic cells induces regulatory T cells in patients with chronic obstructive pulmonary disease by IL‐27/IL‐10/inducible costimulator ligand. J Allergy Clin Immunol 2014; 134:944–54.e8. [DOI] [PubMed] [Google Scholar]
- 32. Kafaja S, Valera I, Divekar AA et al pDCs in lung and skin fibrosis in a bleomycin‐induced model and patients with systemic sclerosis. JCI Insight 2018; 3:e98380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim TG, Kim SH, Lee MG. The origin of skin dendritic cell network and its role in psoriasis. Int J Mol Sci 2017; 19:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kashem SW, Haniffa M, Kaplan DH. Antigen‐presenting cells in the skin. Annu Rev Immunol 2017; 35:469–99. [DOI] [PubMed] [Google Scholar]
- 35. Mokuda S, Miyazaki T, Ubara Y et al CD1a+ survivin+ dendritic cell infiltration in dermal lesions of systemic sclerosis. Arthritis Res Ther 2015; 17:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dieude P, Guedj M, Wipff J et al STAT4 is a genetic risk factor for systemic sclerosis having additive effects with IRF5 on disease susceptibility and related pulmonary fibrosis. Arthritis Rheum 2009; 60:2472–9. [DOI] [PubMed] [Google Scholar]
- 37. Ito I, Kawaguchi Y, Kawasaki A et al Association of a functional polymorphism in the IRF5 region with systemic sclerosis in a Japanese population. Arthritis Rheum 2009; 60:1845–50. [DOI] [PubMed] [Google Scholar]
- 38. Rueda B, Broen J, Simeon C et al The STAT4 gene influences the genetic predisposition to systemic sclerosis phenotype. Hum Mol Genet 2009; 18:2071–7. [DOI] [PubMed] [Google Scholar]
- 39. Gourh P, Agarwal SK, Divecha D, et al. Polymorphisms in TBX21 and STAT4 increase the risk of systemic sclerosis: evidence of possible gene-gene interaction and alterations in Th1/Th2 cytokines. Arthritis Rheum. 2009;60:3794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ciechomska M, Skalska U. Targeting interferons as a strategy for systemic sclerosis treatment. Immunol Lett 2018; 195: 45–54. [DOI] [PubMed] [Google Scholar]
- 41. Barrat FJ, Lu TT. Role of type I interferons and innate immunity in systemic sclerosis: unbalanced activities on distinct cell types? Curr Opin Rheumatol 2019; 31:569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lande R, Lee EY, Palazzo R et al CXCL4 assembles DNA into liquid crystalline complexes to amplify TLR9‐mediated interferon‐alpha production in systemic sclerosis. Nat Commun 2019; 10:1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ah Kioon MD, Tripodo C, Fernandez D et al Plasmacytoid dendritic cells promote systemic sclerosis with a key role for TLR8. Sci Transl Med 2018; 10:eaam8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khanna D, Saggar R, Mayes MD et al A one‐year, phase I/IIa, open‐label pilot trial of imatinib mesylate in the treatment of systemic sclerosis‐associated active interstitial lung disease. Arthritis Rheum 2011; 63:3540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fraticelli P, Gabrielli B, Pomponio G et al Low‐dose oral imatinib in the treatment of systemic sclerosis interstitial lung disease unresponsive to cyclophosphamide: a phase II pilot study. Arthritis Res Ther 2014; 16:R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Bon L, Popa C, Huijbens R et al Distinct evolution of TLR‐mediated dendritic cell cytokine secretion in patients with limited and diffuse cutaneous systemic sclerosis. Ann Rheum Dis 2010; 69:1539–47. [DOI] [PubMed] [Google Scholar]
- 47. Carvalheiro T, Horta S, van Roon JAG et al Increased frequencies of circulating CXCL10‐, CXCL8‐ and CCL4‐producing monocytes and Siglec‐3‐expressing myeloid dendritic cells in systemic sclerosis patients. Inflamm Res 2018; 67:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Higashi‐Kuwata N, Jinnin M, Makino T et al Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthritis Res Ther 2010; 12:R128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bielecki M, Kowal K, Lapinska A, Chyczewski L, Kowal‐Bielecka O. Increased release of soluble CD163 by the peripheral blood mononuclear cells is associated with worse prognosis in patients with systemic sclerosis. Adv Med Sci 2013; 58:126–33. [DOI] [PubMed] [Google Scholar]
- 50. Shimizu K, Ogawa F, Yoshizaki A et al Increased serum levels of soluble CD163 in patients with scleroderma. Clin Rheumatol 2012; 31:1059–64. [DOI] [PubMed] [Google Scholar]
- 51. Terao C, Ohmura K, Kawaguchi Y et al PLD4 as a novel susceptibility gene for systemic sclerosis in a Japanese population. Arthritis Rheum 2013; 65:472–80. [DOI] [PubMed] [Google Scholar]
- 52. Gavin AL, Huang D, Huber C et al PLD3 and PLD4 are single‐stranded acid exonucleases that regulate endosomal nucleic‐acid sensing. Nat Immunol 2018; 19:942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Silvan J, Gonzalez‐Tajuelo R, Vicente‐Rabaneda E et al Deregulated PSGL‐1 expression in B cells and dendritic cells may be implicated in human systemic sclerosis development. J Invest Dermatol 2018; 138:2123–32. [DOI] [PubMed] [Google Scholar]
- 54. Segura E, Touzot M, Bohineust A et al Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 2013; 38:336–48. [DOI] [PubMed] [Google Scholar]
- 55. Silva‐Cardoso SC, Affandi AJ, Spel L et al CXCL4 exposure potentiates TLR‐driven polarization of human monocyte‐derived dendritic cells and increases stimulation of T cells. J Immunol 2017; 199:253–62. [DOI] [PubMed] [Google Scholar]
- 56. Silva‐Cardoso SC, Bekker CPJ, Boes M, Radstake T, Angiolilli C. CXCL4 is a driver of cytokine mRNA stability in monocyte‐derived dendritic cells. Mol Immunol 2019; 114:524–34. [DOI] [PubMed] [Google Scholar]
- 57. Silva‐Cardoso SC, Tao W, Angiolilli C et al CXCL4 links inflammation and fibrosis through transcriptional and epigenetic reprogramming of monocyte-derived cells. BioRxiv 2019. 10.1101/807230 [DOI] [Google Scholar]
- 58. Ding L, Liu T, Wu Z et al Bone marrow CD11c+ cell‐derived amphiregulin promotes pulmonary fibrosis. J Immunol 2016; 197:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mehta H, Goulet PO, Nguyen V et al Topoisomerase I peptide‐loaded dendritic cells induce autoantibody response as well as skin and lung fibrosis. Autoimmunity 2016; 49:503–13. [DOI] [PubMed] [Google Scholar]
- 60. Kavian N, Marut W, Servettaz A et al Arsenic trioxide prevents murine sclerodermatous graft‐versus‐host disease. J Immunol 2012; 188:5142–9. [DOI] [PubMed] [Google Scholar]
- 61. Gerber EE, Gallo EM, Fontana SC et al Integrin‐modulating therapy prevents fibrosis and autoimmunity in mouse models of scleroderma. Nature 2013; 503:126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chia JJ, Zhu T, Chyou S et al Dendritic cells maintain dermal adipose‐derived stromal cells in skin fibrosis. J Clin Invest 2016; 126:4331–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Leveque‐El Mouttie L, Koyama M, Le Texier L et al Corruption of dendritic cell antigen presentation during acute GVHD leads to regulatory T‐cell failure and chronic GVHD. Blood 2016; 128:794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Delaney TA, Morehouse C, Brohawn PZ et al Type I IFNs regulate inflammation, vasculopathy, and fibrosis in chronic cutaneous graft‐versus‐host disease. J Immunol 2016; 197:42–50. [DOI] [PubMed] [Google Scholar]
- 65. Ponsoye M, Frantz C, Ruzehaji N et al Treatment with abatacept prevents experimental dermal fibrosis and induces regression of established inflammation‐driven fibrosis. Ann Rheum Dis 2016; 75:2142–9. [DOI] [PubMed] [Google Scholar]
- 66. Yamamoto T, Takagawa S, Katayama I et al Animal model of sclerotic skin. I: Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J Invest Dermatol 1999; 112:456–62. [DOI] [PubMed] [Google Scholar]
- 67. Affandi AJ, Carvalheiro T, Ottria A et al Low RUNX3 expression alters dendritic cell function in patients with systemic sclerosis and contributes to enhanced fibrosis. Ann Rheum Dis 2019; 78:1249–59. [DOI] [PubMed] [Google Scholar]
- 68. van Laar JM, Farge D, Sont JK et al Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA 2014; 311:2490–8. [DOI] [PubMed] [Google Scholar]
- 69. Sullivan KM, Goldmuntz EA, Keyes‐Elstein L et al Myeloablative autologous stem‐cell transplantation for severe scleroderma. N Engl J Med 2018; 378:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ng SA, Sullivan KM. Application of stem cell transplantation in autoimmune diseases. Curr Opin Hematol 2019; 26:392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kowal‐Bielecka O, Fransen J, Avouac J et al Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 2017; 76:1327–39. [DOI] [PubMed] [Google Scholar]
- 72. Sullivan KM, Majhail NS, Bredeson C et al Systemic sclerosis as an indication for autologous hematopoietic cell transplantation: position statement from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2018; 24:1961–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Peltzer J, Aletti M, Frescaline N, Busson E, Lataillade JJ, Martinaud C. Mesenchymal stromal cells based therapy in systemic sclerosis: rational and challenges. Front Immunol 2018; 9:2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mosanya CH, Isaacs JD. Tolerising cellular therapies: what is their promise for autoimmune disease? Ann Rheum Dis 2019; 78:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bell GM, Anderson AE, Diboll J et al Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann Rheum Dis 2017; 76:227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Khanna D, Spino C, Johnson S et al Abatacept in early diffuse cutaneous systemic sclerosis: results of a Phase II investigator‐initiated, multicenter, double‐blind, randomized, placebo‐controlled trial. Arthritis Rheumatol 2020; 72:125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Khanna D, Denton CP, Jahreis A et al Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a Phase 2, randomised, controlled trial. Lancet 2016; 387:2630–40. [DOI] [PubMed] [Google Scholar]
- 78. Khanna D, Denton CP, Lin CJF et al Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open‐label period of a Phase II randomised controlled trial (faSScinate). Ann Rheum Dis 2018; 77:212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kurasawa K, Hirose K, Sano H et al Increased interleukin‐17 production in patients with systemic sclerosis. Arthritis Rheum 2000; 43:2455–63. [DOI] [PubMed] [Google Scholar]
- 80. Baraut J, Michel L, Verrecchia F, Farge D. Relationship between cytokine profiles and clinical outcomes in patients with systemic sclerosis. Autoimmun Rev 2010; 10:65–73. [DOI] [PubMed] [Google Scholar]
- 81. McInnes IB, Kavanaugh A, Gottlieb AB et al Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the Phase 3, multicentre, double‐blind, placebo‐controlled PSUMMIT 1 trial. Lancet 2013; 382:780–9. [DOI] [PubMed] [Google Scholar]
- 82. Ghosh S, Gensler LS, Yang Z et al Ustekinumab safety in psoriasis, psoriatic arthritis, and Crohn’s disease: an integrated analysis of Phase II/III clinical development programs. Drug Saf 2019; 42:751–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Matsushita T, Date M, Kano M et al Blockade of p38 mitogen‐activated protein kinase inhibits murine sclerodermatous chronic graft‐versus‐host disease. Am J Pathol 2017; 187:841–50. [DOI] [PubMed] [Google Scholar]


